Abstract
Fragmented investigation has masked the overall picture for causes of cardiovascular disease (CVD). Among the risk factors for CVD, high blood pressure (BP) is associated with the strongest evidence for causation and it has a high prevalence of exposure. Biologically normal levels of BP are considerably lower than what has typically been characterized as normal in research and clinical practice. We propose that CVD is primarily caused by a right-sided shift in the population distribution of BP. Our view that BP is the predominant risk factor for CVD is based on conceptual postulates that have been tested in observational investigations and clinical trials. Large cohort studies have demonstrated that high BP is an important risk factor for heart failure, atrial fibrillation, chronic kidney disease, heart valve diseases, aortic syndromes and dementia, in addition to coronary heart disease and stroke. In multivariate modelling, the presumed attributable risk of high BP for stroke and coronary heart disease has increased steadily with progressive use of lower values for normal BP. Meta-analysis of BP-lowering randomized controlled trials has demonstrated a benefit which is almost identical to that predicted from BP risk relationships in cohort studies. Prevention of age-related increases in BP would in large part reduce the vascular consequences usually attributed to aging, and together with intensive treatment of established hypertension would eliminate a large proportion of the population burden of BP-related CVD.
Keywords: blood pressure, cardiovascular disease causation, randomized controlled trials, cartesian evidence, attributable risk
Proposed causation of cardiovascular disease
High blood pressure (BP), cigarette smoking, diabetes mellitus, and lipid abnormalities are major modifiable risk factors for cardiovascular disease (CVD). Among these, high BP is associated with the strongest evidence for causation and has a high prevalence of exposure. However, there is considerable evidence that a biologically normal level of BP in humans is considerably lower than what has been traditionally employed in clinical practice and research, leading to an underrepresentation of the role that BP plays as a risk factor for CVD. We propose the following integrated theory for CVD causation that is supported by a robust body of coherent and consistent evidence:
“Cardiovascular disease in humans is primarily caused by a right-sided shift in the distribution of BP.”
Theories abound in the current era of social networks, but few fulfill the basic requirements for causality. Scientific theories are most credible because they are structured and subject to refutation by systematic observation and experimental hypothesis testing (1). Our theory fulfills virtually all of the criteria for causality proposed by Bradford Hill (2).
SHIFT OF BP DISTRIBUTION IN HUMANS: UNCOVERING A SELECTION BIAS
At the end of the 19th century, Osler did not mention the risks of high BP in his classic “The Principles and Practice of Medicine” textbook because at that time there was no practical way to measure BP using a non-invasive technique (3). Shortly after the development (4,5) and dissemination of noninvasive methods for BP measurement physicians and actuaries deduced that high BP could be a cause of disease, especially CVD events (6,7). In 1913, Janeway’s study of 7872 patients led him to conclude that an average BP above 160 mm Hg was pathological (7).
Despite the merits of early BP-CVD association reports, they compared the risk of CVD in adults identified as having a very high level of BP with the corresponding risk in counterparts with a lower but still high BP. The pioneers of BP measurement could not know that nearly all humans, including most of those in the “lower” BP category, had a level of BP above what is biologically normal and desirable. It took several decades before BP was measured in groups of humans with a true biologically normal BP because they were living in isolated unacculturated societies. Investigators discovered that almost all of those studied had an average BP that was substantially lower than the corresponding levels noted in studies that had been conducted in acculturated societies (8–11).
There are potentially confounding differences between people living in “acculturated” societies and their counterparts living in isolated “unacculturated” societies. Most of these confounding variables were, however, controlled in a seminal study of two Amazonian tribes who had a similar background and cultural habits, with the exception of a difference in sodium intake (8). The Mundurucus, whose lifestyle was influenced by the Franciscans, had incorporated salt into their diet as a means to preserve and season their food. In contrast, the Carajás had little contact with Westerners and consumed almost no salt. Average BP during adult life increased with rising age in the Mundurucus but not in the Carajás (Figure 1), whose mean systolic and diastolic BPs remained about 110 mm Hg and 60 mm Hg, respectively, throughout their adult lifespan.
Figure 1.
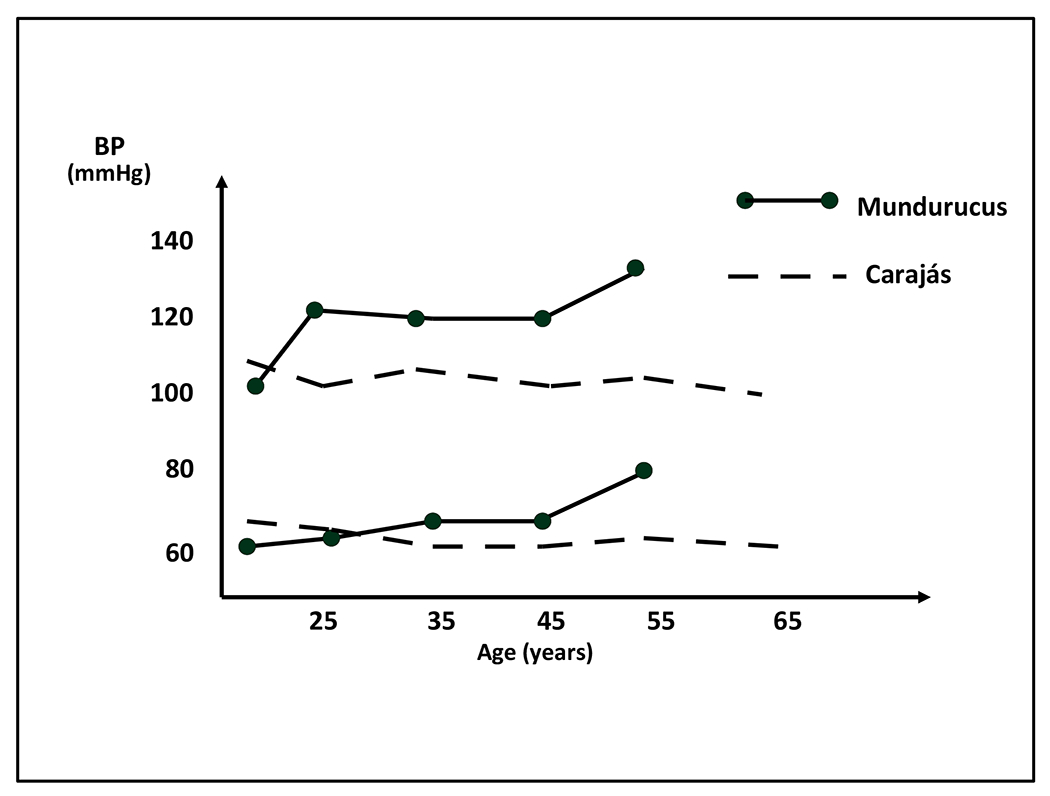
Blood pressure for men by age in Mundurucus and Carajás Indians, showing a rise with aging in the “acculturated” Mundurucus but not in the “unacculturated” Carajás (reprinted with permission from the reference 8).
Another isolated society in Brazil, the Yanomamo Indians, with very limited access to salt also demonstrated little if any rise in BP with aging (10). In addition to excreting very little sodium in their urine, they had high levels of plasma renin activity and aldosterone. These findings suggest biologically normal values of BP, renin activity and aldosterone are very different compared to the relatively high levels of BP and low levels of plasma renin activity and aldosterone identified in acculturated societies, where there is exposure to high levels of dietary sodium. Animal model studies, population studies and clinical trials provide evidence supporting the central role of excessive sodium consumption in causing age-related increases of BP (12).
The consequences of two definitions for identification of an abnormally high BP are depicted in Figure 2. The left-hand panel depicts the rightward shift of systolic and diastolic BP that occurs with acculturation. The shaded area in the distribution for unacculturated populations identifies high BP using a definition (SBP ≥120 mm Hg or DBP ≥70 mm Hg) is based on the BP distribution in observational studies. The corresponding shaded area in the distribution for acculturated populations is based on a definition of high BP (SBP ≥140 mm Hg or DBP ≥90 mm Hg) that has traditionally been used in acculturated populations. The shaded areas in the right-hand panel identifies high BP in acculturated societies using the SBP 120 mm Hg and DBP ≥70 mm Hg cut-points.
Figure 2.
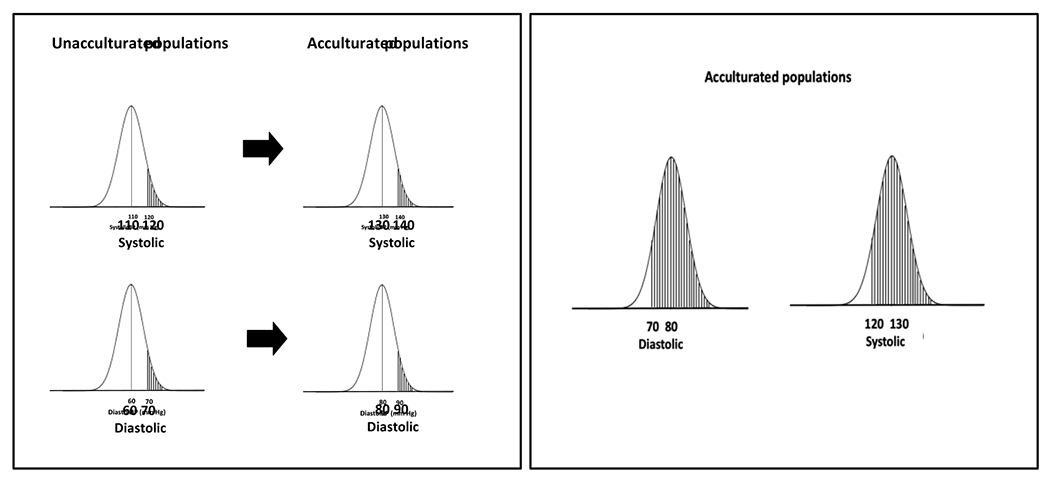
Left-hand panel depicts distribution of systolic and diastolic BP in unacculturated and acculturated populations. Shaded areas identify distribution of a high blood pressure definition (systolic BP ≥120 mm Hg or diastolic BP ≥70 mm Hg) for adults in unacculturated societies and for their counterparts living in acculturated societies using the traditional definition for diagnosis of hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg). Shaded areas in the right-hand panel highlight distribution of high systolic and diastolic blood pressure applying the definition used for high blood pressure in unacculturated societies (SBP ≥120 mm Hg or diastolic BP ≥70 mm Hg).
Around 70% of those living in acculturated societies would be at higher risk of developing CVD according these values. Using the cut-points proposed for diagnosis of hypertension in the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) BP guideline (SBP ≥130 mm Hg or DBP ≥80 mm Hg) (13), around 63% of adults aged 45 to 75 years in the United States (U.S.) and 55% in China have hypertension (14).
In recent decades, a leftward shift in the distribution of BP has been identified in high-income countries (15). As expected, this downward shift in BP has been accompanied by a reduction in the incidence of CVD. The absolute number of individuals with hypertension in the world, however, has increased due to a rightward shift in the distribution of BP in low- and middle-income countries (15).
EVIDENCE FROM COHORT STUDIES
In an early cohort study, Keith, Wagner and Barker reported on BP-related risks, stratified by BP levels, symptoms, electrocardiographic (ECG) abnormalities, albuminuria/hematuria, and optic fundi abnormalities (16). The mortality rate was proportional to severity of illness, being higher than 80% over one year for participants who had BP resistant to treatment, bad general condition, an abnormal ECG, albuminuria, hematuria and optical edema (characterized as class IV). Despite this and other observational reports published in the first half of the 20th century that suggested high BP caused CVD, many opinion leaders believed high BP was an inconsequential finding and use of the term “benign essential hypertension” was commonplace. Paul Dudley White was in the group of influential leaders who believed high BP was a physiological compensatory mechanism and should not be manipulated by treatment (17).
In a series of seminal reports, the Prospective Studies Collaboration pooled data from many cohort studies and accounted for the effects of regression dilution bias in order to generate precise estimates of the relationship between BP and CVD. Their most important report (20) was based on experience in 61 cohort studies that provided 12.7 million person-years of risk experience (56,000 deaths from coronary heart disease [CHD] and stroke). The risk of CVD increased steadily with progressively higher levels of baseline SBP and DBP, above a usual SBP and DBP of 115 and 75 mmHg, respectively. For a 20 mmHg higher level of SBP and 10 mm Hg higher level of DBP the risk of CVD was two-fold higher (Figure 3, left-hand panel, with log-transformed vertical axis). The corresponding pattern for BP-related absolute risk of CVD (Figure 3, righthand panel), revealed an exponential association between increased BP and risk for CHD and stroke. It identified a relatively small increment in absolute risk of CVD with increasing BP levels at lower BP values and at younger age, which likely explains why individual studies in young and middle-aged participants have only identified a CVD risk at higher values of BP (21).
Figure 3.
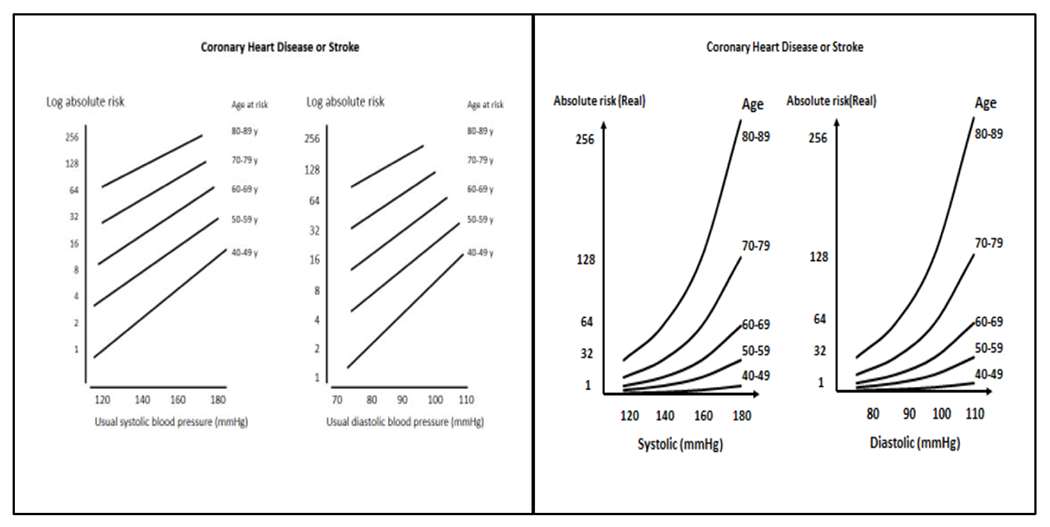
Log transformed (left-hand panel) and untransformed (right-hand panel) absolute risk of coronary heart disease or stroke in adults, by systolic and diastolic blood pressure, stratified by age. (Reprinted with permission from references 20 and 21).
Most of the early observational studies were focused on BP complications (stroke and CHD) which usually occur earlier than other BP clinical consequences and are especially prominent at higher levels of BP. At older age, stroke and CHD are accompanied by several complications that develop over a longer period of exposure to high BP, either because the target organs are more resistant or because more modest BP elevations have been present for a prolonged period of time. The complications of high BP can be classified as short- and long-term consequences (Table). Consistent observational evidence suggests high BP is the leading cause of long-term consequences such as heart failure, with and without preserved ejection fraction, (22), atrial fibrillation (23), valvular heart disease (24,25), peripheral arterial disease and aortic syndromes (26), chronic kidney disease and end stage renal disease (27,28), dementia (29,30) and Alzheimer’s Disease (31). Diabetes (32), erectile dysfunction (33), and age-related macular degeneration (34) are other conditions that likely have high BP as one of their causes.
Table.
Short-term and long-term consequences of high BP
| Short and long-term consequences |
| Stroke |
| Coronary heart disease |
| Heart failure |
| Cardiovascular death |
| Long-term consequences |
| Hypertensive cardiomyopathy |
| Heart failure with preserved ejection fraction |
| Atrial fibrillation |
| Valvular heart disease |
| Aortic syndromes |
| Peripheral arterial disease |
| Chronic kidney disease |
| Dementias |
| Diabetes mellitus |
| Erectile dysfunction |
CENTENARIANS
The primary reason that centenarians reach 100 years of age is an unusually low burden of CVD and cancer. CHD, stroke, dementia, and hypertension, are less frequent in centenarians compared to individuals who die at a younger age (35) (Figure 4). Vascular aging is directly associated with level of BP and not inexorable. Prevention of age-related increases in BP would substantially reduce the vascular consequences usually attributed to aging. Individuals who develop hypertension late in life are not at increased risk for CVD earlier in life (36). Centenarians may be resistant to dietary sodium loads, with inability to excrete sodium in the absence of high pressure natriuresis.
Figure 4.
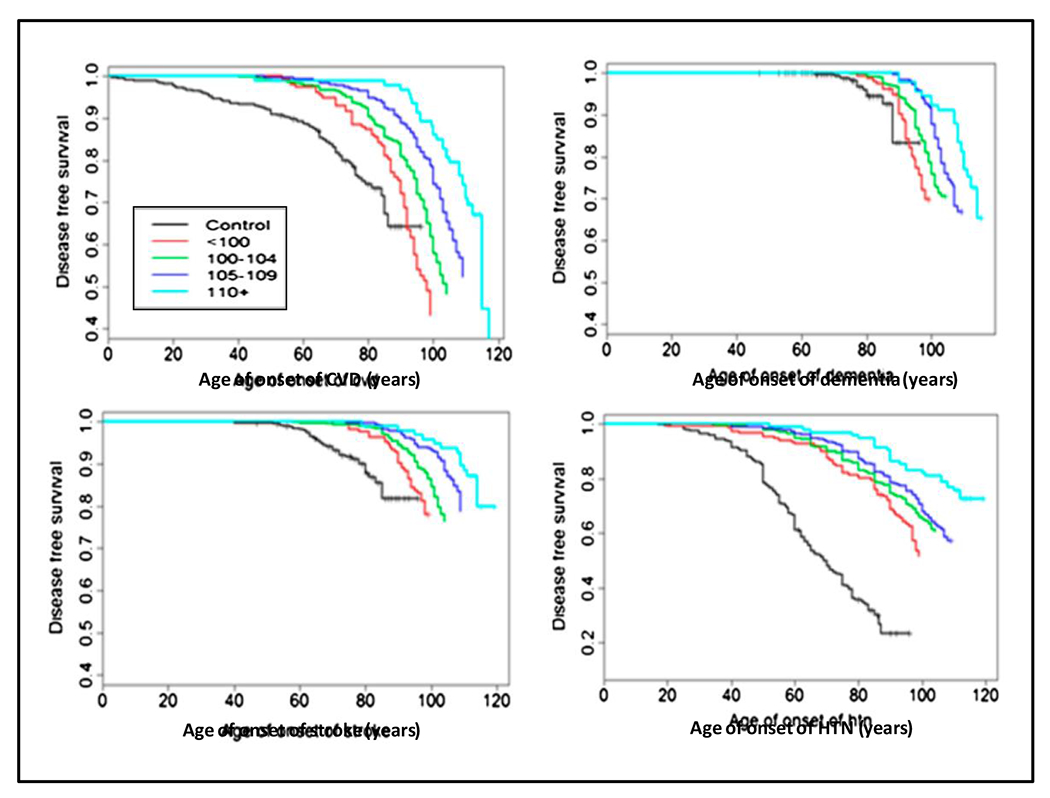
Disease-free age survival for cardiovascular disease (CVD), dementia, stroke and hypertension (HTN) in controls (individuals without a familial predisposition for exceptional longevity (black line), and centenarians (color lines), stratified by age in years at death. The data demonstrate a consistent delay in onset of CVD, dementia and stroke when HTN starts late in life. (Reprinted with permission from reference 35).
CARTESIAN EVIDENCE
Experimental studies, the highest hierarchical method for demonstration of causality, provide strong support for our theory of CVD causation. The 1967 Veterans Administration Cooperative Study Group on Antihypertensive Agents (37), Systolic Hypertension in the Elderly Program (SHEP) trial (38) and Systolic Blood Pressure Intervention Trial (SPRINT) (39) are three of many RCTs that have provided strong evidence regarding the effectiveness of BP lowering for prevention of CVD. Redefining the outcomes according a modern paradigm, the number needed to treat in order to prevent one major CVD event per year in the 1967 Veterans Administration trial was only six patients in those with a baseline diastolic BP averaging 115-129 mm Hg (40). Prior to publication of the SHEP results, many doctors believed isolated systolic hypertension was a natural and benign compensatory consequence of ageing. The trial showed that chlorthalidone-based therapy reduced the incidence of stroke, the primary endpoint, by 36% compared to placebo. SPRINT demonstrated that participants randomized to a systolic BP goal less than 120 mm Hg (intensive treatment) had an incidence of the primary composite CVD endpoint that was 25% lower compared to those randomized to a systolic BP goal less than 140 mm Hg (standard treatment) (39). There were reductions of 43% in CVD mortality and 27% in all-cause mortality. A similar benefit was demonstrated in the relatively large (N=2636) subgroup of participants 75 years or older at baseline, including those with frailty and/or reduced gait speed (41).
High-quality meta-analyses that have demonstrated the effectiveness of BP lowering for prevention of CVD. Additionally, studies have compared the observed benefit of BP reduction in RCTs with the expected benefit based on BP as a risk factor for CVD in observational studies (42–44). In a study that included 147 RCTs, Law et al. calculated the CVD risk reduction for the average active treatment versus control trial difference in systolic BP (10 mm Hg) observed in their meta-analysis (42). As depicted in Figure 5, the reductions in stroke and CHD incidence rates were similar to the benefits expected on the basis of a 10 mm Hg difference in systolic BP in the Prospective Studies Collaboration meta-analysis of observational studies. Benefit for the same difference in BP was greater for stroke, reflecting the higher risk of elevated BP for cerebral vessels compared to the coronary circulation.
Figure 5.
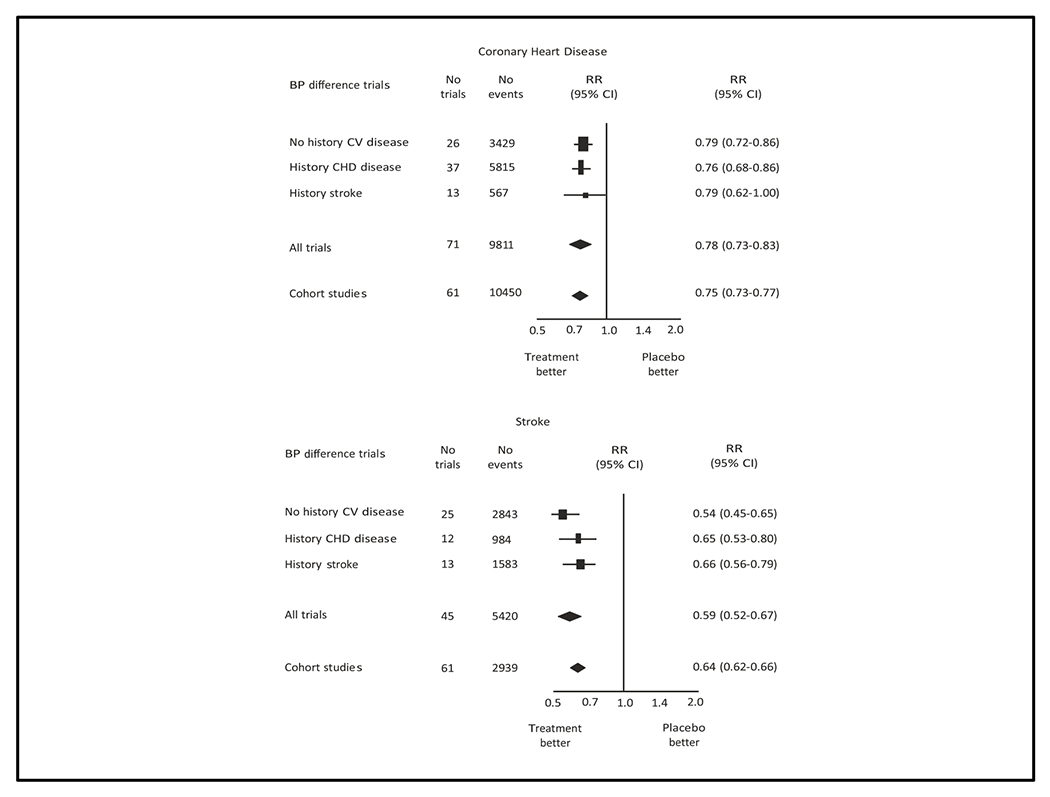
Relative risk estimates of coronary heart disease (top panel) and stroke (bottom panel) for systolic blood pressure reduction of 10 mm Hg or diastolic blood pressure reduction of 5 mm Hg in clinical trials meta-analysis and corresponding difference in meta-analysis of observational cohort studies. (Reprinted with permission from reference 42)
A similar benefit was observed in a network meta-analysis conducted by Bundy et al (44). In this study, the relative risk reduction of major CVD events in trials where the participants had been treated to a systolic BP target between 120-124 mm Hg, compared to a corresponding target of 160 mm Hg or higher, was 64%, which is close to the hypothetical risk reduction of 75% for a 40 mm Hg systolic BP reduction in the Prospective Studies Collaboration meta-analysis (20). The similarity between risk predicted in cohort studies and benefit demonstrated in RCTs fulfills one of the postulates of Descartes (45), i.e., that the sum of the angles of any triangle equals 180 degrees (two right angles). The close approximation between the observed (clinical trials) and expected (observational cohort studies) benefits of BP lowering is impressive, since the expected estimate is based on imperfect measurements of a biological parameter (BP).
Scant experimental evidence exists for prevention of the longer-term consequences of high BP. Designing trials for these endpoints is challenging, because they require a longer duration of treatment than is necessary to demonstrate CVD benefit. Despite this, suggestive benefit of BP lowering in RCTs exists for some of these outcomes. For example, the composite outcome of mild cognitive impairment and dementia was significantly less common during long-term follow-up in the SPRINT study (46,47).
The central role of BP levels has also been demonstrated by studies where BP has risen during treatment and resulted in CVD events. Examples come from studies using celecoxib (48), sibutramine (48) and torcetrapib (50). In contrast, prevention of CVD events by inhibitors of sodium–glucose cotransporter 2 in adults with (51,52), and without diabetes (53) may be due, in part, to BP-lowering.
ATTRIBUTABLE RISKS
Estimation of the high BP contribution to causation of CVD has increased progressively as the definition of hypertension and analytic techniques have evolved. Early estimates of attributable risk were 25% for CHD and 50% for stroke (54). Based on the risks identified by the Prospective Studies Collaboration (20), the attributable risk for BP equal or higher than 115/75 mmHg was estimated to be 49% for CHD and 62% for stroke (55). However, these estimates almost certainly understate the true contribution of high BP to development of CVD. In many of the cohort studies, residual bias in estimation is probable because the risk estimates have been based on only a few BP measurements. Vascular, cardiac and renal damage results from an excessively high vascular load of beat-to-beat elevations in BP over long periods (21). One or two BP measurements provide an insufficient estimate of vascular load. Other CVD risk factors, such as smoking, dyslipidemia and excess body weight, are subject to measurement error but their estimation is much more precise compared to BP readings. More efficient methods for BP measurement, such as ambulatory BP measurement (56), particularly at nighttime (57), home BP monitoring and automated office BP measurement provide more precise estimation of BP-CVD risk, including identification of risk at lower BP values compared to traditional office BP measurements (56). Another reason for underestimation of BPrelated risk is inclusion of intermediate consequences of high BP as causes of CVD in multivariate explanatory equations (58). This is especially true for analyses that have included left ventricular hypertrophy or vascular wall thickening but even overweight and obesity are CVD risk factors that are largely mediated by high BP (59). Recognizing the potential for underestimation, other confounding risks increase concomitantly with BP, but in clinical trials the absolute risk reduction from BP lowering accounts for nearly all of the predicted risk, leaving little residual risk to be explained by the other concomitant risks.
WHAT IS MISSING?
In adults without CVD, a strong BP-CVD risk association has been identified at lower levels of systolic (120-139 mm Hg) and diastolic (80-89 mm Hg) BP (20). Individuals within this BP range are at increased risk for development of higher levels of BP over relatively short periods of follow-up (60,61) and already have evidence of target organ damage (62,63). Moreover, meta-analyses of event-based RCTs have shown the benefit of antihypertensive treatment in adults with CVD (secondary prevention) and an average BP within the same range (42,43). Trials have also shown that low-dose pharmacotherapy in adults without CVD and an average systolic BP of 130-139 mm Hg or diastolic BP ≤89 mm Hg (64,65) and systolic BP 120-139 mm Hg or diastolic BP 8089 mm Hg (66) lowers BP and prevents incident hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg). The PREVER-Prevention trial additionally demonstrated that low dose diuretic therapy (chlorthalidone and amiloride) in adults with a SBP 120139 mm Hg or DBP 80-89 mm Hg prevented incident LVH estimated by ECG (66).
Nonetheless, no BP lowering trial has demonstrated prevention of CVD events in adults without CVD who have a systolic BP <140 mm Hg or diastolic BP <90 mm Hg.
Despite strong evidence of a BP lowering benefit in clinical trials and meta-analyses, direct documentation of intensive BP lowering benefit is lacking in adults with high BP who have diabetes or a history of prior stroke. At least two event-based trials (one in Brazil and the other in China) are addressing this question in diabetics and one trial (in Brazil) is being conducted in stroke survivors.
CONCLUSION AND PERSPECTIVES
Our proposal that CVD is predominantly caused by a rightward shift in the distribution of BP is supported by coherence and factual evidence. As a theory, it is open to refutation and formulation of alternative proposals. Among the existing theories, however, ours is the one that best meets Occam’s razor premise, i.e., it is the hypothesis with the fewest assumptions. Other risk factors, including lipid abnormalities, cigarette smoking, physical inactivity, and dietary influences other than sodium play an important role in CVD causation. Although elevated BP has the greatest effect on population health, prevention of CVD is best achieved by a comprehensive approach aimed at improving CVD risk factors at all stages of life.
Regardless of its exact contribution, high BP is a major risk factor for development of CVD. Prevention of the age-related increase in BP would substantially reduce the vascular consequences usually attributed to aging. It is time to focus greater attention on initiatives for prevention of the typical age-related increase in BP in addition to control of high BP in those with established hypertension. Even partial improvement in the age related increase in BP would eliminate a large proportion of the existing burden of BP-related CVD.
Funding sources:
Dr. Whelton was supported by a Centers for Research Excellence grant from the National Institute of General Medical Sciences (P20GM109036).
Footnotes
Disclosures: none
Conflicts of interest: none
References
- 1.Popper Karl R.. The logic of scientific discovery. Routledge, London, 1959. [Google Scholar]
- 2.Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med. 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osler W The principles and practice of medicine. New York: D Appleton and Company; 1892. [Google Scholar]
- 4.Riva-Rocci S Un nuovo sfigmomanometro. Gazz Medi Torino 1896; 50:981–96. [Google Scholar]
- 5.Korotkov NS. To the question of methods of determining the blood pressure. Rep Imp Military Acad 1905; 11: 365–7. [Google Scholar]
- 6.Fisher JW. The diagnostic value of the sphygmomanometer in examinations for life insurance. JAMA 1914; 63:1752–4. [Google Scholar]
- 7.Janeway TC. A clinical study of hypertensive cardiovascular disease. Arch Intern Med 1913; 12:755–8. [Google Scholar]
- 8.Lowenstein FW. Blood pressure in relation to age and sex in the tropics and subtropics. A review of the literature and an investigation in two tribes of Brazil Indians. Lancet 1961;277 (1):389–92. [Google Scholar]
- 9.Page LB, Damon A, Moellering RC Jr. Antecedents of cardiovascular disease in six Solomon Islands societies. Circulation 1974; 49:1132–46. [DOI] [PubMed] [Google Scholar]
- 10.Oliver WJ, Cohen EL, Neel JV. Blood pressure. Sodium intake and sodium related hormones in the Yanomano Indians, a no-salt culture. Circulation 1975; 52:146–61. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho JJ, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, Marcopito LF, Spooner VJ, Dyer AR, and Elliott P. Blood pressure in four remote populations in the INTERSALT Study. Hypertension 1989;14: 238–46. [DOI] [PubMed] [Google Scholar]
- 12.National Academies of Sciences, Engineering, and Medicine 2019. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press. 10.17226/25353. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 14.Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, Krumholz HM. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative crosssectional study. BMJ 2018; 362:k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCD Risk Factor Collaboration (NCD-RisC). Contributions of mean and shape of blood pressure distribution to worldwide trends and variations in raised blood pressure: a pooled analysis of 1018 population-based measurement studies with 88.6 million participants. Int J Epidemiol. 2018; 47:872–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keith NM, Wegener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Sci 1939; 197:332–43. [DOI] [PubMed] [Google Scholar]
- 17.White P Heart disease. 2nd ed. New York: McMillan Co; 1937. 326 p. [Google Scholar]
- 18.Prospective Studies Collaboration. Age-specific relevance of usual BP to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–13. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs FD, ed. Essentials of hypertension. Cham, Switzerland, Springer; 2018. [Google Scholar]
- 20.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 2016; 9(6):pii:003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emdin CA, Anderson SG, Salimi-Khorshidi G, e Woodward M, MacMahon S, Dwyer T, Rahimi K. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol 2017; 46:162–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimi K, Mohseni H, Kiran A, Tran J, Nazarzadeh M, Rahimian F, Woodward M, Dwyer T, MacMahon S, Otto CM. Elevated blood pressure and risk of aortic valve disease: a cohort analysis of 5.4 million UK adults. Eur Heart J 2018; 39: 3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimi K, Mohseni H, Otto CM, Conrad N, Tran J, Nazarzadeh M, Woodward M, Dwyer T, MacMahon S. Elevated blood pressure and risk of mitral regurgitation: A longitudinal cohort study of 5.5 million United Kingdom adults. PLoS Med 2017;14(10):e1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, Woodward M, Rahimi K. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ 2015;351:h4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005; 165:923–8. [DOI] [PubMed] [Google Scholar]
- 26.Kanno A, Kikuya M, Ohkubo T, Hashimoto T, Satoh M, Hirose T, Obara T, Metoki H, Inoue R, Asayama K et al. Pre-hypertension as a significant predictor of chronic kidney disease in a general population: the Ohasama Study. Nephrol. Dial Transplant 2012; 27:3218–23. [DOI] [PubMed] [Google Scholar]
- 27.Emdin CA, Rothwell PM, Salimi-Khorshidi G, Kiran A, Conrad N, Callender T, Mehta Z, Pendlebury ST, Anderson SG, Mohseni H et al. Blood pressure and risk of vascular dementia: evidence from a primary care registry and a cohort study of transient ischemic attack and stroke. Stroke 2016; 47:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker KA, Sharrett R, Wu A, Schneider AL, Alber M, Lutsey PL, BandeenRoche K, Coresh J, Gross AL, Windham BG et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 2019; 322:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joas E, Bäckman K, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension 2012; 59:796–801. [DOI] [PubMed] [Google Scholar]
- 30.Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual blood pressure and risk of new-onset diabetes evidence from 4.1 million adults and a meta-analysis of prospective studies. J Am Coll Cardiol 2015; 66:1552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning L, Yang L. Hypertension might be a risk factor for erectile dysfunction: a meta-analysis. Andrologia 2017. May;49(4). doi: 10.1111/and.12644 . [DOI] [PubMed] [Google Scholar]
- 32.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010; 10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci 2012; 67:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on Hypertension. Lancet 2016; 388:2665–712. [DOI] [PubMed] [Google Scholar]
- 35.Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mmHg. JAMA. 1967; 202:1028–34. [PubMed] [Google Scholar]
- 36.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1991; 265:3255–64. [PubMed] [Google Scholar]
- 37.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs FD, Klag MJ, Whelton PK. The classics: a tribute to the fiftieth anniversary of the randomized clinical trial. J Clin Epidemiol 2000;53(4):335–42. [DOI] [PubMed] [Google Scholar]
- 39.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.42 Law MR, Morris JK, Wald NJ. Use of BP lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387:957–67. [DOI] [PubMed] [Google Scholar]
- 42.Bundy JD, Li C, Stuchlik P, Bu X, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Descartes René (1637). A Discourse on Method: Meditations and Principles. Translated by Veitch, John. London: Orion Publishing Group, 2004. [Google Scholar]
- 44.SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019; 321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The SPRINT MIND Investigators for the SPRINT Research Group; Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA et al. Association of intensive versus standard blood pressure control with cerebral write matter lesions. JAMA 2019; 322(6):524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, Eagle C, Hawk E, Lechuga M, Zauber AG et al. APC and PreSAP Trial Investigators. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation 2006; 114:1028–35. [DOI] [PubMed] [Google Scholar]
- 47.49 James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA et al. SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010; 363: 905–17. [DOI] [PubMed] [Google Scholar]
- 48.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–22. [DOI] [PubMed] [Google Scholar]
- 49.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 50.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019; 380:347–357. [DOI] [PubMed] [Google Scholar]
- 51.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019. Sep 19. [Epub ahead of print] [Google Scholar]
- 52.Kannel WB. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol 1976; 37:269–82. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. The world health report 2002 - Reducing Risks, Promoting Healthy Life (Internet). Geneva: WHO; 2002. Available in: http://www.who.int/whr/2002. Accessed June 14, 2019. [Google Scholar]
- 54.Niiranen TJ, Mäki J, Puukka P, Karanko H, Jula AM. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension 2014; 64:281–6. [DOI] [PubMed] [Google Scholar]
- 55.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J et al. ABC-H Investigators. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients with Hypertension (ABC-H) meta-analysis. Hypertension 2016; 67: 693–700. [DOI] [PubMed] [Google Scholar]
- 56.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014; 383:970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mallmann AB, Fuchs SC, Gus M, Fuchs FD, Moreira LB. Populationattributable risks for ischemic stroke in a community in South Brazil: a case–control study. PLoS One 2012; 7:e35680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreira LB, Fuchs SC, Wiehe M, Gus M, Moraes RS, Fuchs FD. Incidence of hypertension in Porto Alegre, Brazil: a population-based study. J Hum Hypertens 2008; 22:48–50. [DOI] [PubMed] [Google Scholar]
- 59.Kurioka S, Horie S, Inoue A, Mafune K, Tsuda Y, Otsuji Y. Risk of progression to hypertension in nonhypertensive Japanese workers aged 20–64 years. J Hypertens 2014; 32:236–44. [DOI] [PubMed] [Google Scholar]
- 60.Markus MR, Stritzke J, Lieb W, Mayer B, Luchner A, Döring A, Keil U, Hense HW, Schunkert H. Implications of persistent prehypertension for ageing-related changes in left ventricular geometry and function: the MONICA/KORA Augsburg study. J Hypertens 2008; 26:2040–9. [DOI] [PubMed] [Google Scholar]
- 61.Santos AB, Gupta DK, Bello NA, Gori M, Claggett B, Fuchs FD, Shah AM, Coresh J, Sharrett AR, Cheng S et al. Prehypertension is associated with abnormalities of cardiac structure and function in the Atherosclerosis Risk in Communities study. Am J Hypertens 2016; 29:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH Jr, Messerli FH, Oparil S et al. Schork MA; Trial of Preventing Hypertension (TROPHY) Study Investigators. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354:1685–97. [DOI] [PubMed] [Google Scholar]
- 63.Lüders S, Schrader J, Berger J, Unger T, Zidek W, Böhm M, Middeke M, Motz W, Lübcke C, Gansz A et al. PHARAO Study Group. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal BP—a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens 2008; 26:1487–96. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs SC, Poli-de-Figueiredo Carlos E, Figueiredo-Neto JA, Scala LC, Whelton PK, Mosele F, de Mello RB, Vilela-Martin JF, Moreira LB, Chaves H et al. Effectiveness of chlorthalidone plus amiloride for the prevention of hypertension: the PREVER-Prevention randomized clinical trial. J Am Heart Assoc 2016; 5:e004248. [DOI] [PMC free article] [PubMed] [Google Scholar]


