Abstract
Background
Benzodiazepines are commonly prescribed for insomnia management but are often associated with negative safety outcomes such as falls and abuse, particularly among older adults.
Objective
The purpose of this real-world study was to compare the impact of benzodiazepines, low-dose trazodone, and zolpidem immediate release (IR) on healthcare resource utilization (HCRU), and costs among older adults (age ≥ 65 years) with insomnia in the US.
Methods
Using the IBM MarketScan Medicare Supplemental Database, older adults with >1 physician-assigned diagnosis of insomnia and treated with benzodiazepines were matched 1:1 on age, sex, and index-date to individuals treated with trazodone, and separately matched 1:1 on age and sex, to individuals treated with zolpidem immediate release (IR). Between-groups differences were analyzed using general linear models (GLMs) that controlled for multiple confounders.
Results
Significant between-groups differences in HCRU and costs were observed such that relative to zolpidem IR and separately relative to low-dose trazodone, benzodiazepines were consistently associated with worsened outcomes.
Conclusion
These findings build upon and extend prior knowledge on the negative impact of benzodiazepines and suggest directions for future research.
Keywords: benzodiazepine, trazodone, zolpidem, elderly, insomnia, healthcare resource utilization, cost
Plain Language Summary
Insomnia is common and costly among older adults. Significant differences in healthcare resource utilization and costs were observed between individuals treated with benzodiazepines, low-dose trazodone, and zolpidem IR. Relative to zolpidem IR and separately relative to low-dose trazodone, benzodiazepines were consistently associated with worsened outcomes among older patients.
Introduction
Sleep quality typically worsens with age, and half of older adults report poor sleep quality.1–4 In this population, one of the most common clinical sleep disorder diagnoses is insomnia disorder, defined as difficulty initiating or maintaining sleep with associated daytime consequence.5 Among older adults, the prevalence of insomnia ranges from 25% to 40%,1 a prevalence greater than twice that (9–12%)6 observed in the general population. Importantly, among older adults, insomnia disorder is associated with increased medical and psychiatric morbidity, mortality, and diminished quality of life.7,8 In addition to these key health outcomes among older adults, insomnia is associated with dramatically increased economic burden, including direct medical costs as well as indirect costs that are borne by patients, providers, payers, employers, and society.9,10 Among the general population, total direct and indirect costs of insomnia in the US exceed $100 billion per year,10 and among older adults, insomnia is associated with increased HCRU and can be particularly costly.11,12 For example, a recent study found that relative to non-sleep disordered controls, Medicare beneficiaries with untreated insomnia demonstrated $63,607 (in 2013 USD) greater 11-month, all-cause healthcare costs as well as greater healthcare resource utilization across multiple points of service.13
Pharmacotherapy remains the most prescribed treatment for insomnia by a wide margin. This is true even though consensus recommendations advise cognitive-behavioral treatment – insomnia (CBT-I) as first-line treatment for insomnia.14–18 CBT-I is underutilized primarily due to a shortage of trained specialist providers,19 as well as clinician and patient-level barriers that limit uptake of CBT-I. Multiple medications are used for treatment of insomnia, whether FDA approved to treat the condition (eg, zolpidem) or not (eg, trazodone). In the US, the three most commonly prescribed insomnia medications are z-drugs such as zolpidem (1.23% of US adults), low-dose trazodone (<150 mg daily; 0.97% of adults), and benzodiazepines (0.40% of adults).20 However, these medications incur well-documented increased risk for falls, fractures, and adverse cognitive side effects among older adults.21–26 The Beers Criteria published by the American Geriatrics Society discourages use of benzodiazepines and z-drugs (eg, zolpidem) among older adults,23 and these sleep medications must be used with caution in this population. Yet despite these guidelines, the prevalence of benzodiazepine use among older adult Medicare beneficiaries increased from 1.1% to 17.6% between 2012 and 2013, when benzodiazepines were added to the Medicare formulary.27 Among the general population including older adults, the use of low-dose trazodone increased and use of zolpidem decreased between 2011 and 2018.28
Few studies have sought to compare the impact of these medications on adverse outcomes among older adults with insomnia.29,30 To address this known gap in the literature, the purpose of the present study was to compare the impact of benzodiazepines, low-dose trazodone, and zolpidem IR on HCRU and costs. We hypothesized that relative to trazodone and zolpidem IR, benzodiazepines would be associated with increased HCRU and costs, due to the particularly high risk of negative adverse effects such as falls. Exploratory objectives assessed the impact on HCRU and costs of short- versus long-acting benzodiazepines and of FDA-approved benzodiazepines for insomnia versus benzodiazepines not FDA approved for insomnia.
Methods
Study Design and Data Source
This retrospective cohort study used medical and pharmacy administrative claims data from the IBM® MarketScan® Medicare Supplemental Database. The Medicare Supplemental Database includes all Medicare administrative claims with diagnostic, procedural and medication codes and associated costs for services utilized by individuals with employer-sponsored supplemental coverage to Medicare.
The study period was 01 Jan 2014 through 31 Dec 2019. To test our hypothesis (relative to trazodone and separately to zolpidem IR, benzodiazepines are associated with increased HCRU and costs), three cohorts were developed: a cohort of beneficiaries with insomnia treated with benzodiazepines, a cohort of beneficiaries with insomnia treated with low-dose trazodone (≤150mg daily), and a cohort of beneficiaries with insomnia treated with zolpidem IR (by far the most common formulation of zolpidem). Cohort status was defined based on index medication received. To facilitate comparisons between cohorts, individuals in the benzodiazepine cohort were matched 1:1 on age and sex with individuals treated with low-dose trazodone. Separately, individuals in the benzodiazepine cohort were matched 1:1 on age and sex with individuals treated with zolpidem IR. For all cohorts, the earliest insomnia medication fill date was considered the index date. Note that all regression analyses for the study also included multiple covariates as described below. The reason only age and sex were controlled for in matching was because of the limited sample sizes. Additional inclusion criteria for all analyses included adults ≥18 years old with ≥12 months of continuous health plan enrollment both before (ie, “baseline” period) and after (ie, “follow-up” period) the index date. Exclusion criteria included the presence of a prescription claim for an insomnia medication of interest during baseline; presence of any sleep-related diagnoses other than insomnia (ie, ICD-9-CM or ICD-10-CM diagnostic codes for hypersomnias, sleep-related breathing disorders, circadian rhythm sleep disorders, parasomnias, sleep-related movement disorders, or drug-induced sleep disorders); presence of past sleep-related treatment (ie, Current Procedural Terminology [CPT] codes for sleep study procedures, sleep service codes, home sleep apnea testing, or Healthcare Common Procedure Coding System [HCPCS] codes for durable medical equipment); and missing age or sex. This study meets all ethical requirements for research involving retrospective data. Because this study was a retrospective analysis utilizing fully de-identified administrative claims data, Institutional Review Board (IRB) review and patient consent were not required. This study complied with all relevant data protection and privacy regulations.
Benzodiazepines included in this study were estazolam, flurazepam, temazepam, triazolam, quazepam, alprazolam clonazepam, clorazepate, chlordiazepoxide (alone or in combination with amitriptyline or clidinium), diazepam, lorazepam, and oxazepam. These medications were further classified as short-acting and long-acting. Short-acting benzodiazepines have short-to-intermediate duration of elimination half-life as per the Beers’ criteria: alprazolam, estazolam, lorazepam, oxazepam, temazepam, and triazolam. Long-acting benzodiazepines included clorazepate, chlordiazepoxide (alone or in combination with amitriptyline or clidinium), clonazepam, diazepam, flurazepam, and quazepam. Finally, benzodiazepines were categorized as on- and off-label medications for insomnia. Benzodiazepines with an FDA-approved indication for insomnia were considered on-label: estazolam, flurazepam, temazepam, triazolam, and quazepam. All others were categorized as off-label: alprazolam clonazepam, clorazepate, chlordiazepoxide (alone or in combination with amitriptyline or clidinium), diazepam, lorazepam, and oxazepam.
Healthcare Resource Utilization (HCRU) and Costs
HCRU was determined based on multiple individual points of service, including inpatient hospital stays, emergency department (ED) visits, and outpatient visits, as well as all-cause and insomnia-specific pharmacy claims. Costs were inflated to 2019 costs using the medical care component of the consumer price index, then estimated per patient per month (PPPM) based on these same individual points of service as well as total costs.
Covariates
All regression models for the study analyses controlled for the following covariates: age, sex, geographic region, health plan type, Elixhauser Comorbidity Index (ECI) score, hypertension, type 2 diabetes, depression, osteoporosis, Alzheimer’s disease, antihypertensive use, oral hypoglycemic use and antidepressant use. Note that the ECI is a validated measure that combines weighted scores for 30 comorbidities demonstrated to have an impact on healthcare utilization and costs. The ECI was specifically created for use for administrative claims data and uses original Elixhauser weights for the comorbidities rather than van Walraven weights which are used to calculate mortality. The specific covariates that were selected as the demographic characteristics are commonly used in claims analyses and the clinical characteristics were identified as having a relationship to insomnia.
Analytic Plan
Descriptive statistics (means with standard deviation, proportion for continuous and categorical variables, respectively) were used to describe demographic and clinical characteristics of individuals in each of the three cohorts. To test our hypothesis (relative to trazodone and separately to zolpidem IR, benzodiazepines are associated with increased HCRU and costs), we first created a series of generalized linear models (GLMs) with Poisson distribution and log link with 95% confidence intervals (CI) to evaluate differences in HCRU between cohorts (ie, benzodiazepine vs trazodone; benzodiazepine vs zolpidem IR). Rate ratios (RRs) with 95% confidence intervals (CIs) are reported. Next, to evaluate differences in PPPM costs between cohorts, we created a series of GLMs with gamma distribution and log link. Cost ratios (CRs) with 95% CI are reported.
In addition, we performed an exploratory series of Generalized Linear Models (GLMs) to examine the potential impact of differences in duration of action (short-acting vs long-acting) and FDA-approval for insomnia (yes vs no) between various benzodiazepines. Duration of action of benzodiazepines varies considerably (eg, from 3.5 hours for triazolam to >24 hours for flurazepam);31 in this study short-acting was defined as <11 hours, and long-acting was defined as having a duration of action ≥11 hours.
All analyses were conducted using SAS Software Version 9.4.
Results
Participants
The final sample included N=10,707 older adults with insomnia and treated with benzodiazepines (M age = 76.9 years [SD 7.9 years], 68.6% female). As described above, these individuals were matched 1:1 on age and sex with older adults with insomnia treated with trazodone (n = 9192) and separately, zolpidem IR (n = 9075). In matching trazodone with benzodiazepines and zolpidem IR with benzodiazepines, 1515 benzodiazepine patients (14.1%) and 1632 benzodiazepine patients (15.2%) were lost, respectively. Table 1 presents baseline demographic and clinical characteristics for the matched cohorts.
Table 1.
Patient Demographics and Other Baseline Characteristics, Matched Benzodiazepine–Trazodone Cohorts and Matched Benzodiazepine–Zolpidem IR Cohorts
| Category | Matched Benzodiazepine - Trazodone Cohorts | Matched Benzodiazepine - Zolpidem IR Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Benzodiazepines | Trazodone | p value | Benzodiazepines | Zolpidem IR | p value | |||||
| N | % | N | % | N | % | N | % | |||
| Total | 9192 | 100.00% | 9192 | 100.00% | 9075 | 100.00% | 9075 | 100.00% | ||
| Gender | ||||||||||
| Male | 2864 | 31.16% | 2864 | 31.16% | 1.000 | 2880 | 31.74% | 2880 | 31.74% | 1.000 |
| Female | 6328 | 68.84% | 6328 | 68.84% | 6195 | 68.26% | 6195 | 68.26% | ||
| Age at Index Date | ||||||||||
| Mean (SD) | 76.76 | 7.84 | 76.76 | 7.84 | 1.000 | 76.39 | 7.49 | 76.39 | 7.49 | 1.000 |
| ECI Score (weighted) | ||||||||||
| Mean (SD) | 4.79 | 7.36 | 5.03 | 7.73 | 0.026 | 4.69 | 7.35 | 4.79 | 7.40 | 0.380 |
| Geographic Region | ||||||||||
| Northeast | 2234 | 24.30% | 2057 | 22.38% | 0.000 | 2156 | 23.76% | 2646 | 29.16% | 0.000 |
| North Central | 2169 | 23.60% | 2549 | 27.73% | 2125 | 23.42% | 2139 | 23.57% | ||
| South | 3614 | 39.32% | 3412 | 37.12% | 3615 | 39.83% | 3109 | 34.26% | ||
| West | 1158 | 12.60% | 1159 | 12.61% | 1163 | 12.82% | 1165 | 12.84% | ||
| Unknown | 17 | 0.18% | 15 | 0.16% | 16 | 0.18% | 16 | 0.18% | ||
| Health plan type | ||||||||||
| Comprehensive | 3119 | 33.93% | 4267 | 46.42% | 0.000 | 3141 | 34.61% | 3701 | 40.78% | 0.000 |
| HMO | 1202 | 13.08% | 816 | 8.88% | 1183 | 13.04% | 701 | 7.72% | ||
| PPO | 3981 | 43.31% | 3414 | 37.14% | 3911 | 43.10% | 3828 | 42.18% | ||
| Other | 890 | 9.68% | 695 | 7.56% | 840 | 9.25% | 845 | 9.32% | ||
| Comorbidities* | ||||||||||
| Any Psychiatric disorder | 2450 | 26.65% | 2883 | 31.36% | 0.000 | 2515 | 27.71% | 1685 | 18.57% | 0.000 |
| Diabetes | 1918 | 20.87% | 2411 | 26.23% | 0.000 | 1909 | 21.04% | 2073 | 22.84% | 0.003 |
| Hypertension | 6519 | 70.92% | 6546 | 71.21% | 0.661 | 6382 | 70.33% | 6113 | 67.36% | 0.000 |
| COPD | 945 | 10.28% | 963 | 10.48% | 0.663 | 889 | 9.80% | 855 | 9.42% | 0.392 |
| Osteoporosis | 1593 | 17.33% | 1320 | 14.36% | 0.000 | 1551 | 17.09% | 1418 | 15.63% | 0.008 |
| Alzheimer’s disease | 1014 | 11.03% | 1375 | 14.96% | 0.000 | 1080 | 11.90% | 480 | 5.29% | 0.000 |
| Medication use | ||||||||||
| Antidepressants | 3449 | 37.52% | 3933 | 42.79% | 0.000 | 3500 | 38.57% | 2758 | 30.39% | 0.000 |
| Oral hypoglycemics | 1181 | 12.85% | 1640 | 17.84% | 0.000 | 1173 | 12.93% | 1433 | 15.79% | 0.000 |
| Antihypertensives | 6604 | 71.85% | 7050 | 76.70% | 0.000 | 6474 | 71.34% | 6635 | 73.11% | 0.008 |
| Duration of exposure | ||||||||||
| 0–3 months | 6039 | 65.70% | 5110 | 55.59% | 0.000 | 5933 | 65.38% | 6496 | 71.58% | 0.000 |
| >3–6 months | 1392 | 15.14% | 1189 | 12.94% | 1390 | 15.32% | 1366 | 15.05% | ||
| >6–9 months | 774 | 8.42% | 815 | 8.87% | 775 | 8.54% | 544 | 5.99% | ||
| >9–12 months | 987 | 10.74% | 2078 | 22.61% | 977 | 10.77% | 669 | 7.37% | ||
Notes: *Includes only comorbidities with at least 10% prevalence in one or more medication cohorts.
Abbreviations: ECI, Elixhauser Comorbidity Index; SD, standard deviation; COPD, chronic obstructive pulmonary disease; HMO, health maintenance organization; PPO, preferred provider organization.
For exploratory analyses, 8653 patients (80.8%) were prescribed short-acting and 2054 (19.2%) patients were prescribed long-acting benzodiazepines. In addition, 1964 patients (18.3%) were prescribed benzodiazepines FDA approved for insomnia and 8743 patients (81.7%) were prescribed benzodiazepines not FDA approved for insomnia.
Impact of Insomnia Medication on HCRU and Costs
HCRU: Benzodiazepines vs Trazodone
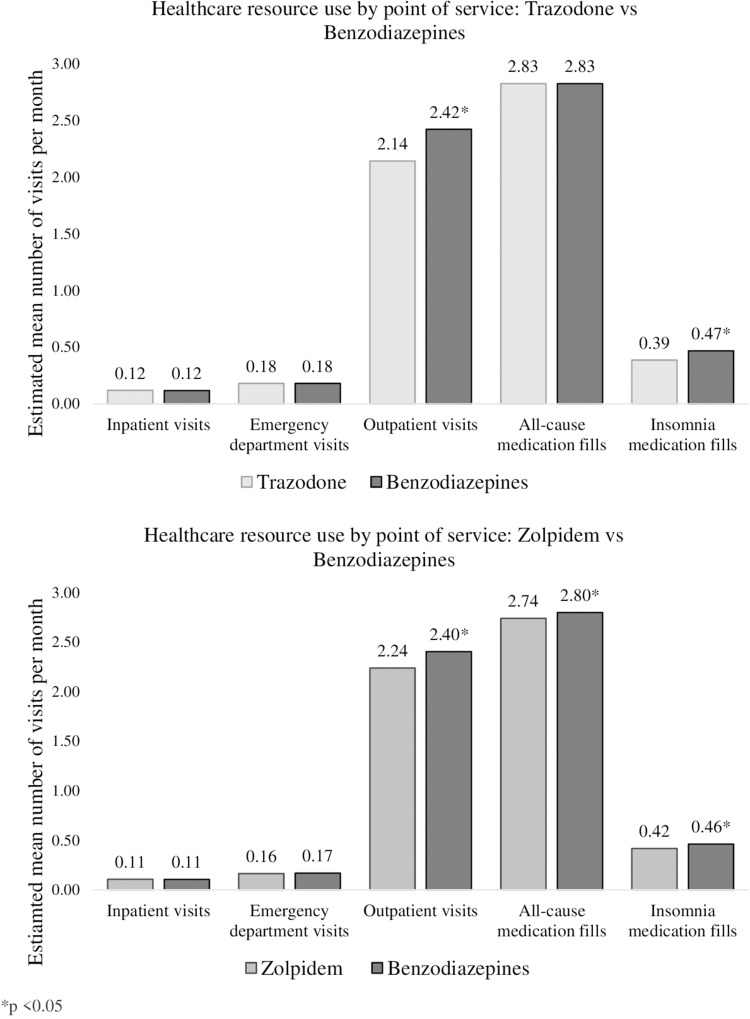
Few differences were observed between individuals treated with benzodiazepines and trazodone in terms of HCRU (Figure 1). Two exceptions were outpatient visits and index insomnia medication fills. Relative to individuals with insomnia treated with benzodiazepines, individuals treated with trazodone demonstrated fewer outpatient visits (2.4 vs 2.1, RR = 0.88 [0.87, 0.90]). Similarly, relative to individuals with insomnia treated with benzodiazepines, individuals treated with trazodone demonstrated fewer index insomnia medication fills (0.5 vs 0.4, RR = 0.83 [0.78, 0.87]). No other significant differences were observed.
Figure 1.
Differences in healthcare resource use between medication groups by point of service *p<0.05.
HCRU: Benzodiazepines vs Zolpidem IR
As presented in Figure 1, relative to individuals with insomnia treated with benzodiazepines, individuals treated with zolpidem IR demonstrated fewer outpatient visits (2.4 vs 2.2, RR = 0.93 [0.91, 0.95]), fewer all-cause medication fills (2.8 vs 2.7, RR = 0.98 [0.96, 1]), and fewer index insomnia medication fills (0.5 vs 0.4, RR = 0.91 [0.85, 0.96]). No other significant differences were observed.
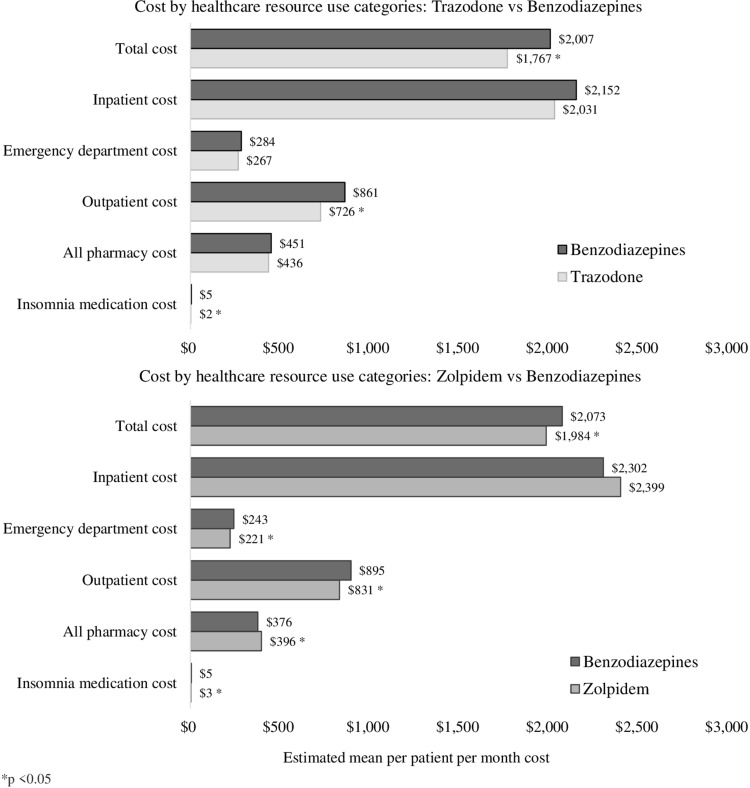
Costs: Benzodiazepines vs Trazodone
Relative to individuals with insomnia treated with benzodiazepines, individuals treated with trazodone demonstrated reduced total PPPM costs ($2006.89 vs $1767.23, CR = 0.88 [0.85, 0.91]), reduced outpatient PPPM costs ($861.05 vs $726.07, CR = 0.84 [0.81, 0.87]), and reduced index insomnia medication-specific PPPM costs ($5.10 vs $1.96, CR = 0.38 [0.37, 0.4]). No other significant differences were observed (see Table 2, Figure 2).
Table 2.
Adjusteda PPPM Costs by Index Medication for 12-Month Follow-Up
| Category | Trazodone vs Benzodiazepinesb | Category | Zolpidem IR vs Benzodiazepinesc | ||||
|---|---|---|---|---|---|---|---|
| N (%) | Estimated Mean | Cost Ratio with 95% CI | N (%) | Estimated Mean | Cost Ratio with 95% CI | ||
| Total Costs | Total Costs | ||||||
| Trazodone | 9192 (100%) | 1767 | 0.88 (0.85,0.91) | Zolpidem IR | 9075 (100%) | 1984 | 0.96 (0.92,0.99) |
| Benzodiazepines | 9192 (100%) | 2007 | Ref | Benzodiazepines | 9075 (100%) | 2073 | Ref |
| Medical Costs | Medical Cost | ||||||
| Inpatient | Inpatient | ||||||
| Trazodone | 1714 (18.65%) | 2031 | 0.94 (0.88,1.01) | Zolpidem IR | 1475 (16.25%) | 2399 | 1.04 (0.98,1.11) |
| Benzodiazepines | 1674 (18.21%) | 2152 | Ref | Benzodiazepines | 1651 (18.19%) | 2302 | Ref |
| ED | ED Costs | ||||||
| Trazodone | 2851 (31.02%) | 267 | 0.94 (0.88,1) | Zolpidem IR | 2469 (27.21%) | 221 | 0.91 (0.85,0.98) |
| Benzodiazepines | 2899 (31.54%) | 284 | Ref | Benzodiazepines | 2893 (31.88%) | 243 | Ref |
| Outpatient | Outpatient | ||||||
| Trazodone | 8965 (97.53%) | 726 | 0.84 (0.81,0.87) | Zolpidem IR | 8868 (97.72%) | 831 | 0.93 (0.89,0.96) |
| Benzodiazepines | 9139 (99.42%) | 861 | Ref | Benzodiazepines | 9021 (99.4%) | 895 | Ref |
| Pharmacy Costs | Pharmacy Costs | ||||||
| All Pharmacy | All Pharmacy | ||||||
| Trazodone | 9192 (100%) | 436 | 0.97 (0.93,1) | Zolpidem IR | 9075 (100%) | 396 | 1.05 (1.02,1.1) |
| Benzodiazepines | 9192 (100%) | 451 | Ref | Benzodiazepines | 9075 (100%) | 376 | Ref |
| Index Drug | Index Drug | ||||||
| Trazodone | 9192 (100%) | 2 | 0.38 (0.37,0.4) | Zolpidem IR | 9075 (100%) | 3 | 0.55 (0.53,0.57) |
| Benzodiazepines | 9192 (100%) | 5 | Ref | Benzodiazepines | 9075 (100%) | 5 | Ref |
Notes: aAll models adjusted for age, sex, geographical region, plan type, Elixhauser Comorbidity Index score, depression, diabetes, hypertension, osteoporosis, Alzheimer’s disease, antidepressant drug use, oral hypoglycemic drug use, and antihypertensive drug use at baseline, as well as index insomnia medication group exposure levels. bBenzodiazepines matched to trazodone. cBenzodiazepines matched to zolpidem IR.
Abbreviations: PPPM, per patient per month; USD, US dollars; CI, confidence interval; ED, emergency department; Ref, reference.
Figure 2.
Differences in healthcare resource use between medication groups by categories of cost *p<0.05.
Costs: Benzodiazepines vs Zolpidem IR
As presented in Table 2 and Figure 2, relative to individuals with insomnia treated with benzodiazepines, individuals treated with zolpidem IR demonstrated reduced total PPPM costs ($2072.96 vs $1983.89, CR = 0.96 [0.92,0.99]), reduced ED PPPM costs ($242.70 vs $221.34, CR = 0.91 [0.85, 0.98]), reduced outpatient PPPM costs ($830.88 vs $895.30, CR = 0.93 [0.89, 0.96]), reduced all-cause pharmacy PPPM costs ($396.11 vs $375.64, CR = 1.05 [1.02, 1.1]), and reduced index insomnia medication PPPM costs ($4.89 vs $2.70, CR = 0.55 [0.53, 0.57]). No other significant differences were observed.
HCRU by Duration of Action and FDA-Approval of Benzodiazepines
In terms of duration of action, relative to short-acting benzodiazepines, long-acting benzodiazepines were associated with increased all-cause pharmacy fills (2.8 vs 3.0, RR = 1.05 [1.02, 1.08]). In terms of FDA approval, relative to benzodiazepines with FDA approval for insomnia, benzodiazepines without FDA approval for insomnia were associated with increased outpatient visits (2.3 vs 2.5, RR = 1.07 [1.03, 1.11]) and increased all-cause pharmacy fills (2.8 vs 2.9, RR = 1.04 [1.01, 1.08]). No other significant differences were observed.
Costs by Duration of Action and FDA-Approval of Benzodiazepines
In terms of duration of action, relative to short-acting benzodiazepines, long-acting benzodiazepines were associated with greater all-cause PPPM pharmacy costs ($470.01 vs $421.80, CR = 1.11 [1.05, 1.18]). In terms of FDA approval, relative to benzodiazepines with FDA approval for insomnia, benzodiazepines without FDA approval for insomnia were associated with increased PPPM outpatient costs ($842.66 vs $756.53, CR = 1.11 [1.05, 1.18]), higher all-cause PPPM pharmacy costs ($443.61 vs $390.10, CR = 1.14 [1.07, 1.21]), and reduced index insomnia medication PPPM costs ($3.29 vs $16.06, CR = 0.2 [0.19, 0.22]). No other significant differences were observed.
Discussion
In this large national study and relative to individuals with insomnia treated with the two most commonly prescribed insomnia medications (zolpidem IR and low-dose trazodone), older adults treated with benzodiazepines demonstrated greater HCRU and higher costs across multiple points of service. These findings suggest that benzodiazepines are associated with adverse outcomes even relative to other commonly prescribed insomnia medications. Overall, these data add to a substantial evidence base that highlights the risks of benzodiazepines among older adults and suggest several directions for future research.
All three drugs examined in this study – benzodiazepines, zolpidem, and trazodone – are not optimal insomnia management and carry significant risk to patients. On one hand, the risks of cognitive and motor impairments, as well as risk for falls, resulting from benzodiazepines and z-drugs (including zolpidem) are well established.21–26 Each medication also carries unique risks, such as physiologic dependence for benzodiazepines (as a class) and dangerous sleep behaviors for zolpidem. On the other hand, relatively less research has examined possible adverse consequences of low-dose trazodone. This older antidepressant is commonly prescribed off-label for treatment of insomnia, possibly due to a lack of anti-cholinergic activity and cardiotoxicity. Scant data support the use of low-dose trazodone for insomnia, and the medication is associated with residual sleepiness and next-day hangover.32 Further, our group has recently published data demonstrating that trazodone is associated with increased risks for falls, greater HCRU, and higher costs across multiple points of service.24,25 The current study builds upon and expands those prior findings to highlight hitherto unstudied risks of trazodone among older adults. Finally, our findings build upon and expand prior knowledge regarding the complex relationship between insomnia medication treatment and economic outcomes among older adults,33 contributing additional needed health economic data from the payer perspective.
Our study has numerous strengths. First, our sample was large and included individuals with a broad range of commercial insurance from around the country, suggesting high generalizability as well as adequate statistical power to evaluate relationships of interest. Second, our research question is timely and builds on prior research into the relative safety of benzodiazepines and two of the most commonly prescribed insomnia medications, zolpidem IR and trazodone. Third, we employed a conservative operational definition of insomnia treatment with benzodiazepines, requiring both ≥1 physician-assigned diagnosis and ≥1 pharmacy claims. Finally, our study is one of the few studies to compare the effects of various insomnia medications among older adults.
At the same time, our administrative claims data methodology has limitations. Most importantly, we were unable to assess detailed sleep information, such as objective or subjective sleep, insomnia severity, daytime symptoms of insomnia, or other patient-level information. Second and related, we were unable to assess lifestyle factors (eg, exercise, alcohol use, smoking) that are known to impact sleep. Third, although our sample was large, it was not randomly selected, and the generalizability of our findings to individuals with other insurance plans (or no insurance) is unknown. Fourth, we were unable to assess medication adherence, instead relying on number of pills prescribed as a proxy for adherence. As a result, we were unable to examine “as needed” use of benzodiazepines or other insomnia medications. Fifth, individuals with insomnia frequently seek relief via over-the-counter (OTC) medications and other remedies that are not captured in administrative claims. Sixth, despite our conservative approach that required both a physician-assigned insomnia diagnosis and ≥1 benzodiazepine medication fills, we were unable to ascertain indications for prescribed medications. Seventh, we did not distinguish between specific molecules of interest. While individual BZDs have much in common, they are ultimately different molecules, and future research should examine molecule-specific differences. Eighth, chlordiazepoxide users also use antidepressants and clidinium which may have an impact on BZD effect. Likewise, we do not evaluate medication discontinuation or switching, which could impact results. Ninth, there is a lack of consensus regarding cutoffs for short vs long-acting medications, and future research should explore various cutoffs beyond the 11h threshold used in this study. Tenth, although we sought to control for a large number of potential confounders, residual confounding could exist. Finally, although we obtained HCRU and costs data from a broad range of points of service, we were unable to ascertain the impact of benzodiazepines, trazodone, and zolpidem IR on key insomnia economic outcomes from other perspectives, such as the employer perspective.34
Conclusions
In conclusion, the three most commonly prescribed older insomnia medications are associated with significantly increased risk for adverse outcomes among older adults.21–26 Results from this study expand prior knowledge by demonstrating that relative to zolpidem and separately, relative to low-dose trazodone (≤150mg/daily), benzodiazepines are associated with worsened outcomes including increased HCRU and costs across a broad range of points of service. CBTI remains the recommended first-line treatment for insomnia among older adults but is underutilized due to an insufficient number of trained providers19 and other system, provider, and patient-level barriers. Pharmacotherapy is by far the most common treatment approach. Thus, our data suggest that clinicians should adhere to consensus recommendations to avoid benzodiazepines in older adults when possible, and exercise caution when prescribing common older insomnia medications including zolpidem and low-dose trazodone, particularly among older adults. Researchers should continue to examine patterns and consequences of insomnia treatment among older adults, including CBTI as well as newer medications with different mechanisms of action.
Funding Statement
The study was funded by Eisai Inc.
Data Sharing Statement
MarketScan is not publicly available; it is only available through license.
Ethics Approval
IRB approval was not required because the analyses used retrospective de-identified data.
Author Contributions
All authors have met the following criteria for authorship:
Made a significant contribution to the work in all of these areas – study conception, study design, study execution, acquisition of data, data analysis and interpretation.
Have drafted or written, or substantially revised or critically reviewed the article.
Have agreed on the journal to which the article will be submitted.
Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
Agree to take responsibility and be accountable for the contents of the article.
Disclosure
EMW’s institution has received research funding from the AASM Foundation, Department of Defense, Merck, ResMed, and ResMed Foundation. EMW has served as a scientific consultant to DayZz, Eisai, EnsoData, Idorsia, Merck, Primasun, Purdue, and ResMed, and is an equity shareholder in WellTap. TRJ and FHF are full-time employees of Eisai Inc., which manufactures insomnia medications. DG and DTA are full-time employees of Genesis Research, which received funding to conduct these analyses. The authors report no other conflicts of interest in this work.
References
- 1.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–S11. doi: 10.1016/j.sleep.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann CN, Spira AP, Alexander GC, Rutkow L, Mojtabai R. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf. 2016;25(6):637–645. doi: 10.1002/pds.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maust DT, Blow FC, Wiechers IR, Kales HC, Marcus SC. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363–e71. doi: 10.4088/JCP.16m10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763 [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric A, American Psychiatric Association DSMTF. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013:947. [Google Scholar]
- 6.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.1989.03430110069030 [DOI] [PubMed] [Google Scholar]
- 7.Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. doi: 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 8.Taylor DJ, Lichstein KL, Durrence HH. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4):227–247. doi: 10.1207/S15402010BSM0104_5 [DOI] [PubMed] [Google Scholar]
- 9.Pollack M, Seal B, Joish VN, Cziraky MJ. Insomnia-related comorbidities and economic costs among a commercially insured population in the United States. Curr Med Res Opin. 2009;25(8):1901–1911. doi: 10.1185/03007990903035505 [DOI] [PubMed] [Google Scholar]
- 10.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. doi: 10.1016/j.smrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Tzuang M, Owusu JT, Huang J, et al. Associations of insomnia symptoms with subsequent health services use among community-dwelling US older adults. Sleep. 2021;44(5). doi: 10.1093/sleep/zsaa251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann CN, Canham SL, Mojtabai R, et al. Insomnia and health services utilization in middle-aged and older adults: results from the health and retirement study. J Gerontol a Biol Sci Med Sci. 2013;68(12):1512–1517. doi: 10.1093/gerona/glt050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS. Untreated insomnia increases all-cause health care utilization and costs among medicare beneficiaries. Sleep. 2019;42(4). doi: 10.1093/sleep/zsz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 15.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–262. doi: 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of H. National institutes of health state of the science conference statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049 [DOI] [PubMed] [Google Scholar]
- 17.VA/DoD. The management of chronic insomnia disorder and obstructive sleep apnea (Insomnia/OSA). Available from: https://www.healthquality.va.gov/guidelines/CD/insomnia/index.asp2019. Accessed May 30, 2023.
- 18.Wilson SJ, Nutt DJ, Alford C, et al. British Association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307 [DOI] [PubMed] [Google Scholar]
- 19.Thomas A, Grandner M, Nowakowski S, Nesom G, Corbitt C, Perlis ML. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med. 2016;14(6):687–698. doi: 10.1080/15402002.2016.1173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349. doi: 10.5665/sleep.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Gutierrez MJ, Martinez-Cengotitabengoa M, Saez de Adana E, et al. Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas. 2017;101:17–22. doi: 10.1016/j.maturitas.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 22.Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(Suppl 2):9–13. [PubMed] [Google Scholar]
- 23.Panel BtAGSBCUE. American geriatrics society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 24.Amari DT, Juday T, Frech FH, et al. Falls, healthcare resources and costs in older adults with insomnia treated with zolpidem, trazodone, or benzodiazepines. BMC Geriatr. 2022;22(1):484. doi: 10.1186/s12877-022-03165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickwire EM, Amari DT, Juday TR, Frech F, Gor D, Malhotra M. Incremental health care resource use and costs among adult patients with depression and treated for insomnia with zolpidem, trazodone, or benzodiazepines. Curr Med Res Opin. 2022;38(5):711–720. doi: 10.1080/03007995.2022.2047537 [DOI] [PubMed] [Google Scholar]
- 26.Tom SE, Wickwire EM, Park Y, Albrecht JS. Nonbenzodiazepine sedative hypnotics and risk of fall-related injury. Sleep. 2016;39(5):1009–1014. doi: 10.5665/sleep.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrecht JS, Wickwire EM, Vadlamani A, Scharf SM, Tom SE. Trends in insomnia diagnosis and treatment among medicare beneficiaries, 2006–2013. Am J Geriatr Psychiatry. 2019;27(3):301–309. doi: 10.1016/j.jagp.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong J, Murray Horwitz M, Bertisch SM, Herzig SJ, Buysse DJ, Toh S. Trends in dispensing of zolpidem and low-dose trazodone among commercially insured adults in the United States, 2011–2018. JAMA. 2020;324(21):2211–2213. doi: 10.1001/jama.2020.19224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamowicz M, Bartlett G, Tamblyn R, du Berger R. Modeling cumulative dose and exposure duration provided insights regarding the associations between benzodiazepines and injuries. J Clin Epidemiol. 2006;59(4):393–403. doi: 10.1016/j.jclinepi.2005.01.021 [DOI] [PubMed] [Google Scholar]
- 30.Sylvestre MP, Abrahamowicz M, Čapek R, Tamblyn R. Assessing the cumulative effects of exposure to selected benzodiazepines on the risk of fall-related injuries in the elderly. Int Psychogeriatr. 2012;24(4):577–586. doi: 10.1017/S1041610211002031 [DOI] [PubMed] [Google Scholar]
- 31.Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. doi: 10.1177/1179573518770672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton RE. The side effect profile of trazodone in comparison to an active control and placebo. J Clin Psychopharmacol. 1981;1:89S–93S. doi: 10.1097/00004714-198111001-00016 [DOI] [Google Scholar]
- 33.Wickwire EM, Vadlamani A, Tom SE, Johnson AM, Scharf SM, Albrecht JS. Economic aspects of insomnia medication treatment among medicare beneficiaries. Sleep. 2020;43(1):zsz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickwire EM. Value-based sleep in the workplace. Sleep. 2016;39(10):1767–1768. doi: 10.5665/sleep.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]