Abstract
The complexity of contaminant mixtures in surface waters has presented long-standing challenges to the assessment of risks to human health and the environment. As a result, novel strategies for both identifying contaminants that have not been routinely monitored through targeted methods and prioritizing detected compounds with respect to their biological relevance are needed. Tracking biotransformation products in biofluids and tissues in an untargeted fashion facilitates the identification of chemicals taken up by the resident species (e.g., fish), so by default ensuring that detected compounds are biologically relevant in terms of exposure. In this study, we investigated xenobiotic glucuronidation, which is arguably the most important phase II metabolism pathway for many pharmaceuticals, pesticides, and other environmental contaminants. The application of an untargeted high-resolution mass spectrometry-based approach tentatively revealed the presence of over 70 biologically relevant xenobiotics in bile collected from male and female fathead minnows exposed to wastewater treatment plant effluents. The majority of these were not targets of conventional contaminant monitoring. These results highlight the utility of biologically based untargeted screening methods when evaluating chemical contaminants in complex environmental mixtures.
Keywords: Glucuronides, phase II metabolites, untargeted metabolomics, high-resolution mass spectrometry, LC–MS, environmental contaminants, neutral loss-dependent MS3, bile
Graphical Abstract

Introduction
Surface waters heavily impacted by human activity often contain a variety of legacy contaminants (polychlorinated biphenyls , dioxins, etc.) along with a burgeoning list of contaminants of emerging concern (pharmaceuticals, personal care products, perfluorinated chemicals, etc.). (1) Unfortunately, adequate toxicity information is available for only a tiny fraction of these contaminants, many of which do not occur on common chemical monitoring lists. For these and other reasons, thorough assessments of risk to humans and resident wildlife (e.g., fish) are difficult to conduct using traditional approaches. Thus, there is a need for high-throughput, untargeted contaminant screening methods that are anchored in the biochemical processes that may impact toxicity to help prioritize the hundreds to thousands of surface water contaminants, regardless of whether the chemicals have been listed for monitoring or studied for toxicity.
The application of an approach that leverages highly sensitive and selective analytical techniques to track biotransformation products within an organism exposed to surface water contaminants is an attractive strategy for addressing this need. At a fundamental level, the detection of a contaminant’s biotransformation product(s) within an organism provides evidence of the chemicals’ bioavailability and uptake and thus potential toxicological relevance. Moreover, this type of monitoring can help elucidate the detoxification/toxification pathways of understudied or unidentified environmental pollutants (and their degradants), including the ways that these pathways vary according to sex, age, species, and so forth. Also, while biotransformation and subsequent excretion often confers resistance to potential xenobiotic-induced toxicity, sustained utilization of these xenobiotic metabolism pathways may incur an unsustainable physiological cost (e.g., consumption of energetic reserves). (2) In other instances, biotransformation can activate compounds to metabolites more toxic than the parent chemicals. (3) As a result, identification of the varied array of contaminant biotransformation products possibly present in an organism may indicate (and help anticipate) the risk of adverse impacts on, for example, reproduction and development, as well as the long-term survival of individuals and populations. Significantly, this type of approach is consistent with and supportive of the eco-exposome concept as a basis for assessing complex contaminant mixtures in ecological risk assessments. (4)
Many of the most important biotransformation pathways for pharmaceuticals, pesticides, and other environmental contaminants involve the addition of a glucuronic acid moiety to an exogenous compound [i.e., the contaminant and/or its phase I metabolite(s)] via uridine 5′-diphospho-glucuronosyltransferase (UGT), resulting in the formation of a glucuronide. (5,6) The addition of the polar glucuronic acid moiety increases the hydrophilicity of the xenobiotic molecule, enhancing the organism’s ability to excrete the contaminant. While glucuronide formation has long been recognized for playing a critical role in mammalian species’ responses to xenobiotic exposures, (7) it is also fundamental to xenobiotic metabolism in fish. Indeed, numerous studies have reported the detection of glucuronides in various fish species exposed to a wide range of environmental contaminants. (8) Furthermore, it appears to be the most important pathway (quantitatively) in fish for detoxification of many xenobiotics. (8)
There are currently multiple methods for the identification and quantitation of glucuronides, including enzymatic hydrolysis assays, acid/base hydrolysis assays, and, more recently, mass spectrometry-based methods. (9) Traditionally, both enzymatic hydrolysis (e.g., β-glucuronidase) and acid/base hydrolysis have been used to de-conjugate the glucuronic acid moiety from the parent xenobiotic, followed by a targeted analytical method to detect and quantify the parent. Although both hydrolysis methods are commonly employed, neither is without significant limitations. For example, enzymatic hydrolysis is highly dependent on the source of the β-glucuronidase, which results in varying efficiencies for different glucuronide substrates, potentially leading to high rates of false negatives for some classes of xenobiotics, as has been reported for opioids. (10,11) Alternatively, acid hydrolysis can result in parent degradation, as has been shown to occur with benzodiazepines. (11)
More recently, triple quadrupole liquid chromatography–mass spectrometry (LC–MS/MS) methods (e.g., employing constant neutral loss, where all targeted pairs of precursor ions and product ions in the experiment differ by a single characteristic neutral loss) have directly measured glucuronides without the need to de-conjugate them prior to analysis. (12) These methods have attracted considerable attention due, in large part, to their high sensitivity and selectivity. However, their low mass resolution still requires some prior knowledge regarding the parent xenobiotic (i.e., known ion transitions for multiple reaction monitoring) to achieve confident identification. Thus, these triple quadrupole MS methods are well suited for suspect screening with a suitable list of MS/MS transitions but not applicable to untargeted analyses. Fortunately, recent advances in techniques based on high-resolution accurate-mass (HRAM) mass spectrometry have demonstrated great potential for untargeted detection of phase II metabolites such as glucuronides. (13–15) Unlike the triple quadrupole methods, these techniques provide sufficient mass accuracy that, along with auxiliary information to constrain candidate structures, can provide chemical identifications without establishing prior targets. (16) While this approach overcomes most of the limitations of these earlier methods for detecting glucuronides, it has not yet, to our knowledge, been demonstrated as a technique for environmental monitoring and surveillance, an application uniquely poised to benefit from such an advance.
In the present study, we evaluate the use of an optimized HRAM-MS method for the untargeted monitoring of glucuronides in fish exposed to complex mixtures of aquatic contaminants. Specifically, we have detected and identified glucuronides in bile samples collected from adult fathead minnows (Pimephales promelas) exposed for 21 d to treated effluent from the Western Lake Superior Sanitary District (WLSSD) wastewater treatment plant in Duluth, MN, USA. These samples were collected as part of a previous study by Cavallin et al. that investigated the presence and concentrations of contaminants in the effluent as well as impacts on a variety of biological endpoints resulting from exposure to the treated effluent. (17) Here, in a proof-of-concept fashion, we compare and contrast measured xenobiotic glucuronides in bile with the corresponding contaminant monitoring data reported by Cavallin et al., and we explore the ability of this untargeted approach to identify differences between male and female fish in the biotransformation of xenobiotics (e.g., pharmaceuticals) and endogenous metabolites (e.g., steroids) to understand potential sex-specific chemical risks.
Experimental Section/Methods
Bile Sample Preparation
Details of the 21 d exposures of male and female fathead minnows to treated wastewater from the WLSSD have been described previously. (17,18) Briefly, fish were housed in on-site aquaria (using laboratory space at the WLSSD) and exposed to final treated wastewater prior to its discharge into the receiving water. After 21 d of exposure, fish were anesthetized with buffered tricaine methanesulfonate (MS-222; Finquel, Argent), and gallbladders were collected and snap-frozen in liquid nitrogen. Frozen gallbladders collected from control fish (exposed to sand-filtered (10 μm), ultraviolet-treated Lake Superior Water) and effluent-exposed fish were homogenized in ice-cold 100 mM ammonium acetate buffer and then cooled on ice. Prior to analysis, extracts were pooled to increase glucuronide concentrations. Specifically, each sample consisted of two gallbladder extracts from fish within the same class (sex and exposure). After pooling, the n for the various classes ranged from 3 to 6 (Table S1). Following pooling, each sample was spiked with isotopically labeled glucuronide standards (final concentration 200 ng/mL of each standard). From each spiked and pooled sample, a 10% (by volume) aliquot was collected and frozen at −80 °C and then shipped on dry ice to the Thermo Fisher facility in San Jose, CA for HRAM-MSn analysis. Additional details on bile preparation are summarized in the Supporting Information.
Description of the HRAM-MSn Method
Untargeted glucuronide HRAM-MSn-based assays were carried out at the Thermo Fisher laboratory using an ID-X (Orbitrap) MS system coupled to a Vanquish UHPLC system via a heated electrospray ionization source (HESI-II) operated in both positive electrospray ionization (+ESI) and negative electrospray ionization (−ESI) modes (Table S2).
Untargeted Glucuronide Screening and Parent Annotation
The presence of a glucuronide was confirmed if (1) a neutral loss matching the mass of anhydrous glucuronic acid (176.0321 amu) was observed in +ESI and/or −ESI and (2) fragment masses characteristic of glucuronic acid were observed using negative higher-energy collisional dissociation (HCD) MS2 (Figure 1; see the Supporting Information for additional details). Only those detections that met both requirements were designated as glucuronides and thus carried forward for parent compound annotation.
Figure 1.

General schematic for HRAM-MSn untargeted analysis of glucuronides. Bile samples were subjected to HRAM-MSn analysis on the ID-X for neutral loss (+ESI and −ESI) and characteristic fragment ion analysis (−ESI). The presence of a neutral loss corresponding to anhydrous glucuronic acid (176.0321) in MS2 CID spectra and the detection of characteristic fragment ions (red spectral peaks) were required to confirm identification of a glucuronide. Detection of a neutral loss at 176.0321 amu triggered a MS3 HCD acquisition of the deconjugated parent, which was searched against MS2 spectral libraries (mzCloud or in-house libraries) for positive matches of parent compounds for annotation. The −ESI MS2 HCD spectra were also searched against MS2 spectral libraries for potential matches of glucuronide compounds for annotation.
Parent compound annotation used a combination of MS2 spectra and neutral loss-triggered MS3 spectra. Specifically, MS2 and MS3 spectra were annotated by comparing fragmentation spectra to authentic standards (in-house library) or mzCloud (Compound Discoverer 3.1) spectra using “Identity” and “Identity Substructure” searches, respectively. Hits with scores ≥ 80 were deemed confident matches for glucuronides (MS2) or parents (MS3), qualifying for Metabolomics Standard Initiative (MSI) level I annotation [for exact mass, MSn (n = 2, 3)] and retention time matches to the authentic glucuronide standard) or MSI level II annotation (for exact mass and MS2 matches to mzCloud or using structural elucidation). (19) When MSI level I or II annotations were not possible, those detections that displayed both requirements for glucuronide confirmation (neutral loss and characteristic fragment ions consistent with glucuronic acid) were designated MSI III (based on glucuronide class identification). Tentative parent structures were then identified for these glucuronides using a combination of database searching (ChemSpider) (20) and the Thermo Scientific fragmentation tool Fragment Ion Search (FISh). Specifically, the predicted MSI III glucuronide formulas were searched using the ChemSpider database to determine tentative parent structures. Those searches that returned matches were ranked based on the number of references (including those originating from the following databases: SureChem (a patent database), PubMed, and Royal Society of Chemistry Publishing found, with the highest ranked glucuronide match used for annotation. This approach has been recommended previously for the annotation of “known unknowns” when using ChemSpider. (20) If no glucuronide matches were found in ChemSpider, the predicted formula was reduced by that of anhydrous glucuronic acid and submitted to ChemSpider to search for tentative parent structures. Hits that could potentially form glucuronides based on the presence of specific functional groups (including hydroxyl, carboxyl, thiol and amino groups) were also ranked by number of references, and proposed glucuronide structures for the top three candidates were manually generated and submitted for scoring. The ChemSpider hit with the most references and for which FISh scoring at least explained the ion fragment pattern at the MS2 level corresponding to the parent was selected as the tentative glucuronide annotation (Scheme S1). (Note: where multiple functional groups were available on the parent structure, we do not specify the exact site of glucuronidation. Glucuronide isomers are designated as isomer A, B, etc.) To indicate that these annotations were assigned tentative parent structures in addition to the glucuronide class designation, they are designated as MSI Level III+.
Results and Discussion
Untargeted Screening of Glucuronides in Fish Bile
A comprehensive analysis of phase II conjugates by Levsen et al. revealed that glucuronides produce a neutral loss of 176 (nominal mass reported) in both +ESI and −ESI, which corresponded to anhydrous glucuronic acid. (21) Given this, the observation of a neutral loss of 176.0321 amu (exact mass) in HRAM-MS2 spectra was used here (after confirmation with labeled and unlabeled standards, see the Supporting Information) as strong evidence of a glucuronide in bile extracts from fish exposed to the WLSSD-treated wastewater effluent (Figure 2A). Using this strategy for both +ESI and −ESI modes, a total of 205 compounds were flagged as tentative glucuronides (Figure 1).
Figure 2.
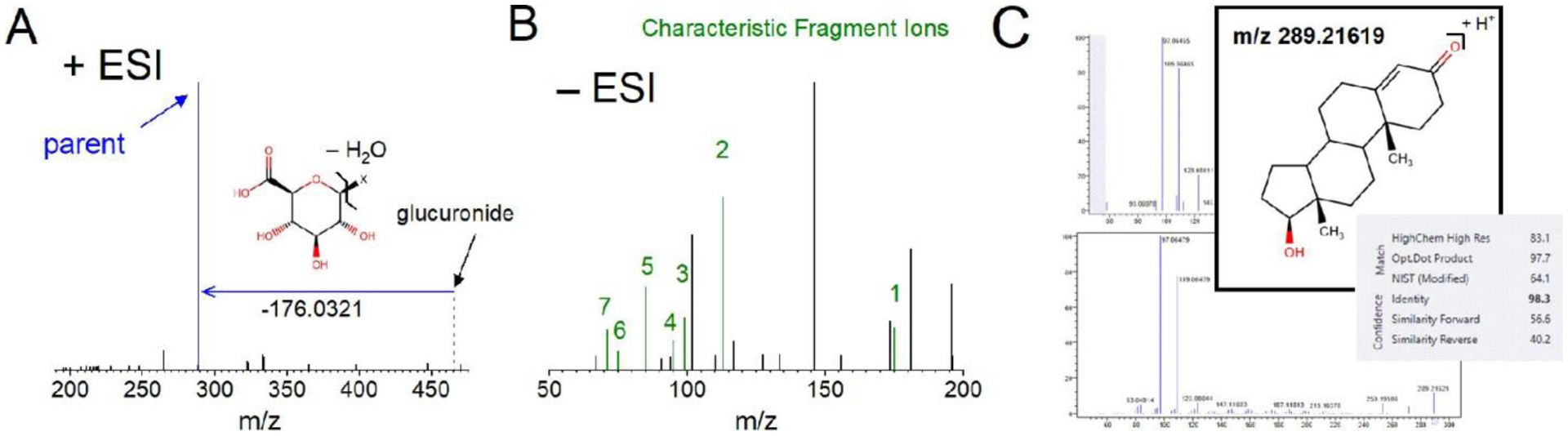
Results of the untargeted screening approach for identifying the testosterone glucuronide in bile of male and female fathead minnows exposed to a WLSSD effluent. (A) The positive mode MS2 CID spectrum shows a neutral loss corresponding to the anhydrous glucuronic acid moiety (structure inset), resulting in the [M + H]+ of the parent xenobiotic (blue spectral peak). (B) The negative mode MS2 HCD spectrum shows seven fragment anions (green and numbered spectral peaks) that are characteristic of glucuronic acid. (C) The presence of a neutral loss at the appropriate mass triggered an MS3 HCD spectrum of the precursor ion (inset, m/z 289.21619 for this example), corresponding to the parent (C, top). The MS3 spectrum was submitted to an in-house or online library such as mzCloud (C, bottom), resulting in a confident match for the identity of the parent (C, gray box). Note that the testosterone glucuronide was also confirmed by a MS2 library search in mzCloud.
However, the presence of an exact mass neutral loss of 176.0321 amu alone is not sufficient to establish with certainty the presence of a glucuronide due to the possibility of other unknown moieties with exact mass coincidences. Indeed, studies have shown that arriving at unambiguous identifications is difficult without supporting evidence. (16) Therefore, in addition to monitoring for a neutral loss of 176.0321 amu, fragment ions characteristic of the glucuronic acid moiety (Table S3) were used as orthogonal information to support glucuronide identification (Figure 2B). Based on Levsen et al., the most commonly reported characteristic glucuronide fragment ion is m/z 175, whereas secondary ions include m/z 113 and 85. (21) We observed these same fragments at m/z 175.0247, 113.0244, and 85.0294 using the authentic standards (ions 1, 2, and 5, respectively, in Table S3; their detection in testosterone glucuronide is shown in Figure 2B). Note that we also routinely detected four fragment ions of comparable or lower intensity at m/z 99.0086, 95.0137, 75.0086, and 71.0137 (Figure 2B and Table S3). The relative intensities of all seven fragment ions vary considerably among the authentic glucuronide standards (Figure S1), pointing to a significant limitation of relying on MS2 similarity library matching alone for annotating unknown glucuronides. Furthermore, other sugars (e.g., glucose) can, and do, produce similar fragments, making fragmentation analysis alone potentially misleading for the identification of glucuronides. (22) For example, focusing solely on the presence of the seven characteristic fragment ions in the current study revealed 380 compounds that were tentatively flagged as glucuronides in −ESI mode (Figure 1). However, when observation of a neutral loss at 176.0321 amu (205 compounds) was required in conjunction with the characteristic fragment ions, this number was reduced to 164. These 164 compounds (henceforth referred to as “candidate glucuronides”) were then prioritized for parent compound identification (Figure 1) and all subsequent analyses described below.
Annotation with HRAM-MSn Fragmentation of Bile Glucuronides
All 164 candidate glucuronides were annotated at one of the top three (of four) annotation confidence levels described in the MSI. (19) Specifically, we report MSI level I (unambiguous parent structure) annotations for three glucuronides [those of triclosan, bisphenol A (BPA), and resveratrol]. Annotation at MSI level II, which required the use of neutral loss-triggered +ESI MS3 spectra to provide putative parent structures, was achieved for 19 glucuronides. In addition, one other glucuronide, that of testosterone, was identified at MSI Level II using either an MS2 or MS3 spectrum. The remaining 141 glucuronides were reported at the MSI III confidence level (compound class). Notably, these glucuronides were only detectable when using −ESI MS3, and their intensities were too low at −ESI MS3 for MSI Level I or II annotation, presumably due, at least in part, to their low abundance in the bile extracts. Despite this, 102 of these 141 had proposed parent structures based on ChemSpider matches and FISh scores and were designated as MSI Level III+ to indicate this additional information.
Note that we attempted library matching or structural elucidation for all 164 proposed glucuronides, or their deconjugated parents, or both. However, the majority of these compounds do not have authentic glucuronide standards or online database glucuronide entries available, as more than half of the proposed xenobiotic glucuronides have not been previously reported. Indeed, as mentioned above, only testosterone glucuronide was annotated in this study by an MS2 library search in mzCloud (> 80 confidence level; Figure 2C). Thus, HRAM-MSn fragmentation methods that do not rely on the presence of library MS2 entries are a marked improvement on current HRAM-MS2 methods for the untargeted detection and annotation of glucuronides. Indeed, the ability of HRAM-MS3 methods to improve the annotation of glucuronides was critical for the current application.
Evaluating Glucuronides in Bile in the Context of Chemical Monitoring Data
Due to the untargeted nature of our approach, we anticipated the presence of both exogenously and endogenously derived glucuronides among the 164 that were detected. After removing 64 glucuronides that were measured in the control fish (and thus operationally defined as endogenous), the remaining 100 candidate glucuronides that were only measured in the exposed fish were classified as exogenous and assumed to be derived from the chemicals in the final treated WLSSD effluent (Tables 1 and S4). We were able to propose unique identifiers for 71 of these 100 exogenous glucuronides, revealing a variety of pharmaceuticals, personal care products, pesticides, and commercial/industrial chemicals as the potential parent xenobiotics (Table S4). However, only five of these were associated with chemicals that were detected at some point during the exposure, (17,18) namely, BPA, estrone, isophorone, propranolol, and triclosan. Apart from these five, none of the xenobiotics from which these exogenous glucuronides were derived was on the contaminant monitoring target list by Cavallin et al. (17) Perhaps this finding should not be surprising, given the relatively limited number of contaminants (190 in total) that were targeted. (17) However, when we also matched our list of 71 proposed xenobiotics (i.e., the parent compounds identified from the exogenous glucuronides) to the much more extensive list of organic chemicals targeted in a recent comprehensive national survey of U.S. streams, (1) only five additional matches were found: esfenvalerate, ibuprofen, isopropylbenzene, testosterone, and tetramethrin. Therefore, 61 tentatively identified xenobiotics from a total of 71 that we detected as glucuronides were not present on any of the targeted chemical lists used for one of the most comprehensive surveys of U.S. surface waters ever published. (1) Together, these findings provide an interesting perspective on the conventional approach to chemical monitoring─namely, there are likely many untargeted xenobiotics that are accumulated and transformed by fish but which would remain unobserved.
Table 1.
Breakdown of Candidate Glucuronidesa in Male and Female Fathead Minnows
| endogenousb | exogenous | |
|---|---|---|
| males only | 21 | 51 |
| females only | 16 | 1 |
| both | 27 | 48 |
| total | 64 | 100 |
Glucuronides were either qualitatively sex-specific (detected in either males or females only) or non-sex-specific (detected in both males and females).
Similarly, glucuronides were either detected in control fish (endogenous) or only in effluent-exposed fish (exogenous).
Eight of the 10 xenobiotics from chemical monitoring lists that we detected as glucuronides are in fact known from the literature to form glucuronides (Table S4). When we expanded our search to include all 71 xenobiotics from the current analysis (i.e., all parent compounds identified from the proposed exogenous glucuronides), we found that slightly less than half (31 out of the 71) had been reported in the literature to form glucuronides. Here, we tentatively identified 38 glucuronides that have not, to our knowledge, been previously reported. Given that glucuronidation is a major pathway for phase II metabolism in vertebrates, (7) this finding highlights the value of this untargeted approach for understanding fish metabolism of xenobiotics.
Detected Contaminants Not Found as Glucuronides
In addition to demonstrating the utility of this approach for identifying biologically relevant, yet typically unobserved contaminants based on occurrence as glucuronides, it is notable that only a relatively small fraction of the total analytes detected in water from the study were found as glucuronides. Specifically, of the 190 organics targeted in the current study, 79 were detected at least once in the composite water sampling conducted for the wastewater effluent over the course of the 21 d exposure. (17) However, we were able to associate only five of these 79 xenobiotics with glucuronides that we found in the fish bile. Although only a rough approximation, using the effluent concentration of the least abundant xenobiotic for which we were able to observe a glucuronide (propranolol) in the fish, we estimate a limit of detection for this technique of 0.05 μg of xenobiotic per liter of effluent. If we focus on only those contaminants detected above this threshold that are also known from the literature to form glucuronides in any animal (note that this includes glucuronides which may not form in fish), we observed glucuronides for about 20% of the contaminants that meet these criteria. Also, recall that there were 17 candidate glucuronides that we classified as exogenous for which we were unable to propose a unique identity. It is possible, if not likely, that some of these also arose from the detected xenobiotics. Furthermore, the pharmacokinetic properties of the contaminants in the fish would be expected to influence this comparison, as the 79 chemical detections were the cumulative result of three 7 day composite sampling events, (17) whereas the bile was extracted from the fish at the end of 21 days of exposure. Therefore, glucuronide detection after 21 days would be limited by the excretion rates of the xenobiotics, along with other temporal factors. Finally, some percentage of the detected contaminants were undoubtedly biotransformed by pathways that did not result in a glucuronide in the bile (e.g., sulfation as a phase II reaction) or were removed using alternate routes of excretion (e.g., urine) and thus would not have been observed with the current approach. Clearly, further research is needed to better interpret and understand the relationship between detected and undetected xenobiotics and glucuronides that are observed in biota using untargeted methods such as the one described here. Our findings of a wide range of glucuronides for parent xenobiotics that are likely toxicologically relevant, yet not targeted in chemical monitoring campaigns, demonstrate the value of pursuing these lines of investigation.
Untargeted Screening of Glucuronides for Evaluating Sex-Specific and Non-Sex-Specific Responses to Contaminant Exposures
While a variety of factors can influence our ability to detect glucuronides (e.g., rates of uptake and excretion), the untargeted approach used here offers an important opportunity to delineate possible sex-specific differences in biotransformation of environmental contaminants, an important consideration for understanding potential risks to exposed populations. We observed sex-specific responses for most of the 100 exogenous glucuronides associated with xenobiotics in the WLSSD effluent, with the majority only detected in male fish (Table 1). Although hepatic gene expression analysis of these fish revealed, for both males and females, significant up-regulation of the transcripts for enzymes involved in general xenobiotic metabolism (uridine diphosphate-glucuronosyltransferase, ugt1a1; cytochrome P4501A1, and cyp1a1), the transcript for cytochrome P4503A (cyp3a) displayed a concentration-dependent up-regulation in males only. (17) (Cavallin et al. observed upregulation of cyp3a in the females only at the lowest effluent concentration [i.e., 5%].) In both mammals and fish, cytochrome P450 enzymes (the most abundant subfamily being CYP3A) are primarily responsible for the oxidative metabolism (phase I reactions) of pharmaceuticals, which can further undergo phase II metabolism including glucuronidation if the phase I metabolite is insufficiently water-soluble. (23) Interestingly, ∼40% of the pharmaceutical-related glucuronides were detected here exclusively in the male fish bile. The remaining ∼60% were detected in the bile of both sexes. The bias observed for pharmaceutical-related glucuronides in the male fish suggests that glucuronidation may be the dominant phase two reaction for this class of contaminants in males.
Looking at individual glucuronidated contaminants or contaminant classes in the dataset was also revealing regarding sex-specific and non-sex-specific glucuronidation. For example, we identified the glucuronide of BPA and tentatively identified glucuronides of two BPA alternatives, bisphenol S (BPS) and 1-hydroxycyclohexyl phenyl ketone (1-HCPK) (Table S4), all of which are used in the manufacturing of thermal papers. (24–26) All three of these glucuronides were only detected in male fish. Furthermore, BPA-glucuronide was previously reported in skin mucus from these same male (but not female) fish. (18) This may be a result of preferential formation of alternate conjugates (e.g., sulfation) of these compounds in the female fish, as different relative concentrations of various BPA conjugates (mono-glucuronide, di-glucuronide, mono-sulfate, and di-sulfate) have been reported in human females versus males. (27) Interestingly, we detected and tentatively identified the glucuronide of another BPA alternative, 4-[(4-isopropoxyphenyl)sulfonyl]phenol (BPS-monoP), associated with the wastewater effluent that was not sex specific.
The glucuronide of 2-mercaptobenzothiazole (2-MBT) also displayed a lack of sex specificity, increasing in the bile of both males and females in relation to the effluent concentration (Figure 3). 2-MBT is used in industrial and consumer products including rubber manufacturing, (28) and, despite being produced in high quantities (500–5000 tons, EPA Toxic Substances Control Act Aggregated Product Volume), is not monitored even in comprehensive studies. (1) This lack of monitoring is unfortunate as 2-MBT is a recognized thyroid disrupting chemical, recently having been reported to reduce thyroid hormone levels and delay inflation of the anterior swim bladder in fathead minnow and zebrafish embryos. (29,30) By using the structural information provided by MS3, we were also able to tentatively identify a previously unreported methoxylated metabolite of 2-MBT, 2-(methylsulfanyl)-1,3-benzothiazol-6-ol (Figure S2), which was also detected in both males and females.
Figure 3.

Box-and-whisker plots for the 2-MBT glucuronide. The mean values (white circles) are overlaid on the boxes and connected by a dashed line. Outliers are denoted by filled diamonds. Statistically significant differences (p < 0.1) are denoted by lowercase letters (male, left) and uppercase letters (female, right) and were determined from ANOVA followed by Tukey’s HSD post-hoc analysis for each sex separately for 0% (0), 5% (L), 20% (M), and 100% (H) effluent exposures.
Monitoring for Glucuronide-Mediated Activation of Contaminants
While it is generally true that glucuronides are less biologically active than their parent precursors, this is not always the case. In some instances, glucuronidation can activate the parent xenobiotic and/or hinder its removal from the organism. (31,32) For example, acyl glucuronidation, the major metabolic pathway for many carboxylic acid-containing drugs (e.g., non-steroidal anti-inflammatory drugs), may result in a more toxic form. (33) Specifically, acyl glucuronides can undergo spontaneous hydrolysis, intramolecular rearrangements, and nucleophilic substitution reactions with proteins. The formation of such protein adducts can reduce the organism’s ability to rapidly clear drugs, producing more-persistent toxins, with half-lives of weeks to months. (32) In the current study, we tentatively identified a variety of glucuronides of drugs that are recognized for their tendency to form acyl glucuronides including ibuprofen, ketoprofen, fenoprofen, and naproxen. Additionally, while the bulk of research concerning acyl glucuronide toxicity has focused on human pharmaceuticals as an explanation for adverse drug reactions, some environmental contaminants have also shown the ability to form acyl glucuronides. For example, the pyrethroid insecticides (e.g., permethrin) have been shown to form acyl glucuronides in humans. (32) Interestingly, we detected the glucuronides of both tetramethrin and 3-phenoxybenzoic acid (3-PBA) in the male fish (Table S4). 3-PBA is a common metabolite of several pyrethroids (e.g., permethrin, l-cyhalothrin, deltamethrin, fenpropathrin, and esfenvalerate) (34) and is known to primarily form an acyl glucuronide as a result of phase II metabolism. Furthermore, its glucuronide has been shown previously to form protein adducts with human plasma albumin. (32)
It is critical to note that only a small percentage of the acyl glucuronide formed for a given xenobiotic is expected to form protein adducts. For example, Bolze et al. reported acyl glucuronide binding (irreversible) to human serum albumin (HSA) as a percentage of total starting glucuronide for a variety of pharmaceuticals, and none of the compounds studied exceeded a 6% binding. (35) Noort et al. conducted a similar evaluation for the glucuronide of 3-PBA, reporting an average of 1.8% bound to HSA. (32) However, these relatively small percentages may be deceptive when evaluating risks associated with exposures to mixtures of environmental contaminants. Fish residing in waters that contain complex mixtures of contaminants would arguably be susceptible to the interactive effect(s) of protein adducts produced by a variety of the acyl glucuronides that are frequently present in wastewater. Additionally, the chemical reactivity (and thus the extent of protein adduct formation) of an acyl glucuronide is a function of the electronic and steric properties of the parent xenobiotic and the surrounding physicochemical conditions. (36) As a result, some acyl glucuronides will no doubt display higher toxicity than others depending on the chemical characteristics of the parent xenobiotic as well as a host of other factors (species, sex, age, etc.) related to the physiology of the exposed fish.
Untargeted Analysis of Endogenous Glucuronides
Although not the primary focus of this study, the approach we used for measuring glucuronides of xenobiotics also enabled untargeted monitoring of endogenous glucuronides. We tentatively identified 64 endogenous glucuronides using the HR-MS-based screening approach (Table 1). While some xenobiotic compounds were without a doubt operationally classified as endogenous due to their presence in the control fish (e.g., butylated hydroxy toluene [BHT], a common fish feed additive), (37) the majority of the remaining glucuronides that we defined as endogenous were assumed to be native to the fish and included conjugates of steroids, prostaglandins, lipids, bile acids, and amino acid derivatives.
Here, again, a substantial number of the endogenous glucuronides displayed sex specificity (Tables 1 and S4). For example, the glucuronide of prostaglandin F2α was only detected in the bile of the female fish, potentially a result of its role as a postovulatory pheromone, acting as an olfactory simulant in male conspecifics. (38) The glucuronide for cortisol was also detected exclusively in the female fish. Cortisol is closely associated with stress in fish and other vertebrates, as its secretion triggers a variety of physiological responses (e.g., mobilization of energy reserves) that prepare the fish to adapt to adverse conditions. (39) Conjugation with glucuronic acid (or sulfate) provides a means to deactivate cortisol and thus reduce the severity of the stress response. Consistent with this, cortisol glucuronide in the female fish trended inversely with effluent concentration (p-value < 0.1) (Figure 4C). This decrease occurred presumably as free (i.e., un-glucuronidated) cortisol occurred at higher effluent concentrations as a generalized stress response. However, the reason that this glucuronide was only detected in the bile of the female fish is uncertain.
Figure 4.

Box-and-whisker plots for the glucuronides related to the endogenous metabolites of 17-β-estradiol (A), testosterone (B), and cortisol (C). The mean values (white circles) are overlaid on the boxes and are connected with a dashed line. Statistically significant differences are denoted by lower case letters (female) and were determined by ANOVA (p < 0.1) followed by Tukey’s HSD post-hoc analysis for each sex separately for 0% (0), 5% (L), 20% (M), and 100% (H) effluent exposures.
Note that the glucuronides of 17β-estradiol and testosterone were observed in the bile of both male and female fish, though 17β-estradiol glucuronide levels were too low to report as detected in males. Further, both these glucuronides decreased with higher effluent exposure in a concentration-dependent manner in the bile of the females (Figure 4A,B). Interestingly, the free plasma concentration of both of these steroids was measured in the same female fish, and no impact of the effluent exposure was observed (no changes in free 17β-estradiol or testosterone were observed in the males either). (17) James has suggested that fish exposed to pollutants may display reduced steroid glucuronidation due to contaminant-mediated inhibition of the relevant UGT isoforms. (40) This may be a plausible explanation for the observed reduction of these glucuronides with increased exposure to the effluent. However, studies reporting UGT inhibition of steroid metabolism were conducted using exposures at high micromolar to low millimolar concentrations, which are unlikely to occur in situ. (40–42) Furthermore, several environmental contaminants have been shown to induce UGT activity, (40,43,44) which, depending on the isoform(s), could result in increased glucuronidation of steroids. As noted previously, Cavallin et al. reported an increase in transcript abundance for UGT1A1 in the livers of the male and female fish included in this study at the highest effluent concentration. It is difficult to ascertain whether this increased gene expression was an unsuccessful attempt to compensate for UGT inhibition (i.e., increase total UGT activity by increasing enzyme synthesis), or did not produce a concomitant increase in hepatic UGT protein levels, or does not represent the relevant isoform for 17β-estradiol and testosterone glucuronides found in bile, or some other explanation. Regardless, though it is beyond the scope of the current study to explore these factors, the use of this approach to identify (in an untargeted fashion) endogenous glucuronides alongside those that are exogenously derived adds additional value to its inclusion in monitoring efforts to assess impacts of contaminant exposures on fish behavior, reproduction, and development.
Conclusions and Implications
By applying the neutral loss approach and fragmentation patterns characteristic of glucuronic acid, we have demonstrated a novel application of an HRAM-MSn method for the untargeted identification of glucuronides in fish exposed to complex chemical mixtures. While we chose to focus on glucuronidation due to its importance in detoxification and excretion, it is important to note that this strategy can be extended to the screening of any phase II metabolite that exhibits neutral loss and characteristic fragmentation patterns observable with HRAM-MSn analysis. Sulfation and glutathione conjugation, for example, are two important modifications (both for exogenous and endogenous compounds) that are logical targets in future applications of this methodology and for which inclusion in untargeted monitoring will no doubt broaden our understanding of fish contaminant metabolism and further enhance the biological relevance of contaminant monitoring efforts in surface waters. Additionally, untargeted monitoring for a variety of conjugates provides a critical path to incorporating the eco-exposome concept into ecological assessments across a range of species.
Even though the present study’s focus was limited to glucuronides, their untargeted analysis resulted in the identification of numerous biologically relevant xenobiotics that do not appear on even the most extensive chemical monitoring target lists. While this may be somewhat surprising, similar findings are likely to become commonplace as untargeted approaches are more widely implemented in environmental monitoring efforts. For example, Gonzalez de Vega et al. recently compared the results of both targeted and untargeted approaches for detecting per- and polyfluoroalkyl substances (PFAS) in water samples. (45) While the targeted analysis resulted in the measurement of only seven of the 24 frequently monitored PFAS, the untargeted method revealed an additional 107 PFAS.
Appreciation for the complexity of contaminant mixtures in surface waters is likely to only increase as similar studies are conducted. As a result, effective chemical risk assessment demands tools that are capable of distinguishing chemical detections that are biologically relevant (and thus worthy of increased attention) from those that are, despite being measured, of lower risk to exposed populations. Including approaches such as the untargeted glucuronide screening method described here provides a route to achieve this, thus expanding beyond contaminant monitoring based on best guesses of likelihood of occurrence, anticipated health concern based on often limited toxicity data, or mere presence as determined by instruments with vanishingly low detection levels.
Supplementary Material
Acknowledgments
We thank Michael J. Cyterski at the U.S. EPA for helpful discussions on statistical analysis and the staff and management at the Western Lake Superior Sanitary District for accommodating this study.
Funding
M. Evich was supported by an Oak Ridge Institute for Science and Education (ORISE) research fellowship award at the Center for Environmental Measurement and Modeling in Athens, Georgia.
Abbreviations
- WWTP
wastewater treatment plant
- LC–HRMS
liquid chromatography high-resolution mass spectrometry
- BPA
bisphenol A
- PCBs
polychlorinated biphenyls
- UGT
uridine 5′-diphospho-glucuronosyltransferase
- LC–MS/MS
liquid chromatography-mass spectrometry
- HRAM
high-resolution accurate-mass
- WLSSD
Western Lake Superior Sanitary District
- ESI
electrospray ionization
- HCD
higher-energy collisional dissociation
- CID
collision-induced dissociation
- MSI
Metabolomics Standard Initiative
- FISh
Fragment Ion Search
- BPS
bisphenol S
- 1-HCPK
1-hydroxycyclohexyl phenyl ketone
- BPS-monoP
4-[(4-isopropoxyphenyl)sulfonyl]phenol
- 2-MBT
2-mercaptobenzothiazole
- NSAIDs
non-steroidal anti-inflammatory drugs
- 3-PBA
3-phenoxybenzoic acid
- HAS
human serum albumin
- BHT
butylated hydroxy toluene
- PFAS
per- and polyfluoroalkyl substances
Footnotes
The authors declare no competing financial interest.
The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency (EPA). Mention of trade names or products does not convey and should not be interpreted as conveying official EPA approval, endorsement, or recommendation.
References
- 1.Bradley PM; Journey CA; Romanok KM; Barber LB; Buxton HT; Foreman WT; Furlong ET; Glassmeyer ST; Hladik ML; Iwanowicz LR; Jones DK; Kolpin DW; Kuivila KM; Loftin KA; Mills MA; Meyer MT; Orlando JL; Reilly TJ; Smalling KL; Villeneuve DL Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams. Environ. Sci. Technol 2017, 51, 4792–4802, DOI: 10.1021/acs.est.7b00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bains OS; Kennedy CJ Energetic costs of pyrene metabolism in isolated hepatocytes of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol 2004, 67, 217–226, DOI: 10.1016/j.aquatox.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 3.Kleinow KM; Melancon MJ; Lech JJ Biotransformation and induction: implications for toxicity, bioaccumulation and monitoring of environmental xenobiotics in fish. Environ. Health Perspect. 1987, 71, 105–119, DOI: 10.1289/ehp.8771105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz S; Nichols JW; Escher BI; Ankley GT; Altenburger R; Blackwell B; Brack W; Burkhard L; Collette TW; Doering JA; Ekman D; Fay K; Fischer F; Hackermüller J; Hoffman JC; Lai C; Leuthold D; Martinovic-Weigelt D; Reemtsma T; Pollesch N; Schroeder A; Schüürmann G; von Bergen M The Eco-Exposome Concept: Supporting an Integrated Assessment of Mixtures of Environmental Chemicals. Environ. Toxicol. Chem 2022, 41, 30–45, DOI: 10.1002/etc.5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutton GJ Developmental Aspects of Drug Conjugation, with Special Reference to Glucuronidation. Annu. Rev. Pharmacol. Toxicol 1978, 18, 17–35, DOI: 10.1146/annurev.pa.18.040178.000313 [DOI] [PubMed] [Google Scholar]
- 6.Rowland A; Miners JO; Mackenzie PI The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol 2013, 45, 1121–1132, DOI: 10.1016/j.biocel.2013.02.019 [DOI] [PubMed] [Google Scholar]
- 7.Ge S; Tu Y; Hu M Challenges and Opportunities with Predicting in Vivo Phase II Metabolism via Glucuronidation from in Vitro Data. Curr. Pharmacol. Rep 2016, 2, 326–338, DOI: 10.1007/s40495-016-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke DJ; George SG; Burchell B Glucuronidation in Fish. Aquat. Toxicol 1991, 20, 35–56, DOI: 10.1016/0166-445x(91)90040-g [DOI] [Google Scholar]
- 9.Trontelj J Quantification of Glucuronide Metabolites in Biological Matrices by LC-MS/MS. Tandem Mass Spectrometry; IntechOpen, 2012; pp 531–558. [Google Scholar]
- 10.Sitasuwan P; Melendez C; Marinova M; Spruill M; Lee LA Comparison of Purified β-glucuronidases in Patient Urine Samples Indicates a Lack of Correlation Between Enzyme Activity and Drugs of Abuse Metabolite Hydrolysis Efficiencies Leading to Potential False Negatives. J. Anal. Toxicol 2019, 43, 221–227, DOI: 10.1093/jat/bky082 [DOI] [PubMed] [Google Scholar]
- 11.Wang P; Stone JA; Chen KH; Gross SF; Haller CA; Wu AH Incomplete recovery of prescription opioids in urine using enzymatic hydrolysis of glucuronide metabolites. J. Anal. Toxicol 2006, 30, 570–575, DOI: 10.1093/jat/30.8.570 [DOI] [PubMed] [Google Scholar]
- 12.Xia YQ; Miller JD; Bakhtiar R; Franklin RB; Liu DQ Use of a quadrupole linear ion trap mass spectrometer in metabolite identification and bioanalysis. Rapid Commun. Mass Spectrom 2003, 17, 1137–1145, DOI: 10.1002/rcm.1037 [DOI] [PubMed] [Google Scholar]
- 13.Ancillotti C; Ulaszewska M; Mattivi F; Del Bubba M Untargeted Metabolomics Analytical Strategy Based on Liquid Chromatography/Electrospray Ionization Linear Ion Trap Quadrupole/Orbitrap Mass Spectrometry for Discovering New Polyphenol Metabolites in Human Biofluids after Acute Ingestion of Vaccinium myrtillus Berry Supplement. J. Am. Soc. Mass Spectrom 2019, 30, 381–402, DOI: 10.1007/s13361-018-2111-y [DOI] [PubMed] [Google Scholar]
- 14.Dai WD; Yin PY; Zeng ZD; Kong HW; Tong HW; Xu ZL; Lu X; Lehmann R; Xu GW Nontargeted Modification-Specific Metabolomics Study Based on Liquid Chromatography-High-Resolution Mass Spectrometry. Anal. Chem 2014, 86, 9146–9153, DOI: 10.1021/ac502045j [DOI] [PubMed] [Google Scholar]
- 15.Rinschen MM; Ivanisevic J; Giera M; Siuzdak G Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol 2019, 20, 353–367, DOI: 10.1038/s41580-019-0108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kind T; Fiehn O Seven Golden Rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry. BMC Bioinf. 2007, 8, 105, DOI: 10.1186/1471-2105-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavallin JE; Jensen KM; Kahl MD; Villeneuve DL; Lee KE; Schroeder AL; Mayasich J; Eid EP; Nelson KR; Milsk RY; Blackwell BR; Berninger JP; LaLone CA; Blanksma C; Jicha T; Elonen C; Johnson R; Ankley GT Pathway-based approaches for assessment of real-time exposure to an estrogenic wastewater treatment plant effluent on fathead minnow reproduction. Environ. Toxicol. Chem 2016, 35, 702–716, DOI: 10.1002/etc.3228 [DOI] [PubMed] [Google Scholar]
- 18.Mosley JD; Ekman DR; Cavallin JE; Villeneuve DL; Ankley GT; Collette TW High-resolution mass spectrometry of skin mucus for monitoring physiological impacts and contaminant biotransformation products in fathead minnows exposed to wastewater effluent. Environ. Toxicol. Chem 2018, 37, 788–796, DOI: 10.1002/etc.4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumner LW; Amberg A; Barrett D; Beale MH; Beger R; Daykin CA; Fan TWM; Fiehn O; Goodacre R; Griffin JL; Hankemeier T; Hardy N; Harnly J; Higashi R; Kopka J; Lane AN; Lindon JC; Marriott P; Nicholls AW; Reily MD; Thaden JJ; Viant MR Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221, DOI: 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little JL; Williams AJ; Pshenichnov A; Tkachenko V Identification of “Known Unknowns” Utilizing Accurate Mass Data and ChemSpider. J. Am. Soc. Mass Spectrom 2012, 23, 179–185, DOI: 10.1007/s13361-011-0265-y [DOI] [PubMed] [Google Scholar]
- 21.Levsen K; Schiebel HM; Behnke B; Dötzer R; Dreher W; Elend M; Thiele H Structure elucidation of phase II metabolites by tandem mass spectrometry: an overview. J. Chromatogr. A 2005, 1067, 55–72, DOI: 10.1016/j.chroma.2004.08.165 [DOI] [PubMed] [Google Scholar]
- 22.Taylor VF; March RE; Longerich HP; Stadey CJ A mass spectrometric study of glucose, sucrose, and fructose using an inductively coupled plasma and electrospray ionization. Int. J. Mass Spectrom 2005, 243, 71–84, DOI: 10.1016/j.ijms.2005.01.001 [DOI] [Google Scholar]
- 23.Burkina V; Zlabek V; Zamaratskaia G Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ. Toxicol. Pharmacol 2015, 40, 430–444, DOI: 10.1016/j.etap.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 24.Goldinger DM; Demierre AL; Zoller O; Rupp H; Reinhard H; Magnin R; Becker TW; Bourqui-Pittet M Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul. Toxicol. Pharmacol 2015, 71, 453–462, DOI: 10.1016/j.yrtph.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 25.Morizane M; Kawasaki Y; Miura T; Yagi K; Esumi S; Kitamura Y; Sendo T Photoinitiator-Initiated Estrogenic Activity in Human Breast Cancer Cell Line Mcf-7. J. Toxicol. Environ. Health, Part A 2015, 78, 1450–1460, DOI: 10.1080/15287394.2015.1094431 [DOI] [PubMed] [Google Scholar]
- 26.Song Y; Xie P; Cai Z Metabolism of bisphenol S in mice after oral administration. Rapid Commun. Mass Spectrom 2018, 32, 495–502, DOI: 10.1002/rcm.8051 [DOI] [PubMed] [Google Scholar]
- 27.Ho KL; Yuen KK; Yau MS; Murphy MB; Wan Y; Fong BMW; Tam S; Giesy JP; Leung KSY; Lam MHW Glucuronide and Sulfate Conjugates of Bisphenol A: Chemical Synthesis and Correlation Between Their Urinary Levels and Plasma Bisphenol A Content in Voluntary Human Donors. Arch. Environ. Contam. Toxicol 2017, 73, 410–420, DOI: 10.1007/s00244-017-0438-1 [DOI] [PubMed] [Google Scholar]
- 28.Liao CY; Kim UJ; Kannan K A Review of Environmental Occurrence, Fate, Exposure, and Toxicity of Benzothiazoles. Environ. Sci. Technol 2018, 52, 5007–5026, DOI: 10.1021/acs.est.7b05493 [DOI] [PubMed] [Google Scholar]
- 29.Nelson KR; Schroeder AL; Ankley GT; Blackwell BR; Blanksma C; Degitz SJ; Flynn KM; Jensen KM; Johnson RD; Kahl MD; Knapen D; Kosian PA; Milsk RY; Randolph EC; Saari T; Stinckens E; Vergauwen L; Villeneuve DL Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquat. Toxicol 2016, 173, 192–203, DOI: 10.1016/j.aquatox.2015.12.024 [DOI] [PubMed] [Google Scholar]
- 30.Stinckens E; Vergauwen L; Schroeder AL; Maho W; Blackwell BR; Witters H; Blust R; Ankley GT; Covaci A; Villeneuve DL; Knapen D Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquat. Toxicol 2016, 173, 204–217, DOI: 10.1016/j.aquatox.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 31.Johnson CH; Wilson ID; Harding JR; Stachulski AV; Iddon L; Nicholson JK; Lindon JC NMR Spectroscopic Studies on the in Vitro Acyl Glucuronide Migration Kinetics of Ibuprofen ((±)-(R,S)-2-(4-Isobutylphenyl) Propanoic Acid), Its Metabolites, and Analogues. Anal. Chem 2007, 79, 8720–8727, DOI: 10.1021/ac071368i [DOI] [PubMed] [Google Scholar]
- 32.Noort D; van Zuylen A; Fidder A; van Ommen B; Hulst AG Protein Adduct Formation by Glucuronide Metabolites of Permethrin. Chem. Res. Toxicol 2008, 21, 1396–1406, DOI: 10.1021/tx8000362 [DOI] [PubMed] [Google Scholar]
- 33.Regan SL; Maggs JL; Hammond TG; Lambert C; Williams DP; Park BK Acyl Glucuronides: The Good, The Bad and The Ugly. Biopharm Drug Dispos. 2010, 31, 367–395, DOI: 10.1002/bdd.720 [DOI] [PubMed] [Google Scholar]
- 34.Richards J; Lu Z; Fu Q; Schlenk D; Gan J Conversion of Pyrethroid Insecticides to 3-Phenoxybenzoic Acid on Urban Hard Surfaces. Environ. Sci. Technol. Lett 2017, 4, 546–550, DOI: 10.1021/acs.estlett.7b00466 [DOI] [Google Scholar]
- 35.Bolze S; Bromet N; Gay-Feutry C; Massiere F; Boulieu R; Hulot T Development of an in Vitro Screening Model for the Biosynthesis of Acyl Glucuronide Metabolites and the Assessment of Their Reactivity toward Human Serum Albumin. Drug Metabol. Dispos 2002, 30, 404, DOI: 10.1124/dmd.30.4.404 [DOI] [PubMed] [Google Scholar]
- 36.Camilleri P; Buch A; Soldo B; Hutt AJ The influence of physicochemical properties on the reactivity and stability of acyl glucuronides. Xenobiotica 2018, 48, 958–972, DOI: 10.1080/00498254.2017.1384967 [DOI] [PubMed] [Google Scholar]
- 37.Lundebye AK; Hove H; Måge A; Bohne VJB; Hamre K Levels of synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) in fish feed and commercially farmed fish. Food Addit. Contam., Part A 2010, 27, 1652–1657, DOI: 10.1080/19440049.2010.508195 [DOI] [PubMed] [Google Scholar]
- 38.Stacey N Hormones, pheromones and reproductive behavior. Fish Physiol. Biochem 2003, 28, 229–235, DOI: 10.1023/b:fish.0000030540.99732.2c [DOI] [Google Scholar]
- 39.Sadoul B; Geffroy B Measuring cortisol, the major stress hormone in fishes. J. Fish. Biol 2019, 94, 540–555, DOI: 10.1111/jfb.13904 [DOI] [PubMed] [Google Scholar]
- 40.James MO Steroid catabolism in marine and freshwater fish. J. Steroid Biochem. Mol. Biol 2011, 127, 167–75, DOI: 10.1016/j.jsbmb.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 41.Jurgella GF; Marwah A; Malison JA; Peterson R; Barry TP Effects of xenobiotics and steroids on renal and hepatic estrogen metabolism in lake trout. Gen. Comp. Endocrinol 2006, 148, 273–281, DOI: 10.1016/j.ygcen.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 42.Thibaut R; Porte C Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J. Steroid Biochem. Mol. Biol 2004, 92, 485–494, DOI: 10.1016/j.jsbmb.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 43.Leaver MJ; Wright J; Hodgson P; Boukouvala E; George SG Piscine UDP-glucuronosyltransferase 1B. Aquat. Toxicol 2007, 84, 356–365, DOI: 10.1016/j.aquatox.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 44.Martin-Skilton R; Saborido-Rey F; Porte C Endocrine alteration and other biochemical responses in juvenile turbot exposed to the Prestige fuel oil. Sci. Total Environ 2008, 404, 68–76, DOI: 10.1016/j.scitotenv.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez de Vega R; Cameron A; Clases D; Dodgen TM; Doble PA; Bishop DP “Simultaneous targeted and non-targeted analysis of per- and polyfluoroalkyl substances in environmental samples by liquid chromatography-ion mobility-quadrupole time of flight-mass spectrometry and mass defect analysis”. J. Chromatogr. A 2021, 1653, 462423, DOI: 10.1016/j.chroma.2021.462423 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


