Abstract
BACKGROUND
Since 2010, Black persons in the United States have had a greater increase in opioid overdose–related mortality than other groups, but national-level evidence characterizing racial and ethnic disparities in the use of medications for opioid use disorder (OUD) is limited.
METHODS
We used Medicare claims data from the 2016–2019 period for a random 40% sample of fee-for-service beneficiaries who were Black, Hispanic, or White; were eligible for Medicare owing to disability; and had an index event related to OUD (nonfatal overdose treated in an emergency department or inpatient setting, hospitalization with injection drug use–related infection, or inpatient or residential rehabilitation or detoxification care). We measured the receipt of medications to treat OUD (buprenorphine, naltrexone, and naloxone), the receipt of high-risk medications (opioid analgesics and benzodiazepines), and health care utilization, all in the 180 days after the index event. We estimated differences in outcomes according to race and ethnic group with adjustment for beneficiary age, sex, index event, count of chronic coexisting conditions, and state of residence.
RESULTS
We identified 25,904 OUD-related index events among 23,370 beneficiaries, with 3937 events (15.2%) occurring among Black patients, 2105 (8.1%) among Hispanic patients, and 19,862 (76.7%) among White patients. In the 180 days after the index event, patients received buprenorphine after 12.7% of events among Black patients, after 18.7% of those among Hispanic patients, and after 23.3% of those among White patients; patients received naloxone after 14.4%, 20.7%, and 22.9%, respectively; and patients received benzodiazepines after 23.4%, 29.6%, and 37.1%, respectively. Racial differences in the receipt of medications to treat OUD did not change appreciably from 2016 to 2019 (buprenorphine receipt: after 9.1% of index events among Black patients vs. 21.6% of those among White patients in 2016, and after 14.1% vs. 25.5% in 2019). In all study groups, patients had multiple ambulatory visits in the 180 days after the index event (mean number of visits, 6.6 after events among Black patients, 6.7 after events among Hispanic patients, and 7.6 after events among White patients).
CONCLUSIONS
Racial and ethnic differences in the receipt of medications to treat OUD after an index event related to this disorder among patients with disability were substantial and did not change over time. The high incidence of ambulatory visits in all groups showed that disparities persisted despite frequent health care contact. (Funded by the National Institute on Drug Abuse and the National Institute on Aging.)
The crisis of opioid overdoses and mortality related to opioid use disorder (OUD) continues to accelerate in the United States. In 2021, an estimated 80,816 overdose deaths involved opioids, a 15% increase as compared with 2020.1 This tragic increase encompasses a rapid demographic shift in overdose deaths. Among Black persons in the United States, opioid overdose mortality increased by a factor of 7.7 from 2010 to 2020, a greater increase than in any other racial or ethnic group tracked by the Centers for Disease Control and Prevention.2,3 In the United States in 2020, annual rates of opioid-related overdose among Black persons exceeded those among White persons for the first time in more than 20 years. Among Hispanic persons, the overall annual rates of overdose have remained below those among White and Black persons since 1999, but rates in the Hispanic population increased by 40% between 2019 and 2021.2
To address this public health crisis, equitable access to medications for OUD, which substantially reduce rates of overdose and death, is vital.4–6 Evidence suggests that Black and other historically marginalized persons are less likely than White persons to receive medications for OUD, to live near a provider of such medications, or to continue OUD treatment.7–9 The roots of these disparities are multifactorial, including stigma, racial segregation of health care, discrepant incarceration rates, disproportionate enrollment in Medicaid,10 and increases in fentanyl use in urban areas, which tend to have larger Black and Hispanic populations.11
Filling the literature gaps on racial disparities in overdose and OUD treatment could inform policy and infrastructure investments in treatment for addiction. Few studies have examined a full range of medications for OUD (buprenorphine, naloxone, naltrexone, and methadone) or the receipt of opioid analgesics or benzodiazepines, drugs that are associated with high risks of overdose and death among persons with OUD.12,13 Many national studies on disparities regarding medications for OUD rely on federal survey data from outpatient offices or substance use treatment centers (which are limited to populations receiving treatment for substance use8,14,15) or use geographic proxies for race.16–18 To address these evidence gaps, we examined racial disparities in the receipt of medications for OUD and treatment retention among a national sample of Medicare beneficiaries with disability who had an acute event related to OUD, a situation in which access to such medications is an urgent priority.
METHODS
STUDY SAMPLE AND DATA SOURCES
We used Medicare claims from 2016 through 2019 for a random 40% sample of U.S.-residing beneficiaries, 18 years of age or older, who were eligible for Medicare owing to disability and had an index event signaling active OUD symptoms warranting treatment (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Medicare beneficiaries with disability are disproportionately affected by addiction and opioid-related adverse events; estimates suggest that one in four opioid overdoses in the United States occurs in this population.19
We required fee-for-service Medicare enrollment in Parts A (inpatient), B (outpatient), and D (prescription) from 180 days before to 30 days after the index event and survival for 30 days after the index event in order to capture data regarding the receipt of medications for OUD. We followed eligible beneficiaries for 180 days after the index event or until death or disenrollment from Medicare Part A, B, or D. We excluded persons who had long-term prescriptions for opioids (defined as ≥90 days’ supply of opioid in the 180 days before the index event), because medications for OUD may not be clinically appropriate for such persons. Because controlled-substance restrictions are not designed to curb end-of-life prescriptions, we excluded patients with end-stage renal disease and those receiving hospice care. This study was approved by the institutional review board at Dartmouth College.
OUD-RELATED INDEX EVENTS
The study sample included all the beneficiaries who met the inclusion criteria above. Index events were defined as follows: a nonfatal opioid overdose treated in the emergency department or inpatient setting, hospitalization with an injection drug use–related infection (e.g., acute hepatitis C, phlebitis, septic arthritis, skin or soft-tissue infection, or endocarditis combined with an OUD diagnosis in the previous 30 days), or inpatient or residential rehabilitation or detoxification care with a primary diagnosis of OUD or receipt of an OUD diagnosis in the previous 30 days.20,21 A patient could contribute more than one event if the subsequent event occurred 12 or more months after the date of the previous event.
STUDY OUTCOMES
The primary outcomes were receipt (i.e., record of a prescription being filled at a pharmacy) of the following drugs in the 180 days after the index event: buprenorphine, naltrexone, naloxone, and high-risk medications (opioid analgesics and benzodiazepines) (Table S2). We captured data on the days’ supply for filled prescriptions of buprenorphine and naltrexone using the Part D “days supply” variable, which defines the intended duration of treatment. Total days’ supply was calculated as the sum of days with any prescription coverage (e.g., two prescriptions for 15 days’ supply that were dispensed on the same day would count as 15 days, not 30 days). Among buprenorphine recipients, we identified those with 150 or more days’ supply in the 180 days after an index event as a measure of treatment retention.22,23
Although methadone is an important type of medication for OUD, we could not capture data on methadone receipt from federally licensed outpatient treatment programs for OUD in the main analysis because our claims data precede the 2020 Medicare coverage expansion to this service.24 In a sensitivity analysis, we used a separate sample of Medicare claims from 2020 through 2021 to assess methadone receipt in conjunction with buprenorphine receipt to estimate bias from unobserved methadone receipt in our main analysis (Table S3). This analysis was not included in our main results because little is known about the accuracy and sensitivity of methadone claims in Medicare given the recent policy change and because of potential confounding effects of the coronavirus disease 2019 pandemic on access to medication for OUD.
We also examined health care use and health outcomes. To assess differences in frequency of contact with medical providers (opportunities for prescription of medications for OUD), we measured the proportion of beneficiaries with one or more claim for evaluation and management (i.e., ambulatory visit) from any provider (including community mental health centers, rural health centers, and federally qualified health centers) in the 180 days after the index event and the mean number of such visits per index event. We measured visits overall and separately for primary care physicians, mental health clinicians, and any clinician recording a primary diagnosis of OUD (Table S4). We similarly captured data on emergency department visits, inpatient admissions, and opioid overdose events (using the index-event definitions).
BENEFICIARY CHARACTERISTICS
For the classifications of race and ethnic group, we used the Research Triangle Institute variable in the Medicare Beneficiary Summary File.25 As compared with beneficiary-reported race, this variable identifies Black beneficiaries with a sensitivity of 96.7% and specificity of 99.4% and Hispanic beneficiaries with a sensitivity of 90.8% and specificity of 98.7%.25 We excluded other racial groups because of misclassification risk and small sample sizes.
We collected data on beneficiary age, sex, state of residence, Medicaid eligibility, eligibility for the Part D low-income subsidy (a prescription cost-share subsidy program for low-income beneficiaries),26 and long-term care status (defined as a beneficiary having ≥50% of prescriptions in the 6 months before the index event filled by a long-term care pharmacy) (Table S5).27,28 Using the Chronic Conditions Data Warehouse algorithm, we identified a total count of 60 coexisting conditions.29 We also quantified patient use of medications for OUD, opioid analgesics, and benzodiazepines before the index event.
STATISTICAL ANALYSIS
The unit of analysis in the models was at the level of the OUD-related index event. We assessed each outcome across three racial and ethnic groups: non-Hispanic Black (Black), Hispanic of any race (Hispanic), and non-Hispanic White (White). We estimated all two-way combinations of groups (Black vs. White, Hispanic vs. White, and Hispanic vs. Black) for each outcome using a set of indicator variables for race and ethnic group in linear regression models (or logistic regression for binary outcomes) and controlling for beneficiary age, sex, chronic condition count (0, 1 or 2, 3 to 5, or ≥6), type of index event (overdose, hospitalization with injection drug use–related infection, or rehabilitation or detoxification), and state of residence. These main models were replicated in a separate analysis of data from the 2020–2021 period to model receipt of any methadone, any buprenorphine, or any methadone or buprenorphine in the 180 days after the index event (see the Supplement Methods section in the Supplementary Appendix).
To examine differences related to race and ethnic group that arise within states (i.e., among individual persons facing similar state policies) as compared with differences across states (i.e., among individual persons facing different state policies), we compared models that did and did not include state-of-residence indicators. Comparison of these sets of models can reveal the extent to which observed differences between racial and ethnic groups are potentially explained by different practices in states across all the groups as compared with differences within states according to race and ethnic group. In separate analyses, we stratified comparisons according to sex and to race and ethnic group. We plotted unadjusted quarterly rates of buprenorphine and naltrexone receipt among White beneficiaries as compared with Black beneficiaries over time at the index-event level for the second quarter of 2016 through the second quarter of 2019.
In addition, we repeated analyses among beneficiaries who had been attributed to providers participating in a Medicare Shared Savings Plan to explore the relationship between value-based payment models and disparities, and we ran a sensitivity analysis using the models described above and including fixed effects for individual Part D plans, for which enrollment may systematically vary according to race and ethnic group. Finally, we repeated main models using a cohort that did not exclude beneficiaries who died in the 30 days after the index event.
We performed analyses using SAS software, version 9.4 (SAS Institute), and Stata/MP software, version 17.0 (Stata). We computed Huber–White adjusted standard error estimates to account for the correlation of observations within states. We report results with point estimates and 95% confidence intervals, which are not adjusted for multiple comparisons and should not be interpreted as hypothesis tests. Additional details are provided in the Supplement Methods section.
RESULTS
CHARACTERISTICS OF THE BENEFICIARIES AND INDEX EVENTS
The study included 23,370 beneficiaries, of whom 3524 were Black, 1858 were Hispanic, and 17,988 were White; there were a total of 25,904 OUD-related index events, with 3937 events (15.2%) occurring among Black patients, 2105 (8.1%) among Hispanic patients, and 19,862 (76.7%) among White patients (Table 1 and Fig. S1). Black beneficiaries were more likely than White beneficiaries to be dually eligible for Medicaid or to have a Part D low-income subsidy. During the 6 months before the index event, Black patients were less likely than other patients to have received buprenorphine (before 9.2% of index events, vs. before 16.0% of those among Hispanic patients and 20.2% of those among White patients), naloxone (10.1%, vs. 16.6% and 19.0%, respectively), or benzodiazepine (25.3%, vs. 30.6% and 41.0%, respectively), as measured by a prescription fill.
Table 1.
Characteristics of OUD-Related Index Events in Medicare Beneficiaries, According to Race and Ethnic Group.*
| Characteristic | Overall (N = 25,904) | Black (N = 3937) | Hispanic (N =2105) | White (N = 19,862) |
|---|---|---|---|---|
| No. of unique beneficiarie† | 23,370 | 3524 | 1858 | 17,988 |
| Index event characteristics | ||||
| Type of OUD-related index event (%)‡ | ||||
| Opioid overdose | 34.1 | 37.1 | 30.5 | 33.9 |
| Inpatient rehabilitation or detoxification | 25.7 | 28.2 | 25.0 | 25.2 |
| Intravenous drug use-related infection with hospitalization | 43.5 | 37.4 | 49.1 | 44.1 |
| Beneficiary characteristics § | ||||
| Age at event (yr) | 50.5±12.4 | 53.4±11.4 | 49.0±11.8 | 50.0±12.5 |
| Female sex (%) | 46.7 | 42.0 | 37.2 | 48.6 |
| Low-income subsidy (%)¶ | 88.6 | 94.3 | 93.9 | 86.8 |
| Dually eligible for Medicaid (%) | 81.8 | 89.4 | 89.0 | 79.6 |
| Long-term care resident (%)|| | 4.3 | 3.9 | 3.8 | 4.5 |
| Coexisting conditions** | ||||
| No. of coexisting conditions | 9.1±5.2 | 9.4±5.5 | 8.7±5.4 | 9.1±5.2 |
| Hepatitis C (%) | 27.7 | 28.3 | 34.6 | 26.8 |
| HIV infection or AIDS (%) | 3.6 | 10.2 | 7.1 | 2.0 |
| Depression (%) | 58.7 | 51.0 | 56.3 | 60.4 |
| Fibromyalgia, chronic pain, or fatigue (%) | 58.5 | 52.7 | 48.6 | 60.6 |
| Schizophrenia or other psychosis (%) | 20.7 | 23.5 | 24.6 | 19.7 |
| Bipolar disorder (%) | 37.4 | 28.0 | 35.0 | 39.4 |
| Alcohol use disorder (%) | 31.1 | 33.8 | 32.7 | 30.4 |
| Drug use disorder (%) | 64.8 | 64.7 | 67.8 | 64.6 |
| OUD (%)†† | 58.1 | 54.8 | 62.0 | 58.3 |
| Cancer (%)‡‡ | 2.9 | 4.0 | 1.7 | 2.7 |
| Prescription fill for medications for OUD within 6 mo before index event (%) | ||||
| Buprenorphine | 18.2 | 9.2 | 16.0 | 20.2 |
| Naloxone | 17.4 | 10.1 | 16.6 | 19.0 |
| Naltrexone | 1.6 | 1.3 | 1.5 | 1.7 |
| Benzodiazepine | 37.7 | 25.3 | 30.6 | 41.0 |
Plus-minus values are means ±SD. The characteristics listed are those of Medicare beneficiaries with an opioid use disorder (OUD)-related index event and are summarized on the basis of index events. Race and ethnic group were determined on the basis of the Research Triangle Institute variable in the Medicare Beneficiary Summary File. Groups were defined as non-Hispanic Black (Black), Hispanic of any race (Hispanic), and non-Hispanic White (White). AIDS denotes acquired immunodeficiency syndrome, and HIV human immunodeficiency virus.
One beneficiary may have contributed more than one index event if 12 months had elapsed since the date of the previous index event.
Index events were defined as a nonfatal opioid overdose treated in the emergency department or inpatient setting, hospitalization with intravenous drug use-related infection (e.g., acute hepatitis C, phlebitis, septic arthritis, or endocarditis, combined with an OUD diagnosis in the previous 30 days), or an inpatient or residential rehabilitation or detoxification stay with a primary diagnosis of OUD. Percentages in this section total more than 100% because a small number of patients had more than one type of event during their index encounter. For example, a patient who had been hospitalized with diagnoses of opioid overdose and an intravenous drug use-related infection would have contributed a single index event but would have had two types of OUD-related index events.
Beneficiary characteristics are reported at the level of the index event, such that the same individual beneficiary may have contributed more than once to the mean or the percentage shown.
Low-income subsidy was defined as the Medicare Part D low-income subsidy, a signal of poverty, which was dichotomized in this study as any or none.
Long-term care resident status was assigned to beneficiaries who had at least 50% of their prescription fills in the 6 months before the index event dispensed from a pharmacy at a long-term care facility.
For the total count, data on the presence of 60 conditions were gathered from the Chronic Condition Data Warehouse, which uses claims since 1999 to describe Medicare beneficiaries’ accumulated chronic disease burden. Ten of the most relevant conditions for OUD are shown here, with the remaining conditions listed in Table S6. Coexisting conditions were defined as any condition that was present by the end of the calendar year before the index event.
Because OUD diagnoses are included in the Chronic Condition Data Warehouse flag for drug use disorder, this flag was not used in the count of coexisting conditions.
The “cancer” designation reflects whether a patient had a flag for breast, colorectal, endometrial, lung, or prostate cancer in the Chronic Condition Data Warehouse file. This flag was not used in the count of coexisting conditions.
RECEIPT OF MEDICATIONS FOR OUD ACCORDING TO RACE AND ETHNIC GROUP
During the 180 days after the index event, Black patients were less likely to receive any buprenorphine (after 12.7% of index events) than Hispanic patients (after 18.7% of index events) or White patients (after 23.3% of index events) (Table 2). In an adjusted analysis, lower percentages of index events among Black and Hispanic patients than of those among White patients were followed by receipt of any buprenorphine (adjusted difference for Black vs. White, −8.7 percentage points; 95% confidence interval [CI] −11.3 to −6.0; adjusted difference for Hispanic vs. White, −4.2 percentage points; 95% CI, −6.7 to −1.8). Black recipients of buprenorphine received a lower days’ supply than White patients (adjusted difference, −23.4 days; 95% CI, −32.5 to −14.2), and a lower percentage of Black recipients of buprenorphine than of White recipients had treatment retention lasting at least 150 days (30.1% vs. 44.8%; adjusted difference, −14.0 percentage points; 95% CI, −20.3 to −7.8).
Table 2.
Receipt of Medications after OUD-Related Event, According to Race and Ethnic Group.*
| Variable | Black (N = 3937) | Hispanic (N =2105) | White (N = 19,862) | Adjusted Difference (95% CI) | ||
|---|---|---|---|---|---|---|
| Black vs. White | Hispanic vs. White | Hispanic vs. Black | ||||
| Buprenorphine | ||||||
| Any receipt in 180 days — no. (%) | 501 (12.7) | 393 (18.7) | 4627 (23.3) | −8.7 (−11.3 to −6.0) | −4.2 (−6.7 to −1.8) | 4.4 (2.0 to 6.9) |
| Total days’ supply received within 180 days, among recipients | 94.1±68.4 | 111.7±66.9 | 118.1±66.0 | −23.4 (−32.5 to −14.2) | −4.0 (−11.3 to 3.3) | 19.4 (8.1 to 30.7) |
| Treatment retention — no./total no. (%)† | 151/501 (30.1) | 160/393 (40.7) | 2073/4627 (44.8) | −14.0 (−20.3 to −7.8) | −1.9 (−7.3 to 3.6) | 12.2 (5.8 to 18.6) |
| Naloxone | ||||||
| Any receipt in 180 days — no. (%) | 568 (14.4) | 435 (20.7) | 4546 (22.9) | −6.7 (−9.5 to −3.7) | −2.3 (−5.1 to 0.5) | 4.3 (1.5 to 7.1) |
| Naltrexone | ||||||
| Any receipt in 180 days — no. (%) | 110 (2.8) | 70 (3.3) | 664 (3.3) | −0.1 (−0.7 to 0.6) | 0.1 (−0.9 to 1.1) | 0.2 (−0.7 to 1.0) |
| Total days’ supply received within 180 days, among recipients | 57.1±49.7 | 45.9±42.9 | 55.9±48.3 | −1.4 (−18.7 to 15.9) | −7 (−16.7 to 2.7) | −5.6 (−24.7 to 13.6) |
| Opioid analgesic | ||||||
| Any receipt in 180 days — no. (%) | 921 (23.4) | 474 (22.5) | 4656 (23.4) | −0.8 (−2.7 to 1.2) | 0.1 (−2.1 to 2.3) | 0.9 (−1.8 to 3.6) |
| Benzodiazepine | ||||||
| Any receipt in 180 days — no. (%) | 921 (23.4) | 623 (29.6) | 7359 (37.1) | −14.1 (−16.7 to −11.6) | −6.7 (−8.4 to −5.1) | 7.4 (4.5 to 10.3) |
The unit of analysis was the index event. Unadjusted outcomes should be interpreted as the occurrence or mean value of each outcome in the 180 days after an index event. Columns show adjusted marginal differences for each possible combination of the three racial and ethnic groups. Differences between percentages are shown in percentage points. We estimated adjusted differences for all two-way combinations of groups (Black vs. White, Hispanic vs. White, and Hispanic vs. Black) for each outcome. Models included a set of indicator variables for race, sex, an interaction term for race by sex, age, chronic condition count (categorized as 0, 1 or 2, 3 to 5, or ≥6), and state of residence. Reported 95% confidence intervals reflect Huber—White adjusted standard errors to account for the correlation of observations within state of residence. Index event types that were included in the models were a nonfatal opioid overdose treated in the emergency department or inpatient setting, hospitalization with an injection drug use-related infection, and inpatient or residential rehabilitation or detoxification stay with a primary diagnosis of OUD.
Treatment retention was defined as the receipt of at least 150 days’ supply of buprenorphine in the 180 days after an index event with any buprenorphine fill.
The results regarding naloxone receipt were similarly disparate: 14.4% among Black patients, as compared with 20.7% among Hispanic patients and 22.9% among White patients. Naltrexone fills were uncommon; fill rates and days’ supply did not differ significantly across the Black, Hispanic, and White groups. Racial and ethnic differences in the receipt of medications for OUD were similar within subpopulations defined according to sex (Table S7).
Most fills of medications for OUD after the index event occurred among patients who had received such medications in the 6 months before the index event: 78.3% of the index events were followed by buprenorphine receipt and 74.3% were followed by naloxone receipt (Table S8). Among patients who had not had fills of medications for OUD before the index event, the differences according to race and ethnic group were smaller in absolute terms. For example, among patients’ index events with no observed preceding receipt of medication for OUD, 6.7% of index events among Black patients and 9.2% of those among White patients were followed by any buprenorphine receipt in the 180 days after the index event.
Adjusted percents of index events followed by receipt of medications for OUD in analyses according to race differed little in models that did and did not control for state-of-residence fixed effects (Table S9), a finding that suggests that disparities represent race differences within states rather than differences across states. Racial differences in the receipt of medications to treat OUD did not change appreciably from 2016 to 2019 (buprenorphine receipt within 180 days after the index event: after 9.1% of events among Black patients vs. 21.6% of events among White patients in the second quarter of 2016, and 14.1% vs. 25.5% in the second quarter of 2019) (Fig. 1 and Table S10).
Figure 1. Quarterly Trends in Buprenorphine and Naloxone Receipt in the 180 Days after an Opioid Use Disorder–Related Event.
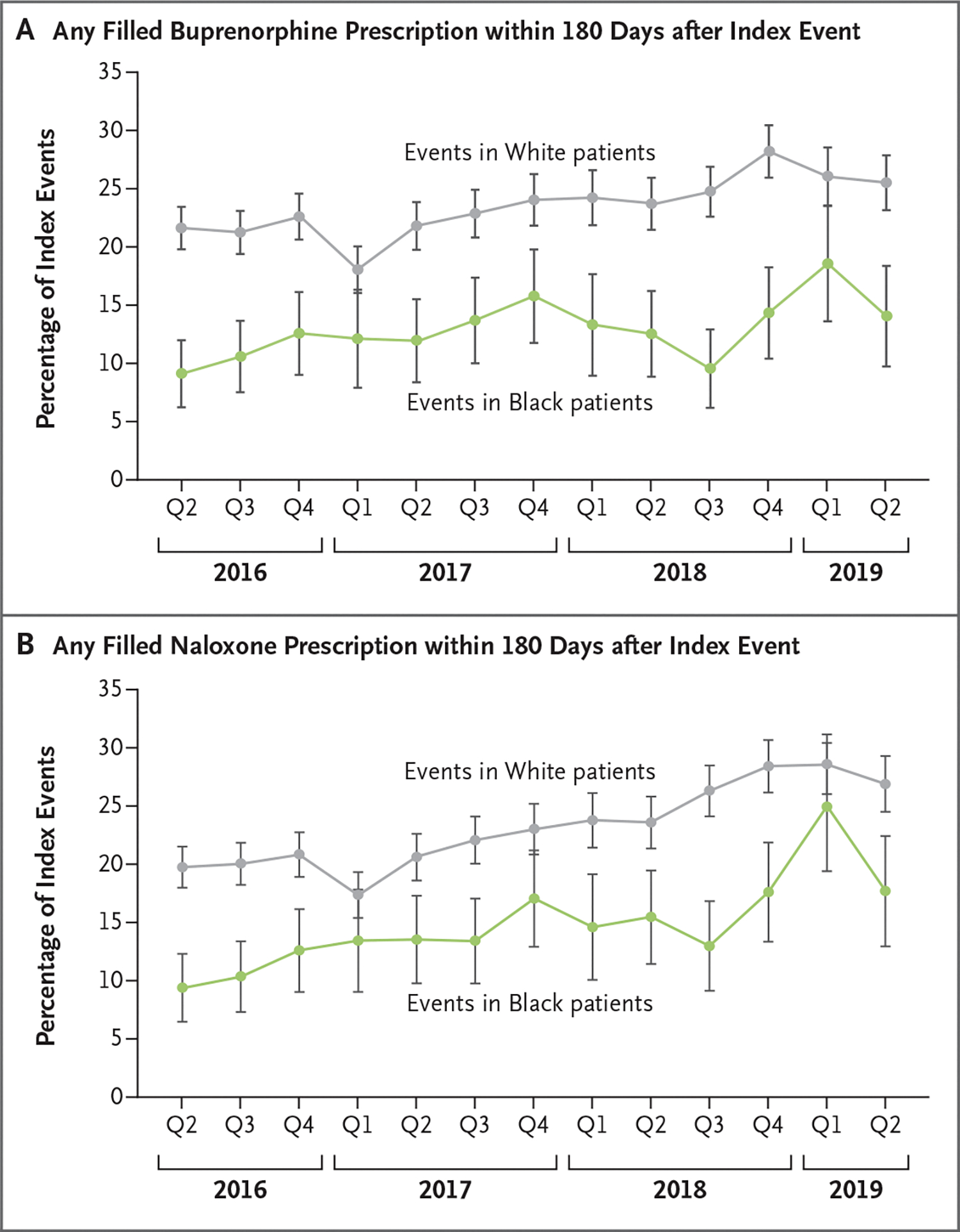
Panel A shows the percentage of opioid use disorder–related index events that were followed by receipt of any filled prescription for buprenorphine within 180 days, according to quarter (Q) from the second quarter of 2016 through the second quarter of 2019 and according to race of the beneficiary. Panel B shows the same for filled prescriptions for naloxone. I bars indicate 95% confidence intervals, under an assumption of a normal approximation.
RECEIPT OF HIGH-RISK MEDICATIONS ACCORDING TO RACE AND ETHNIC GROUP
In the 180 days after the index event, the incidence of opioid analgesic receipt did not differ across the study groups (approximately 23% in all three groups) (Table 2). However, the incidence of receipt of benzodiazepines did differ (after 23.4% of index events among Black patients vs. 29.6% of those among Hispanic patients and 37.1% of those among White patients). Differences persisted after adjustment for state-of-residence fixed effects. In an unadjusted analysis, 32.0% of index events among Black female patients were followed by a benzodiazepine prescription fill, as compared with 43.0% of those among White female patients and 28.4% of those among all male patients.
HEALTH CARE UTILIZATION AND HEALTH OUTCOMES
We measured health care utilization and outcomes in the 180 days after the index event. In this time frame, most index events were followed by at least one ambulatory visit with any clinician (86.9%) or an ambulatory visit with a primary care clinician (76.3%) (Table 3 and Table S11). Black and Hispanic patients’ index events were followed by fewer ambulatory visits than White patients’ index events (mean number of visits, 6.6, 6.7, and 7.6, respectively; adjusted difference for Black vs. White, −0.9 visits; 95% CI, −1.3 to −0.6; adjusted difference for Hispanic vs. White, −0.6 visits; 95% CI, −0.9 to −0.3).
Table 3.
Health Care Utilization and Adverse Outcomes after an OUD-Related Event, According to Race and Ethnic Group.*
| Variable | Black (N = 3937) | Hispanic (N =2105) | White (N = 19,862) | Adjusted Difference (95% CI) | ||
|---|---|---|---|---|---|---|
| Black vs. White | Hispanic vs. White | Hispanic vs. Black | ||||
| All ambulatory visits | ||||||
| ≥1 Visit in 180 days — no. (%) | 3296 (83.7) | 1745 (82.9) | 17,460 (87.9) | −4.9 (−6.9 to −2.9) | −2.6 (−4.4 to −0.8) | 2.3 (−0.2 to 4.8) |
| No. of visits | 6.6±6.7 | 6.7±7.3 | 7.6±7.2 | −0.9 (−1.3 to −0.6) | −0.6 (−0.9 to −0.3) | 0.3 (−0.1 to 0.8) |
| Emergency department visit | ||||||
| ≥1 Visit in 180 days — no. (%) | 2771 (70.4) | 1437 (68.3) | 13,342 (67.2) | 4.9 (3.5 to 6.3) | 0.6 (−1.6 to 2.9) | −4.3 (−6.5 to −2.1) |
| No. of visits | 3.2±6.8 | 2.6±5.0 | 2.4±4.1 | 0.9 (0.5 to 1.2) | 0.02 (−0.2 to 0.2) | −0.8 (−1.2 to −0.4) |
| Hospitalization | ||||||
| ≥1 Hospitalization in 180 days — no. (%) | 1903 (48.3) | 949 (45.1) | 9,033 (45.5) | 3.3 (1.1 to 5.6) | −1.0 (−3.0 to 1.0) | −4.3 (−7.2 to −1.4) |
| No. of visits | 1.2±1.9 | 1.0±1.6 | 0.9±1.5 | 0.2 (0.1 to 0.4) | −0.01 (−0.1 to 0.1) | −0.3 (−0.3 to −0.2) |
| Overdose event | ||||||
| ≥1 Event in 180 days — no. (%) | 291 (7.4) | 153 (7.3) | 1,288 (6.5) | 0.6 (−0.7 to 1.8) | 0.3 (−0.7 to 1.3) | −0.3 (−1.7 to 1.1) |
| No. of events | 0.1±0.4 | 0.1±0.4 | 0.1±0.3 | 0.01 (−0.01 to 0.03) | 0.01 (−0.01 to 0.02) | 0.0 (−0.02 to 0.02) |
The unit of analysis was the index event. Unadjusted outcomes should be interpreted as the occurrence or mean value of each outcome in the 180 days after an index event. Columns show adjusted marginal differences for each possible combination of race and ethnic group. Differences between percentages are in percentage points. We estimated adjusted differences for all two-way combinations of groups (Black vs. White, Hispanic vs. White, and Hispanic vs. Black) for each outcome. Models included a set of indicator variables for race, sex, an interaction term for race by sex, age, chronic condition count out of 60 conditions, and state of residence. Reported 95% confidence intervals reflect Huber-White adjusted standard errors to account for the correlation of observations within state of residence. Index event types that were included in the models were a nonfatal opioid overdose treated in the emergency department or inpatient setting, hospitalization with an injection drug use-related infection, and inpatient or residential rehabilitation or detoxification stay with a primary diagnosis of OUD.
During the 180 days after the index event, Black patients had more emergency department visits than White patients (mean [±SD], 3.2±6.8 vs. 2.4±4.1) and more hospitalizations (mean, 1.2±1.9 vs. 0.9±1.5) (Table 3). During the 180-day window, the overall incidence of overdose was 6.7%; in an adjusted analysis, there were no apparent differences according to race or ethnic group.
ADDITIONAL ANALYSES
Among patients who were attributed to providers in a Medicare Shared Savings Plan (Table S12), disparities in buprenorphine and naloxone receipt were nearly identical to those among patients in the full study cohort. In models that included patients’ Part D drug plan, the differences in the percentages of index events followed by medication receipt were essentially identical to the main model outcomes (Table S13).
Death in the 180 days after the index event was observed after 4.9% of the index events in Black patients, after 3.8% of those in Hispanic patients, and after 4.5% of those in White patients (Table S14). Output from models including patients who died in the first 30 days after the index event did not differ from the main model output (Table S15).
In the 2020–2021 data analyses, Black patients received methadone after 8.3% of the index events, Hispanic patients after 11.2%, and White patients after 8.6%; after the index events, either methadone or buprenorphine was received after 23.6% of index events among Black patients, after 29.1% of those among Hispanic patients, and after 32.4% of those among White patients (Table S16). In an adjusted analysis, Black patients received either buprenorphine or methadone in the 180 days after an index event less frequently than White patients (difference in percentage of index events, −5.1 percentage points; 95% CI, −8.5 to −1.8); the results were similar in the analysis that compared Hispanic patients with White patients (difference in percentage of index events, −4.8 percentage points; 95% CI, −8.2 to −1.4).
DISCUSSION
In this national study of Medicare beneficiaries with disability and active OUD symptoms, we observed substantial racial and ethnic disparities in the receipt of medications for OUD, particularly among Black beneficiaries. The disparities were not explained by state of residence or observable differences in beneficiary age, sex, or burden of chronic conditions across groups. Sensitivity analyses regarding data from the 2020–2021 period, when methadone was covered by Medicare, showed similar absolute differences in the incidences of receipt of medications for OUD owing to similar levels of methadone receipt among Black and White patients.17 These results show that racial and ethnic disparities in the receipt of medications for OUD were large and persistent, but use rates were low overall, including among White patients, who had only had a buprenorphine prescription filled after one in five index events. These disparities are unlikely to close without the structural barriers and structural racism obstructing equitable access to medications for OUD, such as stigma and geographic maldistribution of relevant providers, being addressed.
We observed disparities among racial and ethnic groups despite the fact that all the patients in the study had substantial contact with care providers for ambulatory visits. Black and Hispanic patients had a mean 6.6 and 6.7 ambulatory visits, respectively, in the 180 days after an index event, more than 1 visit per month. Although this frequency was lower than that among White patients, the qualitative difference is small as compared with disparities in the receipt of medications for OUD. This finding challenges the hypothesis that limited care access is a key driver of racial disparities in the receipt of medications for OUD in this population. This hypothesis is further contradicted by the substantially lower incidences of buprenorphine treatment retention and lower days’ supply among Black patients as compared with those among White patients.
These results suggest that to reduce disparities in the receipt of medications for OUD, access to providers and frequency of contact are less important mechanisms to target than racism, stigma (both internalized and external to patients),30 and other structural factors among providers primarily caring for underserved populations. For example, at the patient level, interviews of patients with OUD showed that Black and Hispanic patients were less likely than White patients to trust the safety and effectiveness of medications for OUD.31 This finding is consistent with other research on trust among non-White patients.32–34 In addition, Black and Hispanic patients may be less likely than White patients to believe that treatment with medications for OUD is warranted.35 At the provider level, disparities could emerge from low availability of buprenorphine-prescribing primary care providers,36 bias against the use of medications for OUD for Black or Hispanic patients, or lack of comfort or confidence in treating OUD among providers of care for underserved populations.37 Each of these patient- and provider-level challenges is likely to require community-specific interventions.
The observed disparities in the receipt of medications for OUD after an index event largely reflected patterns of use before the index event. This finding suggests that index events — indicators of active OUD symptoms — provide little boost to the prescribing of medications for OUD or in the closing of disparities. The limited effect of an index event on the prescription of medications for OUD was observed even for naloxone, although this treatment has few regulatory restrictions and is indicated for anyone at high risk for opioid overdose. The low use of medications for OUD among these high-risk patients stands in contrast to their use in other settings, such as the Veterans Health Administration system, in which use is much higher than in the general population.38 We found that the incidence of opioid overdose did not differ across groups despite differences in receipt of medications for OUD. This finding could reflect a lack of statistical power to detect a difference, differences in the real-world effectiveness of medications for OUD across populations, or unmeasured confounding. Regardless, our findings suggest that improvements in access to medications for OUD to achieve equitable outcomes will necessitate ensuring that providers have the right tools and training to facilitate the initiation of such treatment and referral to continuity care.
We also observed high use of opioid analgesics and benzodiazepines in the study population. These drugs are associated with substantial risk among patients with OUD39 and were received in the short timeframe after an OUD-related event. This finding highlights additional concerns about OUD treatment in the United States and emphasizes the potential value of a robust system in which patients with OUD could access mental health and pain specialists so that their needs could be addressed safely and adequately.
This study has several limitations. First, our study of Medicare fee-for-service beneficiaries with disability may not be generalizable to other groups, including Medicare Advantage enrollees. Beneficiaries with disability represent a disproportionate share of persons with opioid-related overdose in the United States.19 Therefore, the 9.1 million Medicare beneficiaries with disability should be a priority for improved addiction treatment. Second, we could not observe receipt of methadone for OUD in the main study period (2016–2019), an important confounder for buprenorphine receipt and potentially for other related services. Analysis of data from the 2020–2021 period that included methadone use showed qualitatively similar disparities. Third, the likelihood of a written prescription being filled may differ according to race or ethnic group. We cannot account for this potential bias because claims data reflect prescriptions that are filled, not prescriptions that are written. Fourth, diagnostic coding of OUD may vary according to racial or ethnic group; the effect of such difference would depend on the direction of bias. Finally, we studied OUD treatment around OUD-related events. Our results may not reflect OUD treatment in stable patients, but OUD-related events indicate important opportunities to intervene.40
In this study, we found large racial and ethnic disparities in the receipt of medications for OUD among Medicare beneficiaries with disability, especially when we compared data regarding White and Black patients. The disconnect between treatment, increasing rates of overdose, and shifting demographic characteristics highlights broad failures and persistent structural racism within our health care delivery system. The prioritization of equitable and sufficient OUD treatment is essential in order to successfully address the growing overdose problem in the United States and to stem its immeasurable societal cost.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute on Drug Abuse (R01 DA049757, to Drs. Meara, Lewinson, and Morden and Ms. Hardy; R01 DA048533, to Drs. Huskamp and Mehrotra; and P30 DA035772, to Dr. Huskamp) and the National Institute on Aging (K23 AG058806-01, to Dr. Barnett).
Footnotes
The views expressed in this article are those of the authors. The National Institutes on Aging and Drug Abuse had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. UnitedHealthcare had no role in the production of this research or the manuscript.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Michael L. Barnett, Department of Health Policy and Management, Harvard T.H. Chan School of Public Health, Boston, Massachusetts Division of General Internal Medicine and Primary Care, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts.
Ellen Meara, Department of Health Policy and Management, Harvard T.H. Chan School of Public Health, Boston, Massachusetts National Bureau of Economic Research, Cambridge, Boston, Massachusetts.
Terri Lewinson, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH
Brianna Hardy, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH
Deanna Chyn, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH
Moraa Onsando, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH
Haiden A. Huskamp, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts
Ateev Mehrotra, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts Division of General Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Nancy E. Morden, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH UnitedHealthcare, Minnetonka, MN.
REFERENCES
- 1.Centers for Disease Control and Prevention. U.S. overdose deaths in 2021 increased half as much as in 2020 — but are still up 15%. May 11, 2022. (https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm).
- 2.Friedman JR, Hansen H. Evaluation of increases in drug overdose mortality rates in the us by race and ethnicity before and during the COVID-19 pandemic. JAMA Psychiatry 2022;79:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KFF. Opioid overdose deaths by race/ethnicity. May 9, 2022. (https://www.kff.org/other/state-indicator/opioid-overdose-deaths-by-raceethnicity/).
- 4.Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry 2019;24:1868–83. [DOI] [PubMed] [Google Scholar]
- 5.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-Year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 2003;361:662–8. [DOI] [PubMed] [Google Scholar]
- 7.Andraka-Christou B Addressing racial and ethnic disparities in the use of medications for opioid use disorder. Health Aff (Millwood) 2021;40:920–7. [DOI] [PubMed] [Google Scholar]
- 8.Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry 2019;76:979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunphy CC, Zhang K, Xu L, Guy GP Jr. Racial-ethnic disparities of buprenorphine and vivitrol receipt in Medicaid. Am J Prev Med 2022;63:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saloner B, Levin J, Chang H-Y, Jones C, Alexander GC. Changes in buprenorphine-naloxone and opioid pain reliever prescriptions after the Affordable Care Act Medicaid expansion. JAMA Netw Open 2018;1(4):e181588–e181588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration. The opioid crisis and the Black/African American population: an urgent issue. April 2020. (https://store.samhsa.gov/product/The-Opioid-Crisis-and-the-Black-African-American-Population-An-Urgent-Issue/PEP20-05-02-001). [Google Scholar]
- 12.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med 2016;164:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NL, Choi S, Herrera C-N. Black clients in expansion states who used opioids were more likely to access medication for opioid use disorder after ACA implementation. J Subst Abuse Treat 2022;133:108533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahler GJ, Mennis J, Baron DA. Racial/ethnic disparities in the use of medications for opioid use disorder (MOUD) and their effects on residential drug treatment outcomes in the US. Drug Alcohol Depend 2021;226:108849. [DOI] [PubMed] [Google Scholar]
- 16.Stein BD, Dick AW, Sorbero M, et al. A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Subst Abus 2018;39:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open 2020;3(4):e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings JR, Wen H, Ko M, Druss BG. Race/ethnicity and geographic access to Medicaid substance use disorder treatment facilities in the United States. JAMA Psychiatry 2014;71:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med 2016;375:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open 2020;3(2):e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larochelle MR, Wakeman SE, Ameli O, et al. Relative cost differences of initial treatment strategies for newly diagnosed opioid use disorder: a cohort study. Med Care 2020;58:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams AR, Nunes EV, Bisaga A, et al. Developing an opioid use disorder treatment cascade: a review of quality measures. J Subst Abuse Treat 2018;91:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams AR, Samples H, Crystal S, Olfson M. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry 2020;177:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis CS. The SUPPORT for Patients and Communities Act — what will it mean for the opioid-overdose crisis? N Engl J Med 2019;380:3–5. [DOI] [PubMed] [Google Scholar]
- 25.Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of race and ethnicity codes in medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care 2020;58(1):e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoemaker JS, Davidoff AJ, Stuart B, Zuckerman IH, Onukwugha E, Powers C. Eligibility and take-up of the Medicare Part D low-income subsidy. Inquiry 2012;49:214–30. [DOI] [PubMed] [Google Scholar]
- 27.Research Data Assistance Center. Pharmacy service type code (https://resdac.org/cms-data/variables/pharmacy-service-type-code).
- 28.Morden NE, Chyn D, Wood A, Meara E. Racial inequality in prescription opioid receipt — role of individual health systems. N Engl J Med 2021;385:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Center for Medicare and Medicaid Services. Welcome to the Chronic Conditions Data Warehouse. 2014. (https://www.ccwdata.org/).
- 30.Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husain JM, Cromartie D, Fitzelle-Jones E, Brochier A, Borba CPC, Montalvo C. A qualitative analysis of barriers to opioid agonist treatment for racial/ethnic minoritized populations. J Subst Abuse Treat 2023;144:108918. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar K, Thomas SB, Musa D, Almario D, Garza MA. Racial differences in parents’ distrust of medicine and research. Arch Pediatr Adolesc Med 2009;163:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med 2008;23:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ 2018;133:407–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinedo M, Villatoro AP. The role of perceived treatment need in explaining racial/ethnic disparities in the use of substance abuse treatment services. J Subst Abuse Treat 2020;118:108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beetham T, Saloner B, Wakeman SE, Gaye M, Barnett ML. Access to office-based buprenorphine treatment in areas with high rates of opioid-related mortality: an audit study. Ann Intern Med 2019;171:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrilla CHA, Coulthard C, Larson EH. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med 2017;15:359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finlay AK, Harris AHS, Timko C, et al. Disparities in access to medications for opioid use disorder in the Veterans Health Administration. J Addict Med 2021;15:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroll DS, Nieva HR, Barsky AJ, Linder JA. Benzodiazepines are prescribed more frequently to patients already at risk for benzodiazepine-related adverse events in primary care. J Gen Intern Med 2016;31:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larochelle MR, Bernstein R, Bernson D, et al. Touchpoints — opportunities to predict and prevent opioid overdose: a cohort study. Drug Alcohol Depend 2019;204:107537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


