Abstract
Purpose
To examine whether nighttime dexmedetomidine infusion improved sleep quality in patients after laryngectomy.
Patients and Methods
Thirty-five post-laryngectomy patients admitted to the intensive care unit (ICU) were randomly assigned to a 9-h (from 2100 h on surgery day to 0600 h the morning after laryngectomy) dexmedetomidine (0.3 μg/kg/h continuous infusion) or placebo group. Polysomnography results were monitored during the dexmedetomidine infusion period. The percentage of stage 2 non-rapid eye movement (stage N2) sleep was the primary outcome measure.
Results
Thirty-five patients (18 placebo group; 17 dexmedetomidine group) had complete polysomnogram recordings. The percentage of stage N3 sleep was significantly increased in the dexmedetomidine infusion group (from median 0% (0 to 0) in placebo group to 0% (interquartile range, 0 to 4) in dexmedetomidine group (difference, −2.32%; 95% CI, −4.19 to −0.443; P = 0.0167)). Infusion had no effect on total sleep time, stage N1 or N2 sleep percentages, or sleep efficiency. It decreased muscle tensity and snore non-rapid eye movement. Subjective sleep quality improved. Hypotension incidence increased in the dexmedetomidine group, but significant intervention was not required.
Conclusion
Dexmedetomidine infusion improved overall patient sleep quality in the ICU after laryngectomy.
Keywords: dexmedetomidine infusion, postoperative, polysomnography, sleep disordered
Introduction
The prevalence of sleep disturbances, including sleep deprivation and abnormal architecture, is high in post-surgical patients, particularly in those who are admitted to the intensive care unit (ICU).1 Sleep disturbance is also prevalent in patients after laryngectomy.2 Since after laryngectomy, the upper and lower airway tracts are separated, colder and dryer air directly enters into the trachea and causes troublesome respiratory problems (ie excessive sputum production and involuntary coughing). Disturbed sleep and sleep-wake rhythms are serious problems in postoperative patients and may lead to prolonged postoperative recovery time,3 increased incidence of cardiovascular disease, cognitive dysfunction,4 and impaired immune function.5
Nonpharmacologic interventions, such as elimination of unnecessary noise and light, consolidation of patient care interactions, use of earplugs and eye masks, relaxation techniques, and addition of white noise, have been implemented to improve patients’ sleep quality in the ICU.6,7 However, the effects of these strategies are limited, and adjunctive drug therapy is often needed in some circumstances.8
Dexmedetomidine has increasingly been used in ICU patients to improve sleep quality.9 Dexmedetomidine exerts its sedative effects through an endogenous sleep-promoting pathway and preserves sleep architecture to some degree in the preclinical settings.10 In mechanically ventilated ICU patients, night-time infusion of dexmedetomidine 0.6 μg kg−1 h−1 induced a sedation level from −1 to −2 on the Richmond Agitation Sedation Scale (RASS) and improved the sleep architecture by increasing sleep efficiency and stage N2 sleep.11 A similar phenomenon was also observed in non-ventilation patients even with night-time infusion of none sedative dose of dexmedetomidine 0.1 μg kg−1 h−1.12 Our previous study demonstrated that the combination of dexmedetomidine/sufentanil in patients undergoing partial laryngectomy significantly improves subjective sleep quality, reduced the dosage of sufentanil and the frequency of postoperative coughing episodes.13 We hypothesized that dexmedetomidine might also improve sleep architecture in patients after laryngectomy. The purpose of this study was to investigate the effect of dexmedetomidine infusion on the sleep architecture of patients who were admitted to the ICU after laryngectomy.
Materials and Methods
Study Design
This pilot study used a controlled randomized trial design. It aimed to evaluate nighttime dexmedetomidine infusion efficacy on objective and subjective sleep quality variables. The study period was from September 2021 to June 2022.
Patient Recruitment
Male patients who underwent total/partial laryngectomy with general anesthesia and who were admitted to the post-surgery ICU before 2000 h were included in the study. The exclusion criteria were: (1) A history of mental disease; (2) A history of sleep disorders (requirement for hypnotic/sedative use during the previous month); (3) A history of obstructive sleep apnea syndrome; (4) Severe sinus bradycardia (heart rate ≤50 beats/min), or atrioventricular block (second degree or greater) without a pacemaker; or (5) Serious hepatic or renal dysfunction. Baseline demographic data and perioperative variables were obtained after enrollment.
Drug Administration and Procedures
During the study period, consecutively enrolled patients were randomly assigned to a dexmedetomidine (200 μg/2 mL, Jiangsu Hengrui Medicine Co, Ltd, China) or normal saline (placebo) group, based on a schedule obtained via computer-generated randomization. The dexmedetomidine was diluted to 50 mL with normal saline and was administered via the intravenous route at a 0.3 μg/kg/h infusion rate for 9 h (from 21:00 on surgery day to 6:00 the next morning).
Patient-controlled analgesia was used for routine postoperative analgesia. Sufentanil, 150 μg, was diluted to 150 mL with normal saline, and the programmed delivery protocol was a 1.5 mL intravenous bolus (10-min lockout interval) and background infusion of 1.5 mL/h. Hydromorphone (0.2 mg injected at 10-min intervals, intravenous route, up to five times per hour) was used as a supplemental analgesic, when necessary. The healthcare teams, patients, and study personnel were all blinded to treatment group assignments. Termination of study-drug infusion or unmasking of blinding, or both, could be performed at the request of on-duty attending physicians in cases of emergency. Emergencies included rapid, unexpected deterioration of the clinical condition of the patient. These cases were recorded on the case report form.
Sleep Quality Evaluation
Standard polysomnographic measurements (Embla systems N7000 or S4500, Natus Medical Inc., Pleasanton, CA, USA) were used for objective total sleep evaluations. Following guidelines of the American Academy of Sleep Medicine, bilateral electrooculogram, three pairs of electroencephalograms, bipolar chin electromyogram, and modified lead II electrocardiogram results were recorded. Data on thoracic and abdominal respiratory effort, oronasal airflow, blood oxygen saturation, posture, and snoring were also obtained.
A trained technician who was blinded to the assignment performed the PSG. Sleep recording results were manually diagnosed. Other two skilled technicians who did not participate in patient care or data collection and who were blinded to the study protocol manually checked the results. Based on American Academy of Sleep Medicine study results, the adult sleeping period was divided into wakefulness (W), non-rapid eye movement (NREM, including NREM 1 (N1), NREM 2 (N2), NREM 3 (N3)), and rapid eye movement (REM) periods.14 Summation of N1, N2, N3, and REM was used to calculate total sleep time. The ratio of total sleep time and total recording time was used to calculate sleep efficiency; the results were presented as percentage values. Sleep stage duration divided by total sleep time was used to calculate sleep stage percentages. The mean numbers of arousals and awakenings per hour of sleep were used to calculate the sleep fragmentation index. Sleep quality was assessed at 6:00 on the morning after surgery. A 0–100 visual analog scale, with 0 and 100 representing the poorest and the best sleep, respectively, was used to calculate a subjective sleep quality score.
Other Outcome Assessments
After drug infusions ended at 6:00, RASS scoring was used to assess sedation levels.15 An 11-point numeric rating scale (0, no pain; 10, worst pain) was used for subjective assessment of pain scores with movement and at rest at 3 h, 6 h, and 24 h after surgery.
After surgery, adverse events (eg, hypotension, hypertension, bradycardia, tachycardia, respiratory depression, desaturation) were monitored for 24 h. Interventions for adverse events included one or more of the following: interruption of the study-drug infusion, intravenous fluid bolus, medication administration, oxygen administration, noninvasive/invasive ventilation, and physical therapy. During the first 5 days after laryngectomy, confusion assessment16 was performed once per day (between 18:00–20:00) to evaluate postoperative delirium.
Statistical Analysis
During the nighttime, 15% of the sleep experienced by non-ventilated, post-intraabdominal surgery patients in the ICU is stage N2 sleep.12 We assumed that giving an intravenous dexmedetomidine infusion (0.3 μg/kg/h) would increase this percentage by 45%, compared with the placebo. Seventeen patients per group was the calculated sample size required to achieve 80% power to detect this difference (two-tailed significance level of 0.05). The percentage of stage N2 sleep was the primary endpoint. Secondary endpoints included sleep duration and efficiency, total sleep time, stages N1, N3, and REM sleep percentages, the sleep fragmentation index, and subjective sleep quality. Postoperative complications were also recorded.
Statistical analysis was performed with SPSS 14.0 (IBM, Armonk, NY, USA). and SAS 9.2 (SAS Institute, Cary, NC, USA). The D’Agostino & Pearson normality test was used to test whether a sample differs from a normal distribution. Normally distributed continuous variables (age, BMI, anesthesia duration, subjective sleep quality, PCA consumption, total sleep time, total awake time, sleep efficiency, duration of N1, N2%, duration of N2, REM%, snoring related arousal index NREM, snoring index NREM, arousal index NREM, snoring index REM, arousal index REM, tension muscle activity REM, arousal index) were summarized through means and standard deviations and analyzed by the Student’s t tests. Mann–Whitney U-tests was used to analyze the data that were not normally distributed (N1%, N3%, duration of N3, snoring related arousal index REM, phase muscle activity NREM, tension muscle activity NREM, phasic muscle activity REM, snoring index, phasic muscle activity, tension muscle activity, miniSpO2, the minimum pulse frequency during sleep time, the maximum pulse frequency during sleep time, RASS, pain score). Chi-square analysis (tumor stage) or Fisher exact tests (surgical type, number of patients with N3) was used to analyze categorical variables. Median (IQR) were used for description. Two-way repeated measures analysis of variance was used for between-group analysis (ie, dexmedetomidine group vs placebo group) of interactions for time and group factors (ie, differences in hemodynamic profiles (heart rate and mean blood pressure)). All tests were two-sided, and a cutoff of P < 0.05 was used to determine statistical significance.
Results
Patient Characteristics
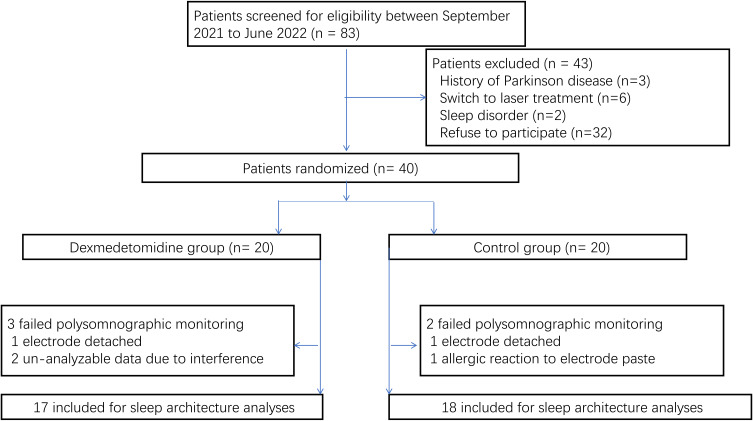
During the period of study, 40 male patients were enrolled in the study. In three of these patients, electrode detachment or allergic reaction to the electrode paste (dexmedetomidine group, one patient; placebo group, two patients) resulted in failure of polysomnographic monitoring. In two patients, signal interference (dexmedetomidine group, two patients) resulted in unanalyzable polysomnographic data. The sleep architecture analyses did not include the data associated with these patients (Figure 1). Blinding was maintained throughout the study period. The results for baseline variables were similar between the two groups (Table 1).
Figure 1.
Flowchart of the study. Forty patients were randomized. Data of five patients (three from dexmedetomidine group, two from control group) were excluded from the sleep architecture analyses.
Table 1.
Baseline Variables Between the Two Groups
| Factors | All Enrolled Patients | P value | Patients Included for Sleep Architecture Analyses | P value | ||
|---|---|---|---|---|---|---|
| Dexmedetomidine (N=20) | Control (N=20) | Dexmedetomidine (N=17) | Control (N=18) | |||
| Age (years) | 65.8 ± 8.3 | 65.6 ± 7.7 | 0.840a | 65.4 ± 8.5 | 65.6 ± 7.9 | 0.781a |
| BMI (kg/m2) | 21.9 ± 2.8 | 23.4 ± 2.7 | 0.090a | 22.0 ± 2.9 | 23.4 ± 2.8 | 0.167a |
| Tumor stage (1/2/3/4) | 6/8/3/3 | 7/7/4/2 | 0.922b | 4/8/2/3 | 6/7/3/2 | 0.840b |
| Surgical type (partial/ laryngectomy) | 14/6 | 14/6 | 0.999c | 12/5 | 13/5 | 0.999c |
| Anesthesia duration (min) | 199.3 ± 63.3 | 166.1 ± 71.1 | 0.151a | 203.8 ± 66.1 | 175.6 ± 72.1 | 0.267a |
Notes: Data are shown as mean (SD) or number. aStudent’s t-test; bMann–Whitney U; cFisher’s exact test.
Objective and Subjective Sleep Analyses
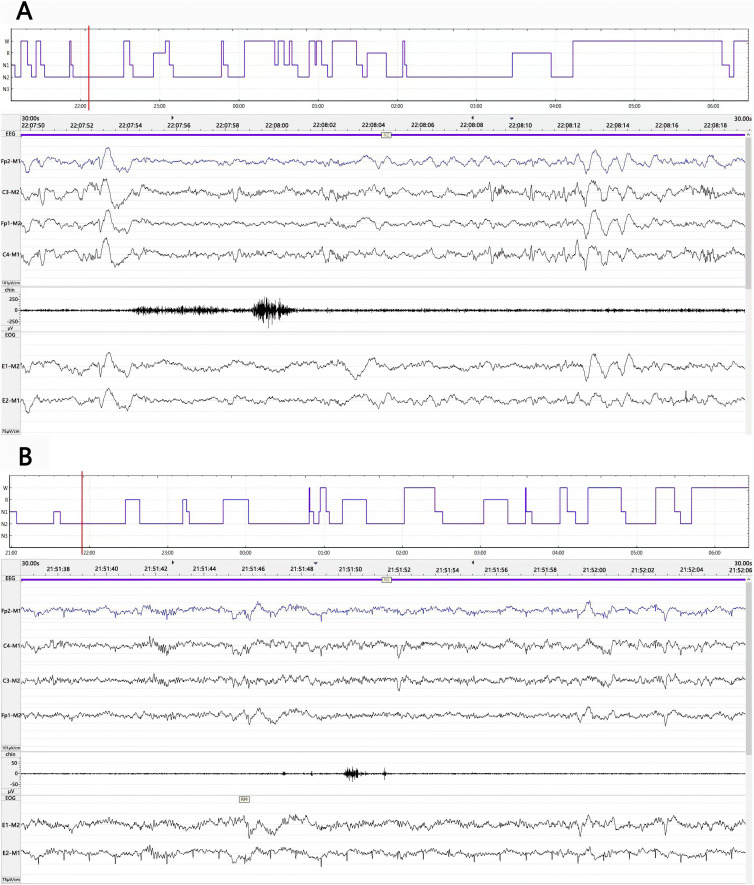
The results of the objective sleep analyses are presented in Table 2 and Figure 2. During the entire study period, total sleep time, sleep efficiency, and REM sleep time were similar between groups. In the control group patients, the polysomnography results revealed the presence of severely abnormal sleep architecture (ie, decreased percentages of non-REM sleep, increased percentages of stage REM sleep, a high snoring high sleep arousal index, and a high maximum pulse frequency) (Figure 2A). Stage N1 and N2 sleep were seen in all patients. Stage N3 sleep was only seen in 2 patients in the control group and in 7 patients in the dexmedetomidine group (P= 0.0599). The percentages of stage N1 and stage N2 sleep were similar between the groups. However, dexmedetomidine significantly increased the percentage of N3 sleep, compared with that during natural sleep (P= 0.0169). Although the correlation was not significant relative to the chosen alpha level of 0.05, dexmedetomidine infusion decreased the total arousal index from a mean value of 14.8 in the control group to a mean value of 9.7 in the dexmedetomidine group (P= 0.0922). The results were similar for the arousal index during NREM sleep (P=0.0633). Low-dose dexmedetomidine infusion also decreased the total snoring index, from a median value of 47.7 (30.7 to 70.0) in the control group to a median value of 27.5 (22.1 to 51.1) in the dexmedetomidine group (P=0.0537). While the snoring index during REM sleep was similar between the dexmedetomidine and placebo groups (28.5 ± 15.5 vs 35.6 ± 23.0, respectively, P= 0.2906), dexmedetomidine significantly decreased the snoring index (38.6 ±23.1 vs 55.4 ± 21.2, respectively, P= 0.0319) and snoring related arousal index (0.2 ± 0.1 vs 0.5 ± 0.4, respectively, P= 0.0092) during NREM sleep, compared with the control group. Dexmedetomidine also significantly decreased the total tension muscle activity index from a median value of 5.3 (3.5–9.1) in the control group to a median value of 3.0 (2.1–4.0) in the dexmedetomidine group (P= 0.0156) (Figure 2B).
Table 2.
Sleep Architecture Analyses Between the Two Groups
| Factors | Dexmedetomidine | Control | P value |
|---|---|---|---|
| (N=17) | (N=18) | ||
| Total sleep time (min) | 441.8 ± 60.52 | 435.3 ± 114.3 | 0.833a |
| Total wake time (min) | 124.2 ± 65.73 | 123.3 ± 84.4 | 0.509a |
| Sleep efficiency (%) | 78.32 ±11.14 | 78.24 ± 14.4 | 0.988a |
| N1% | 4.1 (2.4, 6.8) | 5.4 (3.2, 10.0) | 0.314b |
| Duration of N1 (min) | 21.58 ±13.52 | 28.67 ±21.79 | 0.259a |
| N2% | 76.77±8.45 | 75.3 ±7.0 | 0.573a |
| Duration of N2 (min) | 341.0 ± 15.93 | 329.0 ±23.78 | 0.681a |
| N3% | 0 (0, 4.0) | 0 (0, 0) | 0.017b,* |
| Duration of N3 (min) | 0 (0, 17.22) | 0 (0, 0) | 0.019b,* |
| Number of patients with N3 | 7 (41.18) | 2 (11.11) | 0.060c |
| REM% | 15.5 ± 5.6 | 17.3 ± 6.6 | 0.385a |
| Snoring related arousal index, NREM (per hour) | 0.2 ± 0.1 | 0.5 ± 0.4 | 0.009a,** |
| Snoring related arousal index, REM (per hour) | 0 (0, 0) | 0 (0, 0) | 0.104b |
| Snoring index, NREM (per hour) | 38.6 ±23 0.1 | 55.4 ± 21.2 | 0.032a,* |
| Arousal index, NREM (per hour) | 9.8 ± 7.2 | 15.9 ± 11.2 | 0.063a |
| Phasic muscle activity, NREM (per hour) | 9.7 (7.7, 19.8) | 13.1 (8.3, 26.7) | 0.458b |
| Tension muscle activity, NREM (per hour) | 3.3 (2, 4.0) | 5.8 (2.6, 10.2) | 0.068b |
| Snoring index, REM (per hour) | 28.5 ± 15.5 | 35.6 ± 23.0 | 0.291a |
| Arousal index, REM (per hour) | 9.6 ± 9.0 | 10.2 ± 7.3 | 0.833a |
| Phasic muscle activity, REM (per hour) | 10.2 (5.1, 17.7) | 10.5 (5.2, 16.8) | 0.929b |
| Tension muscle activity, REM (per hour) | 3.4 ± 3.5 | 4.5 ± 4.4 | 0.409a |
| Snoring index (per hour) | 27.5 (22.1, 51.1) | 47.7 (30.7, 70.0) | 0.054b |
| Arousal index (per hour) | 9.7 ± 7.1 | 14.8 ± 10.0 | 0.092a |
| Phasic muscle activity (per hour) | 10.5 (7.9, 18.8) | 12.5 (8.1, 24.1) | 0.574b |
| Tension muscle activity (per hour) | 3.0 (2.1, 4.0) | 5.3 (3.5, 9.1) | 0.016b,* |
| MiniSpO2 (%) | 91.8 ± 4.0 | 89.7 ± 3.7 | 0.119a |
| The minimum pulse frequency during sleep time (per hour) | 56.8 ± 12.4 | 59.9 ± 6.7 | 0.355a |
| The maximum pulse frequency during sleep time (per hour) | 91.4 ± 13.0 | 99.8 ± 13.7 | 0.062a |
Notes: Data are shown as mean (SD) or median (IQR) or number (percentage). aStudent’s t-test; bMann–Whitney U; cFisher’s exact test. *P<0.05, **P<0.01.
Abbreviations: NREM, Non-Rapid Eye Movement; REM, Rapid Eye Movement; N1%, the percent of NREM 1; N2%, the percent of NREM 2; N3%, the percent of NREM3.
Figure 2.
Representative sleep architecture during the period of study-drug infusion and polysomnographic traces at the time point indicated by the cursor (red line) of patients in the control (A) and dexmedetomidine (B) groups. Severe sleep fragmentation and sleep architecture disorganization were seen in both patients. Compared with the patient in the control group (A), patients in dexmedetomidine (B) showed decreased sleep stage N1, total arousal index and arousal index of NREM sleep, increased N2 and N3 sleep. N1= stage N1 sleep; N2= stage N2 sleep; N3= stage N3 sleep; R= stage REM sleep; W=wakefulness.
On the first postoperative morning, the mean subjective sleep quality score was higher in the dexmedetomidine group than in the placebo group (75.3 vs 56.8, respectively, 20 patients per group, P=0.004) (Table 3).
Table 3.
Subjective Sleep Quality and Other Outcomes Between the Two Groups
| Factors | All Enrolled Patients | P value | Patients Included for Sleep Architecture Analyses | P value | ||
|---|---|---|---|---|---|---|
| Dexmedetomidine (N=20) | Control (N=20) | Dexmedetomidine (N=17) | Control (N=18) | |||
| Subjective sleep quality on the first postoperative morning | 73.5 ± 4.2 | 56.8 ± 4.2 | <0.0001a | 73.4 ± 4.6 | 57.0 ± 4.1 | <0.0001a |
| RASS | 2(2, 2) | 2(2, 2) | 0.738b | 2(2, 2) | 2(2, 2) | 0.968b |
| Pain score | 21.9 ± 2.8 | 23.4 ± 2.7 | 0.090a | 22.0 ± 2.9 | 23.4 ± 2.8 | 0.167a |
| At rest | ||||||
| 3 | 1(1, 2) | 2(2, 3) | <0.0001b | 1(1, 1) | 2(2, 3) | <0.0001b |
| 6 | 1(1, 1) | 2(2, 2) | <0.0001b | 1(1, 1) | 2(2, 2) | <0.0001b |
| 24 | 1(1, 1) | 2(2, 2) | <0.0001b | 1(1, 1) | 2(2, 2) | <0.0001b |
| At active | ||||||
| 3 | 2(2, 2) | 3(3, 3) | <0.0001b | 2(2, 2) | 3(3, 3) | <0.0001b |
| 6 | 2(2, 2) | 3(3, 3) | <0.0001b | 2(2, 2) | 3(3, 3) | <0.0001b |
| 24 | 2(1.3, 2) | 3(3, 3) | <0.0001b | 2(1, 2) | 3(3, 3) | <0.0001b |
| PCA consumption on the first 24h postoperatively (mL) | 36.8 ± 1.2 | 41.7 ± 1.0 | <0.0001a | 36.8 ± 1.3 | 41.7 ± 1.0 | <0.0001a |
| Desaturation | 0 | 0 | – | 0 | 0 | – |
| Occurrence of delirium | 0 | 0 | – | 0 | 0 | – |
Notes: Data are shown as mean (SD) or median (IQR) or number. aStudent’s t-test; bMann–Whitney U.
Abbreviations: RASS, Ramsay sedation scale; PCA, patient controlled analgesia; ICU, Intensive care unit; RASS, Richmond Agitation Sedation Scale; PSG, Polysomnography; NREM, Non-Rapid Eye Movement; REM, Rapid Eye Movement.
Other Outcomes
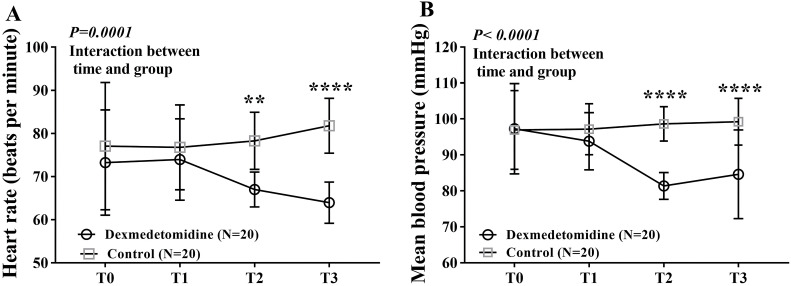
At the end of the study-drug infusion, the RASS scores were not significantly different between groups. At 3, 6, and 24 h after surgery, the subjective pain scores, both with movement and at rest, were significantly different between groups (all P<0.0001). Patient-controlled analgesia consumption during the first postoperative 24 h was less in the dexmedetomidine group than in the placebo group (36.8 mL vs 41.7 mL, respectively, P<0.0001). However, no patients in either group experienced desaturation or required intervention because of adverse events. Postoperative delirium was not observed in either group (Table 3). Heart rate and mean blood pressure values were lower in the dexmedetomidine group than in the placebo group at several timepoints (P=0.0001 and P<0.0001, respectively) (Figure 3). Post-surgery ICU stay length was not analyzed because all patients were transferred to the ward at 1000 h on the first postoperative morning.
Figure 3.
Changes in HR (A) and MBP (B) at different post-operative timepoints. Repeated-measurement ANOVA shows significant difference in HR over times between the dexmedetomidine group and the control group, with HR at T2 and T3 lower in dexmedetomidine group than in control group. There was a significantly decreased MBP in the dexmedetomidine group vs control group over time. T0, baseline value before anesthesia; T1, at admission to intensive care unit; T2, at zero o’clock on the first postoperative day; T3, at the end of dexmedetomidine infusion, respectively; **P<0.01, ****P<0.0001.
Discussion
In this investigation, we studied the effect of nighttime dexmedetomidine infusion on objective and subjective sleep quality in patients who admitted to the ICU after laryngectomy. Our major finding was that 0.3 μg-1kg-1h-1 dexmedetomidine infusion increased the percentage of non-REM stage 3 sleep and improved patients’ subjective sleep quality.
Studies using polysomnography showed that the sleep pattern of ICU patients was characterized as disorganized circadian rhythm, prolonged sleep latencies, fragmented sleep, decreased sleep efficiency, abnormally increased stages N1 and N2 sleep, and decreased or absent of N3 sleep and REM sleep.17,18 Sleep deprivation is prevalent in patients after laryngectomy.2 In this study, we found that patients in the treatment group had improved quality of sleep shown by decreased sleep stage N1 and REM, increased N2 and N3 sleep, and decreased total arousal index and arousal index of NREM sleep values. A trend that more patients in the treatment group presenting stage N3 sleep group also evidenced that dexmedetomidine does improve sleep quality in patients after laryngectomy.
Dexmedetomidine is an alpha-2 adrenergic agonist sedative that is approved for intravenous use. Studies have demonstrated that dexmedetomidine improved sleep quality by modulating non-REM sleep circuitry.11,12,19 The effects on sleep are likely associated with its pharmacologic activation of the endogenous sleep-promoting pathway to produce a state resembling physiologic stage N2 sleep.20 The treatment effect in the current study was not observed (stage N2 sleep was not increased in the dexmedetomidine group). The finding is in contrast to a previous study,12 in which they found that a continuous infusion of dexmedetomidine promotes N2 percentage in patients after non-cardiac surgery. The discrepancy may be due to the dose used (0.01 ug kg-1h-1 vs 0.03 ug kg-1h-1) and surgical procedure (intra- abdominal surgery vs laryngectomy).
Akeju et al found that a single nighttime loading dose of intravenous dexmedetomidine in healthy volunteers promotes non-rapid eye movement stage 3 sleep in a dose dependent manner.19 A recent study demonstrated that oral dexmedetomidine could improve N3 sleep and have a negative impact on REM sleep.21 Consistent with the study mentioned above.19,21 We found that dexmedetomidine infusion 0.03 ug kg-1 h-1 improved N3 sleep. Considering the importance and significance of stage N3 sleep, the restorative sleep,22 the change in this stage of sleep implies that the beneficial effects of dexmedetomidine infusion is obvious.
Dexmedetomidine can improve sedation and analgesia, reduces the incidence of postoperative delirium, and improves sleep quality.23 Previous study with dexmedetomidine infusion a non-sedative dose (0.01 ug kg-1 h-1) results in a RASS score of 2 in patients after intra-abdominal surgery.12 However, dexmedetomidine infusion 0.06 ug kg-1 h-1 was related to a RASS score ranging from −1~ −2 in mechanically ventilated patients.11 In our study, dexmedetomidine infusion 0.03 μg kg-1 h-1 was applied for patients after laryngectomy and the median RASS score was 2. Patients undergoing laryngectomy are suffering continuous airway stimuli from the tracheostomy tube and cold air. Dexmedetomidine can attenuate airway irritation during airway manipulation.24 This relatively higher dose of dexmedetomidine used in our study is needed to counteracts the undesirable stimuli due to anatomic and physiological change of airway.
That dexmedetomidine promoted N3 sleep without impairing psychomotor vigilance is meaningful. Firstly, N3 sleep is associated with improved cognition and synaptic plasticity,25 suggesting that dexmedetomidine may confer cognitive benefits for patients after laryngectomy. Secondly, sleep deprivation is associated with increased pro-inflammatory cytokine levels26 which is the neuropathogenesis of delirium in the elderly.27 Combined together, night intravenous administration of dexmedetomidine is feasible to enhance sleep with neurocognitive sparing benefits.
In our study, we found that dexmedetomidine 0.03 μg kg-1 h-1 infusion improved the effect of analgesia and decreased the requirement of opioids. Our finding was consistent with previous study.13,23,28,29 The side effect of dexmedetomidine, i.g. hypotension and/or bradycardia, on human hemodynamic changes is dose-dependent.30 In our study, we found that 0.03 ug kg-1 h-1 dexmedetomidine infusion decreased HR and blood pressure, but this decrease is not clinically significant. Dexmedetomidine reduces delirium incidence in elderly ICU patients after noncardiac surgery.28 The incidence of postoperative delirium was not improved by the treatment in our patients. This is likely due to a small sample size of the current study.
There are weakness in our study. First, the sample size is small. Second, we used a fixed dose of dexmedetomidine and did not consider the individual variability. An individualized dose-response trial is needed to explore the optimal dosing regimen in similar patient populations. Third, the polysomnographic monitoring was only performed on the first night after surgery. The administration of dexmedetomidine is limited to highly monitored care settings because it is only available for use as an intravenous medication. A capsule-based solid oral dosage formulation of dexmedetomidine increased the duration of non-rapid eye movement stage 2 sleep by 63 (95% CI, 19 to 107) min.21 Thus, an oral formulation may broaden the use and benefits of dexmedetomidine to patients in general medical and surgical units.
Conclusion
In conclusion, this pilot study found that a 0.03 μg/kg/h dexmedetomidine infusion improved objective and subjective sleep quality in patients admitted to the ICU after laryngectomy. The dosing regimen used was safe and feasible for use in clinical practice.
Acknowledgments
The authors gratefully acknowledge Weixing Li, M.D., Yanzhe Huang, M.D., and Zhiwei Tao, M.D. (Department of Anesthesiology and Critical Care Medicine) for their help with data collection.
Funding Statement
This research was supported by Eye & ENT Hospital’s double priority project A (SYA202010 to Dr Shen).
Data Sharing Statement
Data will be available from the corresponding author on reasonable request.
Ethics Approval
Our study was performed in accordance with Declaration of Helsinki and its amendments. The study protocol was approved by the Clinical Research Ethics Committee of the Eye & ENT Hospital, Fudan University (2020008-2); it was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-2100051438). Written informed consent was obtained from each patient.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Elliott R, McKinley S, Cistulli P. The quality and duration of sleep in the intensive care setting: an integrative review. Int J Nurs Stud. 2011;48(3):384–400. doi: 10.1016/j.ijnurstu.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 2.Hilgers FJ, Ackerstaff AH, Aaronson NK, Schouwenburg PF, Van Zandwijk N. Physical and psychosocial consequences of total laryngectomy. Clin Otolaryngol Allied Sci. 1990;15(5):421–425. doi: 10.1111/j.1365-2273.1990.tb00494.x [DOI] [PubMed] [Google Scholar]
- 3.Gögenur I, Bisgaard T, Burgdorf S, van Someren E, Rosenberg J. Disturbances in the circadian pattern of activity and sleep after laparoscopic versus open abdominal surgery. Surg Endosc. 2009;23(5):1026–1031. doi: 10.1007/s00464-008-0112-9 [DOI] [PubMed] [Google Scholar]
- 4.Ni P, Dong H, Zhou Q, et al. Preoperative sleep disturbance exaggerates surgery-induced neuroinflammation and neuronal damage in aged mice. Mediators Inflamm. 2019;2019:8301725. doi: 10.1155/2019/8301725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz FS, Andersen ML, Guindalini C, Araujo LP, Lopes JD, Tufik S. Sleep influences the immune response and the rejection process alters sleep pattern: evidence from a skin allograft model in mice. Brain Behav Immun. 2017;61:274–288. doi: 10.1016/j.bbi.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 6.Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95. doi: 10.1093/bja/aet304 [DOI] [PubMed] [Google Scholar]
- 7.Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423–428. doi: 10.1016/j.sleep.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 8.Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial. J Am Geriatr Soc. 2006;54(1):38–47. doi: 10.1111/j.1532-5415.2005.00562.x [DOI] [PubMed] [Google Scholar]
- 9.Reardon DP, Anger KE, Adams CD, Szumita PM. Role of dexmedetomidine in adults in the intensive care unit: an update. Am J Health Syst Pharm. 2013;70(9):767–777. doi: 10.2146/ajhp120211 [DOI] [PubMed] [Google Scholar]
- 10.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–436. doi: 10.1097/00000542-200302000-00024 [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou C, Kondili E, Diamantaki E, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121(4):801–807. doi: 10.1097/ALN.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 12.Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125(5):979–991. doi: 10.1097/ALN.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 13.Qin M, Chen K, Liu T, Shen X. Dexmedetomidine in combination with sufentanil for postoperative analgesia after partial laryngectomy. BMC Anesthesiol. 2017;17(1):66. doi: 10.1186/s12871-017-0363-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciołek M, Niedźwiecki M, Sieklicki S, Drozdowski J, Siebert J. Automated detection of sleep apnea and hypopnea events based on robust airflow envelope tracking in the presence of breathing artifacts. IEEE J Biomed Health Inform. 2015;19(2):418–429. doi: 10.1109/JBHI.2014.2325997 [DOI] [PubMed] [Google Scholar]
- 15.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Yu H, Qiao H, Li C, Chen K, Shen X. Risk factors and incidence of postoperative delirium in patients undergoing laryngectomy. Otolaryngol Head Neck Surg. 2019;161(5):807–813. doi: 10.1177/0194599819864304 [DOI] [PubMed] [Google Scholar]
- 17.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105–1114. doi: 10.5665/sleep.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. doi: 10.1186/cc12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akeju O, Hobbs LE, Gao L, et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. 2018;129(1):69–78. doi: 10.1016/j.clinph.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52(2):289–294. doi: 10.1111/j.1399-6576.2007.01537.x [DOI] [PubMed] [Google Scholar]
- 21.Chamadia S, Hobbs L, Marota S, et al. Oral dexmedetomidine promotes non-rapid eye movement stage 2 sleep in humans. Anesthesiology. 2020;133(6):1234–1243. doi: 10.1097/ALN.0000000000003567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterhouse J, Fukuda Y, Morita T. Daily rhythms of the sleep-wake cycle. J Physiol Anthropol. 2012;31(1):5. doi: 10.1186/1880-6805-31-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song B, Li Y, Teng X, Li X, Yang Y, Zhu J. The effect of intraoperative use of dexmedetomidine during the daytime operation vs the nighttime operation on postoperative sleep quality and pain under general anesthesia. Nat Sci Sleep. 2019;11:207–215. doi: 10.2147/NSS.S225041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: a double-blinded randomized controlled trial. J Anesth. 2013;27(2):211–217. doi: 10.1007/s00540-012-1499-y [DOI] [PubMed] [Google Scholar]
- 25.Prehn-Kristensen A, Munz M, Göder R, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7(6):793–799. doi: 10.1016/j.brs.2014.07.036 [DOI] [PubMed] [Google Scholar]
- 26.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Shen X. Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. 2018;30(11):1287–1295. doi: 10.1007/s40520-018-1008-8 [DOI] [PubMed] [Google Scholar]
- 28.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 29.Nie Y, Liu Y, Luo Q, Huang S. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2014;31(4):197–203. doi: 10.1097/EJA.0000000000000011 [DOI] [PubMed] [Google Scholar]
- 30.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77(6):1134–1142. doi: 10.1097/00000542-199212000-00014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available from the corresponding author on reasonable request.