Abstract
Background:
Risk stratifying patients with cardiogenic shock (CS) is a major unmet need. The recently proposed Society for Cardiovascular Angiography and Interventions (SCAI) Stages as an approach to identify patients at risk for in-hospital mortality remains under investigation. We studied the utility of the SCAI Stages and further explored the impact of hemodynamic congestion on clinical outcomes.
Methods:
The cardiogenic shock working group registry includes CS patients from 8 medical centers enrolled between 2016 and 2019. Patients were classified by the maximum SCAI Stage (B-E) reached during their hospital stay according to drug and/or device utilization. In-hospital mortality was evaluated for association with SCAI Stages and hemodynamic congestion.
Results:
Of the 1414 CS patients, the majority were due to decompensated heart failure (HF; 50%) or myocardial infarction (MI; 35%). In-hospital mortality was 31% for the total cohort, but higher among MI patients (41% vs 26%, MI vs HF, p<0.0001). Risk for in-hospital mortality was associated with increasing SCAI stage [OR (95% CI): 3.25 (2.63–4.02)] in both MI and HF cohorts. Hemodynamic data was available in 1116 (79%) patients. Elevated biventricular filling pressures were common among CS patients and right atrial pressure was associated with increased mortality and higher SCAI Stage.
Conclusions:
Our findings support an association between the proposed SCAI staging system and in-hospital mortality among patient with HF and/or MI. We further identify that venous congestion is common and identifies CS patients at high risk for in-hospital mortality. These findings provide may inform future management protocols and clinical studies.
Keywords: Cardiogenic shock, hemodynamics, acute myocardial infarction, heart failure
Introduction
Cardiogenic shock (CS) is a complex clinical syndrome that begins with impaired cardiac function leading to systemic hypoperfusion and results in hemodynamic, neurohormonal and metabolic changes that progressively worsen without treatment. Despite major advances in drug and short-term mechanical circulatory support (MCS) device therapies over the past two decades, reported 30-day mortality due to CS remains largely unchanged, ranging between 30 and 60% 1–4. One explanation for the broad range and inconsistent mortality over time may be that the lack of clear criteria for risk stratification of patients at the time of presentation obscures survival trends over time in specific subgroups, be they at low, intermediate or high risk. This issue is further complicated by the fact that most studies involving CS focus on patients with myocardial infarction (MI) 5–7. However, the number of patients with CS in the setting of decompensated heart failure (HF) has grown, owing to exponential growth of the heart failure population 8. Survival trends and risk stratification for CS have not been adequately investigated in the HF population.
The use of short-term MCS devices has also increased and device options for CS now include the intra-aortic balloon pump (IABP), trans-valvular axial flow pumps (Impella; Abiomed Inc), left atrial to femoral artery pumps (TandemHeart; LivaNova Inc), veno-arterial extracorporeal membrane oxygenation (VA-ECMO), and extracorporeal centrifugal flow pumps 9, 10. With an increasing number of device options for these critically ill patients, risk stratification of patients presenting with CS is now more important than ever, since clarification of mortality and hemodynamic deficits in risk subsets may inform the development of treatment algorithms and the design of registry studies and randomized controlled trials which are necessary to evaluate clinical benefits.
Recently, a proposed staging system for CS based on input from a multi-disciplinary panel of clinical experts was proposed by the Society for Coronary Angiography and Intervention (SCAI) and endorsed by 4 other American medical associations 11. The SCAI system includes 5 classes of CS: A) at risk for CS, B) beginning CS, C) classic CS, D) deteriorating CS, and E) extreme CS. Each stage is defined by physical exam, biochemical and hemodynamic findings and were intentionally left as general definitions to accommodate the variability among clinical parameters available at the time of presentation. The SCAI staging system also proposes that increasing intensity of drug and device treatment over time accompanies clinical deterioration.
Two recent studies used markers of hypoperfusion and lactate levels respectively to define SCAI stages and showed a direct association between mortality and increasing SCAI stage. Limitations of these studies include the single-center study design, the lack of invasive hemodynamic data, and skewed distribution of short-term MCS devices in the study population 12, 13. Accordingly, additional studies are required to explore the utility of SCAI stages with contemporary real-world experience and to further determine the importance of hemodynamic parameters in risk stratifying CS patients.
To begin addressing these critical gaps in knowledge, we employed a multicenter registry of patients with CS due to decompensated HF, MI or other causes, hospitalized at 8 medical centers in the United States. The primary objective of this study was to test whether the SCAI Classification system successfully stratifies patients at risk of all-cause in-hospital mortality and to further assess associations between hemodynamic parameters at presentation with mortality.
Methods:
Data Source
The authors declare that all supporting data are available within the article and its online supplementary files to the methods. The cardiogenic shock working group (CSWG) is an academic research consortium of hospitals in the United States inclusive of a national registry of all-cause CS that began in 2016 with 4 initial sites across the United States contributing data on at least 100 adult refractory CS patients annually. The registry grew to include 8 total contributing sites by 2019. The registry includes a standardized set of data elements which were defined by principal investigators from the CSWG. These include patient, procedural, and hospital characteristics. Data represent discrete CS in-patient cases treated at each institution between 2016 and 2019 Patient demographic, laboratory and hemodynamic data were collected at a single time point as close to admission as possible, prior to initiation of mechanical support, in the hospital records. Information about pharmacologic and device therapies represented the maximum therapies provided during the hospitalization (detailed further below). CS diagnosis was physician-adjudicated at each site and was defined as a sustained episode of systolic blood pressure <90 mmHg for at least 30 minutes and/or a cardiac index (CI) <2.2 L/min/m2 determined to be secondary to cardiac dysfunction, and/or the requirement for either pharmacologic support (vasopressors or inotropes) or short-term MCS (i.e. IABP, Impella, VA-ECMO, or extracorporeal centrifugal flow pumps) at any time throughout a patient’s hospitalization. Quality assurance was achieved through adjudication at each site by the respective clinical coordinators and principal investigator. In addition, values were centrally audited and screened by the CSWG research team (KT, SN, LJ, JH, NH, MA, EZ, NKK) for any discrepancies or major outliers and resolved with the submitting site.
Study Population
Between 2016 and 2019, data from 1565 individual patient hospital admissions with a diagnosis of CS were collected. Proper IRB approval was obtained to access this data from medical records and patient consent was not required. CS etiology was reported by each site as due to MI, HF, or other. MI was defined as any primary diagnosis of either non-ST-segment elevation or ST-segment elevation MI (NSTEMI, STEMI). HF was defined as any primary diagnosis of acute on chronic HF, not otherwise related to MI. Other causes included post-cardiotomy, myocarditis, or not otherwise specified CS. Patients under the age of 18 years (n=1, 0.06%) and those with unknown mortality status at the time of hospital discharge (n=150, 9.6%) were excluded leaving a study population of 1414 patients with CS from 8 hospitals for analysis.
SCAI Classification
Patients were stratified according to the maximum SCAI classification stage reached during hospitalization to assess CS severity compared to in-hospital mortality11. According to the SCAI definition of stages, clinical deterioration based on persistent hypotension and hypoperfusion is the main determinant of a patient’s SCAI stage and is associated with a need for intensification of treatment. Therefore, treatment escalation during hospitalization for CS was used as a proxy for persistent hypotension and hypoperfusion to retrospectively define maximum deterioration since hemo-metabolic parameters were only assessed at admission. A CSWG-adapted definition of SCAI stages was applied in our study cohort based on total use of vasopressors, inotropes and MCS across a patient’s hospital stay as follows (Figure 1): SCAI defines Stage A patients as those at risk for CS and stage A was therefore not captured in our study population. Stage B patients are those exhibiting early symptoms not including hypo-perfusion and therefore do not require pharmacological or mechanical support. Stage C patients are those with hypoperfusion requiring initial intervention with up to either one drug or one MCS device. Stage D patients are those whose condition deteriorates despite initial intervention, defined in our dataset by the need for additional drugs or MCS treatment. Finally, Stage E patients are those who have deteriorated further and require maximal support, defined in our dataset as requiring at least two MCS devices and two drugs during their hospitalization. While timing of maximal vasopressor/inotrope treatment is not known in comparison to the timing of device treatment, each progression of treatment is considered a form of escalation and therefore, deterioration as defined by SCAI, so can be assessed independently when assigning maximal patient SCAI stage.
Figure 1.
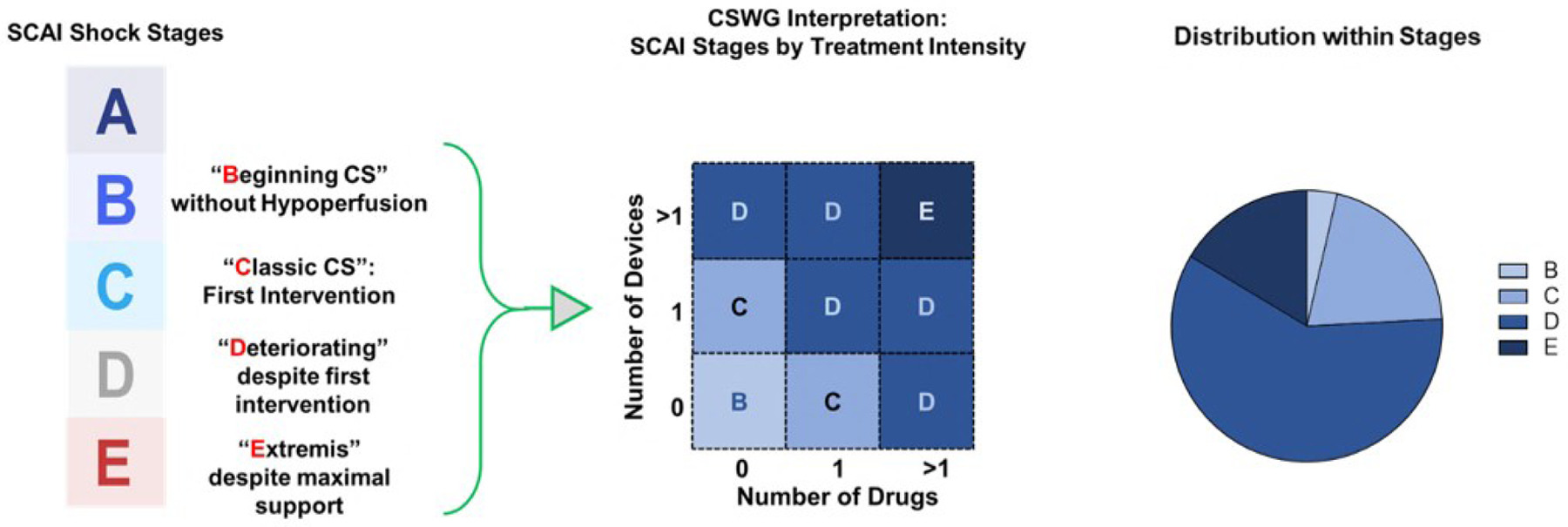
Definition, retrospective adjudication and distribution of SCAI stages within the CSWG registry
A sensitivity analysis incorporating lactate into SCAI stage definitions was performed. Stage B was defined as having a baseline lactate < 2 meq/L and having received no drugs or devices throughout hospitalization; Stage C was defined by a baseline lactate < 5 meq/L and having received either 1 drug or 1 device; Stage D patients had a baseline lactate < 5 meq/L but received greater than 1 drug or 1 device; Stage E patients were defined by a baseline lactate of ≥ 5 (Supplemental Figure 1).
Hemodynamic Congestion and Clinical Outcomes
Hemodynamic associations with mortality and their distribution across SCAI stages were evaluated according to 4 specific profiles of congestion. Pulmonary capillary wedge pressure (PCWP) was considered elevated at ≥18 mmHg and right atrial pressure (RAP) was considered elevated when ≥12 mmHg. Values of RAP and PCWP in excess of these upper limits were used to stratify patients into 1 of the following 4 congestion profiles: right-sided (RV, elevated RAP) congestion, left-sided (LV, elevated PCWP) congestion, bi-sided (BiV, both RAP and PCWP elevated) congestion, or euvolemic (EuV, both RAP and PCWP below cutoff values).
Statistical Analyses
The primary outcome of interest was all-cause, in-hospital mortality; all mortality outcomes analyzed in this report refer exclusively to in-hospital mortality. Secondary analyses explored descriptive statistics comparing characteristics and outcomes of SCAI stages and congestion profiles (as described above). All analyses were performed on an all-cause CS cohort, an MI CS subcohort, and an HF CS subcohort. Univariate logistic regression models were used to estimate odds and 95% confidence intervals of mortality in association with SCAI stages and congestion profile. Multivariate analyses were then performed, adjusting by significant comorbidities, to assess the independent associations of SCAI, congestion profile, and shock etiology. Descriptive statistics for categorical variables were reported as percentages and compared by χ2 tests and continuous variables were reported as means with standard deviations and were compared using t tests or ANOVA as appropriate to report p-values with a significance level of α=0.05.
Results
Patient Characteristics
Data from a total of 1414 patient hospitalizations for CS were analyzed. Patient characteristics are summarized in Table 1. Mean cardiac index in the total cohort and across each SCAI subcohort ranged between 1.8–1.9 L/min/m2. The majority of the study population was male and white. From the total population, the primary cause of CS was identified as HF in 50.4% (n=712), MI in 34.9% (n=494), and other causes in 14.71% (n=208). Stage B, C, and D patients were also more commonly HF patients while Stage E was primarily MI patients. While short-term MCS devices were broadly represented in different treatment combinations among the overall study ( Figure 2) cohort, intra-aortic balloon pump (IABP) was the most commonly used device in the overall cohort (n=770, 54.5%). This was also the case in Stage C (n=121, 46.0%) and D (n=464, 61.2%) and ECMO devices were the most commonly used devices in stage E patients (n=154, 72.6%). Prior percutaneous coronary intervention (PCI), hypertension (HTN), elevated AST, elevated lactate, and elevated filling pressures were also more common among Stage E patients. Characteristics of shock etiology sub-cohorts are presented in Table 2. Compared to HF-CS patients, MI patients were older with higher lactate and lower serum creatinine (sCr) levels. Additionally, LV ejection fraction (EF) was higher among MI patients and mean pulmonary arterial (PA) pressure was lower. No differences in cardiac filling pressures, cardiac output (CO), mean arterial pressure (MAP), or heart rate (HR) were noted between the HF and MI cohorts. Characteristics of congestion sub-cohorts are presented in Table 3.
Table 1.
Baseline descriptive statistics of CSWG study population by SCAI stage
| All (N=1414) |
SCAI Stage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| B (n=46) |
C (n=263) |
D (n=758) |
E (n=212) |
p-value | ||||||||||||
|
| ||||||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||||||
| Non-Survivors | 431 | (30.4) | 0 | (0) | 28 | (10.7) | 250 | (33.0) | 117 | (55.2) | <0.001 | |||||
| Male | 1025 | (72.5) | 33 | (71.7) | 199 | (75.7) | 540 | (71.2) | 155 | (73.1) | 0.58 | |||||
| Shock Etiology | ||||||||||||||||
| MI | 494 | (34.9) | 2 | (4.4) | 81 | (30.8) | 244 | (32.3) | 130 | (61.32) | <0.001 | |||||
| HF | 712 | (50.4) | 40 | (87.0) | 149 | (56.7) | 432 | (57.2) | 55 | (25.9) | ||||||
| Other | 208 | (14.7) | 4 | (8.7) | 33 | (12.6) | 79 | (10.5) | 27 | (12.7) | ||||||
| # Pressors/Inotropes | <0.001 | |||||||||||||||
| 0 | 236 | (16.7) | 46 | (100.0) | 171 | (65.0) | 19 | (2.5) | 0 | (0) | ||||||
| 1 | 393 | (27.8) | 0 | (0) | 92 | (35.0) | 301 | (39.7) | 0 | (0) | ||||||
| 2+ | 650 | (46.0) | 0 | (0) | 0 | (0) | 438 | (57.8) | 212 | (100.0) | ||||||
| # Devices | <0.001 | |||||||||||||||
| 0 | 224 | (15.8) | 46 | (100.0) | 92 | (35.0) | 61 | (8.1) | 0 | (0) | ||||||
| 1 | 882 | (62.4) | 0 | (0) | 171 | (65.0) | 620 | (81.8) | 0 | (0) | ||||||
| 2 + | 308 | (21.8) | 0 | (0) | 0 | (0) | 77 | (10.2) | 212 | (100.0) | ||||||
| Type of MCS | ||||||||||||||||
| Impella | 410 | (29.0) | 0 | (0) | 38 | (14.5) | 186 | (24.5) | 137 | (64.62) | <0.001 | |||||
| ECMO | 333 | (23.6) | 0 | (0) | 12 | (4.6) | 127 | (16.8) | 154 | (72.6) | <0.001 | |||||
| IABP | 770 | (54.5) | 0 | (0) | 121 | (46.0) | 464 | (61.2) | 145 | (68.4) | <0.001 | |||||
| Race | 0.002 | |||||||||||||||
| White | 647 | (45.8) | 32 | (69.6) | 152 | (57.8) | 306 | (40.4) | 98 | (46.3) | ||||||
| Hispanic/Latino | 31 | (2.2) | 1 | (2.2) | 9 | (3.4) | 13 | (1.7) | 3 | (1.4) | ||||||
| African-American | 31 | (2.2) | 0 | (0) | 2 | (0.8) | 15 | (2.0) | 3 | (1.4) | ||||||
| Asian | 28 | (2.0) | 0 | (0) | 8 | (3.0) | 11 | (1.5) | 7 | (3.3) | ||||||
| Other | 82 | (5.8) | 13 | (28.3) | 19 | (7.2) | 44 | (5.8) | 4 | (1.9) | ||||||
| Medical History | ||||||||||||||||
| HTN | 681 | (48.2) | 12 | (26.1) | 118 | (44.9) | 380 | (50.1) | 115 | (54.3) | <0.001 | |||||
| DM2 | 489 | (34.6) | 11 | (23.9) | 87 | (33.1) | 262 | (34.6) | 89 | (42.0) | 0.06 | |||||
| Afib/Flutter | 296 | (20.9) | 14 | (30.4) | 49 | (18.6) | 168 | (22.2) | 46 | (21.7) | 0.08 | |||||
| CKD (any stage) | 323 | (22.8) | 14 | (30.4) | 64 | (24.3) | 182 | (24.0) | 34 | (16.0) | 0.24 | |||||
| PVD | 60 | (4.2) | 1 | (2.2) | 12 | (4.6) | 33 | (4.4) | 10 | (4.7) | 0.55 | |||||
| COPD | 101 | (7.1) | 6 | (13.0) | 16 | (6.1) | 56 | (7.4) | 16 | (7.6) | 0.55 | |||||
| CVA/TIA | 159 | (11.2) | 4 | (8.7) | 28 | (10.7) | 101 | (13.3) | 15 | (7.1) | 0.01 | |||||
| Valvular Disease | 214 | (15.1) | 12 | (26.1) | 51 | (24.5) | 126 | (25.8) | 19 | (15.1) | 0.09 | |||||
| PCI | 293 | (20.7) | 11 | (23.9) | 43 | (21.6) | 136 | (29.7) | 87 | (44.9) | <0.001 | |||||
| CABG | 114 | (8.1) | 3 | (6.5) | 16 | (7.3) | 64 | (11.5) | 21 | (10.5) | 0.29 | |||||
| VT | 216 | (15.3) | 11 | (23.9) | 39 | (18.5) | 107 | (20.7) | 45 | (32.6) | 0.01 | |||||
| ICD | 329 | (23.3) | 23 | (50.0) | 71 | (34.0) | 173 | (33.5) | 42 | (30.7) | 0.11 | |||||
| CRT | 97 | (6.9) | 7 | (15.2) | 13 | (6.2) | 65 | (12.6) | 10 | (7.3) | 0.03 | |||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
|
| ||||||||||||||||
| Demographic | ||||||||||||||||
| Age | 1412 | 59.9 | (14.8) | 46 | 54.6 | 16.0 | 263 | 60.5 | 15.2 | 758 | 60.5 | 14.6 | 212 | 57.6 | 13.6 | 0.004 |
| Weight (kg) | 1138 | 85.3 | (22.6) | 46 | 87.0 | 20.2 | 231 | 87.4 | 22.2 | 589 | 83.7 | 23.6 | 155 | 86.1 | 19.9 | 0.15 |
| Metabolic | ||||||||||||||||
| AST | 788 | 459.4 | (1492.6) | 37 | 32.0 | 19.9 | 124 | 153.5 | 547.8 | 424 | 355.6 | 1168.9 | 174 | 1023.4 | 2446.2 | <0.001 |
| BUN | 1026 | 32.4 | (20.5) | 46 | 28.6 | 16.6 | 196 | 29.8 | 19.9 | 538 | 33.6 | 21.2 | 199 | 33.2 | 20.9 | 0.08 |
| Lactate | 676 | 4.4 | (4.2) | 1 | 1.4 | 0 | 62 | 4.2 | 4.0 | 401 | 3.7 | 3.8 | 165 | 5.9 | 4.9 | <0.001 |
| HCO3 | 836 | 22.1 | (5.4) | 44 | 25.7 | 3.0 | 159 | 23.8 | 4.8 | 444 | 22.0 | 5.3 | 170 | 20.0 | 5.9 | <0.001 |
| Serum Creatinine | 1295 | 1.8 | (1.1) | 46 | 1.3 | 0.4 | 248 | 1.5 | 0.8 | 739 | 1.8 | 1.2 | 203 | 1.9 | 1.1 | <0.001 |
| pH | 577 | 7.3 | (0.2) | 2 | 7.4 | 0.1 | 51 | 7.3 | 0.1 | 312 | 7.3 | 0.1 | 168 | 7.3 | 0.1 | 0.18 |
| Hemodynamic | ||||||||||||||||
| Admission EF (%) | 771 | 24.9 | (15.5) | 1 | 65.0 | 0 | 126 | 28.1 | 16.5 | 490 | 24.2 | 15.0 | 111 | 24.1 | 17.1 | 0.005 |
| RAP | 1037 | 14.2 | (6.9) | 44 | 8.8 | 6.2 | 177 | 12.9 | 6.7 | 619 | 14.3 | 6.9 | 165 | 16.2 | 6.3 | <0.001 |
| PCWP | 847 | 24.5 | (8.9) | 45 | 16.5 | 7.3 | 177 | 24.3 | 8.3 | 473 | 25.2 | 8.8 | 131 | 24.6 | 9.2 | <0.001 |
| Mean PAP | 904 | 32.8 | (9.8) | 44 | 27.0 | 11.3 | 178 | 33.3 | 9.5 | 646 | 33.5 | 9.6 | 169 | 30.8 | 9.4 | <0.001 |
| CO | 1062 | 3.8 | (2.4) | 45 | 3.8 | 0.7 | 188 | 3.5 | 1.2 | 651 | 3.8 | 2.4 | 153 | 4.4 | 3.6 | 0.003 |
| CPO | 999 | 0.6 | (0.4) | 45 | 0.6 | 0.1 | 178 | 0.6 | 0.3 | 607 | 0.6 | 0.4 | 146 | 0.7 | 0.6 | 0.44 |
| Heart Rate | 1248 | 92.0 | (22.7) | 46 | 75.2 | 12.8 | 234 | 85.9 | 19.7 | 685 | 93.8 | 22.2 | 193 | 97.3 | 24.8 | <0.001 |
| Cardiac Index | 1071 | 1.9 | (0.6) | 45 | 1.9 | 0.3 | 191 | 1.8 | 0.5 | 659 | 1.9 | 0.6 | 151 | 1.9 | 0.6 | 0.09 |
| MAP | 1230 | 74.5 | (14.7) | 46 | 71.8 | 7.5 | 250 | 80.3 | 15.7 | 724 | 74.4 | 14.3 | 205 | 67.8 | 12.9 | <0.001 |
p-values calculated using chi-sq test of independence or ANOVA as appropriate. Abbreviations: Afib: Atrial fibrillation; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPO: cardiac power output; CRT: cathode-ray tube; CVA: cerebrovascular accident; HCO3: Bicarbonate; HTN: Hypertension; ICD: implantable cardioverter defibrillator; MAP: mean arterial pressure; PAP: pulmonary arterial pressure; PCI: percutaneous coronary intervention; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; RAP: right arterial pressure; TIA: transient ischemic attack; VT: ventricular tachycardia
Figure 2.
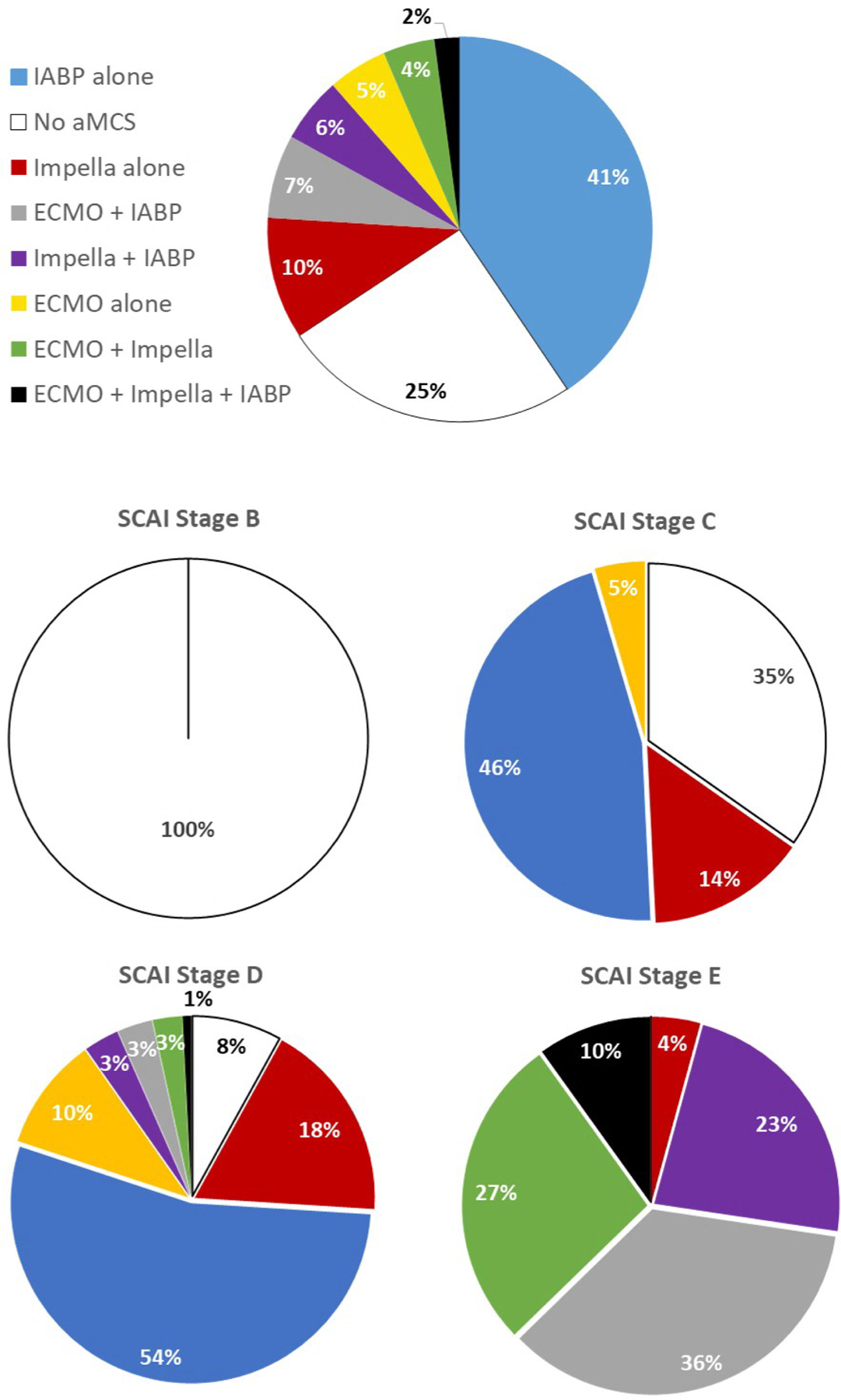
Device usage among CSWG patients with available hemodynamic data among the entire study cohort and each SCAI stage
Table 2.
Baseline descriptive statistics of the CSWG study population by shock etiology
| Overall (N=1414) |
Shock Etiology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| MI (N=494) | HF (N=712) | p-value | ||||||||
|
| ||||||||||
| n | (%) | n | (%) | n | (%) | |||||
| SCAI Stage | <0.001 | |||||||||
| B | 1 | (0.1) | 0 | (0) | 1 | (0.34) | ||||
| C | 232 | (16.4) | 75 | (26.0) | 139 | (47.9) | ||||
| D | 220 | (15.6) | 114 | (39.6) | 85 | (29.3) | ||||
| E | 192 | (13.6) | 99 | (34.4) | 65 | (22.4) | ||||
| # Pressors/Inotropes | <0.001 | |||||||||
| 0 | 236 | (16.7) | 86 | (18.8) | 119 | (17.6) | ||||
| 1 | 393 | (27.8) | 115 | (25.2) | 241 | (35.7) | ||||
| 2+ | 650 | (46.0) | 256 | (56.0) | 316 | (46.8) | ||||
| # MCS Devices | <0.001 | |||||||||
| 0 | 224 | (15.8) | 21 | (4.3) | 161 | (22.6) | ||||
| 1 | 882 | (62.4) | 294 | (59.5) | 465 | (65.3) | ||||
| 2+ | 308 | (21.8) | 179 | (36.2) | 86 | (12.1) | ||||
| Type of MCS | ||||||||||
| Impella | 410 | (29.0) | 210 | (42.5) | 148 | (20.8) | <0.001 | |||
| ECMO | 333 | (23.6) | 169 | (43.2) | 106 | (14.9) | <0.001 | |||
| IABP | 770 | (54.5) | 292 | (59.1) | 382 | (53.7) | 0.06 | |||
| Gender | 0.005 | |||||||||
| Female | 387 | (27.4) | 153 | (31.0) | 169 | (23.7) | ||||
| Male | 1025 | (72.5) | 340 | (68.8) | 543 | (76.3) | ||||
| Race | <0.001 | |||||||||
| White | 647 | (45.8) | 175 | (35.4) | 321 | (45.1) | ||||
| Hispanic/Latino | 31 | (2.2) | 16 | (3.2) | 7 | (1.0) | ||||
| Asian | 31 | (2.2) | 18 | (3.6) | 4 | (0.6) | ||||
| African-American | 28 | (2.0) | 8 | (1.6) | 13 | (1.8) | ||||
| Other | 82 | (5.8) | 15 | (3.0) | 55 | (7.7) | ||||
| Medical History | ||||||||||
| HTN | 681 | (48.2) | 321 | (65.0) | 276 | (38.8) | <0.001 | |||
| DM | 489 | (34.6) | 220 | (44.5) | 222 | (31.2) | <0.001 | |||
| Afib/Flutter | 296 | (20.9) | 37 | (7.5) | 227 | (31.9) | <0.001 | |||
| CKD (any stage) | 323 | (22.8) | 84 | (17.0) | 207 | (29.1) | <0.001 | |||
| PVD | 60 | (4.2) | 27 | (5.5) | 22 | (3.1) | 0.0177 | |||
| COPD | 101 | (7.1) | 27 | (5.5) | 62 | (8.7) | 0.0028 | |||
| CVA/TIA | 159 | (11.2) | 60 | (12.1) | 88 | (12.4) | 0.2023 | |||
| Valvular Disease | 214 | (15.1) | 24 | (4.9) | 154 | (21.6) | <0.001 | |||
| PCI | 293 | (20.7) | 160 | (32.4) | 101 | (14.2) | <0.001 | |||
| CABG | 114 | (8.1) | 40 | (8.1) | 60 | (8.4) | 0.0135 | |||
| VT | 216 | (15.3) | 37 | (7.5) | 154 | (21.6) | <0.001 | |||
| ICD | 329 | (23.3) | 15 | (3.0) | 287 | (40.3) | <0.001 | |||
| CRT | 97 | (6.9) | 10 | (2.0) | 86 | (12.1) | <0.001 | |||
| n | Mean | (SD) | n | Mean | (SD) | n | Mean | (SD) | ||
|
| ||||||||||
| Demographic | ||||||||||
| Age | 1412 | 59.9 | (14.8) | 493 | 64.9 | (12.8) | 712 | 57.9 | (14.1) | <0.001 |
| Weight (kg) | 1138 | 85.3 | (22.6) | 403 | 83.2 | (19.5) | 534 | 86.5 | (24.6) | 0.027 |
| Metabolic | ||||||||||
| AST | 788 | 459.4 | (1492.6) | 345 | 448.5 | (1066.3) | 328 | 441.4 | (1805.2) | 0.950 |
| BUN | 1026 | 32.4 | (20.5) | 416 | 28.3 | (17.8) | 456 | 37.7 | (22.6) | <0.001 |
| Lactate | 676 | 4.4 | (4.2) | 292 | 4.7 | (4.1) | 307 | 3.8 | (4.1) | 0.011 |
| HCO3 | 836 | 22.1 | (5.4) | 367 | 20.2 | (4.9) | 330 | 24.3 | (5.3) | <0.001 |
| Serum Creatinine | 1295 | 1.8 | (1.1) | 448 | 1.7 | (1.2) | 687 | 1.9 | (1.1) | 0.003 |
| pH | 577 | 7.3 | (0.2) | 306 | 7.3 | (0.2) | 179 | 7.3 | (0.1) | <0.001 |
| Hemodynamic | ||||||||||
| Admission EF | 771 | 24.9 | (15.5) | 260 | 30.9 | (15.9) | 429 | 20.2 | (12.4) | <0.001 |
| RAP | 1037 | 14.2 | (6.9) | 303 | 14.6 | (6.5) | 626 | 14.0 | (7.2) | 0.176 |
| PCWP | 847 | 24.5 | (8.9) | 271 | 24.2 | (9.2) | 486 | 24.8 | (8.8) | 0.354 |
| Mean PAP | 904 | 32.8 | (9.8) | 257 | 30.2 | (9.0) | 549 | 34.4 | (9.8) | <0.001 |
| Cardiac Output | 1062 | 3.8 | (2.4) | 329 | 3.8 | (2.1) | 630 | 3.8 | (2.4) | 0.826 |
| CPO | 999 | 0.6 | (0.4) | 314 | 0.6 | (0.4) | 584 | 0.6 | (0.4) | 0.638 |
| Heart Rate | 1248 | 92.0 | (22.7) | 407 | 91.2 | (23.0) | 660 | 92.2 | (22.1) | 0.474 |
| Cardiac Index | 1071 | 1.9 | (0.6) | 335 | 1.9 | (0.6) | 635 | 1.8 | (0.6) | 0.096 |
| MAP | 1230 | 74.5 | (14.7) | 433 | 74.9 | (16.9) | 628 | 74.0 | (12.7) | 0.483 |
p-values calculated using chi-sq test of independence or t-test as appropriate. Abbreviations: Afib: Atrial fibrillation; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPO: cardiac power output; CRT: cathode-ray tube; CVA: cerebrovascular accident; HCO3: Bicarbonate; HTN: Hypertension; ICD: implantable cardioverter defibrillator; MAP: mean arterial pressure; PAP: pulmonary arterial pressure; PCI: percutaneous coronary intervention; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; RAP: right arterial pressure; TIA: transient ischemic attack; VT: ventricular tachycardia
Table 3.
Baseline characteristics of CSWG study population by congestion profile
| Congestion Profile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Euvolemic | Left Ventricular | Right Ventricular | Biventricular | ||||||
|
| |||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | p-value | |
| Mortality | 24 | (16.9) | 35 | (18.8) | 23 | (34.9) | 143 | (36.9) | <0.001 |
| Shock Etiology | 0.01 | ||||||||
| MI | 39 | (27.5) | 45 | (24.2) | 29 | (43.9) | 111 | (28.6) | |
| HF | 88 | (62.0) | 120 | (64.5) | 25 | (37.9) | 238 | (61.5) | |
| Other | 15 | (10.6) | 21 | (11.3) | 12 | (18.2) | 38 | (9.8) | |
| SCAI Stage | 0.06 | ||||||||
| B | 1 | (2.4) | 0 | (0) | 0 | (0) | 0 | (0) | |
| C | 14 | (33.3) | 29 | (43.9) | 9 | (22.0) | 75 | (35.9) | |
| D | 17 | (40.5) | 23 | (34.9) | 14 | (34.2) | 71 | (34.0) | |
| E | 10 | (23.8) | 14 | (21.2) | 18 | (43.9) | 63 | (30.1) | |
| # Pressors/Inotropes | <0.001 | ||||||||
| 0 | 40 | (29.0) | 25 | (13.6) | 7 | (10.8) | 60 | (15.9) | |
| 1 | 49 | (35.5) | 75 | (40.8) | 19 | (29.2) | 116 | (30.7) | |
| 2+ | 49 | (35.5) | 84 | (45.7) | 39 | (60.0) | 202 | (53.4) | |
| # Devices | <0.001 | ||||||||
| 0 | 64 | (45.1) | 50 | (26.9) | 10 | (15.2) | 60 | (15.5) | |
| 1 | 56 | (39.4) | 104 | (55.9) | 30 | (45.5) | 233 | (60.1) | |
| 2+ | 22 | (15.5) | 32 | (17.2) | 26 | (39.4) | 95 | (24.5) | |
| Device Type | |||||||||
| Impella | 29 | (20.4) | 48 | (25.8) | 24 | (36.4) | 116 | (30.0) | 0.06 |
| ECMO | 19 | (13.4) | 25 | (13.4) | 22 | (33.3) | 89 | (22.9) | <0.001 |
| IABP | 52 | (36.6) | 96 | (51.6) | 36 | (54.6) | 223 | (57.5) | <0.001 |
| Male | 99 | (69.7) | 141 | (75.8) | 45 | (68.2) | 267 | (68.8) | 0.35 |
| Race | 0.10 | ||||||||
| White | 79 | (71.8) | 105 | (80.8) | 38 | (84.4) | 183 | (77.2) | |
| Hispanic/Latino | 1 | (0.9) | 1 | (0.8) | 2 | (4.4) | 10 | (4.2) | |
| African-American | 4 | (3.6) | 3 | (2.3) | 1 | (2.2) | 8 | (3.4) | |
| Asian | 3 | (2.7) | 1 | (0.8) | 2 | (4.4) | 9 | (3.8) | |
| Other | 23 | (20.9) | 20 | (15.4) | 2 | (4.4) | 27 | (11.4) | |
| Medical History | |||||||||
| HTN | 59 | (43.1) | 76 | (43.7) | 32 | (50.8) | 180 | (51.9) | 0.35 |
| DM2 | 41 | (29.1) | 57 | (30.7) | 22 | (33.9) | 141 | (36.4) | 0.33 |
| Afib/Flutter | 33 | (27.1) | 51 | (33.3) | 18 | (33.3) | 123 | (40.2) | 0.07 |
| CKD (any stage) | 36 | (26.9) | 45 | (26.6) | 11 | (18.3) | 104 | (30.6) | 0.24 |
| PVD | 6 | (4.6) | 6 | (3.7) | 5 | (8.6) | 20 | (6.4) | 0.44 |
| COPD | 12 | (8.8) | 12 | (6.9) | 3 | (4.8) | 40 | (11.1) | 0.23 |
| CVA/TIA | 22 | (16.1) | 28 | (16.2) | 6 | (9.5) | 52 | (14.4) | 0.60 |
| Valvular Disease | 25 | (21.2) | 39 | (26.5) | 16 | (29.6) | 75 | (26.3) | 0.61 |
| PCI | 25 | (20.8) | 43 | (29.5) | 23 | (42.6) | 71 | (25.7) | 0.02 |
| CABG | 9 | (6.8) | 13 | (8.2) | 8 | (13.8) | 32 | (10.4) | 0.39 |
| VT | 36 | (29.5) | 41 | (26.6) | 15 | (27.8) | 71 | (23.1) | 0.54 |
| ICD | 54 | (44.3) | 77 | (50.0) | 11 | (20.0) | 116 | (37.9) | <0.001 |
| CRT | 16 | (13.1) | 18 | (11.7) | 1 | (1.8) | 36 | (11.8) | 0.14 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Demographic | |||||||||
| Age | 56.5 | 15.4 | 57.6 | 15.4 | 60.6 | 14.9 | 59.4 | 14.7 | 0.11 |
| Weight (kg) | 81.6 | 20.1 | 82.5 | 20.3 | 83.2 | 22.8 | 87.1 | 22.1 | 0.03 |
| Metabolic | |||||||||
| AST | 452.3 | 1552.6 | 340.2 | 1067.9 | 860.9 | 3070.4 | 447.0 | 1447.0 | 0.29 |
| BUN | 28.3 | 15.7 | 31.2 | 19.2 | 32.2 | 22.1 | 37.6 | 22.8 | <0.001 |
| Lactate | 3.6 | 3.3 | 3.7 | 4.5 | 5.1 | 3.4 | 4.7 | 3.4 | 0.13 |
| HCO3 | 23.7 | 4.9 | 23.9 | 5.2 | 20.0 | 5.5 | 22.2 | 5.7 | <0.001 |
| Serum Creatinine | 1.6 | 0.8 | 1.5 | 1.0 | 1.8 | 1.5 | 1.9 | 1.2 | 0.002 |
| pH | 7.3 | 0.1 | 7.4 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | <0.001 |
| Hemodynamic | |||||||||
| EF (%) | 22.6 | 12.2 | 24.0 | 16.8 | 25.2 | 16.8 | 23.5 | 15.2 | 0.85 |
| RAP | 6.7 | 3.1 | 8.7 | 2.9 | 16.7 | 3.5 | 19.2 | 5.1 | <0.001 |
| PCWP | 13.2 | 3.9 | 25.6 | 5.3 | 15.8 | 2.4 | 29.3 | 7.4 | <0.001 |
| Mean PAP | 23.3 | 6.8 | 33.6 | 6.9 | 25.8 | 8.3 | 37.3 | 9.0 | <0.001 |
| Cardiac Output | 4.0 | 1.6 | 3.6 | 0.9 | 4.2 | 2.9 | 3.9 | 3.3 | 0.40 |
| CPO | 0.6 | 0.3 | 0.6 | 0.2 | 0.7 | 0.5 | 0.6 | 0.5 | 0.57 |
| Heart rate | 83.8 | 19.0 | 90.2 | 19.2 | 91.6 | 23.9 | 93.8 | 21.4 | <0.001 |
| Cardiac Index | 2.0 | 0.5 | 1.9 | 0.5 | 1.9 | 0.6 | 1.8 | 0.6 | <0.001 |
| Mean arterial pressure | 73.2 | 13.6 | 74.7 | 13.6 | 71.9 | 19.1 | 73.7 | 13.1 | 0.53 |
p-values calculated using chi-sq test of independence or ANOVA as appropriate. Abbreviations: Afib: Atrial fibrillation; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPO: cardiac power output; CRT: cathode-ray tube; CVA: cerebrovascular accident; HCO3: Bicarbonate; HF: heart failure; HTN: Hypertension; ICD: implantable cardioverter defibrillator; MI: myocardial infarction; MAP: mean arterial pressure; PAP: pulmonary arterial pressure; PCI: percutaneous coronary intervention; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; RAP: right arterial pressure; TIA: transient ischemic attack; VT: ventricular tachycardia
In-Hospital Outcomes
In-hospital mortality in the study cohort was 30.5%. In-hospital mortality was higher among MI patients (39.5%) than HF patients (25.3%; p<0.0001) (Table 4). Overall, survivors were younger and exhibited lower prevalence of arterial HTN, type 2 diabetes mellitus (DM2), and prior coronary artery bypass grafting (CABG) compared to non-survivors (Table 4). Clinical variables stratified by survivorship among MI and HF patients are shown in Supplemental Tables 1 and 2. MI survivors were less likely to receive ventricular assist devices (VADs) or heart transplant compared to HF survivors (Supplemental Figure 2).
Table 4.
Differences between survivors and non-survivors in the overall CSWG study cohort
| Mortality | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Survivors (n=938) | Non-Survivors (n=431) | p-value | |||||
|
| |||||||
| n | % | n | % | ||||
| SCAI Stage | <0.001 | ||||||
| B | 1 | 0.3 | 0 | 0 | |||
| C | 157 | 44.5 | 75 | 25.7 | |||
| D | 113 | 32.0 | 107 | 36.6 | |||
| E | 82 | 23.2 | 110 | 37.7 | |||
| # Pressors/Inotropes | <0.001 | ||||||
| 0 | 206 | 23.3 | 30 | 7.6 | |||
| 1 | 305 | 34.5 | 88 | 22.3 | |||
| 2+ | 373 | 42.2 | 277 | 70.1 | |||
| # MCS Devices | <0.001 | ||||||
| 0 | 197 | 20.0 | 27 | 6.3 | |||
| 1 | 631 | 64.2 | 251 | 58.2 | |||
| 2+ | 155 | 15.8 | 153 | 35.5 | |||
| Types of MCS | |||||||
| Impella | 218 | 22.2 | 192 | 44.6 | <0.001 | ||
| ECMO | 168 | 17.1 | 165 | 38.3 | <0.001 | ||
| IABP | 560 | 57.0 | 210 | 48.7 | 0.004 | ||
| Etiology | <0.001 | ||||||
| MI | 299 | 30.4 | 195 | 45.2 | |||
| HF | 532 | 54.1 | 180 | 41.8 | |||
| Gender | 0.236 | ||||||
| Female | 260 | 26.5 | 127 | 29.5 | |||
| Male | 722 | 73.5 | 303 | 70.3 | |||
| Race | 0.259 | ||||||
| White | 460 | 46.8 | 187 | 43.4 | |||
| Hispanic/Latino | 20 | 2.0 | 11 | 2.6 | |||
| Asian | 24 | 2.4 | 7 | 1.6 | |||
| African-American | 18 | 1.8 | 10 | 2.3 | |||
| Other | 66 | 6.7 | 16 | 3.7 | |||
| Medical History | |||||||
| HTN | 426 | 43.3 | 255 | 59.2 | <0.001 | ||
| Diabetes | 310 | 31.5 | 179 | 41.5 | <0.001 | ||
| Afib/Flutter | 207 | 21.1 | 89 | 20.6 | 0.462 | ||
| CKD (any stage) | 218 | 22.2 | 105 | 24.4 | 0.339 | ||
| PVD | 37 | 3.8 | 23 | 5.3 | 0.040 | ||
| COPD | 68 | 6.9 | 33 | 7.7 | 0.883 | ||
| CVA/TIA | 109 | 11.1 | 50 | 11.6 | 0.682 | ||
| Valvular Disease | 161 | 16.4 | 53 | 12.3 | 0.296 | ||
| PCI | 187 | 19.0 | 106 | 24.6 | 0.040 | ||
| CABG | 59 | 6.0 | 55 | 12.8 | <0.001 | ||
| VT | 143 | 14.5 | 73 | 16.9 | 0.049 | ||
| ICD | 250 | 25.4 | 79 | 18.3 | 0.020 | ||
| CRT | 69 | 7.0 | 28 | 6.5 | 0.985 | ||
| n | Mean | SD | n | Mean | SD | ||
|
| |||||||
| Demographic | |||||||
| Age | 982 | 58.3 | 15.0 | 430 | 63.6 | 13.5 | <0.001 |
| Weight (kg) | 803 | 85.3 | 22.8 | 335 | 85.2 | 22.1 | 0.970 |
| Metabolic | |||||||
| AST | 526 | 364.1 | 1324.0 | 262 | 650.8 | 1771.0 | 0.011 |
| BUN | 703 | 30.3 | 18.7 | 323 | 37.0 | 23.3 | <0.001 |
| Lactate | 377 | 3.6 | 3.4 | 299 | 5.4 | 4.9 | <0.001 |
| HCO3 | 576 | 23.2 | 5.2 | 260 | 19.8 | 5.3 | <0.001 |
| Serum Creatinine | 907 | 1.7 | 1.0 | 388 | 2.0 | 1.3 | <0.001 |
| pH | 336 | 7.3 | 0.1 | 241 | 7.3 | 0.2 | <0.001 |
| Hemodynamic | |||||||
| Admission EF | 522 | 24.6 | 15.2 | 249 | 25.7 | 16.1 | 0.335 |
| RAP | 747 | 13.2 | 6.5 | 290 | 16.6 | 7.4 | <0.001 |
| PCWP | 595 | 24.0 | 8.9 | 252 | 25.6 | 8.8 | 0.018 |
| Mean PAP | 665 | 32.8 | 9.8 | 239 | 32.9 | 10.0 | 0.899 |
| Cardiac Output | 747 | 3.7 | 2.0 | 315 | 4.1 | 3.2 | <0.001 |
| CPO | 704 | 0.6 | 0.4 | 295 | 0.6 | 0.5 | 0.381 |
| Heart Rate | 894 | 91.0 | 22.3 | 354 | 94.6 | 23.5 | 0.012 |
| Cardiac Index | 760 | 1.8 | 0.6 | 311 | 1.9 | 0.7 | 0.120 |
| MAP | 849 | 76.3 | 14.2 | 381 | 70.6 | 15.2 | <0.001 |
p-values calculated using chi-sq test of independence or t-test as appropriate. Abbreviations: Afib: Atrial fibrillation; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPO: cardiac power output; CRT: cathode-ray tube; CVA: cerebrovascular accident; HCO3: Bicarbonate; HF: heart failure; HTN: Hypertension; ICD: implantable cardioverter defibrillator; MI: myocardial infarction; MAP: mean arterial pressure; PAP: pulmonary arterial pressure; PCI: percutaneous coronary intervention; PCWP: pulmonary capillary wedge pressure; PVD: peripheral vascular disease; RAP: right arterial pressure; TIA: transient ischemic attack; VT: ventricular tachycardia
Association of SCAI Stages with Outcomes
Patients with known drug and device data (n=1279) were classified into SCAI stages based on the number of drug and/or device treatments (Figure 1). Increasing drug or device treatment was directly associated with in-hospital mortality (Supplemental Figure 3, Table 4). All Stage B patients survived to hospital discharge. Thereafter, each increased stage was associated with an increased risk of in-hospital mortality (Figure 3). Compared to SCAI stage C, stage D had 4.1 (95% CI: 2.7–6.3) times the odds of in-hospital mortality while stage E had 10.3 (95% CI: 6.4–16.6) times the odds of in-hospital mortality. Additionally, stage D had less than half the odds of in-hospital mortality of stage E (OR: 0.4, 95% CI: 0.29–0.55). This was also true among the MI cohort with mortality ORs of 3.9 (95% CI: 2.0–7.6) and 8.1 (95% CI: 4.0–16.3) among stage D and E patients, respectively, compared to stage C patients and an OR of 0.49 (95% CI: 0.32–0.75) among stage D patients compared to those in stage E. The same trend was also observed in HF patients. HF stage D and E patients had ORs of 3.5 (95% CI: 2.0–6.1) and 10.0 (95% CI: 4.7–21.0) compared to stage C patients and stage D patients had 0.35 times the odds of mortality (95% CI: 0.20–0.61) compared to stage E patients.
Figure 3.
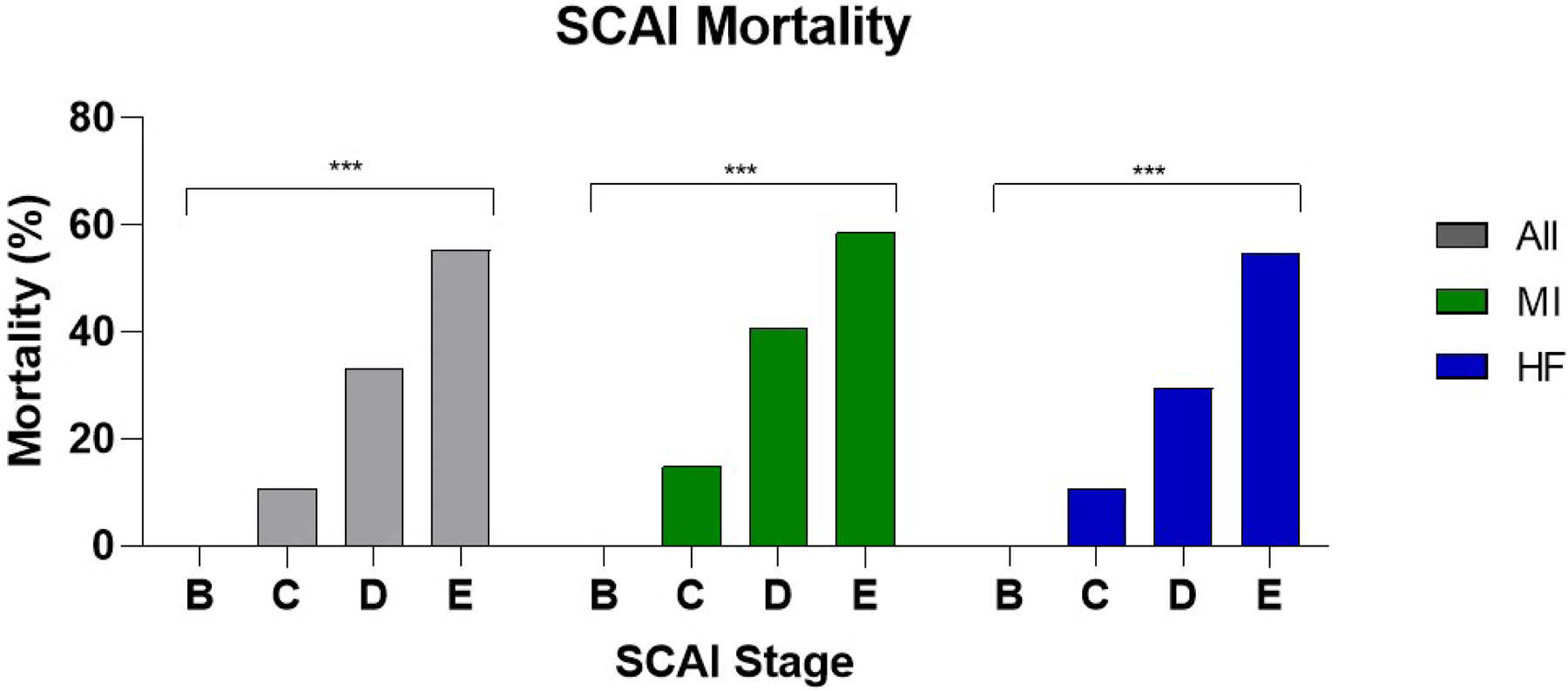
In-hospital mortality by SCAI stage among different etiologies of shock. ***: p ≤ 0.001
Lactate and drug and device data were available in 645 patients and were used to perform a sensitivity analysis of SCAI staging incorporating lactate levels. Of these 645 patients, 1 patient (0.1%) was classified as Stage B, 232 (35.9%) were Stage C, 220 (34.1%) were Stage D, and 192 (29.8%) were Stage E. This distribution differed significantly from the entire study cohort using only drug and device escalation. However, a similar trend in mortality was observed in this sensitivity analysis with 0% mortality in Stage B, 32.3% in Stage C, 48.6% in Stage D and 57.3% in Stage E (Supplemental Figure 1).
Association of Congestion Profiles with Outcomes
We next explored the impact of hemodynamic congestion on mortality. PACs were used to collect any hemodynamic data in 79% of the total study population (n=1116) with both RAP and PCWP assessed in 55% of the total population (n=781). Mean cardiac index (CI) was 1.9±0.6 across the study population. A positive correlation between RAP and PCWP (R2=0.26, p<0.001) was observed in these patients. Using these data, we grouped CS patients into one of four “congestion” profiles as defined above (Figure 4). BiV congestion (i.e., elevated right and left heart filling pressures) was most commonly observed (50%, n=390). Both BiV and RV congestion profiles were associated with the highest in-hospital mortality among the total cohort and among either MI or HF subgroups (Figure 5). Stage B patients were comprised mainly of EuV patients. The frequency of BiV congestion increased with increased SCAI stage among the entire cohort and in the MI and HF subgroups (Figure 5).
Figure 4.
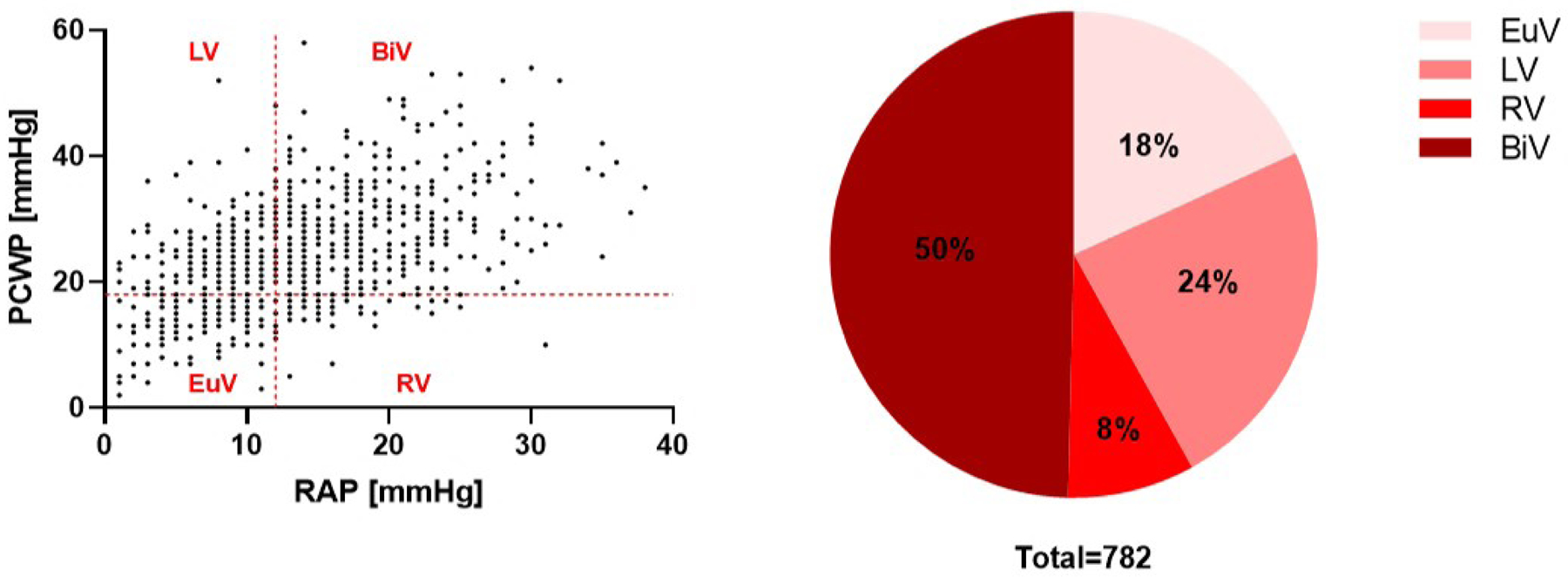
Adjudication and distribution of congestion profiles among the CSWG study population with available hemodynamic data
Figure 5.
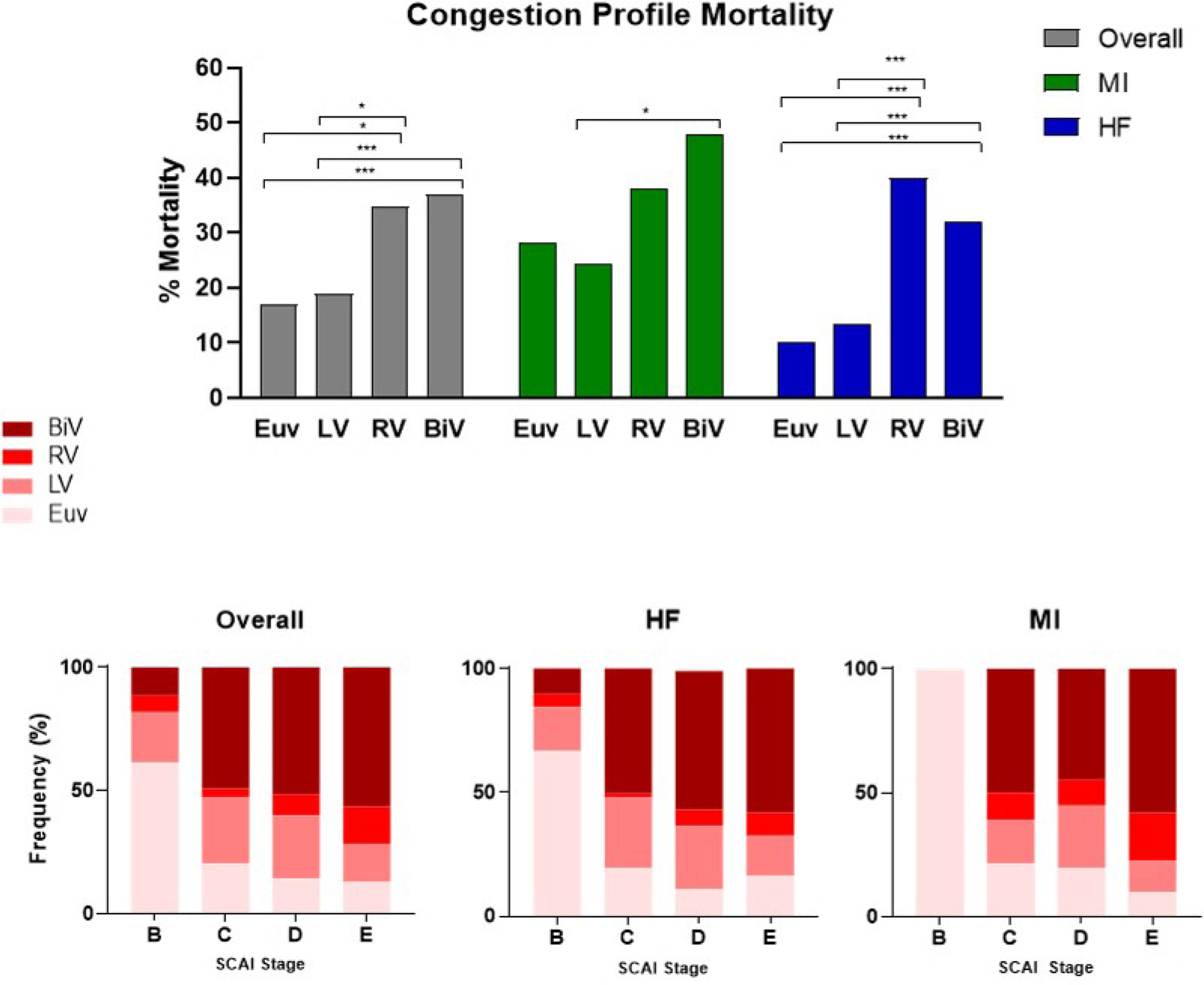
A. Comparisons of mortality across congestion profiles among he overall, MI, and HF study cohorts. Comparisons adjusted by Bonferroni. B. Distribution of congestion profiles across SCAI stages. *: p ≤ 0.05, ***: p ≤ 0.001
Multivariate Analyses
To better understand the relationship between SCAI stages, shock etiology, and hemodynamics with in-hospital mortality, we ran several multivariate analyses. In the entire study cohort, after adjusting for shock etiology, congestion profile, and other comorbidities (HTN, age, DM2, Prior PCI, and ventricular tachycardia (VT)) we found that SCAI stages were still a significant predictor of mortality with stage D and E patients having aORs of 11.8 (95% CI: 4.6–30.5) and 21.3 (95% CI: 7.7–59.0), respectively, compared to Stage C patients and Stage D patients having an aOR of 0.6 (95% CI: 0.3–0.9) compared to stage E patients. After adjustment, shock etiology was no a significant independent predictor of mortality while biventricular congestion remained a signifcant independent predictor of mortality compared to left ventricular congestion or no congestion (BiV v. LV aOR: 2.4, 95% CI: 1.4–3.7) (BiV v. EuV aOR: 2.1, 95% CI: 1.1–4.0). Additionally, after adjusting for SCAI stage in a separate multivariable model, RAP remained a significant predictor of mortality (OR: 1.06 95% CI: 1.03–1.08).
Discussion
Using a large, multi-center registry inclusive of invasive hemodynamics and contemporary short-term MCS strategies, we identified that the proposed SCAI staging system is associated with in-hospital mortality among patients with CS due to HF and/or MI. Compared to HF, MI patients have higher mortality, but MI survivors have a greater likelihood of recovery to discharge, with few patients bridging to durable ventricular assist devices or orthotopic heart transplantation. Given the availability of hemodynamic data, we confirmed a low mean cardiac index in the study population and observed a high prevalence of both right- and left-sided (biventricular) congestion in the study population. Worsening congestion was associated with both increasing SCAI stages and in-hospital mortality. These findings address critical gaps in our understanding of cardiogenic shock by confirming not only that SCAI stages identify patients at risk for in-hospital mortality in a population that reflects contemporary clinical practice, but also that basic hemodynamic data may be used to further stratify risk among patients with cardiogenic shock.
We assigned SCAI stages based on the consensus statement parameters focused on treatment intensity, defined by the number of drug and device therapies utilized during admission for CS (Figure 1). Drug or device escalation were each directly associated with in-hospital mortality. This observation is particularly important given the broad range of short-term MCS devices included in the analysis and supports the need for future prospective studies exploring the utility of device-based CS algorithms. Progression from one SCAI classification to the next represents a deteriorating clinical course of CS as indicated by increasing intensity of medical and/or device-based therapies to stabilize a critically ill patient, ultimately ending with use of ‘all resources at hand’ in Stage E. Therefore, we employed a matrix of drug and device escalation and identified that SCAI stages are directly associated with in-hospital mortality.
To validate our method of assigning SCAI stages, a sensitivity analysis incorporating lactate levels was performed. While a different distribution of patients across SCAI stages was observed, the relationship between SCAI stage and mortality remained unchanged (Supplemental Figure 1). Patient characteristics for this alternate definition of SCAI stages are displayed in supplemental table 3. Since lactate levels are not uniformly collected, these observations suggest that stratifying patients based on maximal drug or device utilization may be a reasonable approach to defining SCAI stages for the purpose of data analysis. Clinically, a more uniform definition for SCAI stages is needed and prospective registries should incorporate lactate levels in addition to measures of hypotension, hypoperfusion and drug/device utilization.
Recent reports exploring the utility of SCAI stages have employed different definitions for each stage. Jentzer and colleagues defined SCAI stages based on clinical indices of hypotension and hypoperfusion with inclusion of a change in lactate from admission to maximal value recorded as a marker of deterioration 12. This group showed a correlation with in-hospital mortality in a single-center database that largely focused on IABP use with minimal exposure to other short-term MCS devices. Schrage and colleagues defined SCAI stages based primarily on lactate levels 13. This single-center study also identified a correlation between SCAI stages and in-hospital mortality. Neither study included invasive hemodynamic data. Our findings now employ a multi-center registry inclusive of contemporary short-term MCS devices and provide new information derived from invasive hemodynamic data that support our distinct approach to assigning SCAI stages.
We observed 0% mortality in SCAI Stage B patients, who represent patients with early stage shock. Since our report evaluated maximal SCAI stage during a patient’s hospitalization, these patients did not progress into CS and a low mortality rate may be expected and is consistent with prior reports 12, 13. Furthermore, in our sensitivity analysis incorporating lactate levels, SCAI Stage B patients continued to have the lowest mortality rate of 0%. More study of this unique population of ‘pre-shock’ patients is required.
Accordingly, our findings strengthen the proposed SCAI classification structure by: 1) providing contemporary evidence that treatment escalation may be an objective means of defining deterioration irrespective of the cause of CS, 2) enabling future analyses to evaluate both escalation and de-escalation of therapies, and 3) informing the development of future registry and randomized clinical trials where different CS strategies can be tested in patient populations with similar expected outcomes.
A unique aspect of the CSWG Registry is the availability of invasive hemodynamic data for analysis. Across survivors and non-survivors, cardiac filling pressures were elevated and CO, CI and cardiac power output (CPO) were low. Non-survivors had higher filling pressures and no significant difference in CPO or CI compared to survivors. CPO and CI were also not significantly changed across SCAI stages, but CO was paradoxically higher among Stage E patients and among non-survivors. This may reflect variability in how CO is calculated (i.e. Fick or thermodilution method) and the impact of maximal drug and/or device treatment to increase CO in sicker patients (ie Stage E). Furthermore, CPO has been validated primarily in MI populations 14 but is less well understood in HF populations, where low CO does not always correlate with low MAP. The SHOCK trial also did not include multiple short-term MCS approaches. Our data suggest that CPO requires further validation in MI and HF shock populations treated with contemporary short-term MCS devices.
In contrast to CO measurements, cardiac filling pressures were consistently elevated across all shock cohorts. Both RAP and PCWP were significantly higher among non-survivors and increased across SCAI stages. We further characterized the impact of cardiac filling pressures on clinical outcomes by defining congestive profiles based on RAP and PCWP. In-hospital mortality was highest among patients with biventricular or right ventricular congestive profiles. Furthermore, the distribution of congestive profiles across SCAI stages suggests that sicker patients are more likely to have biventricular congestion. These findings suggest that venous congestion is potentially an important determinant of clinical outcomes and may be explained by the fact that venous congestion is associated with worsening renal function and congestive hepatopathy 15, 16, which may exacerbate metabolic derangement. Prior reports have also illustrated the association between venous congestion and poor outcomes in heart failure and myocardial infarction 17. These observations suggest that approaches to ‘decongest’ patients with cardiogenic shock may improve clinical outcomes.
We further observed that the presence of biventricular congestion was associated with worsening kidney and liver function and elevated lactate levels compared to other congestive profiles. These data are also consistent with studies suggesting that the presence of right heart failure in the setting of CS is associated with increased mortality 18. Recent data from prospective shock registries utilizing congestive profiles as part of a treatment strategy algorithm showed improvement in mortality due to acute myocardial infarction and CS 19, 20. These findings suggest that CS algorithms that include an assessment of congestive profile may lead to improved outcomes by identifying and managing patients with venous congestion before metabolic failure worsens.
Limitations
The retrospective nature of the registry limits the ability to account for missing data elements, is subject to clinical and selection bias, and further limits our ability to adjust for metabolic and hemodynamic indicators of prognosis. Since the exact timing of data collection cannot be ascertained it would be inappropriate to assess these measures as confounders relative to each other. A limitation of the current analysis is the lack of detail regarding drug dosage, sequence of device application, and timing of therapy as well as specific vasopressors or inotropes used. Future studies specifically looking at drug and/or device escalation across a patient’s hospitalization for cardiogenic shock are required. Furthermore, information about cardiac arrest was not available for analysis. However, even without serial data available, maximal escalation of treatment serves a reasonable marker of overall clinical deterioration. Though, this approach prevents drawing inferences about treatment strategies at each SCAI stage and may be influenced by other factors including institutional availability of devices, physician preference, variations in shock treatment algorithms, and other clinical or anatomic limitations to drug or device implementation. For these reasons, an additional sensitivity analysis incorporating baseline lactate levels into the SCAI staging scheme was performed. As hemodynamic data in this study were assessed after index hospital admission, data was most likely acquired after initiation of drug or device therapy in the case of transfer patients. Future studies involving a more granular retrospective dataset or prospective studies are required to put these findings into context.
Conclusions
In this large, multi-center analysis of a national registry we provide new insight into the characteristics, contemporary treatment strategies, and predictors of in-hospital mortality among patients with all-cause CS or CS due to MI or HF. We provide real-world validation of the SCAI staging scheme as an approach to identify CS patients at risk of in-hospital mortality. We also identified venous congestion as a critical marker of risk, thus potentially identifying an important target of therapy for patients with CS. Future prospective studies are required to confirm the long-term prognostic significance of these findings.
Supplementary Material
What is new:
Using data from the Cardiogenic Shock Working Group Registry inclusive of contemporary short-term, percutaneous mechanical circulatory support (MCS) devices and invasive hemodynamic data, we report a novel validation analysis showing that SCAI stages directly associate with in-hospital mortality
We provide new insight into the distribution of short-term MCS use across SCAI stages.
We show that elevated right heart filling pressures (venous congestion) are common and associated with worsening shock severity and in-hospital mortality.
What are the clinical implications
Our findings suggest that more clinical data inclusive of hemodynamic and metabolic variables are required to confirm the specific definitions of SCAI stages for patients with cardiogenic shock due to myocardial infarction or heart failure and further identify venous congestion as an important marker of risk for in-hospital mortality.
Future studies exploring whether a strategy of early venous decongestion improves clinical outcomes in cardiogenic shock are required.
Sources of Funding
This work was supported by a NIH RO1 grant to NKK (RO1HL139785–01) and institutional grants from Abiomed Inc (Danvers, MA), Boston Scientific Inc (Minneapolis, MN), and Abbott Laboratories (Abbott Park, IL) to Tufts Medical Center.
Abbreviations:
- CS
Cardiogenic Shock
- CSWG
Cardiogenic Shock Working Group
- HF
Heart Failure
- MCS
mechanical circulatory support
- MI
Myocardial Infarction
- PAC
Pulmonary Artery Catheter
- SCAI
Society for Cardiovascular Angiography and Interventions
Footnotes
Disclosures
NKK receives consulting/speaker honoraria and institutional grant support from: Abbott Laboratories, Abiomed Inc., Boston Scientific, Medtronic, LivaNova, MDStart, and Precardia. JHM is a consultant for Abiomed Inc. JA is a consultant for Abbott Laboratories, Abiomed Inc. DB reports an unrestricted, educational grant from Abiomed Inc. to Cardiovascular Research Foundation. SSS is a consultant for Abiomed Inc. (Critical Care Advisory Board). WON receives consulting/speaker honoraria from Abiomed Inc. KLT, EZ, MA, ARG, JHM, CM, KM, SN, LJ, MLE, CDD, DW, EV, NMH, JLH have nothing to disclose.
References
- 1.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH and Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical C, Council on C, Stroke N, Council on Quality of C, Outcomes R and Mission L. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D and Gore JM. Decade-Long Trends (2001–2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes 2016;9:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becher PM, Schrage B, Sinning CR, Schmack B, Fluschnik N, Schwarzl M, Waldeyer C, Lindner D, Seiffert M, Neumann JT, Bernhardt AM, Zeymer U, Thiele H, Reichenspurner H, Blankenberg S, Twerenbold R and Westermann D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiopulmonary Support. Circulation 2018;138:2298–2300. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Bohm M, Ebelt H, Schneider S, Schuler G, Werdan K and Investigators I-SIT. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 6.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM and LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625–34. [DOI] [PubMed] [Google Scholar]
- 7.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BA, Tijssen JG and Henriques JP. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ and Yeh RW. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention 2018;13:e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 10.Stretch R, Sauer CM, Yuh DD and Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 11.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S and Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 12.Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS and Baran DA. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J Am Coll Cardiol 2019;74:2117–2128. [DOI] [PubMed] [Google Scholar]
- 13.Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sorensen NA, Gossling A, Becher PM, Grahn H, Wagner T, Seiffert M, Kluge S, Reichenspurner H, Blankenberg S and Westermann D. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv 2020; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G and Investigators S. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–8. [DOI] [PubMed] [Google Scholar]
- 15.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB and Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG and Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013;61:2397–2405. [DOI] [PubMed] [Google Scholar]
- 17.Brinkley DM Jr., Ho KKL, Drazner MH and Kociol RD. The prognostic value of the relationship between right atrial and pulmonary capillary wedge pressure in diverse cardiovascular conditions. Am Heart J 2018;199:31–36. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs AK, Leopold JA, Bates E, Mendes LA, Sleeper LA, White H, Davidoff R, Boland J, Modur S, Forman R and Hochman JS. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003;41:1273–9. [DOI] [PubMed] [Google Scholar]
- 19.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, Rosner C, Raja A, Barnett SD, Saulino P, deFilippi CR, Gurbel PA, Murphy CE and O’Connor CM. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol 2019;73:1659–1669. [DOI] [PubMed] [Google Scholar]
- 20.Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, Kolski B, Todd J, Senter S, Marso S, Lasorda D, Wilkins C, Lalonde T, Attallah A, Larkin T, Dupont A, Marshall J, Patel N, Overly T, Green M, Tehrani B, Truesdell AG, Sharma R, Akhtar Y, McRae T 3rd, O’Neill B, Finley J, Rahman A, Foster M, Askari R, Goldsweig A, Martin S, Bharadwaj A, Khuddus M, Caputo C, Korpas D, Cawich I, McAllister D, Blank N, Alraies MC, Fisher R, Khandelwal A, Alaswad K, Lemor A, Johnson T, Hacala M, O’Neill WW and National Cardiogenic Shock Initiative I. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv 2019;93:1173–1183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


