Abstract
Purpose
The main aim of the study was to compare the effectiveness of the dietary patterns studied in the context of multiple sclerosis (MS), including anti-inflammatory, Mediterranean diet (MD), Mediterranean-DASH intervention for neurodegenerative delay (MIND), intermittent fasting (IF), gluten-free and ketogenic diets. In addition, another aim was to verify or otherwise the efficacy of other alternative dietary models, which include the Paleo diet, the Wahls diet, the McDougall diet and the Swank diet. Whether and to what extent the use of different dietary regimens can affect the course and reduction of individual MS symptoms was also examined. The advantages and disadvantages of selected diets and dietary patterns in the context of MS are discussed.
Views
Autoimmune diseases are estimated to affect more than 3% of the world’s people, the majority of whom are of working age. Therefore, delaying the first manifestation of the disease, reducing the number of relapses and alleviating symptoms are particularly welcome developments. In addition to finding effective pharmacotherapy, high hopes for patients lie in nutritional prevention and diet therapy. For years the medical literature has discussed supporting the treatment of diseases caused by an impairment of the body’s immune system with the help of nutrition.
Conclusions
An appropriate and balanced diet can be extremely helpful in improving the condition and well-being of patients with MS, and effectively support drug therapy.
Keywords: nutrition, MS, dietary patterns, multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disease characterised by the demyelination of nerve cells in the central nervous system (CNS) [1, 2]. Because of its chronic inflammatory process, leading to gradual but permanent neuronal damage, it is also often referred to as a neurodegenerative disease [3]. The main problem in successfully treating MS remains the brain’s limited capacity to repair itself [3]. Oligodendrocytes are able to produce insulating myelin sheaths, which play a critical role in repairing the damage caused by the disease. In contrast, the second type of glial cells, astrocytes, have a destructive effect on nerve fibers through a rapid scarring mechanism that likely disturbs the further repair of myelin sheaths [1, 3]. There are currently about 2.8 million people with MS worldwide, mostly in North America, Western Europe and Scandinavia [4]. The disease usually begins between the ages of 20 and 40, and the average age at which patients are diagnosed is 32 [4, 5]. In addition, the disease, like other autoimmune diseases, affects women more often than men. Globally, women are on average affected by MS at twice the rate men are, while in some countries the rate is higher [4, 5]. Since the disease mostly affects women of reproductive age, the potential impact on family planning is therefore also an issue. MS is not a contraindication to pregnancy, and it is also known that during pregnancy, especially in the third trimester, the likelihood of a flare-up decreases compared to the time prior to pregnancy. However, already in the postpartum period the risk of a relapse increases again by up to a third [6]. It remains the case that no drug or effective therapy has been invented to stop the progression of the disease or cure the patient completely [4]. The clear mechanisms of the disease are also unknown, although much attention has been paid to genetics, infections and environmental factors [1, 7]. A higher prevalence of MS has been reported in the US and Western Europe or Scandinavia than elsewhere which may be related to regional dietary habits [4]. Such a relationship was confirmed by Esposito et al., who indicated the prevalence of numerous unhealthy dietary habits among MS patients at the onset of the disease [8]. One hypothesis links the Western diet (Western diet) and Western lifestyle (Western lifestyle) to an increase in autoimmune diseases, including MS [7]. The Western lifestyle is associated with, among other things, low physical activity, poor eating habits, a high intake of saturated fatty acids (SFAs) and simple sugars, and low levels of vitamin D [7-10]. Data provided by Cayre et al. report that repair of myelin sheaths occurs only in the early stages of MS, which may support the need for nutritional prophylaxis and other measures to prevent disease progression [3]. To counteract the negative impact of the Western lifestyle several dietary patterns have been proposed to mitigate the course of the disease. Over the years, researchers have proposed and studied a wide variety of diets and dietary factors with beneficial or adverse effects on people with MS [11, 12]. Among the recommended diets are dietary patterns that have proven effects on various aspects of human health, such as the Mediterranean, anti-inflammatory, Mediterranean-DASH intervention for neurodegenerative delay (MIND), ketogenic, intermittent fasting (IF) and gluten-free diets, among others. However, there is also no shortage of modified types of diets used in research studies exclusively in the context of MS. Among them are the Wahls, McDougall, Swank diets. A proper and well-balanced diet can also counteract the occurrence of malnutrition in patients, which can progress as the patient’s mobility is reduced [1]. Indeed, similar relationships have been noted in patients with other neurodegenerative diseases including Alzheimer’s and Parkinson’s disease [13, 14].
The aim of the present study was to examine via a literature review whether and to what extent the use of different dietary patterns can affect the course and reduction of individual MS symptoms.
MATERIAL AND METHOD
Online databases were searched including PubMed, Google Scholar, Researchgate and Science Direct. The following keywords were used: “multiple sclerosis (MS)”, “nutrition and multiple sclerosis”, “diet” and “multiple sclerosis, diet” and “autoimmune diseases”. The papers considered were mostly research papers, meta-analyses and original papers, and systematic reviews. Those included in the review were predominantly published between 2017 and 2023.
Inclusion criteria: articles in English; articles about diet or nutritional pattern; research with adult patients; animal studies; meta-analyses; systematic reviews; clinical studies, including randomized controlled trials; observational studies; and reviews. Exclusion criteria: articles in a language other than English; articles on single nutrients; historical data; research on children and adolescents (< 18 years old); case studies; scoping reviews; narrative reviews; and articles on microbiota and probiotic therapy in the context of MS.
The paleo diet and its modification (Wahls diet)
The Paleolithic (paleo) diet in MS was popularized and modified by Dr. Terry Wahls (Wahls Paleo™) [15]. The Wahls™ diet differs from the traditional paleo diet in that it does not allow the consumption of eggs, dairy, grains, legumes and solanaceous plants and allows two servings of gluten-free grains (such as rice) per week, recommends more than nine cups of fruits and vegetables per day, with one-third each of dark green leafy and sulphur-rich vegetables, encourages the consumption of seaweed, algae and yeast, and limits animal and fish protein. According to some sources, modified Paleolithic diets carry the risk of nutritional deficiencies such as vitamin B, as a result of limited cereal consumption, and calcium and vitamin D deficiencies as a result of not consuming dairy [16]. A recently published study found that a paleo diet may be useful in the treatment of MS, reducing perceived fatigue, improving mental and physical quality of life, increasing exercise capacity, and improving arm and leg function [17]; however, some researchers have suggested this work as having a high risk of bias [18]. According to other researchers, similar dietary interventions can reduce fatigue and improve quality of life in patients with progressive MS [19, 20]. A study by Titcomb et al. evaluated the safety of a modified paleo diet as part of a 12-month intervention among people with progressive MS. The average kilocalorie intake was 1820 ± 506 kilocalories/day and consisted of approximately 38% carbohydrate, 18% protein and 44% fat. Micronutrient intake from food exceeded individual recommendations for most vitamins and minerals. However, vitamin D, choline and calcium were not consumed in sufficient amounts, indicating nutrients of concern. Many people did not meet individual recommendations for vitamin E (so far, it is not known what effect inadequate vitamin E intake may have on MS). The modified Paleolithic diet used in this study was associated with an adequate intake of most micronutrients and favorable changes among people with progressive MS [21].
The ketogenic diet
In addition to the proven efficacy and established use of the ketogenic diet in intractable childhood epilepsy, new applications are increasingly being suggested given its effectiveness in various experimental models, including animal models showing marked inflammatory changes. A systematic review by Koh et al. described the effects of the ketogenic diet based on studies in animal models [22]. They described results that indicated the effects of the ketogenic diet in relation to a reduction in inflammatory mediators. Similar results were obtained by Bock et al., suggesting that a ketogenic diet can reduce the expression of enzymes involved in the biosynthesis of pro-inflammatory eicosanoids [23]. A pilot study reported by Brenton et al. suggests that a modified ketogenic diet may benefit patients with MS [24]. Despite a small study sample, it has been shown to alleviate fatigue and depressive symptoms, reduce body fat and pro-inflammatory adipokines. More recently, the results of a study by Brenton et al. were published in which 53 patients with relapsing-remitting MS (RRMS) completed a 6-month intervention involving a ketogenic diet. Significant improvements in the patients’ disability status, improved quality of life while on the diet, and reduced fatigue and body fat levels were reported [25]. A pilot study by Benlloch et al. that analyzed the dietary habits of 27 MS patients showed that MS patients who followed a ketogenic, isocaloric diet based on the principles of the Mediterranean diet, rich in medium-chain triglycerides (MCTs), for 4 months showed an increase in lean body mass, an improvement in the patients’ metabolic profile (reduced inflammation) and an increase in satiety [26]. A randomized controlled trial examined the effects of intermittent fasting and a ketogenic diet on serum neurofilament light chain (sNfL) levels in patients with MS [27]. Through this, it has been shown that the ketogenic diet may be a promising and effective therapeutic strategy in neuroinflammatory diseases such as MS. One theory supporting the use of the ketogenic diet in MS is also the effect on the microbiota of increasing gut microbial diversity [28]. A study by Swidsinski et al. compared the composition of the colonic microbiota in 25 patients with RRMS and 14 healthy controls. The results of the study proved that in patients with MS, the total concentrations and diversity of important bacterial groups were reduced (p < 0.001). The Bac303 (Bacteroides) and Fprau (Faecalibacterium prausnitzii) bacterial groups were most reduced. The average macronutrient distribution in the control group was as follows: < 50 g carbohydrate, > 160 g fat and < 100 g protein per day. The ketogenic diet intervention in MS patients resulted in an increase in the concentrations of significant microbial groups after 6 months, to the extent that no differences were observed between MS patients and a healthy group. However, the study did not evaluate the clinical effects on MS due to the small number of patients [28]. Several ongoing randomized, controlled trials are listed on Clinicaltrials.gov as testing the safety, tolerability, and efficacy of the ketogenic diet as a therapeutic intervention in MS.
Low-fat diets: McDougall and Swank
The McDougall is a very low-fat diet (10% of energy comes from fat, 14% from protein, 76% from carbohydrates) consisting mainly of plant-based starchy foods, vegetables, and fruits. Animal products, including eggs, dairy products and oils are not allowed [29]. There are no current scientific studies during the period under review on its use and effectiveness in MS, although some researchers have previously identified it as a potential strategy for managing fatigue in MS.
The Swank diet was developed by Dr. Roy Swank, and is based on the observation that the incidence of MS is higher in areas with higher saturated fat intake. Accordingly, the Swank diet is very low in saturated fat (up to 15 g per day), fatty meat, and processed foods [30, 31]. A recent study by Wahls et al. compared a modified paleo diet (Wahls) and the Swank diet in RRMS [32]. Both the low saturated fat diet and the modified Paleolithic diet are popular among people with MS [33]. The Wahls group experienced a 24-week average reduction according to the fatigue scale, which was significantly greater than the Swank group. Adherence to both diets was associated with reduced fatigue and improved quality of life in patients with RRMS. However, due to the lack of sufficient data and high-quality randomized controlled trials it is not possible for either diet to be recommended in clinical practice [34]. A review by Simpson-Yap et al. analyzed the use of the above-described diets by patients with MS. Greater adherence to MS-specific diets was associated with higher socioeconomic status and higher quality of life. Adherence to any diet program was associated with higher overall diet quality [35].
This review also described other dietary programs used in MS such as the Best Bet diet and the Overcoming MS Diet (OMS diet) [35]. The OMS diet program does not allow the consumption of meat, poultry, eggs, dairy, coconut oil or palm oil, but recommends a plant-based diet of whole foods and seafood. Adherence to the OMS program has been positively linked to improved physical and mental quality of life and reduced symptoms of fatigue and depression in people with MS.
The Best Bet diet program aims to reduce the effects of leaky gut syndrome by avoiding proteins that trigger an immune response; however, no studies have examined its effects in people with MS. It allows the consumption of meat (red meat in moderation), poultry, seafood, eggs, vegetables, and fruits; legumes and grains are not allowed.
More recently, dietary adherence was assessed in the Australian MS Longitudinal Study (AMSLS), which found that 21.2% of the 1,490 participants reported following a specific diet, and about 7.7% followed an MS-specific diet, including 6.3% on the OMS diet, 0.9% on Wahls and 0.5% of other Swank diet programs [36]. In the Simpson-Yap et al. cohort, 48.8% of participants used an MS-specific diet program, with the largest percentage – 38.1% – using the OMS diet program, followed by 6.3% the Swank and 3.1% the Wahls, and a smaller percentage (< 1.5%) using other types of diet [35]. Chenard et al. found that the Swank diet program was not associated with any deficiencies compared to general dietary recommendations in the US [37]. On the other hand, the Wahls program already promoted deficiencies in some nutrients and contained excessive amounts of SFAs and sodium [38]. Also, work by a team led by Fitzgerald indicated that the diets of those following both dietary programs were of better quality, as opposed to those with no dietary intervention [39]. Nutrient intakes from specific food groups or selected products are shown in Table 1.
Table 1.
Intake of specific nutrients in selected dietary models dedicated to multiple sclerosis patients
| Nutrient | McDougall diet | OMS diet | Swank diet | Wahls elimination diet |
|---|---|---|---|---|
| Fruits and vegetables | + *In moderation: avocado, non-starchy vegetables/soy-based products *Except: vegetable oil |
+ *In moderation: avocado |
+ | + “Except: white fruits or vegetables, legumes, nightshades, vegetable oil |
| Olives | N/I | N/I | N/I | + |
| Cereals and grains | + | + | + | - |
| Meat, poultry | + “Permitted: only skinless poultry *SFA from animals limit to 15 g/day |
+ Permitted: rendered animal fat/bacon grease |
||
| Seafood | - | + | + | + |
| Eggs | - | - | + “Yolks only |
- |
| Dairy | - | - | N/I | - |
| Nuts/seeds | In moderation | In moderation | N/I | Permitted: flax, hemp, walnut, sesame, tahini |
| Margarine | - | - | - | N/I |
| Butter | N/I | N/I | - | - |
| Coconut oil | N/I | - | - | N/I |
| Palm oil | N/I | - | N/I | N/I |
| Ghee | N/I | N/I | N/I | + |
N/I - no information
Fast mimicking diet
Fast mimicking diet (FMD) does not refer to a specific dietary protocol but encompasses numerous dietary interventions leading to a state of fasting for an extended period and low- or no-energy intake. In particular, common protocols include calorie restriction (CR), e.g., day 1 fasting, followed by day 2-8 very low-calorie diet and IF diet. Cignarella et al. conducted a study on an animal model in which they transplanted microbiota from a mouse fed on an IF regimen to one fed normally. The researchers concluded that intermittent fasting had an effect on the composition of the microbiota, enriching the Bacteroidaceae, Lactobacillaceae and Prevotellaceae families. The number of interleukin 17-producing T cells (IL-17) decreased, along with an increase in the number of Treg cells in the gut-associated lymphoid tissue (GALT). IF has an immunomodulatory effect that is at least partially mediated by the gut microbiome [40]. Fitzgerald et al. in a clinical trial examined the effects of intermittent fasting and a kilocalorie-restricted diet in patients with MS [41]. Both intermittent and daily calorie restriction were shown to be unlikely to be harmful methods of weight loss in this group, at least in the short term. Both CR diets were associated with trends toward improved cardiometabolic outcomes, and CR diets were associated with improved emotional well-being. The impact of the diets needs to be evaluated in the long term.
Anti-inflammatory diets
It has been suggested that oxidative stress also contributes significantly to MS. In addition, serum levels of antioxidant vitamins have been shown to be lower in MS patients compared to controls [42]. Recent work has shown a decrease in levels of the brain’s main antioxidant, glutathione, in progressive MS. However, the timing, extent, and mechanisms by which antioxidants contribute to MS are still unclear and need to be clarified [43]. A recent study by Armon-Omer et al. found that patients with MS had reduced levels of total serum antioxidant capacity [44]. Poor antioxidant capacity in severe MS may play a role in disease progression. A randomized clinical trial by Mousavi-Shirazi-Fard et al. showed that patients with MS who followed an anti-inflammatory diet showed improvements in quality of life and fatigue levels. The results also showed a significant increase in IL-4 levels (p = 0.022) [45]. A modified anti-inflammatory diet model characterized by a higher intake of vegetables, fruits, legumes, nuts, whole grains, olive oil and fish, and limited consumption of red meat, fats and sugars has been shown to reduce fatigue and improve quality of life in MS patients.
Polyphenols have anti-inflammatory, antimicrobial, antioxidant, anti-cancer, anti-diabetic, and neuroprotective effects. They can also counteract cytotoxicity and apoptosis through their immunomodulatory properties and by regulating innate and acquired immunity. Polyphenols have also been shown to reduce oxidative stress and inflammation [46]. Antioxidants can affect dendritic cell (DC) differentiation. In fact, resveratrol has been identified as affecting the differentiation of human DCs into monocytes, with a strong potential for regulatory action. Similarly, epigallocatechin gallate (EGCG) induces apoptosis and affects the phenotype of developing DCs [47]. A study of 66 patients with MS who were treated with grape seed capsules for one month found that the capsules positively affected physical and mental functioning, improving quality of life [48].
Flavonoid-rich foods may show potential to reduce fatigue in MS. It has been suggested that the functional properties of flavonoids allow them to penetrate the blood-brain barrier, potentially leading to improved neurosignaling, as well as the rehabilitation of neuronal function [49]. The results of this study conclusively showed that a cocoa drink rich in flavonoids caused a slight reduction in the effect of perceived fatigue in MS patients compared to a control drink low in flavonoids.
A study by Coe et al. determined the effect of flavonoids on fatigue and tiredness in RRMS. During the 6-week intervention, participants with RRMS (n = 40) consumed a high or low flavonoid cocoa beverage daily. Fatigue and tiredness were measured at three visits (weeks 0, 3 and 6). It was shown that the high-flavonoid beverage showed the potential to reduce fatigue and fatigability in patients with RRMS [50].
Mediterranean diet
In MS, the Mediterranean diet (MD) may work directly by modulating chronic inflammation or indirectly by reducing chronic comorbidities [8]. The MD is a well-studied dietary pattern that has been linked to a reduced risk of developing MS, a reduction in disability during the course of the disease, which is associated with an overall improvement in the course of MS [51, 52]. A study involving a small group of MS patients (n = 20) found that those who consumed a MD rich in fruits and vegetables had reduced levels of IL-17 produced by the expression of T helper cells (Th cells), also known as CD4+ T-cells, and improve after 12 months [53]. A cross-sectional study involving adult patients with MS (n = 102) found no association between adherence to a MD and disease symptoms [54]. However, it was shown that participants who consumed less than one serving of red meat per day and consumed more fish had significantly lower fatigue scores. Interestingly, in this study omega-3 supplementation was positively associated with the severity of fatigue.
Mediterranean-DASH intervention for neurodegenerative delay diet
The MIND diet is a dietary pattern called the Mediterranean-DASH intervention for neurodegenerative delay. A key tenet of the MIND diet is the consumption of low-processed foods, particularly those of plant origin (i.e., an increase in the consumption of dark green leafy vegetables, berries, pulses, whole grain cereal products and nuts), and a reduction in animal products rich in saturated fatty acids [55]. In a study by Noormohammadi et al. a higher MIND diet score was statistically significantly associated with a reduced likelihood of MS [56]. This finding describes that adherence to the MIND diet and a higher intake of green leafy vegetables and beans is associated with a reduced likelihood of MS. In addition, a high intake of cheese, cookies and sweets, and fried/fast foods leads to an increased risk of MS. Poultry as a healthy ingredient was also associated with higher odds. Therefore, it seems that following the MIND diet may be a beneficial way to reducing the odds of experiencing MS [56].
Elimination diets – gluten-free diet
A recent publication reported that many websites offering dietary advice for MS patients recommend abstaining from gluten [57]. Eliminating gluten from the diet is also part of the “Wahls diet”, which as we have seen includes following a modified paleo diet [58]. There is growing evidence that gluten can affect the human body by increasing intestinal permeability, activating the innate immune system, and increasing blood brain barrier (BBB) permeability, causing cross-reactivity with nerve protein and activating autoreactive T cells. The incidence and risk of developing MS in patients with celiac disease has been evaluated, but no significant association has been demonstrated [59]. There is still no evidence of a strong enough association to recommend a gluten-free diet for patients with MS [60].
CONCLUSIONS
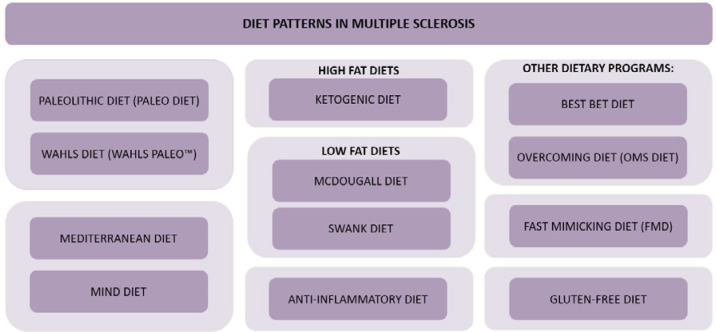
There is still a lack of good-quality research on not only nutrition and the effect of diet on the development of MS, but especially describing the exact mechanisms underlying the disease. Perhaps a more thorough understanding of the mechanisms of myelin regeneration will make it possible to increase the effectiveness of treatment by increasing the number of myelin-producing cells or to stimulate these cells to make myelin more efficiently. In the wake of these discoveries, as in diseases such as Alzheimer’s and Parkinson’s, the role of nutrition may become dramatically more important over the next few years. Figure I summarizes the diets and dietary patterns discussed so far in the scientific literature in the context of MS.
Figure I.
Diets and nutritional patterns discussed in the context of multiple sclerosis
The following table (Table 2) shows the advantages and disadvantages of the dietary models discussed in the context of MS and the overall health of patients.
Table 2.
Advantages and disadvantages of selected diets and dietary patterns discussed in the context of multiple sclerosis (MS)
| Diet or nutritional pattern | Convenience | Drawbacks |
|---|---|---|
| Anti inflammatory diet |
There are many reports of the positive effects of antioxidants and an anti-inflammatory diet on the quality of life and reduced fatigue of MS patients (44-46, 48, 50). Polyphenols affect MS patients on many levels, as the range of their actions is wide and includes, among others: antiinflammatory, neuroprotective, immunomodulatory effects; they counteract cytotoxicity and apoptosis, and can affect dendritic cell differentiation (46, 47). | Lack of clear explanations regarding, among other things, the timing, extent, and mechanisms by which antioxidants affect MS (43). |
| Fast mimicking diet (FMD) | A study in animal models has provided promising findings on the effects of FMD on microbiota composition, a decrease in the number of T lymphocytes and an increase in Treg cells in the GALT (40). A study involving MS patients showed no harm from short-term FMDs (41). |
FMDs do not have clearly defined rules; rather, they are a collection of different dietary interventions related to fasting and low energy intake, which can be problematic to apply and cause possible nutritional deficiencies (especially in overweight patients) (61). There are few studies examining this eating model in MS patients, including animal studies (40, 41). |
| Gluten-free diet | There are reports that gluten may affect the increase of intestinal permeability through activation of the innate immune system (59). Increases BBB permeability, causes cross-reactivity with nerve protein and activates autoreactive T cells (59). |
Increased risk of deficiencies (elimination diet) (62-64). There is no evidence of a sufficiently strong relationship between this diet and MS (60). |
| High fat diets: ketogenic diet | In clinical trials involving MS patients, the following have been noted: decreased disability, improved quality of life, decreased fatigue, decreased body fat and increased lean body mass, and decreased inflammation (through effects on enzymes involved in antioxidant processes) (23, 25, 26). The ketogenic diet in the study was based on the principles of the Mediterranean diet (26). |
Previous studies have often been conducted on a small sample of MS patients or on animal models (22, 25, 26, 28). The ketogenic diet may be associated with nutritional deficiencies due to its affiliation with elimination diets (64). |
| Low fat diets: -McDougall diet -Swank diet |
Low saturated fat intake in both of these diets (29-31). Some researchers have indicated the potential of the McDougall diet to manage fatigue in MS, currently there are no such reports (29). |
Both of these dietary models, due to their very low fat content in the diet, can affect deficiencies in fat-soluble vitamins (A, D, E, K) (29-31). These diets involve numerous restrictions and the exclusion of quite a range of products. The McDougall diet, for example, excludes dairy, which contains many minerals important in MS patients (calcium, vitamin D) due to weakened muscle strength or a greater risk of developing osteoporosis (29, 65). |
| Mediterranean diet |
A well-studied dietary pattern (51, 52, 66). The diet has been linked to a reduced risk of MS and improved course of MS and disability (53). Elements of the Mediterranean diet, such as reduced intake of red meat, saturated fatty acids, sweets and increased fish consumption, appear promising for reducing MS symptoms or fatigue severity (66). |
Inconclusive findings for MS patient (54). The findings on the effectiveness of the Mediterranean diet in MS patients should be confirmed by further intervention studies (66). |
| MIND diet | High antioxidant potential (67). Dietary cognitive enhancement, proven effective in reducing the risk of Alzheimer's disease, MS and their progression, among other things (55, 56, 68). |
A small number of studies involving this diet (a relatively new dietary model). Larger and more thorough studies are still needed for it to be widely recommended. Studies mainly focus on the effect of this diet on Alzheimer's disease (55, 56). |
| The paleo diet and its modification (Wahls Paleo™) | Reports of reducing perceived fatigue and improving mental and physical quality of life in patients with progressive MS or increasing exercise capacity (17, 19, 20). | The risk of nutritional deficiencies, such as B vitamins (limited consumption of cereals) and calcium and vitamin D deficiencies (not consuming dairy products) (16). High risk of bias in the cited study (17, 18). Wahls' program was associated with deficiencies in some nutrients and contained excessive amounts of SFA and sodium (38). |
| Other dietary programs: -Best Bet diet -Overcoming MS diet (OMS diet) |
It has been reported that OMS was associated with improved physical and mental quality of life and reduced symptoms of fatigue and depression in people with MS (35). | Long-term studies on these programs are lacking, and the patient groups studied were few (35). Both programs involved numerous dietary restrictions (35, 36). No effects have been reported in the effects of the Best Bet diet program on the immune response in MS patients (35). |
Conflict of interest
Absent.
Financial support
Absent.
References
- 1.Male D, Peebles RS, Male V. Immunology, 9th edition. London: Elsevier; 2020, pp. 202, 229, 638-440, 732-733, 738-739, 1047, 1057, 1099-1100. [Google Scholar]
- 2.Jones HR, Srinivasan J, Allam GJ, Baker RA. Netter’s Neurology, 2nd edition. Section IX. Multiple Sclerosis and Other Autoimmune CNS Demyelinating Disorders. Philadelphia: Elsevier; 2012, pp. 386-402. [Google Scholar]
- 3.Cayre M, Falque M, Mercier O, Magalon K, Durbec P. Myelin repair: from animal models to humans. Front Cell Neurosci 2021; 15: 604865. 10.3389/fncel.2021.604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton C, King R, Rechtman L, Kaye W, Leray E, Ann Marrie R, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816-1821. 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alroughani R, Akhtar S, Ahmed S, Behbehani R, Al-Hashel J. Is time to reach EDSS 6.0 faster in patients with late-onset versus young-onset multiple sclerosis? PLoS One 2016; 11: e0165846. 10.1371/journal.pone.0165846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pozzilli C, Pugliatti M; Paradig MS Group . An overview of pregnancy-related issues in patients with multiple sclerosis. Eur J Neurol 2015; 22 Suppl 2: 34-39. 10.1111/ene.12797. [DOI] [PubMed] [Google Scholar]
- 7.Jörg S, Grohme DA, Erzler M, Binsfeld M, Haghikia A, Müller DN, et al. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell Mol Life Sci 2016; 73: 4611-4622. 10.1007/s00018-016-2311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito S, Bonavita S, Sparaco M, Gallo A, Tedeschi G. The role of diet in multiple sclerosis: a review. Nutritional Neurosci 2018; 21: 377-390. 10.1080/1028415X.2017.1303016. [DOI] [PubMed] [Google Scholar]
- 9.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of. “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 2014; 14: 404. 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014; 40: 833-842. 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Altowaijri G, Fryman A, Yadav V. Dietary interventions and multiple sclerosis. Curr Neurol Neurosci Rep 2017; 17: 28. 10.1007/s11910-017-0732-3. [DOI] [PubMed] [Google Scholar]
- 12.Riccio P, Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro 2015; 7: 1-20. 10.1177/1759091414568185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Malnutrition in a sample of community-dwelling people with Parkinson’s disease. PLoS One 2013; 8: e53290. 10.1371/journal.pone.0053290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos R, Bretón I, Cereda E, Desport JC, Dziewas R, Genton L, et al. ESPEN guideline clinical nutrition in neurology. Clin Nutr 2018; 37: 354-396. 10.1016/j.clnu.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr 2015; 102: 922-932. 10.3945/ajcn.115.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanara S, Aprile M, Iacono S, Schirò G, Bianchi A, Brighina F, et al. The role of nutritional lifestyle and physical activity in multiple sclerosis pathogenesis and management: a narrative review. Nutrients 2021; 13: 3774. 10.3390/nu13113774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irish AK, Erickson CM, Wahls TL, Snetselaar LG, Darling WG. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis 2017; 7: 1-18. 10.2147/DNND.S116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks NE, Jackson-Tarlton CS, Vacchi L, Merdad R, Johnston BC. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst Rev 2020; 5: CD004192. 10.1002/14651858.CD004192.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JE, Bisht B, Hall MJ, Rubenstein LM, Louison R, Klein DT, Wahls TL. A multimodal, nonpharmacologic intervention improves mood and cognitive function in people with multiple sclerosis. J Am Coll Nutr 2017; 36: 150-168. 10.1080/07315724.2016.1255160. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell KF, Wahls T, Browne RW, Rubenstein L, Bisht B, Chenard CA, et al. Lipid profile is associated with decreased fatigue in individuals with progressive multiple sclerosis following a diet-based intervention: results from a pilot study. PLoS One 2019; 14: e0218075. 10.1371/journal.pone.0218075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titcomb TJ, Bisht B, Moore DD 3rd, Chhonker YS, Murry DJ, Snetselaar LG, Wahls TL. Eating pattern and nutritional risks among people with multiple sclerosis following a modified Paleolithic diet. Nutrients 2020; 12: 1844. 10.3390/nu12061844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh S, Dupuis N, Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res 2020; 167: 106454. 10.1016/j.eplepsyres.2020.106454. [DOI] [PubMed] [Google Scholar]
- 23.Bock M, Karber M, Kuhn H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine 2018; 36: 293-303. 10.1016/j.ebiom.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenton JN, Banwell B, Bergqvist AGC, Lehner-Gulotta D, Gampper L, Leytham E, et al. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2019; 6: e565. 10.1212/NXI.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenton JN, Lehner-Gulotta D, Woolbright E, Banwell B, Bergqvist AGC, Chen S, et al. Phase II study of ketogenic diets in relapsing multiple sclerosis: safety, tolerability and potential clinical benefits. J Neurol Neurosurg Psychiatry 2022; 93: 637-644. 10.1136/jnnp-2022-329074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benlloch M, López-Rodríguez MM, Cuerda-Ballester M, Drehmer E, Carrera S, et al. Satiating effect of a ketogenic diet and its impact on muscle improvement and oxidation state in multiple sclerosis patients. Nutrients 2019; 11: 1156. 10.3390/nu11051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock M, Steffen F, Zipp F, Bittner S. Impact of dietary intervention on serum neurofilament light chain in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021; 9: e1102. 10.1212/NXI.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol 2017; 8: 1141. 10.3389/fmicb.2017.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altowaijri G, Fryman A, Yadav V. Dietary interventions and multiple sclerosis. Curr Neurol Neurosci Rep 2017; 17: 28. 10.1007/s11910-017-0732-3. [DOI] [PubMed] [Google Scholar]
- 30.Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet 1990; 336: 37-39. 10.1016/0140-6736(90)91533-g. [DOI] [PubMed] [Google Scholar]
- 31.Swank RL, Goodwin J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003; 19: 161-162. 10.1016/s0899-9007(02)00851-1. [DOI] [PubMed] [Google Scholar]
- 32.Wahls TL, Titcomb TJ, Bisht B, Eyck PT, Rubenstein LM, Carr LJ, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the WAVES randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin 2021; 7: 20552173211035399. 10.1177/20552173211035399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald KC, Tyry T, Salter A, Cofield SS, Cutter G, Fox RJ, et al. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult Scler Relat Disord 2018; 22: 12-18. 10.1016/j.msard.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Wahls T, Scott MO, Alshare Z, Rubenstein L, Darling W, Carr L, et al. Dietary approaches to treat MS-related fatigue: comparing the modified Paleolithic (Wahls Elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: study protocol for a randomized controlled trial. Trials 2018; 19: 309. 10.1186/s13063-018-2680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson-Yap S, Nag N, Jakaria M, Jelinek GA, Neate S. Sociodemographic and clinical characteristics of diet adherence and relationship with diet quality in an international cohort of people with multiple sclerosis. Mult Scler Relat Disord 2021; 56: 103307. 10.1016/j.msard.2021.103307. [DOI] [PubMed] [Google Scholar]
- 36.Marck CH, Probst Y, Chen J, Taylor B, van der Mei I. Dietary patterns and associations with health outcomes in Australian people with multiple sclerosis. Eur J Clin Nutr 2021; 75: 1506-1514. 10.1038/s41430-021-00864-y. [DOI] [PubMed] [Google Scholar]
- 37.Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient composition comparison between the low saturated fat Swank diet for multiple sclerosis and healthy U.S.-style eating pattern. Nutrients 2019; 11: 616. 10.3390/nu11030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient composition comparison between a modified Paleolithic diet for multiple sclerosis and the recommended healthy U.S.-style eating pattern. Nutrients 2019; 11: 537. 10.3390/nu11030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald KC, Tyry T, Salter A, Cofield SS, Cutter G, Fox R, Ann Marrie R. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018; 90: e1-e11. 10.1212/WNL.0000000000004768. [DOI] [PubMed] [Google Scholar]
- 40.Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab 2018; 27: 1222-1235.e6. 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzgerald KC, Vizthum D, Henry-Barron B, Schweitzer A, Cassard SD, Kossoff E, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord 2018; 23: 33-39. 10.1016/j.msard.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besler HT, Comoğlu S, Okçu Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci 2002; 5: 215-220. 10.1080/10284150290029205. [DOI] [PubMed] [Google Scholar]
- 43.Choi IY, Lee P, Adany P, Hughes AJ, Belliston S, Denney DR, Lynch SG. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult Scler 2018; 24: 1029-1038. 10.1177/1352458517711568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armon-Omer A, Waldman C, Simaan N, Neuman H, Tamir S, Shahien R. New insights on the nutrition status and antioxidant capacity in multiple sclerosis patients. Nutrients 2019; 11: 427. 10.3390/nu11020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mousavi-Shirazi-Fard Z, Mazloom Z, Izadi S, Fararouei M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing-remitting multiple sclerosis patients: a randomized clinical trial. Int J Neurosci 2021; 131: 657-665. 10.1080/00207454.2020.1750398. [DOI] [PubMed] [Google Scholar]
- 46.Shakoor H, Feehan J, Apostolopoulos V, Platat C, Salem Al Dhaheri A, Ali HI. Immunomodulatory effects of dietary polyphenols. Nutrients 2021; 13: 728. 10.3390/nu13030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoneyama S, Kawai K, Tsuno NH, Okaji Y, Asakage M, Tsuchiya T, et al. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J Allergy Clin Immunol 2008; 121: 209-214. 10.1016/j.jaci.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 48.Siahpoosh A, Majdinasab N, Derakhshannezhad N, Khalili HR, Malayeri A. Effect of grape seed on quality of life in multiple sclerosis patients. J Contemp Med Sci 2018; 4: 148-152. [Google Scholar]
- 49.Coe S, Axelsson E, Murphy V, Santos M, Collett J , Clegg M. Flavonoid rich dark cocoa may improve fatigue in people with multiple sclerosis, yet has no effect on glycaemic response: an exploratory trial. Clin Nutr ESPEN 2017; 21: 20-25. 10.1016/j.clnesp.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Coe S, Cossington J, Collett J, Soundy A, Izadi H, Ovington M, et al. A randomised double-blind placebo-controlled feasibility trial of flavonoid-rich cocoa for fatigue in people with relapsing and remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2019; 90: 507-513. 10.1136/jnnp-2018-319496. [DOI] [PubMed] [Google Scholar]
- 51.Katz Sand I, Benn EKT, Fabian M, Fitzgerald KC, Digga E, Deshpande R, et al. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: a pilot study. Mult Scler Relat Disord 2019; 36: 101403. 10.1016/j.msard.2019.101403. [DOI] [PubMed] [Google Scholar]
- 52.Razeghi-Jahromi S, Doosti R, Ghorbani Z, Saeedi R, Abolhasani M, Akbari N, et al. A randomized controlled trial investigating the effects of a mediterranean-like diet in patients with multiple sclerosis-associated cognitive impairments and fatigue. Curr J Neurol 2020; 19: 112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saresella M, Mendozzi L, Rossi V, Mazzali F, Piancone F, LaRosa F, et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front Immunol 2017; 8: 1391-1402. 10.3389/fimmu.2017.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ertaş Öztürk Y, Helvaci EM, Sökülmez Kaya P, Terzi M. Is Mediterranean diet associated with multiple sclerosis related symptoms and fatigue severity? Nutr Neurosci 2022: 1-7. 10.1080/1028415X.2022.2034241. [DOI] [PubMed] [Google Scholar]
- 55.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015; 11: 1007-1014. 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noormohammadi M, Ghorbani Z, Naser Moghadasi A, Saeedirad Z, Shahemi S, Ghanaatgar M, et al. MIND diet adherence might be associated with a reduced odds of multiple sclerosis: results from a case-control study. Neurol Ther 2022; 11: 397-412. 10.1007/s40120-022-00325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckett JM, Bird ML, Pittaway JK, Ahuja KD. Diet and multiple sclerosis: scoping review of web-based recommendations. Interact. J Med Res 2019; 8: e10050. 10.2196/10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passali M, Josefsen K, Frederiksen JL, Antvorskov JC. Current Evidence on the efficacy of gluten-free diets in multiple sclerosis, psoriasis, type 1 diabetes and autoimmune thyroid diseases. Nutrients 2020; 12: 2316. 10.3390/nu12082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgerald KC, Munger KL, Hartung HP, Freedman MS, Montalbán X, Edan G, et al. BENEFIT Study Group . Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol 2017; 82: 20-29. 10.1002/ana.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomsen HL, Jessen EB, Passali M, Frederiksen JL. The role of gluten in multiple sclerosis: a systematic review. Mult Scler Relat Disord 2019; 27: 156-163. 10.1016/j.msard.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Damms-Machado A, Weser G, Bischoff SC. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr J 2012; 11: 34. 10.1186/1475-2891-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis M, Lee AR, McCarthy T. Nutritional considerations of the gluten-free diet. Gastroenterol Clin North Am 2019; 48: 53-72. 10.1016/j.gtc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: a review. Clin Nutr 2016; 35: 1236-1241. 10.1016/j.clnu.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Andrewski E, Cheng K, Vanderpool C. Nutritional deficiencies in vegetarian, gluten-free, and ketogenic diets. Pediatr Rev 2022; 43: 61-70. 10.1542/pir.2020-004275. [DOI] [PubMed] [Google Scholar]
- 65.Gupta S, Ahsan I, Mahfooz N, Abdelhamid N, et al. Osteoporosis and multiple sclerosis: risk factors, pathophysiology, and therapeutic interventions. CNS Drugs 2014; 28: 731-742. 10.1007/s40263-014-0173-3. [DOI] [PubMed] [Google Scholar]
- 66.Ertaş Öztürk Y, Helvaci EM, Sökülmez Kaya P, Terzi M. Is Mediterranean diet associated with multiple sclerosis related symptoms and fatigue severity? Nutr Neurosci 2023; 26: 228-234. 10.1080/1028415X.2022.2034241. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Morris MC, Dhana K, Ventrelle J, Johnson K, Bishop L, et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials 2021; 102: 106270. 10.1016/j.cct.2021.106270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015; 11: 1015-1022. 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]