Abstract
Background:
Myocardial damage due to acute ST-segment elevation myocardial infarction (STEMI) remains a significant global health problem. New approaches to limit myocardial infarct size and reduce progression to heart failure after STEMI are needed. Mechanically reducing left ventricle workload (LV unloading) before coronary reperfusion is emerging as a potential approach to reduce infarct size.
Objective:
Given the central importance of mitochondria in reperfusion injury, we hypothesized that compared to immediate reperfusion (IR), LV unloading before reperfusion improves myocardial energy substrate utilization and preserves mitochondrial structure and function.
Methods:
To explore the effect of LV unloading duration on infarct size, we analyzed data from the STEMI-Door to Unload pilot trial and then tested the effect of LV unloading on ischemia and reperfusion injury, cardiac metabolism and mitochondrial function in swine models of acute myocardial infarction.
Results:
The duration of LV unloading before reperfusion was inversely associated with infarct size in patients with large anterior STEMI. In preclinical models, LV unloading reduced expression of hypoxia-sensitive proteins and myocardial damage due to ischemia alone. LV unloading with a trans-valvular pump (TV-P), but not with veno-arterial extracorporeal membrane oxygenation (ECMO) reduced infarct size. Using unbiased and blinded metabolic profiling, TV-P improved myocardial energy substrate utilization and preserved mitochondrial structure including cardiolipin content after reperfusion compared to IR or ECMO. Functional testing in mitochondria isolated from the infarct zone showed intact mitochondrial structure including cardiolipin content, preserved activity of the electron transport chain including mitochondrial Complex I, and reduced oxidative stress with TV-P supported reperfusion, but not with IR or ECMO.
Conclusion:
These novel findings identify that trans-valvular unloading limits ischemic injury before reperfusion, improves myocardial energy substrate utilization and preserves mitochondrial structure and function after reperfusion.
Keywords: acute myocardial infarction, cardioprotection, circulatory support, mitochondria
Condensed Abstract:
New approaches to limit myocardial infarct size and reduce progression to heart failure after STEMI are needed. We identified that the duration of left ventricular unloading is inversely associated with infarct size in patients with anterior STEMI. Using preclinical models, we observed that trans-valvular unloading reduces myocardial ischemia before reperfusion, improves energy substrate utilization and preserves mitochondrial structural and functional integrity after reperfusion. These findings provide new mechanistic insight into the cardioprotective effect of trans-valvular unloading and suggest that unloading before reperfusion may attenuate reperfusion injury and allow for myocardial recovery by preserving mitochondrial structure and function after STEMI.
Tweet:
Transvalvular unloading before reperfusion preserves mitochondrial structure and function – a critical component for myocardial recovery after AMI
Myocardial damage due to acute ST-segment elevation myocardial infarction (STEMI) remains a significant global health problem. For every 30-minute delay from symptom onset to reperfusion, 1-year mortality increases by 7.5% and infarct size increases by nearly 30%(1,2). For every 5% increase in infarct size 1-year mortality and 1-year heart failure hospitalization increase by 20% in STEMI(3). These data are particularly sobering given that the prevalence of heart failure is projected to exceed 8 million individuals by 2030 in the United States alone(4). New approaches to limit infarct size and reduce progression to heart failure after STEMI are needed.
Within minutes of coronary occlusion, oxygen delivery is reduced, and cardiac metabolism switches from high energy producing fatty acid oxidation to low energy-yielding anaerobic glycolysis, thereby limiting energy production through the citric acid cycle and electron transport chain (ETC). Reperfusion begins to recover glucose and fatty acid oxidation, however a burst of mitochondria-generated reactive oxygen species (ROS) combined with calcium overload increases permeability of the inner mitochondrial membrane (IMM) leading to mitochondrial swelling and rupture. Reperfusion also reduce levels of cardiolipin, an integral lipid that maintains IMM structure and ETC function(5-7).
Mitochondrial Complex I (CI; NADH-ubiquinone oxidoreductase) is a multi-unit protein that transfers electrons from NADH to ubiquinone, which triggers a conformational change in CI thereby facilitating proton transfer across the IMM. During myocardial ischemia, CI becomes deactivated which limits proton transfer and dissipates the trans-membrane proton gradient that drives mitochondrial ATP generation. Upon reperfusion, re-activation of CI increases ROS production and cellular damage(5,8).
Use of percutaneous circulatory support pumps that decrease left ventricular (LV) workload and increase systemic perfusion is increasing. Trans-valvular pumps (TV-P) displace blood from the LV into the ascending aorta. Veno-arterial extracorporeal membrane oxygenation pumps (ECMO) displace and oxygenate venous blood into the aorta. Recent reports identified that compared to immediate reperfusion, first unloading the LV with a TV-P and delaying reperfusion reduces infarct size in preclinical models of STEMI(9-11). Proposed mechanisms for reduced infarct size with trans-valvular unloading include reduced LV work and oxygen consumption; activation of cardioprotective signaling(10); and increased collateral coronary blood flow and myocardial perfusion(12, 13). Given the central importance of mitochondria in the pathogenesis of reperfusion injury, studies exploring the impact of LV unloading on mitochondrial integrity in acute myocardial infarction are required.
The recently completed STEMI-Door to Unload pilot study randomized 50 patients with anterior STEMI to LV unloading and immediate reperfusion (U-IR) or unloading followed by a 30-minute delay before reperfusion (unloading and delayed reperfusion; U-DR)(14). Despite delayed reperfusion, the U-DR arm showed no increase in infarct size compared to the U-IR arm. Among large anterior STEMIs, defined by a sum of precordial ST-elevation (STE)≤6 millimeters (mm), U-DR decreased infarct size normalized to the area at risk compared to U-IR. These results support the need for mechanistic studies to understand how LV unloading attenuates myocardial damage despite a delay to reperfusion.
We hypothesized that compared to immediate reperfusion, mechanically unloading the LV before reperfusion improves myocardial energy substrate utilization and preserves mitochondrial structural and functional integrity.
Materials and Methods
Chemicals and Reagents
Chemicals were purchased from the following suppliers: Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone FCCP (C2920, Sigma), Antimycin A (A8674, Sigma), Rotenone (R8875, Sigma), MPD (87890, Sigma), Sodium Ascorbate (PHR 1279, Sigma), NADH (606-60-8, Sigma), Ru360 (557440, Sigma), Cyclosporine A (59865-13-3, Sigma)
Sub-analysis of the Door to Unload STEMI Pilot Trial
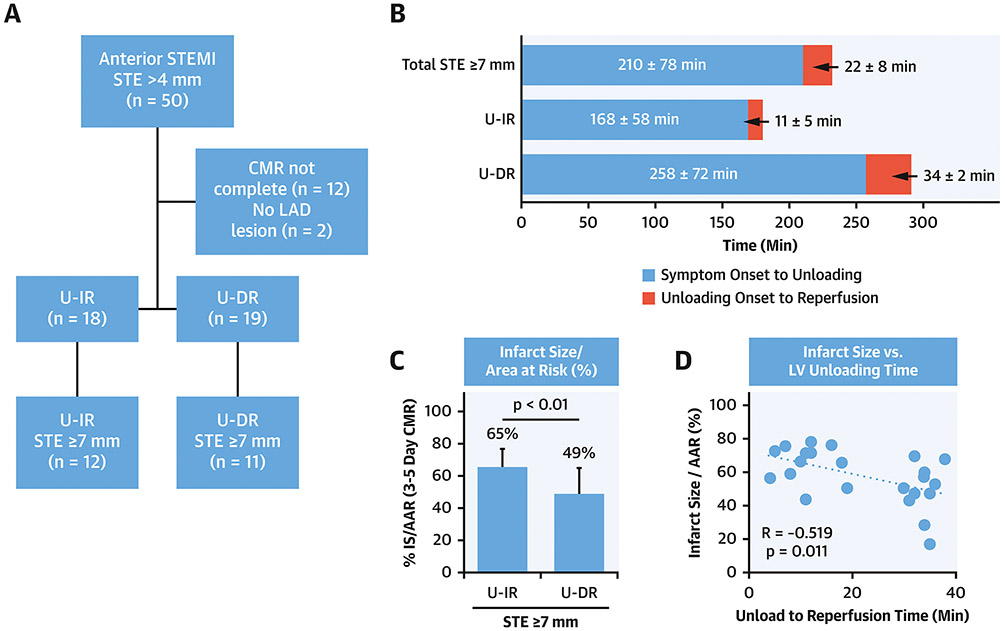
To study the relationship between time from symptom onset to balloon angioplasty, duration of LV unloading, and the magnitude of infarct size normalized to the area at risk as determined by 3 to 5-day cardiac magnetic resonance (CMR) imaging, we analyzed data from patients enrolled in the STEMI-DTU pilot study. STE served as a surrogate for the area at risk. We included patients with the STE≤7mm, available CMR data and documented left anterior descending (LAD) artery occlusion in the analysis (n=23/50; Figure 1A).
Figure 1. Sub-analysis of the STEMI-DTU Pilot Trial.
A)Flow diagram, B)Duration in minutes for total ischemic time, symptom onset to left ventricular unloading (white bars), and unloading to reperfusion (gray bars) for the total group with ST-segment elevation (STE)>7mm and unloading followed by immediate reperfusion (U-IR) or delayed reperfusion (U-DR); C)Infarct size normalized to the area at risk (IS/AAR); D)Plot comparing IS/AAR and the duration of unloading before reperfusion.
Institutional review boards at each site approved the trial, and each enrolled patient provided written informed consent. The study was designed by the principal investigators and Abiomed. The study steering committee members and an independent Data and Safety Monitor oversaw the safety and feasibility of the trial. A clinical events committee, blinded to randomization group, independently adjudicated clinical safety end points in the study. The sponsor was responsible for the study conduct, reporting, and monitoring, and managed the database. Angiographic, cardiovascular magnetic resonance (CMR), echocardiography, and ECG studies were analyzed by core laboratories blinded to the treatment allocation. The study design was approved by the Food and Drug Administration and registered accordingly (NCT03000270).
Swine Models of Myocardial Ischemia and Reperfusion Injury
Preclinical study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Tufts Medical Center. All experiments were performed according to the committee’s guidelines. Adult male Yorkshire swine were anesthetized, mechanically ventilated, and prepped for myocardial infarction studies as described in Supplemental Methods. Using a coronary catheter and wire an angioplasty balloon was deployed in the mid-LAD after the first diagonal branch with angiographic confirmation of occlusion. Angiography performed immediately after reperfusion and at the end of the study protocol confirmed LAD patency.
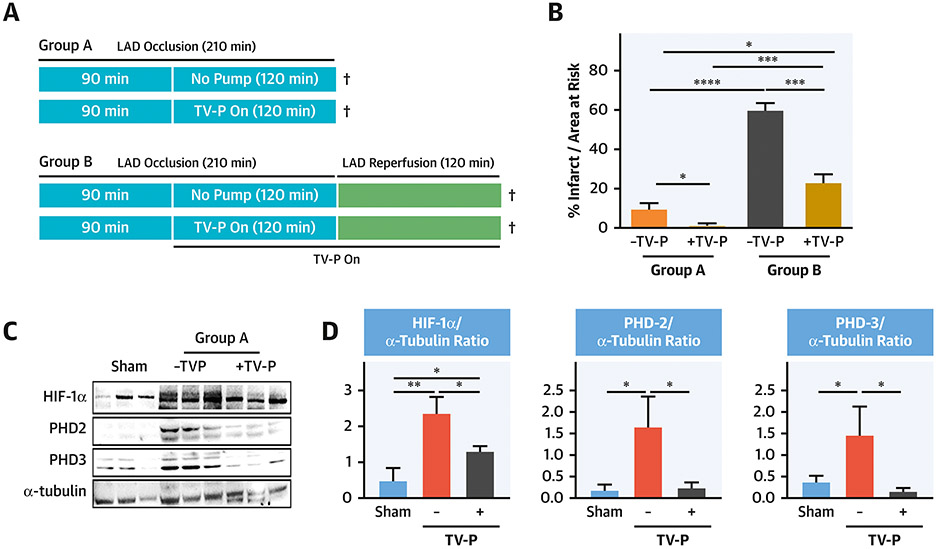
To determine the impact of circulatory support on infarct size during prolonged LAD occlusion with and without reperfusion, 4 groups of adult male swine (n=5/group) were studied (Figure 2A). In Group A, animals were subjected to 210 minutes of LAD occlusion without reperfusion. In half of these animals, a TV-P (Impella CP, Abiomed) was initiated 120 minutes before termination of the protocol. In Group B, 210 minutes of LAD occlusion was followed by 120 minutes of reperfusion with and without TV-P activation 120 minutes before reperfusion.
Figure 2. LV Unloading with Prolonged Coronary Occlusion Model.
A)Experimental Design; B) IS/AAR for Groups A and B with and without trans-valvular pump (TV-P) activation before reperfusion; C-D)Immunoblots and quantification graphs for HIF-1a, PHD-2, and PHD-3. ¥ indicates timing of tissue sampling.
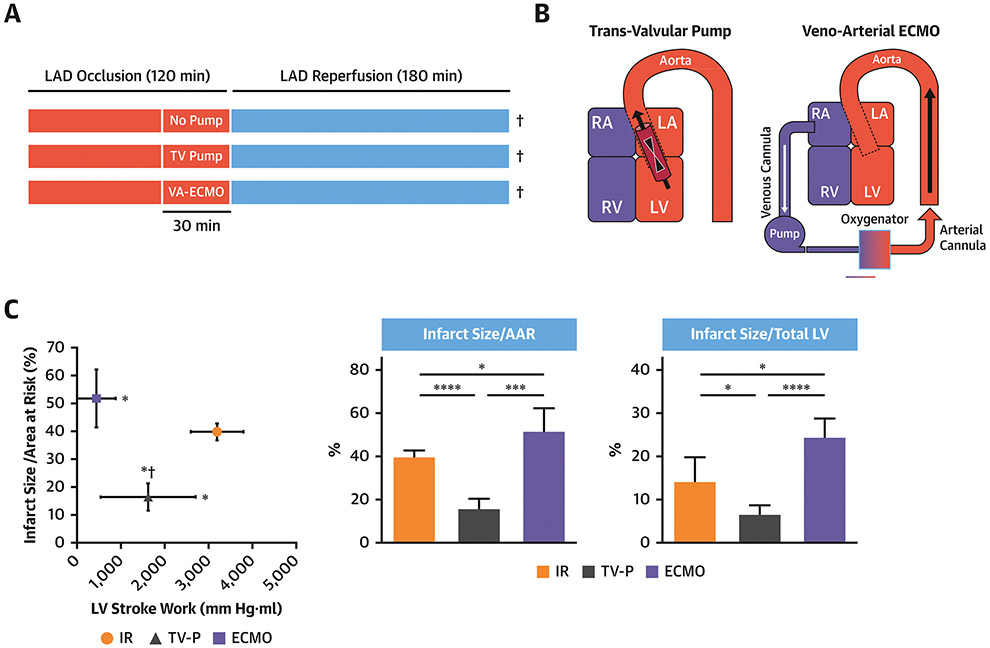
To investigate the effects of TV-P or veno-arterial ECMO prior to reperfusion in AMI, 18 swine underwent 120 minutes of LAD occlusion followed by 180 minutes reperfusion (Figure 3A-B). Following 90 minutes of LAD occlusion, subjects were randomly assigned to continued occlusion for 30 minutes (immediate reperfusion; IR) or continued occlusion for 30 minutes with activation of a TV-P or ECMO (n=6/group). In the two device arms, pumps remained active throughout the 180 minutes after reperfusion. The TV-P was inserted via the right carotid artery and activated at maximal support (44000 rotations/minute; RPM). ECMO was initiated using a 19-French arterial cannula and 25-French multi-stage venous cannula in the left femoral artery and right femoral vein respectively. ECMO was activated at 7500 RPM using a centrifugal pump (CardiacAssist) and membrane oxygenator (Maquet).
Figure 3. LV Unloading with a trans-valvular pump (TVP) or veno-arterial extracorporeal membrane oxygenation (ECMO).
A) Experimental design; B) TV-P or ECMO configurations; C) Plot comparing IS/AAR versus LV stroke work and bar graphs showing IS/AAR and IS/Total LV area. ¥ indicates timing of tissue sampling.
LV pressure and volume relationships were measured using a 5Fr-conductance catheter system (CD Leycom) as previously described (9).
Myocardial Infarct size Quantification
Upon study protocol completion, balloon occlusion was performed within the mid LAD and 0.5% Evan’s blue injected into both coronary vessels to depict the area-at-risk. The LV was removed and sectioned into four 1cm slices from the antero-apical LV distal to the site of stent deployment (infarct zone). Tissue samples were consistently collected from the center of the infarct zone and remote non-infarct zone. LV slices were stained, and total myocardial area, area-at-risk, and infarct zone quantified(9).
RNA Isolation
For RNA isolation, tissue samples were homogenized using a POWERGEN 125 (Fischer scientific) homogenizer in ice-cold 600μl RTL buffer provided by Qiagen RNeasy Mini kit. Isolated RNA was transcribed using the First Strand cDNA Synthesis Kit (Applied biosystems) and transcript levels analyzed by qRT-PCR (Supplemental Table 1) as previously described(9).
Immunoblot analyses
Protein from homogenized tissue (Protein Extraction Reagent;ThermoScientific) was supplemented with protease and phosphatase inhibitors. Protein concentrations were determined and analyzed as previously described(9). Succinate levels were quantified using a commercial kit. Supplemental Methods and Table 2 lists assays and antibodies used.
Mitochondrial Isolation, Oxygen Consumption Rate, and Complex I Activity Analysis
Mitochondrial isolation and oxygen consumption rate (OCR) analysis using the Seahorse XF96 extracellular flux analyzer (Seahorse Bioscience, Billerica, MA) and direct Mitochondrial CI activity analyses are described in Supplemental Methods.
Quantification of GSH/GSSG ratio detection Assay
Oxidative stress reduces the ratio of reduced versus oxidized glutathione (GSH:GSSG). To quantify levels of oxidative stress, we employed a fluorometric glutathione assay. Homogenates from the infarct and non-infarct zones were treated with a dye that fluoresces when reacting with glutathione (ab138881,Abcam).
Catalase Activity Assay
Catalase activity in tissue isolated from infarct zone core were determined as per manufacture’s instruction (ab83464,Abcam). Catalase reacts with hydrogen peroxide (H202) to produce H2O and oxygen. Unconverted H2O2 is measured calorimetrically at OD 570 nm.
Metabolomic screen
Tissue samples were inventoried and stored at −80°C, processed and submitted to Metabolon Inc in blinded fashion for analysis as described in Supplemental Methods.
A/D state of Mitochondrial Complex I
To analyze the activated and deactivated state of mitochondrial CI, oxidation of NADH was determined spectrophotometrically as a decrease in absorption at 340nm as described in Supplemental Methods.
Statistics
Data are presented as mean±SD Statistical analyses were performed using Student 2-tailed t-test. One-way ANOVA analysis (Bonferroni post hoc test) was performed in cases of comparisons with more than 2 groups. Values of P<0.05 were considered statistically significant. Every calculation is based on n=4-6 animals from each group as indicated in the figure legends. P values presented in this report have not been adjusted for multiplicity, and therefore inferences drawn from these statistics may not be reproducible. For analysis of the DTU-STEMI Pilot trial, all continuous variables were compared between treatment groups using appropriate parametric or nonparametric tests. Categorical variables were compared between treatment groups using the Pearson X2 test for contingency tables or the Fisher exact test. All statistical tests and confidence intervals were performed at α=0.05 (2-sided).
Results
Duration of Left Ventricular Unloading Before Reperfusion is Inversely Associated with Infarct Size in Large Anterior ST-Segment Elevation Myocardial Infarction
To explore the relationship between ischemic time, duration of LV unloading before reperfusion, and reduced infarct size, we analyzed data from patients enrolled in the STEMI-DTU pilot study with documented LAD occlusion, STE≥7mm, and available 3-5 day infarct size quantification data by CMR (n=23). Total symptom onset to angioplasty time was 210±78 minutes (Figure 1A-B). Time from symptom onset to angioplasty was longer in the U-DR than U-IR group (p<0.01; Figure 1B). Despite increased ischemic time in the U-DR arm, infarct size normalized to the area at risk (IS/AAR) was smaller in the U-DR group (p<0.01; Figure 1C). The duration of LV unloading before reperfusion was inversely associated with infarct size (R=−0.52, p<0.01; Figure 1D). These findings suggest that LV unloading may attenuate myocardial injury by reducing ischemia before reperfusion.
Trans-valvular unloading limits myocardial injury before reperfusion
To explore mechanisms by which prolonged unloading reduces infarct size before reperfusion, we performed LAD occlusion for a total of 210 minutes without reperfusion in adult male swine (Group A;Figure 2A). Compared to LAD occlusion alone, activation of a TV-P beginning after 90 minutes of LAD occlusion reduced infarct size (9.75±5.9% vs 2±1.9%, p<0.01;Figure 2B). We further quantified levels of proteins known to increase within minutes of tissue hypoxia including hypoxia inducible factor 1-a (HIF-1a), and prolyl hydroxylase domain enzymes (PHD)-2 and PHD-3 without reperfusion (Group A)(15).LAD occlusion without TV-P activation increased levels of all three hypoxia-sensitive proteins in the infarct zone. No increase in these proteins was observed after 210 minutes of LAD occlusion with TV-P activation. Compared to LAD occlusion without TV-P activation, succinate levels were lower with TV-P activation (Supplemental Figure 1).
To study the impact of prolonged unloading on myocardial injury after reperfusion, two additional groups were subjected to 210 minutes of LAD occlusion followed by 120 minutes of reperfusion with and without unloading beginning after 90 minutes of LAD occlusion (Group B;Figure 2A). Compared to reperfusion alone, activation of a TV-P before reperfusion reduced infarct size (60.1±7.04% vs 27.3±9.7%, p<0.001;Figure 2B). Compared to ischemic injury alone, reperfusion increased infarct size by 7.4-fold without TV-P versus 3-fold with TV-P activation (p<0.001). These findings suggest that TV-P activation may reduce myocardial injury by first reducing ischemia before reperfusion.
LV Unloading via ECMO does not reduce Infarct Size
To study whether ECMO can reduce infarct size, we employed a large-bore multi-porous venous cannula that unloads the LV by reducing cardiac preload. Adult male swine were subjected to LAD occlusion for 120 minutes followed by 180 minutes of reperfusion (ischemia-reperfusion; IR). In two separate groups, either a TV-P or ECMO was activated 30 minutes prior to reperfusion followed by 180 minutes of reperfusion (Figure 3A-B). Compared to IR alone, TV-P support reduced LV stroke work and decreased both IS/AAR and IS normalized to the total LV area (IS/Total LV;Figure 3C). ECMO activation reduced right atrial pressure and LV stroke work, but increased IS/AAR and IS/Total LV compared to IR(Table 1).
Table 1.
Hemodynamic Data
| IR (n=4) | Trans-valvular Pump (n=4) | VA-ECMO (n=4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAD occlusion |
LAD occlusion | LAD occlusion | ||||||||||
| Baseline | 90 min |
120 min |
Reperfusion | Baseline | 90 min (Pre- Pump) |
120 min (On- Pump) |
Reperfusion (On-Pump) |
Baseline | 90 min (Pre- Pump) |
120 min (On- Pump) |
Reperfusion (On-Pump) |
|
| Heart Rate (bpm) | 68.5 ± 2.1 | 67.75 ± 2.8 | 69.25 ± 3.2 | 79.75 ± 6 | 72 ± 2.7 | 69.75 ± 1.8 | 75.5 ± 3.6 | 85.25 ± 6.6 | 70.2 ± 3.4 | 72 ± 4.4 | 76.6 ± 6.4 | 92.8 ± 6.5 ** # |
| MAP (mmHg) | 83.5 ± 7.2 | 81.75 ± 8.3 | 79.75 ± 7.3 | 71 ± 5.5 | 83 ± 5.4 | 87.25 ± 7.4 | 93.25 ± 6.7 | 83.5 ± 6.5 | 89.4 ± 3.2 | 85.4±3.8 | 67.4 ± 5.5 ** | 58.4 ± 5.8 ** # |
| RA (mmHg) | 8 ± 1 | 7.5 ±1.6 | 6.75 ± 1.84 | 7 ± 2.3 | 6.25 ± 1.6 | 7 ± 2 | 7.25 ± 1.8 | 6 ± 1.7 | 6.4 ±1.02 | 7.6 ± 0.8 | 2.2 ± 0.6 | 3.2 ± 1.2 # |
| LVEDV | 263 ± 10 | 256 ± 10 | 256 ± 12 | 290±10 #‡ | 168.3 ± 14 | 167 ± 11 | 144 ± 13 | 169 ± 17 | 271 ± 34 | 266 ± 35 | 203 ± 31 | 218 ± 30 |
| LVESV | 220 ± 7 | 215 ± 9 | 211 ± 9 | 237 ± 8 | 137 ± 14 | 137 ± 13 | 125 ± 12 | 152 ± 12 | 210 ± 25 | 203 ± 27 | 180 ± 24 | 202 ± 25 |
| LVESP | 86 ± 8 | 80 ± 8 | 84 ± 10 | 72 ± 4 | 92 ± 9 | 98 ± 7 | 90 ± 6 | 78 ± 5 | 91 ± 5 | 78 ± 4 | 63 ± 5 *# | 56 ± 7 *# |
| LVEDP | 11 ± 2 | 14±1 | 14 ± 1 * | 13 ± 1 | 12 ± 1 | 13 ± 0.2 | 10 ± 0.2 # | 10 ± 1 # | 12 ± 1 | 15 ± 2 | 4 ± 2*# | 3.5 ± 1*# |
| LVSW (mmHg) | 3214 ± 600 | 2728 ± 548 | 3727 ± 550 | 3147± 297 | 2980 ± 826 | 2828 ± 458 | 1435±440 *# | 2125±564 | 4374 ± 724 | 3607 ± 618 | 298 ± 70 # * | 599±209 *# |
| +dp/dt | 761 ± 75 | 715 ± 84 | 733 ± 81 | 772 ± 54 | 800 ± 105 | 806 ± 44 | 757 ± 73 | 667 ± 57 | 958 ± 53 | 790 ± 43* | 447 ± 82*# | 637±67* |
| Device Flow (L/min) | 3.05 ± 0.05 | 3.1 ± 0.07 | 4.6 ± 0.3 | 5.1 ± 0.5 | ||||||||
p<0.05 versus baseline
p<0.05 versus 90 minutes
p<0.05 versus 120 minutes
MAP: mean arterial pressure; RA: right atrial pressure; LV-EDV: end-diastolic volume; LV-ESV: end-systolic volume; LV-ESP: end-systolic pressure; LV-EDP: end-diastolic pressure; LV-SW: stroke work
Trans-valvular unloading preserves myocardial energy substrate levels
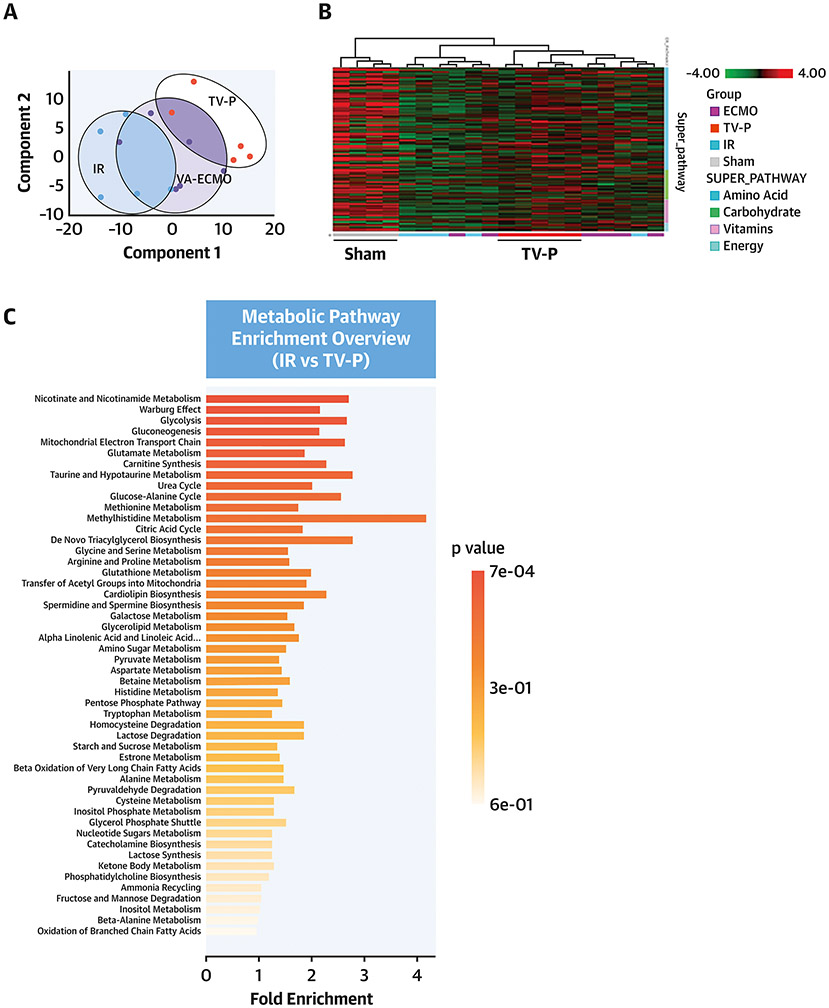
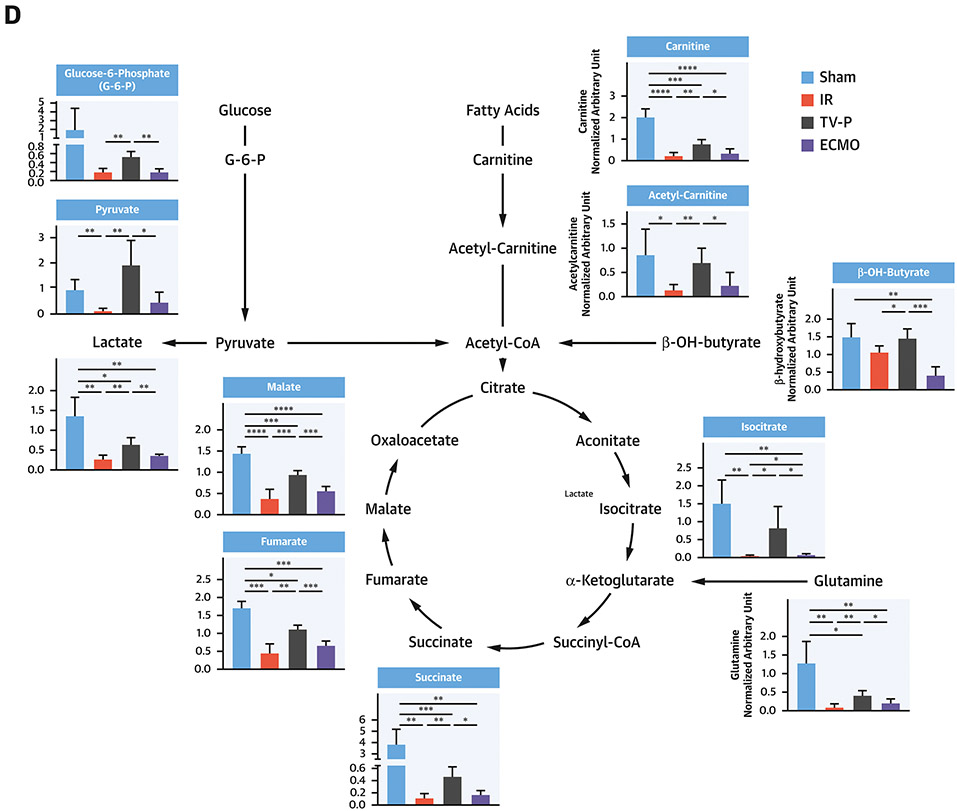
To explore mechanisms governing the cardioprotective effect of unloading, we performed untargeted metabolomics using tissue samples from the infarct zone after reperfusion. Principal component analysis identified distinct metabolite profiles between samples from the three groups (Figure 4A). Hierarchical analysis identified distinct metabolite profiles between TV-P and sham-controls, IR, and ECMO (Figure 4B). Pathway analysis identified that compared to IR, TV-P significantly altered multiple metabolic pathways involving glycolysis, the citric acid cycle, and ETC function (Figure 4C). Compared to sham controls, IR reduced levels of key metabolites associated with glycolysis, glucose oxidation, fatty acid transport and oxidation, and amino acid utilization (Figure 4D and Supplemental Figure 2). Compared to IR, TV-P activation preserved levels of these metabolites, but ECMO did not. These findings suggest that compared to IR or ECMO, trans-valvular unloading preserves substrate levels for oxidative phosphorylation.
Figure 4. Metabolic Profiles with LV Unloading.
A) Principal component analysis for IR, TV-P or ECMO activation before reperfusion; B) Heatmap of metabolite levels for sham, IR, TV-P, or ECMO across metabolic super-pathways; C) Enrichment pathway analysis comparing IR and TV-P; D) Illustration and bar-graphs showing levels of representative metabolites for glycolysis, fatty acid oxidation, and the citric acid cycle. All analyses were performed on tissue isolated from the infarct zone after reperfusion.
Trans-valvular unloading preserves mitochondrial structure
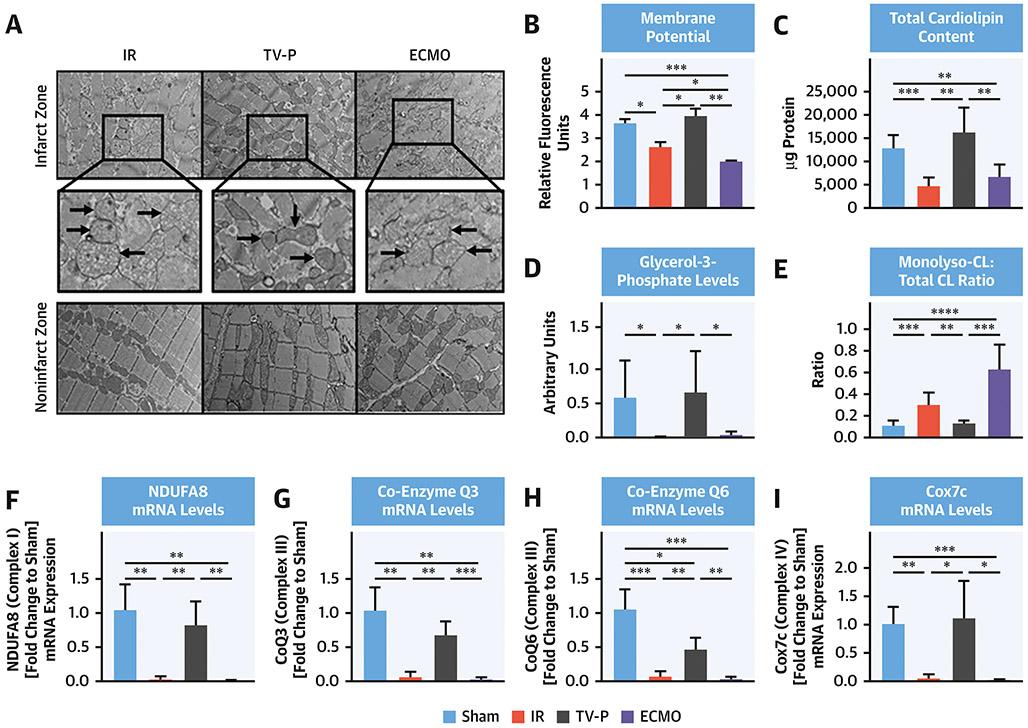
Compared to the non-infarct zone, electron micrographs showed that mitochondria from the infarct zone were swollen and disrupted with reduced membrane potential in the IR and ECMO groups but preserved in the TV-P group (Figure 5A-B). Compared to sham controls, total cardiolipin content and glycerol 3-phosphate levels (G3P; a cardiolipin precursor) were reduced in the infarct zone of both the IR and ECMO groups (Figure 5C-D). In contrast, total cardiolipin content and G3P levels were preserved in the TV-P group. Compared to sham-controls, levels of monolysl-cardiolipin (MLCL, an intermediate of cardiolipin remodeling) normalized to total cardiolipin were increased in with IR and ECMO but unchanged in the TV-P group (Figure 5E). Compared to sham controls, mRNA levels of ETC complex subunits were decreased with IR and ECMO but preserved with TV-P (Figure 5F-I). Supplemental Figure 3 shows comparisons with samples from non-infarct zones. These findings suggest that transvalvular unloading preserves mitochondrial structural integrity.
Figure 5. Mitochondrial Structure with LV Unloading.
A) Electron micrographs of mitochondrial structure from the infarct and non-infarct zones (black arrows and magnification call-out boxes) from IR, TV-P and ECMO; bar graphs showing B) mitochondrial membrane potential, C) total cardiolipin levels, D) glycerol-3-phosphate levels, E) ratio of monolysl-cardiolipin (MLCL) levels versus total cardiolipin levels in mitochondria isolated from the infarct zone; and mRNA levels of F) NDUFA8; G) Coenzyme Q3, H) Coenzyme Q6; and I) Cox7c in tissue samples isolated from the infarct zone after reperfusion.
Trans-valvular unloading preserves mitochondrial function
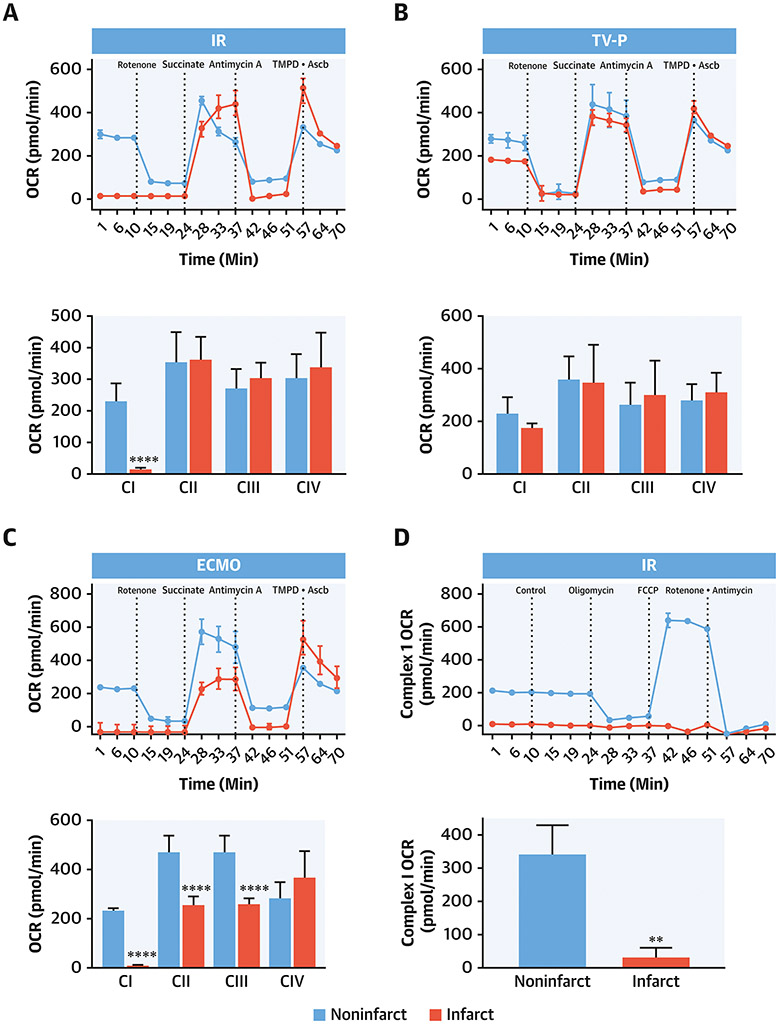
To explore the functional integrity of the ETC we used specific agonists and antagonists for ETC complexes. Compared to non-infarct zones, the rate of basal oxygen consumption in the presence of Complex I substrates required was reduced in the infarct zone by IR (Figure 6A). The CI inhibitor, rotenone, reduces the rate of oxygen consumption in both the infarct and non-infarct zones, thereby confirming intact CI function in the non-infarct zones. Functions of CII, III and IV were intact in both the non-infarct and infarct zones after IR. In contrast to IR, CI-IV function remained intact in both the infarct and non-infarct zones in the TV-P group (Figure 6B). In the ECMO group, functions of CI, II, and III were impaired in the infarct zone compared to the non-infarct zone (Figure 6C).
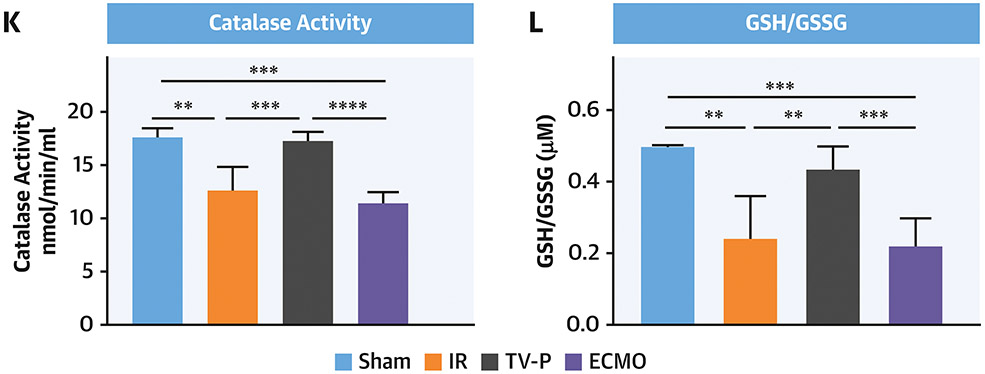
Figure 6. Mitochondrial Function and Oxidative Stress with LV Unloading.
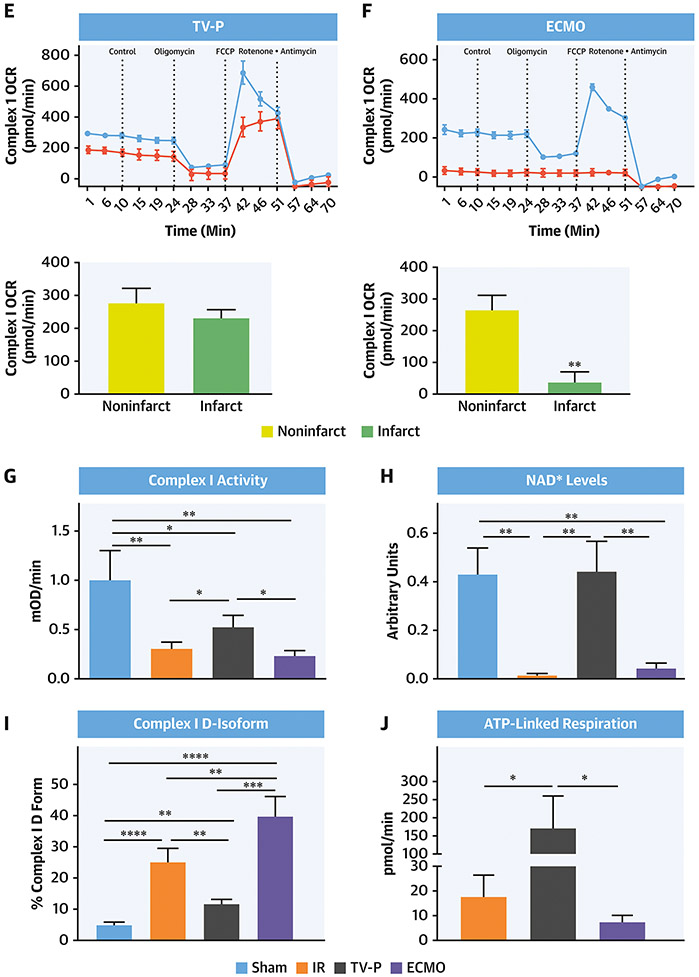
Seahorse plots with quantification bar graphs showing CI, II, III and IV function in mitochondria isolated from the non-infarct (red) and infarct (blue) zones after A) IR, B) TV-P, and C) ECMO. Seahorse plots with quantification bar graphs showing CI function in mitochondria isolated from the non-infarct (red) and infarct (blue) zones after D) IR, E) TV-P, and F) ECMO. Bar graphs showing G) CI activity, H) NAD+ levels, I) percent of deactivated (D)-CI, J) ATP-linked respiration, K) catalase activity, and L) reduced versus oxidized glutathione (GSH:GSSG) ratios.
Myocardial ischemia-reperfusion injury reduces CI-(NADH)-linked state 3 respiration. Compared to mitochondria isolated from non-infarct zones, CI state 3 respiration was reduced in the infarct zone in the IR and ECMO groups, but not the TV-P group (Figure 6D-F). Treatment with FCCP uncouples oxidative phosphorylation and maximizes oxygen consumption. FCCP increased the rate of oxygen consumption in non-infarct zones across all three groups. Compared to non-infarct zones, FCCP treatment failed to increase the rate of oxygen consumption in the infarct zone in the IR and ECMO groups, suggesting that impaired oxidative phosphorylation. In comparison, FCCP increased oxygen consumption in the infarct zone from the TV-P group, suggesting intact oxidative phosphorylation (Figure 6D-F).
Compared to sham controls, additional assays demonstrated reduced CI function and NAD+ levels, a product of CI activity, in the IR, ECMO, but not TV-P group (Figure 6G-H). Compared to sham controls, deactivated CI levels were increased in all three groups (4.8% vs 25.1% vs 11.8% vs 39.9%; sham vs IR vs TV-P vs ECMO, One-way ANOVA <0.01). Compared to IR, TV-P had lower deactivated CI levels (p<0.004;Figure 6I). Next, we treated isolated mitochondria with the ATP synthase inhibitor oligomycin and quantified the rate of oxygen consumption as a correlate of ATP production. Compared to mitochondria isolated from the infarct zone from the IR group, the TV-P, but not the ECMO group, had higher levels of basal respiration for ATP production (Figure 6J). Compared to sham controls, tissue from the infarct zone of the IR and ECMO groups demonstrated reduced catalase activity and GSH:GSSG ratios, but TV-P activation did not (Figure 6K-L). These findings indicate that trans-valvular unloading before reperfusion preserves CI and ETC activity and reduces oxidative stress.
Discussion:
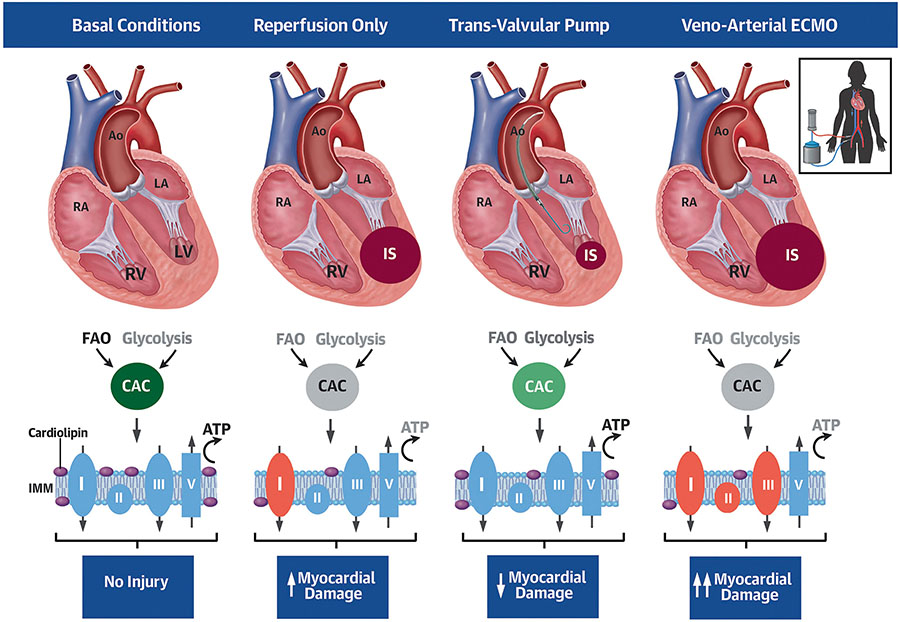
Myocardial infarct size is directly associated with increased mortality and heart failure after acute myocardial infarction. Mechanically reducing LV workload prior to reperfusion is a potentially viable approach to limit infarct size. We provide new mechanistic insight into the cardioprotective effect of LV unloading before reperfusion by first identifying an inverse relationship between the duration of unloading and infarct size in patients with anterior STEMI. Using preclinical models, trans-valvular unloading prior to reperfusion reduced expression of proteins associated with myocardial ischemia, attenuated reperfusion injury, and protected mitochondrial structure including levels of cardiolipin. Using an unbiased metabolomics approach and functional testing in isolated mitochondria, trans-valvular unloading preserved key energy substrate utilization pathways, maintained oxidative phosphorylation, and reduced oxidative stress compared to reperfusion alone (Figure 7; Central Illustration). These findings suggest that trans-valvular unloading limits myocardial ischemia before reperfusion and protects myocardial metabolism and mitochondrial function, which are key requirements for myocardial recovery after STEMI.
Central Illustration: Trans-valvular Unloading Protects Mitochondrial Function in Acute Myocardial Infarction.
Compared to basal conditions, reperfusion alone reduces fatty acid oxidation (FAO), glycolysis and the citric acid cycle (CAC) and further disrupts integrity of the inner mitochondrial membrane (IMM) and Complex I (CI) function, thereby leading to myocardial damage. Trans-valvular pump, but not ECMO, activation before reperfusion preserves cardiac metabolism, mitochondrial function, and reduces infarct size (IS).
The STEMI-DTU pilot study was the first clinical investigation to trans-valvular unloading before reperfusion in anterior STEMI(14). No prohibitive safety signal associated with TV-P use was observed. Despite a more prolonged ischemic time compared to unloading and immediate reperfusion, patients with the largest area at risk had significantly reduced infarct size with unloading and delayed reperfusion. We identified an inverse relationship between the duration of LV unloading before reperfusion and infarct size. These observations generated several important questions about the mechanism(s) underlying unloading as a therapeutic strategy in STEMI. First, does trans-valvular unloading attenuate myocardial injury before reperfusion? Second, would more prolonged LV unloading before reperfusion further decrease infarct size? Third, can other contemporary circulatory support systems such as ECMO unload the LV and reduce infarct size?
Under normoxic conditions, HIF-1α is rapidly degraded by oxygen-dependent PHD enzymes. Tissue hypoxia impairs PHD activity and increases HIF-1α levels within minutes, which return to basal levels upon re-oxygenation(15). As part of a negative feedback loop, PHD-2 and PHD-3 are HIF-target genes whose levels are also increased during hypoxia. We observed that LAD occlusion for 210 minutes without reperfusion increased HIF-1α, PHD-2 and PHD-3 levels, while TV-P activation after 90 minutes of LAD occlusion followed by an additional 120 minutes of occlusion without reperfusion significantly reduced these levels and infarct size. Consistent with these observations, succinate levels were lower after LAD occlusion with trans-valvular unloading(16). These findings suggest that TV-P activation reduces myocardial ischemia in the absence of epicardial coronary reperfusion.
One explanation for this observation is that TV-Ps increase myocardial perfusion by enhancing microcirculatory and collateral blood flow without epicardial reperfusion. Mehrige and Wampler first reported that the trans-valvular Hemopump increased myocardial perfusion before coronary reperfusion in canine models(16). Recent reports support this concept of functional reperfusion (12). These findings open opportunities to test whether trans-valvular unloading reduces ischemic burden during a 30-minute period to allow for drug delivery before reperfusion.
In contrast to cardiogenic shock, ECMO may reduce cardiac preload and unload the LV in euvolemic subjects. We employed ECMO with a large venous drainage cannula and observed reduced right atrial pressures and decreased LV stroke work. However, despite reducing LV stroke work, ECMO increased infarct size and failed to preserve myocardial energetics or mitochondrial function. One explanation may be that extracorporeal circuits with a large surface area may activate neutrophils, which mediate reperfusion injury. This possibility is supported by data showing that left atrial-to-femoral artery bypass without an oxygenator, reduces LV stroke work and infarct size(18). Recent studies report improved survival in cardiogenic shock with the use of Impella to decompress the LV with ECMO(19). Whether initiating LV unloading with Impella before ECMO can protect mitochondria from damage remains unknown and requires further study.
During myocardial ischemia, reduced fatty acid oxidation and increased glycolysis leads to inefficient energy production and uncouples glycolysis from glucose oxidation in the citric acid cycle. Depending on the duration of ischemia, reperfusion may partially restore energy substrate utilization. In our study, principal component and ingenuity pathway analysis of tissues after reperfusion showed clear metabolic profile separation between the IR and TV-P groups among key energy producing pathways involving mitochondrial function. Compared to IR, TV-P activation before reperfusion recovered glycolytic flux and increased pyruvate production, thereby maintaining the citric acid cycle. These findings support that trans-valvular unloading improves substrate utilization for oxidative phosphorylation or potential myocardial recovery.
Consistent with prior reports, we observed preservation of gross mitochondrial structure and membrane potential in tissues after reperfusion with trans-valvular unloading (9). Several studies have also shown that reduced cardiolipin content impairs ETC activity and increases ROS (5-7). We now introduce that compared to IR, TV-P activation preserved cardiolipin content. ECMO increased the ratio of MLCL:cardiolipin, an intermediate of cardiolipin synthesis associated with an inherited cardiac and skeletal myopathy known as Barth syndrome. ECMO further disrupted function of CI, II, and III, suggesting broader impairment of the ETC compared to IR. Recently, the EMBRACE-STEMI trial tested the utility of MTP-131, a peptide that preserves cardiolipin, among STEMI patients and failed to show a reduction in infarct size(20). Whether compounds such as MTP-31 improve infarct size when combined with trans-valvular unloading requires further study.
Mitochondrial CI accounts for 40% of proton motive force required to maintain ATP synthase in the ETC. During myocardial ischemia, CI transforms from an active to deactivated state, thereby limiting ATP synthesis. Upon reperfusion, CI contributes to oxidative damage. We observed reduced CI function, increased levels of deactivated-CI, and increased oxidative stress after reperfusion from the IR and ECMO groups, but not the TV-P group. Specifically, we observed that mitochondria isolated from the IR or ECMO groups, but not TV-P group, did not respire in the presence of substrate for CI, but were able to respire in response to substrates for Complexes II or IV, suggesting that these mitochondria were not fully uncoupled, but rather exhibited impaired CI activity. These findings suggest that trans-valvular unloading limits mitochondrial damage. Adjunct approaches to protect CI and mitochondrial function may further reduce infarct size. Furthermore, whether mitochondrial function continues to improve and is associated with myocardial recovery after trans-valvular unloading remains unknown.
Limitations
Limitations include the relatively small number of large animals studied per group. Healthy swine without comorbidities were studied which may limit clinical applicability of the findings. Sex differences and myocardial salvage also require further study. Since mitochondria are subject to quality control and renewal, the long-term effects of TV-P and ECMO support on mitochondrial function also require further study. Future studies exploring the impact of combined TV-P and ECMO on mitochondrial function are required. Finally, we employed balloon mediated coronary occlusion without coronary thrombus which may further limit the clinical applicability of the findings.
Conclusion
We provide new mechanistic insight into the cardioprotective effects of LV unloading by suggesting that trans-valvular unloading and delayed reperfusion limits infarct size by preserving myocardial energetics and mitochondrial function in acute myocardial infarction. These findings support ongoing studies LV unloading in STEMI and identifies potential drug targets to synergistically reduce infarct size with LV unloading in STEMI.
Supplementary Material
Perspectives:
Competency in Medical Knowledge:
Transvalvular unloading of the left ventricle before coronary reperfusion attenuates myocardial ischemia, reduces reperfusion injury, reduces energy substrate utilization and preserves mitochondrial integrity in an animal model of acute myocardial infarction.
Translational Outlook:
Clinical studies comparing the effects of early myocardial perfusion versus transvalvular unloading and delayed reperfusion on myocardial function in patients with acute myocardial infarction.
Acknowledgments:
Metabolon Inc
Sources of Funding:
This work was supported by a grant from the National Institutes of Health (R01HL139785-01 and R01HL133215-01) to N.K.K. and from Abiomed Inc to Tufts Medical Center
Abbreviations:
- STEMI
ST-segment elevation myocardial infarction
- ETC
Electron transport chain
- ROS
Reactive oxygen species
- CI
Mitochondrial Complex I
- TV-P
Trans-valvular pumps
- ECMO
Extracorporeal membrane oxygenation
- LAD
Left anterior descending
- DTU
Door to unload
- HIF1a
Hypoxia inducible factor 1-a
- PHD
Prolyl hydroxylase domain enzymes
Footnotes
Disclosures: Dr. Kapur receives institutional grant support and speaker/consulting honoraria from: Abbott, Abiomed, Boston Scientific, LivaNova, Medtronic, MD Start, and Precardia. Dr. O’Neill reports consulting fees from Edwards and Abiomed. LS, LR, SB, XQ, CT, EZ, PC, CB, ME, MC, and RK do not have any relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.De Luca G, van 't Hof AW, de Boer MJ, et al. Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J. 2004;25:1009–13. [DOI] [PubMed] [Google Scholar]
- 2.Tarantini G, Cacciavillani L, Corbetti F et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol. 2005;46:1229–35. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Selker HP, Thiele H, et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674–83. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther. 2013;140:258–266. [DOI] [PubMed] [Google Scholar]
- 6.Lesnefsky EJ, Chen Q, Slabe TJ, et al. Ischemia, rather than reperfusion inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 2004;287:H258–H267. [DOI] [PubMed] [Google Scholar]
- 7.Paradies G, Petrosillo G, Pistolese M et al. Decrease in mitochondrial, complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 2004;94:53–59. [DOI] [PubMed] [Google Scholar]
- 8.Chouchani ET, Pell VR, James AM, et al. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016;23:254–263. [DOI] [PubMed] [Google Scholar]
- 9.Kapur NK, Qiao X, Paruchuri V, et al. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015;3:873–82 [DOI] [PubMed] [Google Scholar]
- 10.Esposito ML, Zhang Y, Qiao X, et al. Left Ventricular Unloading Before Reperfusion Promotes Functional Recovery After Acute Myocardial Infarction. J Am Coll Cardiol. 2018;72:501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Li J, Zhao W, et al. Early Assistance With Left Ventricular Assist Device Limits Left Ventricular Remodeling After Acute Myocardial Infarction in a Swine Model. Artif Organs. 2016;40:243–251. [DOI] [PubMed] [Google Scholar]
- 12.Briceno N, Annamalai SK, Reyelt L, et al. Left Ventricular Unloading Increases the Coronary Collateral Flow Index Before Reperfusion and Reduces Infarct Size in a Swine Model of Acute Myocardial Infarction. J Am Heart Assoc. 2019;8:e013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Fish K, Kovacic JC, et al. Left Ventricular Unloading Using an Impella CP Improves Coronary Flow and Infarct Zone Perfusion in Ischemic Heart Failure. J Am Heart Assoc. 2018;7:e006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur NK, Alkhouli MA, DeMartini TJ, et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment-Elevation Myocardial Infarction. Circulation. 2019;139:337–346. [DOI] [PubMed] [Google Scholar]
- 15.Howell NJ, Tennant DA. The role of HIFs in ischemia-reperfusion injury. Hypoxia (Auckl). 2014;2:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pell VR, Spiroski AM, Mulvey J, et al. Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J Mol Cell Cardiol. 2018;123:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merhige ME, Smalling RW, Cassidy D, et al. Effect of the Hemopump left ventricular assist device on regional myocardial perfusion and function. Reduction of ischemia during coronary occlusion. Circulation. 1989;80(5):II158–III166. [PubMed] [Google Scholar]
- 18.Kapur NK, Paruchuri V, Urbano-Morales JA et al. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation. 2013;128:328–36 [DOI] [PubMed] [Google Scholar]
- 19.Kapur NK, Davila CD, Chweich H. Protecting the Vulnerable Left Ventricle: The Art of Unloading With VA-ECMO. Circ Heart Fail. 2019. Nov;12(11):e006581. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CM, Giugliano RP, Kloner RA, et al. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J. 2016;37:1296–1303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.