Abstract
Regiocontrol in traditional cycloaddition reactions between unsaturated carbon compounds is often challenging The increasing focus in modern medicinal chemistry on scaffolds speaks to the need for alternative, more selective routes to diverse rigid carbocycles rich in C(sp3) character. Here we report a Pd-catalyzed double C–H activation of two adjacent methylene units in carboxylic acids, enabled by bidentate amide-pyridone ligands, to achieve a regio-controllable synthesis of BCBs through a formal [2+2] cycloaddition involving σ-bonds only (two C–H bonds and two aryl–halogen bonds). A wide range of cyclic and acyclic aliphatic acids, as well as dihaloheteroarenes, are compatible, generating diversely functionalized BCBs and hetero-BCBs present in drug molecules and bioactive natural products.
One Sentence Summary:
Twofold β,γ-methylene C(sp3)−H activation/C−C bond formation was realized using a Pd(II) catalyst bound to amide-pyridone ligands, leading to regio-controllable [2+2] annulation between aliphatic acids and dihaloarenes.
Benzocyclobutenes (BCBs) represent an important class of rigid four-membered carbocycles found in natural products (1, 2) that have demonstrated great potential as therapeutical molecular scaffolds (3, 4), versatile synthons (5), and functional motifs in material science and mechanochemistry (6, 7) (Fig.1A). BCB-based rigid and three-dimensional pharmacophores have led to the discovery of Ivabradine, an FDA-approved drug for the treatment of heart failure and heart-related chest pain (3). In addition, the BCB-analogue of the psychoactive 2C-B (4-Bromo-2,5-dimethoxyphenethylamine) was found to show superior affinity for the human 5-HT2A receptor compared with the conformationally flexible parent compound and the benzocyclopentane analogue (Fig. 1A) (8). Currently, the [2+2] cycloaddition of alkenes and benzynes is one of the most common synthetic routes to BCBs (9). However, controlling the regioselectivity of this cycloaddition reaction is an unsolved problem. As illustrated in Fig. 1B, the [2+2] cycloadditions between a substituted alkene and benzyne typically lead to mixtures of head-to-head and head-to-tail regioisomers as well as multiple diastereoisomers. A number of alternative intramolecular processes, such as cyclization of o-quinodimethane (10), cyclative C−H arylations (11–14), visible-light induced radical processes (15) and others (16) have been developed. A modular two-component intermolecular synthesis of BCBs has recently been reported using an elegant Pd-catalyzed [2+2] annulation of alkenes bearing an amide directing group and arylboronic acids (17); however, this method does not allow regiocontrol. In addition, existing methods for BCB synthesis suffer from scope and efficiency limitations due to the requirement of preinstallation of reactive functional groups such as double or triple bonds. Notably, the synthesis of medicinally important heterocyclic BCBs using these reactions is challenging (18, 19). Our recently demonstrated Pd-catalyzed activation of β- or γ-C−H bonds of aliphatic acids (20–22) inspired us to explore a formal [2+2] annulation of aliphatic acids with dihaloarenes through a dual sequential methylene C−H activation at the β- and γ-positions. The state of the art of dual functionalization of two adjacent methylene C−H bonds is limited to allylic and benzylic positions, or sites α- to a heteroatom (23–26). Here we report successful palladium-catalyzed BCB synthesis through the annulation of aliphatic acids with dihaloarenes enabled by amide-pyridone ligands, in which the exclusive regiocontrol is achieved through the differentiation between the aryl iodide and bromide sites. The direct use of abundant and structurally varied acyclic and cyclic acids as substrates without pre-functionalization substantially expands access to diverse BCBs, including heterocyclic BCB scaffolds (Fig.1C).
Fig. 1. [2+2] Annulation Reaction via Dual Methylene C−H Bond Functionalization.
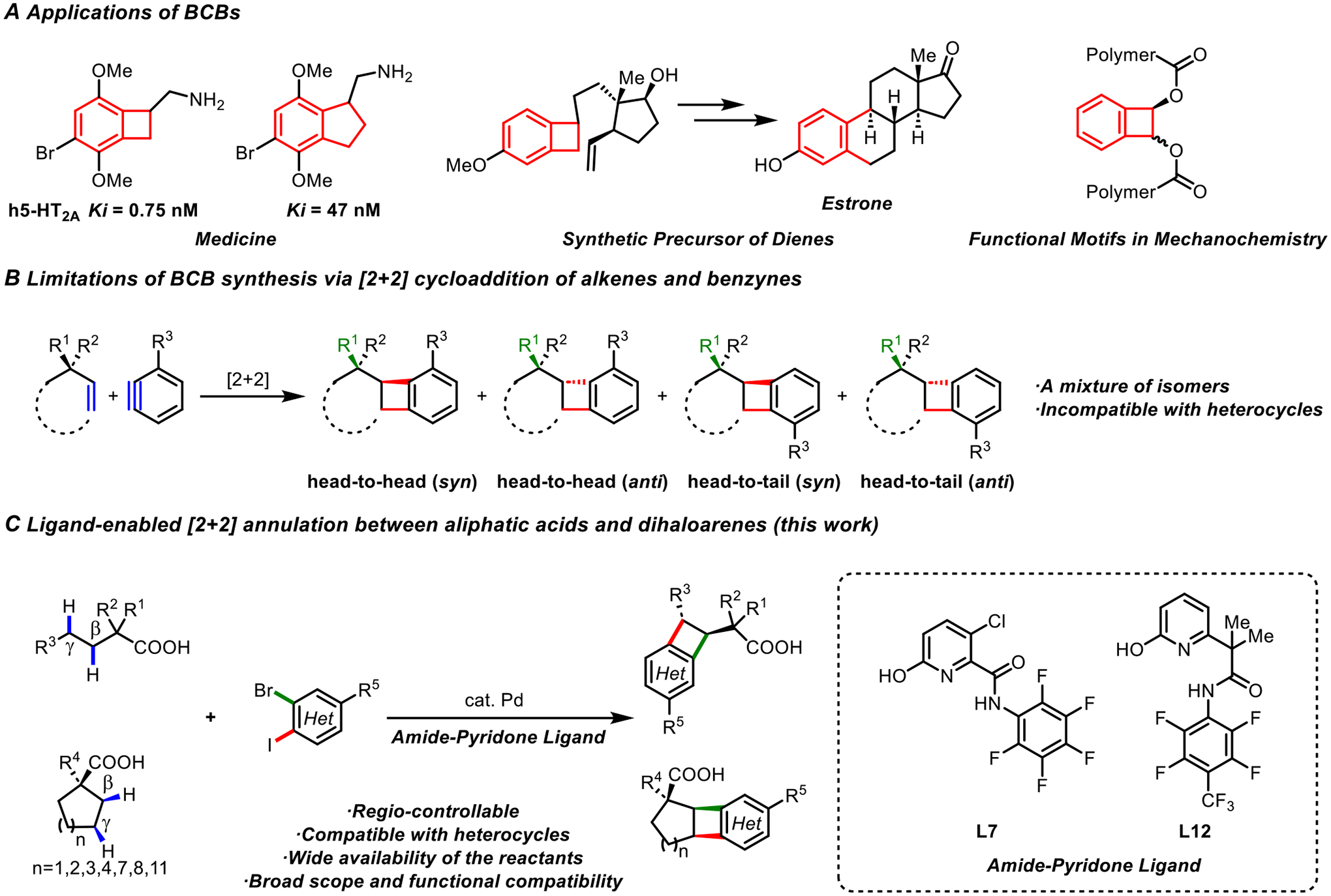
(A) Applications of BCBs. Ki is the inhibitor constant. (B) Limitations of BCB synthesis via [2+2] cycloaddition of alkenes and benzynes. Syn, anti refers to the conformation of R1 group and the adjacent aryl group. (C) Ligand-enabled [2+2] annulation between aliphatic acids and dihaloarenes (this work).
Reaction development.
During our mechanistic investigations of the methylene C–H lactonization of dicarboxylic acids, we observed the β,γ-C–H deuteration of 7-ethoxy-7-oxoheptanoic acid (20). This observation prompted us to wonder whether a sequential twofold C–H coupling process with ortho-dihaloarenes could be developed to construct BCB scaffolds. Using 1-propyl-1-cyclopentanecarboxylic acid 1a and dihaloarene coupling partner 1-bromo-4-chloro-2-iodobenzene 2a with Ag2CO3 as a scavenger for halides, we began to test the feasibility of this transformation with a series of pyridine-pyridone ligands recently developed in our laboratory (Table S1). Encouragingly, the desired BCB 3a was indeed formed as a single isomer (Figure S1). However, attempts to improve the reaction yield using pyridine-pyridone ligands and other known ligand scaffolds (L27 to L36) under various conditions proved unsuccessful (≤ 22% yield).
These initial results with pyridine-pyridone ligands, while promising, pointed to the need for more effective ligands. Since the 2-pyridone serves a critical role as the internal base for C–H cleavage (27), we focused on developing alternatives for the pyridine arm of the scaffold. Inspired by the superior reactivity of the electron-deficient amide motif as a directing group for Pd(II) in C(sp3)−H activation (28), we synthesized a class of bidentate ligands bearing both a pyridone and an electron-deficient amide derived from perfluorinated anilines. Amide-pyridone ligand L1, posited to coordinate to Pd(II) as five-membered chelate, afforded significantly improved yield in the annulation reaction (38%). Through extensive structural tuning, we identified ligand L7 as optimal, providing BCB 3a in 90% isolated yield.
Cyclic acids substrate scope.
Under these optimized conditions, we subsequently evaluated the substrate scope of the [2+2] annulation reaction (Fig. 2). A wide range of cyclic aliphatic acids including five- (1a-l), six- (1m-t), seven- (1u-v), and eight-membered (1w) rings were compatible to afford cis-BCB products as single regioisomers (3a-3w). Larger cyclic aliphatic acids including 11- (1x), 12- (1y) and 15-membered (1z) rings produced trans-BCB products as major isomers. Remaining unreacted aliphatic acid reactants were recovered when the yields are relatively low. Notably, ligand L7 allowed us to reduce Pd loading from 10% to 5% or 1% while maintaining synthetically useful yields for the [2+2] annulation between dihaloarene 2a and acids (1a-1i) as selected examples. Various substitutions at the α-position of carboxylic acid were tested in the reaction. Alkyl groups (3a-3f, 3l-q, 3s, 3u) and aryl groups (3g-3j, 3r) with electronically diverse substituents were compatible with this protocol despite the presence of reactive methyl and aryl C−H bonds. Functionalities such as methoxy (3f), OTBS (3q), phenyl (3d-e, 3p) were well-tolerated. Bicyclic aliphatic acid (1l) was converted to the corresponding fused 6-5-4-6 ring (3l) in 32% yield. Cyclohexanecarboxylic acid bearing substitutions at the 3-position (1s) consistently provided the corresponding product (3s) in moderate yield. In addition to the aliphatic acids containing an α-quaternary center, aliphatic acids containing an α-hydrogen (1k, 1t, 1v-1z) afforded the products (3k,3t,3v-3z) in 20%−52% yield. Quinoline-pyridone ligand L28 was more suitable for 8–15-membered rings (1w-1z). The structures of 3h and trans-3y were confirmed by single-crystal x-ray diffraction analysis. We also investigated the scalability of this [2+2] annulation reaction, in which 1.05 gram of the desired product 3a could be obtained in 88% isolated yield (see supplementary materials for experimental details).
Fig. 2. Cyclic aliphatic acid scope for the [2+2] annulation reaction.
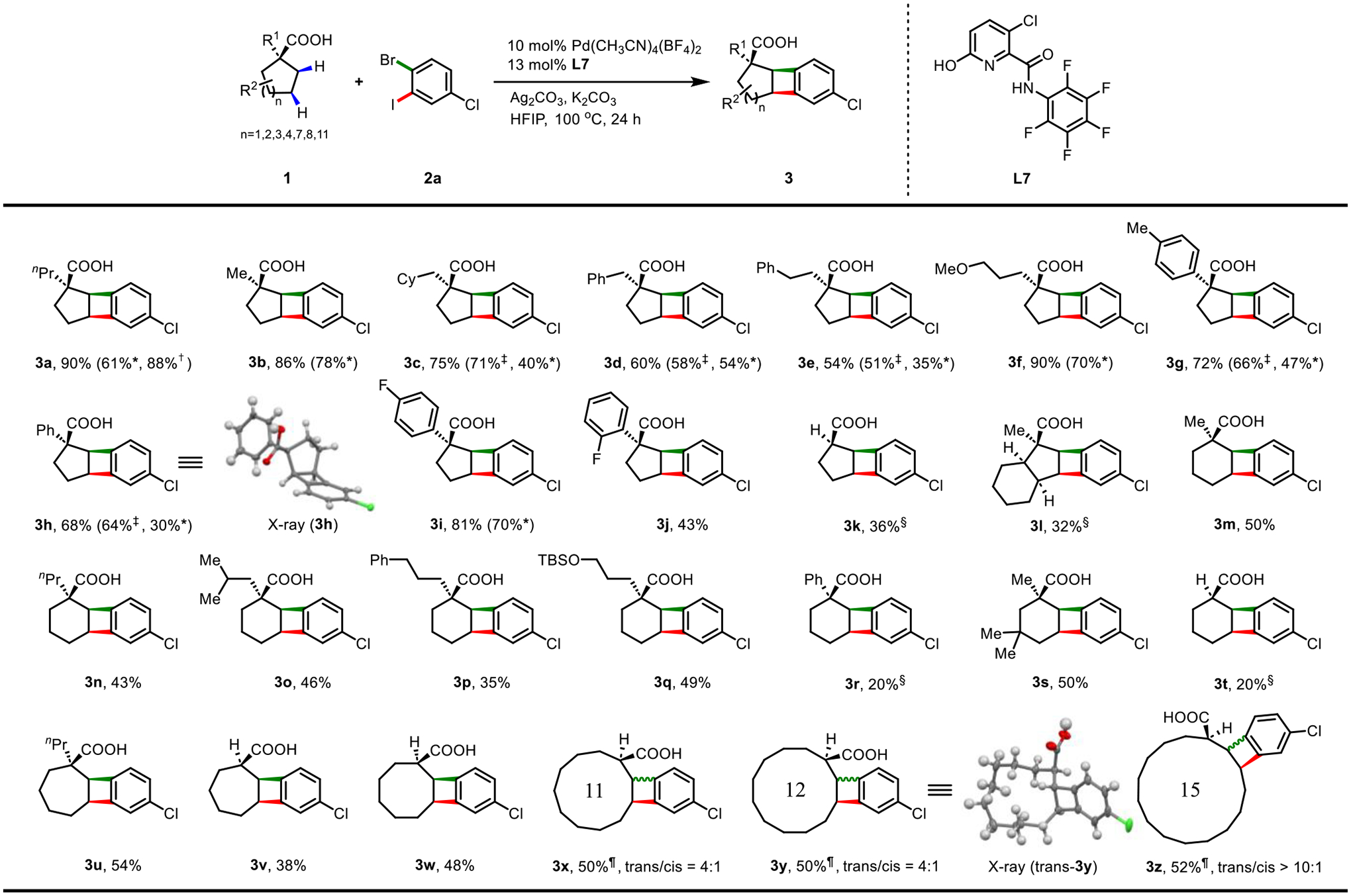
Reaction conditions: aliphatic acid (0.1 mmol), 2a (0.2 mmol), Pd(CH3CN)4(BF4)2 (10 mol%), L7 (13 mol%), K2CO3 (2.5 equiv), Ag2CO3 (2.0 equiv), HFIP (1.0 ml), at 100 °C for 20 hours. Isolated yields are reported. Relative configurations are shown, as the synthesis is racemic. See supplementary materials for details. *Pd(CH3CN)4(BF4)2 (1 mol%) was used, reaction time 56 hours. †Reaction conducted with of aliphatic acid 1a (4.5 mmol), 1.05 g of 3a was obtained. ‡ Pd(CH3CN)4(BF4)2 (5 mol%) was used, reaction time 40 hours. §Aliphatic acid (0.2 mmol), 2a (0.1 mmol), K2CO3 (3.5 equiv).¶L28 instead of L7, KHCO3 instead of K2CO3.
Acyclic acids substrate scope.
Acyclic aliphatic acid 4a only gave low yield with L7 (2%) (Table S6). We found that six-membered chelating amide-pyridone ligand L12 emerged as the most effective ligand for these linear substrates, affording diverse trans-BCBs (Fig. 3). A wide range of functional groups, such as methoxy (5d), chloro (5e-5f), phosphonate (5g), and substituted aryl groups (5h-l) were compatible. Aliphatic acids bearing α-gem-dimethyl groups (4a-l) or a single methyl group (4n-o) were also compatible despite the presence of the typically more accessible α-methyl C−H bonds. In addition to the substrates containing an α-quaternary center (4a-o), aliphatic acids containing an α-hydrogen (4p-q) were also found to be viable, as exemplified with products 5p and 5q, generated in moderate yields. A number of synthetic elaborations of benzocyclobutyl acid products were also demonstrated. Heating a solution of 5a as an o-quinodimethane precursor with N-methylmaleimide in o-dichlorobenzene at 180 °C provided Diels-Alder adduct 6 in 60% yield. 5a could be readily transformed to medicinally important amine 7. The benzocyclobutyl amide 11, a patented bioactive molecule for crop protection (29), could be rapidly synthesized by [2+2] annulation of aliphatic acid 4a and N-dihaloaryl amide 10 in a two-step sequence.
Fig. 3. Acyclic aliphatic acids scope for the [2+2] annulation reaction and transformations of the products.
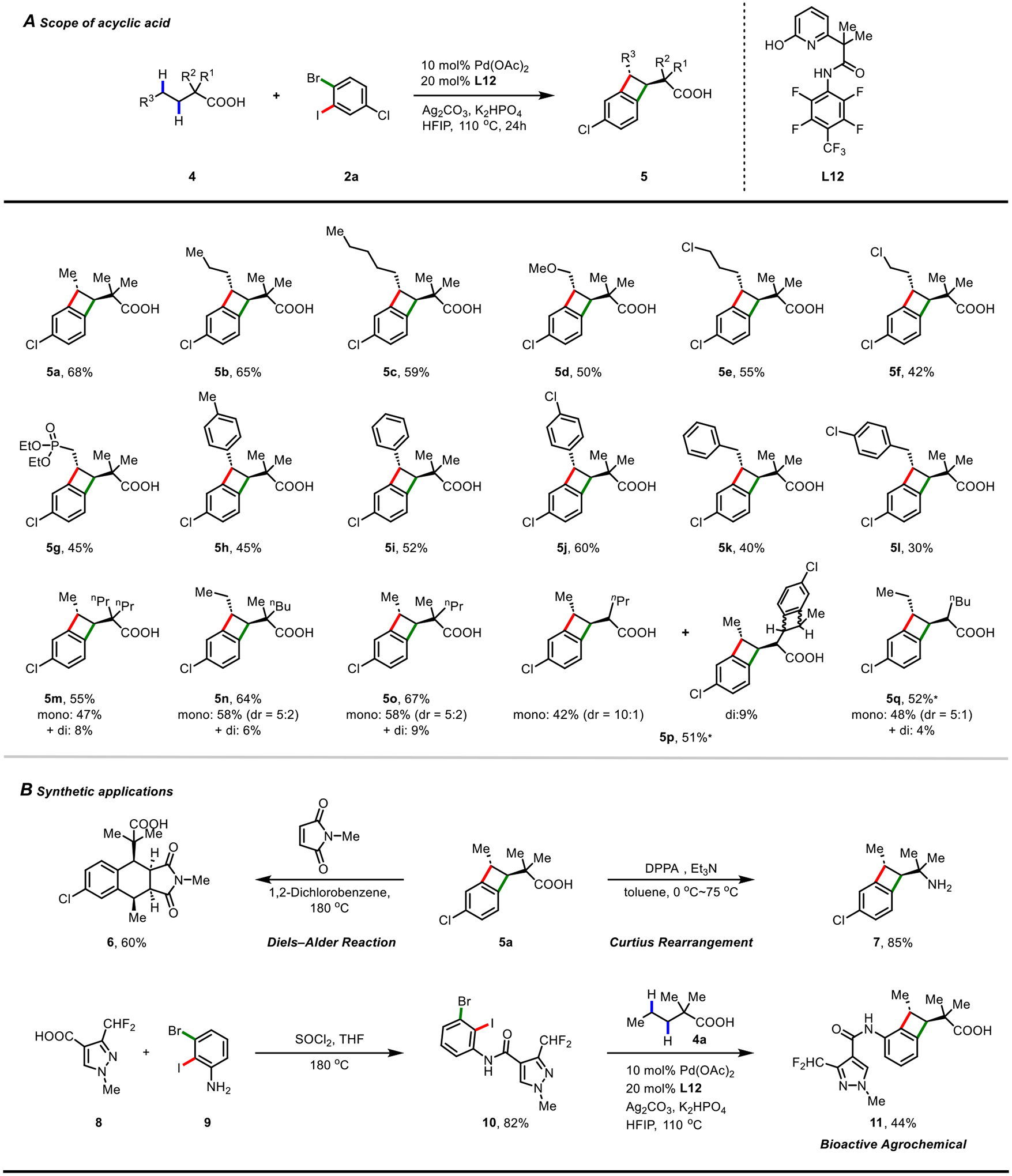
(A) Acyclic aliphatic acid scope. Reaction conditions: aliphatic acid (0.1 mmol), 2a (0.3 mmol), Pd(OAc)2 (10 mol%), L12 (20 mol%), K2HPO4 (3.5 equiv), Ag2CO3 (2.0 equiv), HFIP (1.2 ml), at 110 °C for 24 hours. Isolated yields are reported. Relative configurations are shown, as the synthesis is racemic. Di-5p was isolated and drawn as a representative of di-BCB products. *2a (0.35 mmol), K2HPO4 (3.0 equiv), HFIP (1.8 ml) as a replacement. (B) Synthetic applications. DPPA = Diphenylphosphoryl azide.
Dihaloarenes substrate scope.
The broad scope of bromoiodoarenes further expands the diversity of BCBs (12b-12u) (Fig. 4A). Substituents at various positions were compatible with this catalytic system (12c-12f). Halogens such as fluorine (12g), chlorine (12h), and bromine (12i-j) were compatible, providing the desired BCBs in good yields. Arenes bearing electron-donating (12k-l) and electron-withdrawing (12m-t) groups were also tested, affording the products in good to excellent yields. The amide (12s-t) and carboxylic acid (12u) groups, widely used in C–H activations as directing groups, were also viable for this protocol. An aryl triflate, a highly reactive site in Pd cross-coupling chemistry, was also tolerated (12m). Moreover, dibromoarenes (2bBr, 2v-y) and diiodoarene (2bI) were amenable to the standard conditions, generating the desired BCBs in moderate to good yields (Fig. 4B). As expected, reaction of 1b with 2,3-dibromotoluene (2cBr) afforded a mixture of two regioisomers (12c and 12d, rr = 2.5 : 1), indicating that bromoiodoarenes with two different active sites are vital for controlling regioselectivity.
Fig. 4. Dihaloarenes scope for the [2+2] annulation reaction.
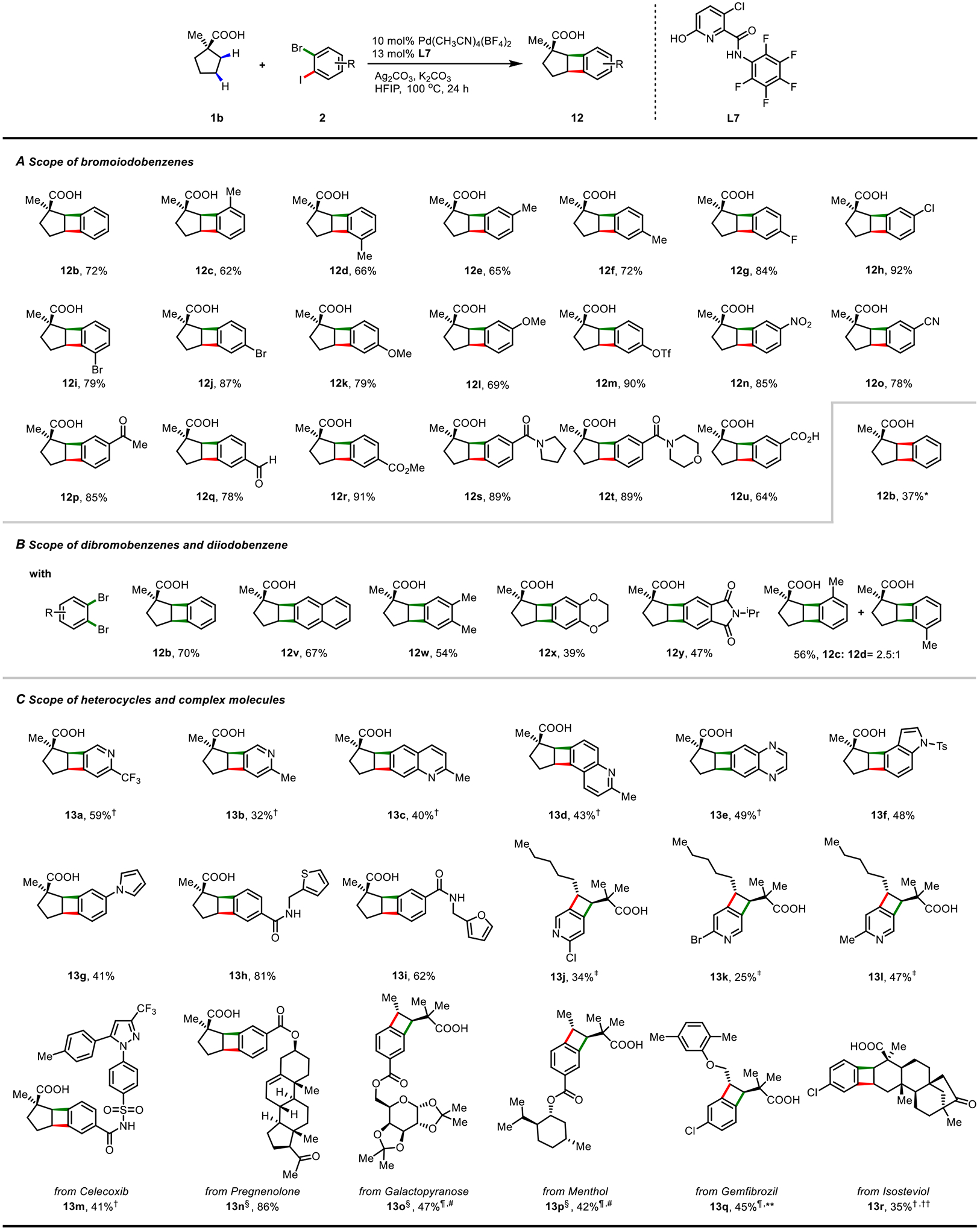
(A) Scope of bromoiodobenzenes; (B) Scope of dibromobenzenes and diiodobenzene; (C) Scope of heterocycles and complex molecules. Reaction conditions: aliphatic acid (0.1 mmol), dihaloarene (0.2 mmol), Pd(CH3CN)4(BF4)2 (10 mol%), L7 (13 mol%), K2CO3 (2.5 equiv), Ag2CO3 (2.0 equiv), HFIP (1.0 ml), at 100°C for 20 hours. Isolated yields are reported. Relative configurations are shown, as the synthesis is racemic. *1,2-diiodobenzene (2bI) as substrate. †Aliphatic acid (0.2 mmol), dihaloarene (0.1 mmol), K2CO3 (3.5 equiv). ‡L28 instead of L7, 4c instead of 1b, KHCO3 instead of K2CO3. §Diastereoisomers. ¶Dihaloarene (0.3 mmol), Pd(OAc)2 (10 mol%), L12 (20 mol%), K2HPO4 (3.5 equiv), Ag2CO3 (2.0 equiv), HFIP (1.2 ml), at 110 °C for 24 hours. #4a instead of 1b. **Gemfibrozil instead of 1b. ††Isosteviol instead of 1b.
Heteroaromatics and complex molecules.
Modular synthesis of hetero-BCBs has been a longstanding challenge using various methods (22, 23). Gratifyingly, pyridine (13a-b, 13j-l), quinoline (13c-d), quinoxaline (13e), and indole (13f) derivatives were also competent in this process, affording the desired hetero-BCBs in 25–59% yield (Fig. 4C). Heterocycles such as pyrrole (13g), thiophene (13h) and furan (13i) were well tolerated, providing the desired products in good to excellent yields. Dihaloarenes bearing bioactive structures such as celecoxib (13m), pregnenolone (13n), galactose (13o), and menthol (13p) were also compatible. This protocol could also be applied in late-stage modification of bioactive molecules, such as gemfibrozil and isosteviol, leading to products 13q and 13r.
Mechanistic study.
The sequential activation of two C–H and two aryl halide bonds in surprisingly exclusive regioselectivity calls for mechanistic investigation into this complex catalytic cycle.Reactivity of various possible intermediates (Fig. S2–3) is consistent with a highly complex Pd(II)/Pd(0)/Pd(II)/Pd(IV) catalytic reaction pathways (Fig. S4). β,γ-dehydrogenation occurred via ligand enabled β- or γ-C−H bond activation accompanied by the formation of Pd(0) species. Oxidative addition of the more reactive aryl iodide with Pd(0) species followed by directed carbopalladation afforded a key intermediate bearing the aryl on the γ-position with all regioselectivity defined. Next, intramolecular oxidative addition of the aryl bromide with the alkyl Pd(II) led to Pd(IV) intermediate, which underwent reductive elimination to afford the desired BCB product and Pd(II) catalyst. The plausibility of the dehydrogenation pathway is supported by the catalytic formation of the desired BCB product using β,γ-unsaturated aliphatic acid S5a as substrate (For various control experiments see Fig. S4A–G). Notably, the following two elementary steps must be exclusively regioselective to form a single regioisomer of BCB: oxidative addition with Pd(0) occurs preferentially with the more reactive aryl iodide bond; the subsequent carbopalladation step is then governed by the directing effect to place the aryl on the γ-position and Pd(II) on the β-position.
Beyond the realization of regioselective functionalization of two adjacent methylene C–H bonds, the modular synthesis of diverse BCBs with exclusive regioselectivity is applicable to pharmaceuticals, material science and mechanochemistry, as well as natural product synthesis. Further studies on the reaction mechanism are under way.
Supplementary Material
Acknowledgements:
We thank Kevin Wu for proofreading and providing helpful suggestions in preparing the manuscript. We thank Zhen Li for proofreading supplementary information and repeating reactions. We thank Md Emdadul Hoque for sharing substrates. We also thank the X-ray Crystallography Facility (UCSD) for x-ray crystallography. Y.-K. L. thanks Quynh Nguyen Wong for the help on purification and HRMS.
Funding:
We acknowledge The Scripps Research Institute, NIH (NIGMS, 2R01GM084019) for financial support.
Footnotes
Competing interests: J.-Q. Y., J.-M. Y. and Y.-K. L. are inventors on a patent application related to this work (US Patent application 63/487,381) filed by The Scripps Research Institute. The authors declare no other competing interests.
Supplementary Materials:
Data and materials availability:
All data are in the supplementary materials. Crystallographic data for compounds 3h and trans-3y are available from the CambridgeCrystallographic Data Center under reference numbers CCDC 2220709 and 2246693.
References:
- 1.Sadana AK, Saini RK, Billups WE, Cyclobutarenes and related compounds. Chem. Rev 103, 1539–1602 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Elnaggar MS, Ebrahim W, Mándi A, Kurtán T, Müller WEG, Kalscheuer R, Singab A, Lin W, Liu Z, Proksch P, Hydroquinone derivatives from the marine-derived fungus Gliomastix sp. RSC Advances 7, 30640–30649 (2017). [Google Scholar]
- 3.Psotka MA, Teerlink JR, Ivabradine: Role in the chronic heart failure armamentarium. Circulation 133, 2066–2075 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Tsotinis A, Afroudakis PA, Garratt PJ, Bocianowska-Zbrog A, Sugden D, Benzocyclobutane, benzocycloheptane and heptenederivatives as melatonin agonists and antagonists. ChemMedChem 9, 2238–2243 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Segura JL, Martín N, o-Quinodimethanes: Efficient intermediates in organic synthesis. Chem. Rev 99, 3199–3246 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Kouznetsova TB, Niu Z, Ong MT, Klukovich HM, Rheingold AL, Martinez TJ, Craig SL, Inducing and quantifying forbidden reactivity with single-molecule polymer mechanochemistry. Nat. Chem 7, 323–327 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Hickenboth CR, Moore JS, White SR, Sottos NR, Baudry J, Wilson SR, Biasing reaction pathways with mechanical force. Nature 446, 423–427 (2007). [DOI] [PubMed] [Google Scholar]
- 8.McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE, 1-Aminomethylbenzocycloalkanes: Conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J. Med. Chem 49, 5794–5803 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Kotha S, Lahiri K, Tangella Y, Recent advances in benzocyclobutene chemistry. Asian J. Org. Chem 10, 3166–3185 (2021). [Google Scholar]
- 10.Schiess P, Heitzmann M, Rutschmann S, Stäheli R, Preparation of benzocyclobutenes by flash vacuum pyrolysis. Tetrahedron Letters, 19, 4569–4572 (1978). [Google Scholar]
- 11.Chaumontet M, Piccardi R, Audic N, Hitce J, Peglion J-L, Clot E, Baudoin O, Synthesis of benzocyclobutenes by palladium-catalyzed C−H activation of methyl groups: method and mechanistic study. J. Am. Chem. Soc 130, 15157–15166 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Ye J, Shi Z, Sperger T, Yasukawa Y, Kingston C, Schoenebeck F, Lautens M, Remote C−H alkylation and C−C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem 9, 361–368 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Provencher PA, Hoskin JF, Wong JJ, Chen X, Yu J-Q, Houk KN, Sorensen EJ, Pd(II)-catalyzed synthesis of benzocyclobutenes by β-methylene-selective C(sp3)−H arylation with a transient directing group. J. Am. Chem. Soc 143, 20035–20041 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Wei W-X, Li Y, Wen Y-T, Li M, Li X-S, Wang C-T, Liu H-C, Xia Y, Zhang B-S, Jiao R-Q, Liang Y-M, Experimental and computational studies of palladium-catalyzed spirocyclization via a Narasaka-Heck/C(sp3 or sp2)−H activation cascade reaction. J. Am. Chem. Soc 143, 7868–7875 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Hao T, Qian L, Shi M, Wei Y, Construction of benzocyclobutenes enabled by visible-light-induced triplet biradical atom transfer of olefins. Angew. Chem. Int. Ed 61, e202204515 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Talbot FJT, Zhang S, Satpathi B, Howell GP, Perry GJP, Crisenza GEM, Procter DJ, Modular synthesis of stereodefined benzocyclobutene derivatives via sequential Cu- and Pd-catalysis. ACS Catalysis 11, 14448–14455 (2021). [Google Scholar]
- 17.Fujii T, Gallarati S, Corminboeuf C, Wang Q, Zhu J, Modular synthesis of benzocyclobutenes via Pd(II)-catalyzed oxidative [2+2] annulation of arylboronic acids with alkenes. J. Am. Chem. Soc 144, 8920–8926 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Rieman JM, Trahanovsky WS, Formation of cyclobuta[b]pyridine and cyclobuta[c]pyridine by the pyrolysis of propargyl 4-pyridyl ether. Tetrahedron Letters, 18, 1867–1870 (1977). [Google Scholar]
- 19.Naiman A, Vollhardt KPC, 1, 2, 4, 5-Tetrahydrodicyclobuta[b,e]pyridine. Angew. Chem 91, 440–441 (1979). [Google Scholar]
- 20.Chan HSS, Yang J-M, Yu J-Q, Catalyst-controlled site-selective methylene C–H lactonization of dicarboxylic acids. Science 376, 1481–1487 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Meng G, Yu J-Q, Ligand-enabled Pd(II)-catalyzed β-methylene C(sp3)−H arylation of free aliphatic acids. J. Am. Chem. Soc 144, 20550–20553 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Hu L, Chekshin N, Zhuang Z, Qian S, Qiao JX, Yu J-Q, Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 374, 1281–1285 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang EM, White MC, Molecular complexity via C–H activation: a dehydrogenative Diels-Alder reaction. J. Am. Chem. Soc 133, 14892–14895 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prusinowski AF, Twumasi RK, Wappes EA, Nagib DA, Vicinal, Double C–H Functionalization of alcohols via an imidate radical-polar crossover cascade. J. Am. Chem. Soc 142, 5429–5438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen T, Lamber TH, Electrophotocatalytic diamination of vicinal C–H bonds. Science 371, 620–626 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen T, Li Y-L, Ye K-Y, Lamber TH, Electrophotocatalytic oxygenation of multiple adjacent C–H bonds. Nature 614, 275–280 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Wang Z, Chekshin N, Qian S, Qiao JX, Cheng PT, Yeung K-S, Ewing WR, Yu J-Q, A tautomeric ligand enables directed C‒H hydroxylation with molecular oxygen. Science 372, 1452–1457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasa M, Chan KSL, Zhang X-G, He J, Miura M, Yu J-Q, Ligand-enabled methylene C(sp3)−H bond activation with a Pd(II) catalyst. J. Am. Chem. Soc 134, 18570–18572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubost C, Wachendorff-Neumann U, Winter P, Brunet S, Vors J-P, Montagne C, “Benzocyclobutane(Thio) Carboxamides,” U.S. Patent 9878985 B2 (2018).
- 30.Oldenziel OH, Van Leusen D, Van Leusen AM, Chemistry of sulfonylmethyl isocyanides. 13. A general one-step synthesis of nitriles from ketones using tosylmethyl isocyanide. Introduction of a one-carbon unit. J. Org. Chem 42, 3114–3118 (1977). [Google Scholar]
- 31.Muth A, Pandey V, Kaur N, Wason M, Baker C, Han X, Johnson T, Altomare DA, Phanstoel IV O, Synthesis and biological evaluation of antimetastatic agents predicated upon Dihydromotuporamine C and its carbocyclic derivatives. J. Med. Chem 57, 4023–4034 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Hu M, Zhao X, Li C, Yuan Z, Liu X, Cai C, Zhang Y, Hu Y, Chen Y, Subphthalocyanine triimides: solution processable bowl-shaped acceptors for bulk heterojunction solar cells. Org. Lett 21, 3382–3386 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Hennessy EJ, Oza V, Adam A, Byth K, Castriotta L, Grewal G, Hamilton GA, Kamhi VM, Lewis P, Li D, Lyne P, Oster L, Rooney MT, Saeh JC, Sha L, Su Q, Wen S, Xue Y, Yang B, Identification and optimization of benzimidazole sulfonamides as orally bioavailable sphingosine 1-phosphate receptor 1 antagonists with in vivo activity. J. Med. Chem 58, 7057–7075 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Belley M, Bayly CI, Colucci J, Dufresne C, Giroux A, Lau CK, Leblanc Y, McKay D, Therien M, Wilson MC, Skorey K, Chan CC, Scapin G, Kennedy BP, Discovery of [(3-bromo-7-cyano-2-naphthyl)(difluoro)methyl]phosphonic acid, a potent and orally active small molecule PTP1B inhibitor. Bioorg. Med. Chem. Lett, 18, 3200–3205 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Winiewska-Szajewska M, Maciejewska AM, Speina E, Poznański J, Paprocki D, Synthesis of novel halogenated heterocycles based on o-phenylenediamine and their interactions with the catalytic subunit of protein kinase CK2. Molecules 26, 3163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming I, Williams DH, The NMR spectra of four-membered carbocyclic ring system. Tetrahedron 23, 2747–2765 (1967). [Google Scholar]
- 37.Nishimoto Y, Yasuda M, Baba A, Coupling reaction of alkyl chlorides with silyl enolates catalyzed by indium trihalide. Org. Lett 9, 4931–4934 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Ikuo U, Suminori U, The synthesis of 11-(2’-dimethylaminoethyl)-5-methyl-5,11 dihydrodibenzo[b,e][1,4]thiazepin and related compounds. neurotropic and psychotropic agents. Bulletin of the Chemical Society of Japan, 48, 2323–2327 (1975). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are in the supplementary materials. Crystallographic data for compounds 3h and trans-3y are available from the CambridgeCrystallographic Data Center under reference numbers CCDC 2220709 and 2246693.


