Abstract
Catalyst-controlled site-selective activation of β- and γ-methylene C─H bonds of free carboxylic acids is a long-standing challenge. Here we show that with a pair of palladium catalysts assembled with quinoline-pyridone ligands of different chelate ring sizes, it is possible to perform highly site-selective monolactonization reactions with a wide range of dicarboxylic acids, generating structurally diverse and synthetically useful γ- and δ-lactones via site-selective β- or γ-methylene C─H activation. The remaining carboxyl group serves as a versatile linchpin for further synthetic applications as demonstrated by the total synthesis of two natural products, myrotheciumone A and pedicellosine, from abundant dicarboxylic acids.
One Sentence Summary:
A pair of palladium catalysts enable site-selective activation of β- and γ-methylene C─H bonds for the synthesis of γ- and δ-lactones from abundant dicarboxylic acids.
Achieving site-selective methylene C─H activation of aliphatic carboxylic acids is a long-standing challenge in organic synthesis. The freedom to site-selectively functionalize particular methylene C─H bonds in these ubiquitous molecular backbones would enable rapid construction of a great variety of molecular scaffolds, and usher in retrosynthetic pathways that were previously deemed implausible. Although directed C─H activation of aliphatic carboxylic acids has been achieved with platinum (1, 2) and palladium catalysis (3-11), development of catalysts for the activation of methylene C─H bonds is nascent, and methods for distinguishing between the similar C─H bonds of multiple adjacent methylene units is an enduring challenge. Recent breakthroughs in ligand development have provided a glimmer of hope for the palladium-catalyzed, carboxylic acid-directed activation of β-methylene C─H bonds, culminating in the reports of a dehydrogenation reaction and a deuteration reaction (12, 13). However, no other C–heteroatom bond formation reaction nor activation of the γ-methylene C─H bonds are hitherto known using this approach. Although alternative strategies for the functionalization of methylene C─H bonds of free carboxylic acids using high-valent, electrophilic Fe or Mn catalysts exist (14, 15), these methods display limited differentiation between methine and methylene C─H bonds. Hence, the challenge of highly methylene-selective, catalyst-controlled site-selective C─H functionalization between adjacent methylene units remains unmet.
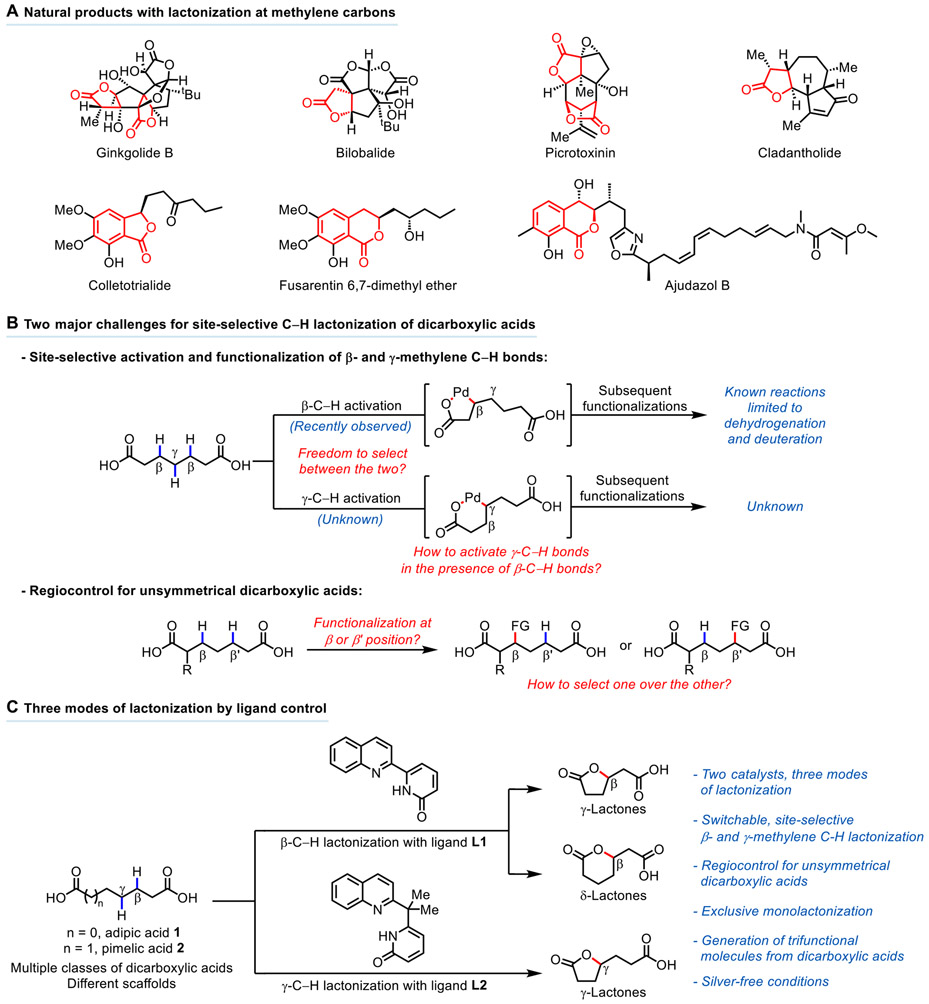
We set our sights on developing site-selective intramolecular C─O bond formation reactions of dicarboxylic acids through lactonization, driven by the fact that lactones are ubiquitous in organic chemistry, and the monolactonization of dicarboxylic acids would leave the remaining carboxyl group as a versatile handle for further synthetic elaborations (16). It is estimated that more than 3,000 γ-lactones exist in nature (17, 18), and the formation of lactones at methylene carbons is commonplace in natural products, as demonstrated in the structures and total syntheses of ginkgolide B (19, 20), bilobalide (21, 22), picrotoxinin (23-25), and cladantholide (26). Benzo-fused lactones such as phthalides and isochromanones are also recurring structures in bioactive natural products, such as colletotrialide, fusarentin 6,7-dimethyl ether, and ajudazol B (27, 28) (Fig. 1A). The principal challenge for the development of the desired lactonization reactions was to achieve tunable site-selective activation of either β- or γ-methylene C─H bonds by catalyst design. Another challenge was to realize regiocontrol for unsymmetrical dicarboxylic acids, thereby precluding the unselective generation of mixtures of regioisomeric products (29) (Fig. 1B).
Fig. 1. Lactonization via activation of methylene C─H bonds.
(A) Natural products with lactonization at methylene carbons (B) Two major challenges for site-selective C─H lactonization of dicarboxylic acids (C) Three modes of lactonization by ligand-control.
Here we report two distinct catalysts for site-selective β- and γ-C─H activation of dicarboxylic acids using quinoline-pyridone ligands, leading to three modes of lactonization for the synthesis of γ- and δ-lactones from various dicarboxylic acids (Fig. 1C). Symmetrical dicarboxylic acids such as adipic acid and pimelic acid could be converted into unsymmetrical lactone acids using this methodology. Unsymmetrical dicarboxylic acids generally provided lactone acids where the more substituted fraction of the molecule preferentially lactonized. These lactonization reactions convert dicarboxylic acids into trifunctional molecules with three orthogonal sites of reactivity that are amenable to chemoselective transformations for complex molecule synthesis. We also discovered that the use of cheap and abundant MnO2 as the oxidant is compatible with the lactonization reactions, demonstrating the possibility of using silver-free conditions for palladium-catalyzed, carboxylic acid-directed lactonization of methylene C─H bonds. The utility of this methodology was demonstrated with two total syntheses, forging the natural products pedicellosine and myrotheciumone A from dicarboxylic acids.
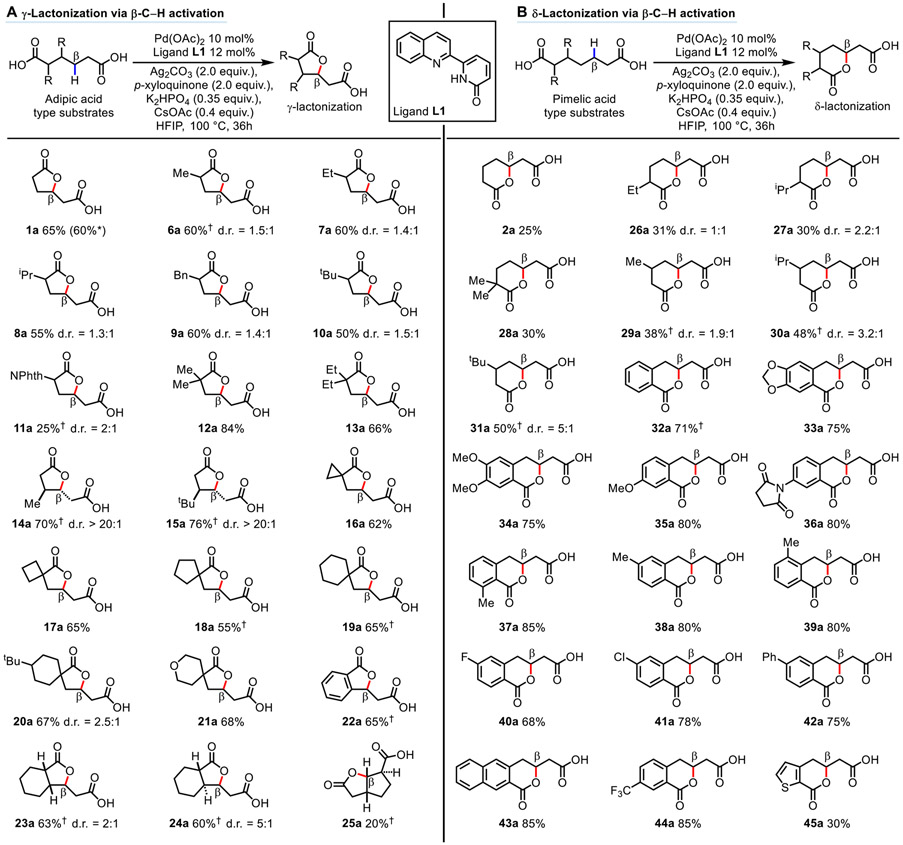
The development of the lactonization reaction began with exploratory studies using adipic acid 1 as the standard substrate. We were mindful that bis-carboxylate chelation by the dicarboxylic acid could prevent the desired directed C─H activation, and thus a tightly bound bidentate ligand would be required to overcome this potential hurdle. Building on the precedent that pyridine-pyridone type ligands could promote β-methylene C─H activation in a palladium-catalyzed, carboxylic acid-directed dehydrogenation reaction (13), a series of pyridine-pyridone type ligands were investigated for the desired C─O bond forming reactivity (see tables S1-S11). Following extensive optimization of reaction parameters, the five-membered chelating quinoline-pyridone ligand L1 emerged as the most effective at initiating the lactonization of 1 to provide γ-lactone 1a in 65% isolated yield (Fig. 2A, first product) with p-xyloquinone as an additive. The rationale for the use of p-xyloquinone comes from the literature precedent that it may assist reductive elimination at the palladium center (30). The formation of γ-lactone 1a from adipic acid 1 implied two mechanistic possibilities: γ-lactonization via γ-C─H activation or γ-lactonization via β-C─H activation. In other words, the observed γ-lactonization could occur via directed γ-C─H activation followed by γ-lactonization by the same carboxyl group, or it could occur via directed β-C─H activation by one of the carboxyl groups followed by γ-lactonization by the remaining carboxyl group. To distinguish between these two possibilities, a control experiment using pimelic acid 2 as the substrate was carried out. We found that δ-lactone 2a was generated in this experiment in 25% isolated yield (Fig. 2b, first product). This observation suggests carboxylic acid-directed β-C─H activation with ligand L1 is more probable, which is then followed by δ-lactonization using the carboxylic acid at the other end of the molecule. The other possibility for δ-lactonization, via a δ-C─H activation pathway, was deemed highly unlikely. Consistent with this hypothesis, the mono methyl ester of adipic acid 1, monomethyl adipate 3, was found to be unreactive for γ-lactonization. In the absence of the second carboxyl group in 3, dehydrogenation of 3 to give the corresponding α,β-unsaturated acid 4 was observed in ca. 17% NMR yield under the γ-lactonization conditions (see fig. S2). However, the use of (E)-hex-2-enedioic acid 5 as the substrate, which is the α,β-dehydrogenation product of adipic acid 1, provided significantly poorer yields of γ-lactone 1a (24% NMR yield) under the γ-lactonization conditions (see fig. S3). These observations suggest that one carboxyl group is responsible for directed β-C─H activation and the other carboxyl group is responsible for γ-lactonization, and this lactonization process is unlikely to occur solely via a dehydrogenation/intramolecular lactonization sequence.
Fig. 2. Substrate scope of γ- and δ-lactones from β-C─H activation.
(A) γ-Lactonization via β-C─H activation. (B) δ-Lactonization via β-C─H activation. Reaction conditions: Substrate (0.1 mmol), Pd(OAc)2 10 mol%, Ligand L1 12 mol%, Ag2CO3 (2.0 equiv.), p-xyloquinone (2.0 equiv.), K2HPO4 (0.35 equiv.), CsOAc (0.4 equiv.), HFIP (1.0 mL), 100 °C, 36h. Isolated yields are reported. *Reaction conducted with 1.0 g of adipic acid 1. †Isolated as the corresponding benzyl ester.
The generality of these two modes of lactonization via β-C─H activation was investigated with a series of dicarboxylic acids with various substituents and topologies. For γ-lactonization reactions via β-C─H activation with ligand L1 (Fig. 2A), substrates possessing α–hydrogens that generally correlate with less efficient C─H activation all provided the desired products in synthetically useful yields (5). The α-mono substituted products with increasing steric demand of the substituent (Me 6a, Et 7a, iPr 8a, Bn 9a, tBu 10a) were isolated in 50-60% yield. The product 11a with a NPhth substituent at its α-position, however, only provided a 25% isolated yield of the benzyl ester. The low yield of 11a was due to low conversion as the unreacted starting material was the major species observed on crude 1H NMR. For the product 12a that bears the gem-dimethyl substituent at its α-position, it was isolated in 84% yield. Increasing the substituent size to a gem-diethyl system, as for product 13a, resulted in a decrease in isolated yield to 66%. Products 14a and 15a possessing substitution at their β-position but with fully unsubstituted α-positions displayed high reactivity and diastereoselectivity, giving isolated yields around 70% as their benzyl esters and favoring the anti-isomer with a d.r. > 20:1. Spirocyclic structures at the α-position provided lactones possessing spirocyclic cyclopropyl (16a), cyclobutyl (17a), cyclopentyl (18a), cyclohexyl (19a, 20a), and 4-tetrahydropyranyl (21a) systems in 55-68% yield. Fused structures were also found to be well tolerated for this lactonization methodology, providing benzofused product 22a in 65% isolated yield as its benzyl ester, and fused 5,6-bicyclic systems with a cis (23a) and a trans (24a) junction in 63% and 60% yield as their benzyl esters, respectively. The more strained 5,5-bicyclic system 25a with endocyclic β-methylene C─H bonds was isolated in 20% yield as its benzyl ester, the low yield of 25a was again due to low conversion. The scalability of this γ-lactonization reaction via β-C─H activation was also investigated, in which the lactone 1a could be obtained in 60% isolated yield with 1.0 g of adipic acid 1 as starting material (see Supplementary Materials for details on experimental set-up).
For δ-lactonization reactions via β-C─H activation (Fig. 2B), aliphatic systems were challenging substrates (2a, 26a-31a), and substitution at the β-position was required to elevate the isolated yield to 38-50% (29a-31a). However, the performance of the reaction for the synthesis of benzo-fused products was superior, providing the products 32a-45a in 68-85% yield, tolerating a range of aromatic substituents such as oxygen (33a-35a), nitrogen (36a), alkyl (37a-39a, 42a), halide (40a, 41a) and a trifluoromethyl group (44a). Modifying the identity of the aromatic ring was also possible, as demonstrated by the formation of naphthalene-fused system 43a and the thiophene-fused system 45a, though the latter was only isolated in 30% yield.
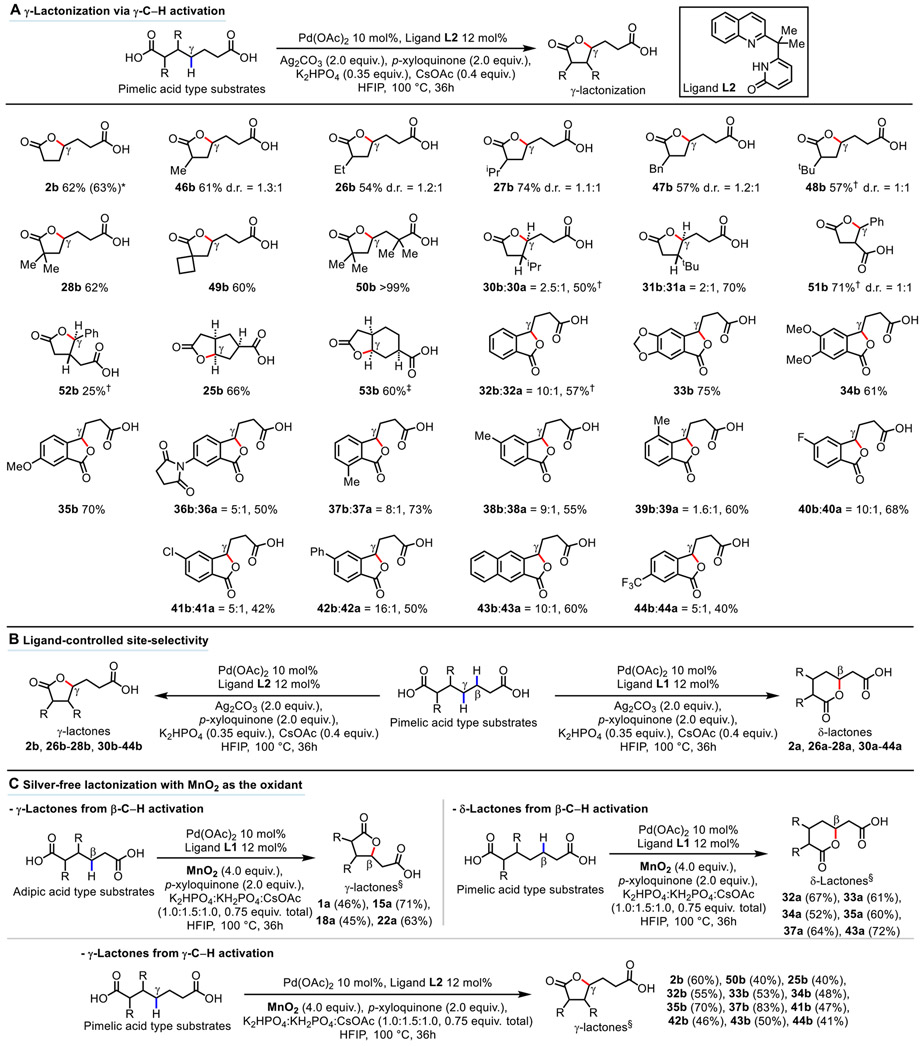
Next, we wondered if the γ-methylene C─H bonds of pimelic acid 2 could also be activated for γ-lactonization. This selectivity presented a particular challenge for a C─H activation approach because activation of the competing β-methylene C─H bonds that would result in five-membered cyclopalladation would tend to be both kinetically and thermodynamically preferred (31). Overcoming this hurdle for C─H activation could lead to two distinct site-selective lactonization pathways via β- or γ-methylene C─H activation. With this objective in mind, we further explored other types of quinoline-pyridone ligands, and we discovered that ligand L2 provided the desired γ-lactone 2b in 62% isolated yield (Fig. 3A). Evidence to support a γ-C─H activation process was obtained from H/D exchange and a series of control experiments (see figs. S1 and S4). For this mode of lactonization via γ-C─H activation, substrates possessing α–hydrogens that generally correlate with less efficient C─H activation all provided the desired products in synthetically useful yields (5). The α-mono substituted products with increasing steric demand of the substitutent (Me 46b, Et 26b, iPr 27b, Bn 47b, tBu 48b) were isolated in 54-74% yield. For the products that are quaternized at their α-positions, the product 28b with single gem-dimethyl substitution was isolated in 62% yield. For the product 50b with double gem-dimethyl substitutions, the isolated yield increased to >99%. The formation of spirocyclic systems were also tested with this lactonization methodology; however, only the cyclobutyl system was amenable to lactonization, giving 49b in an isolated yield of 60%. γ-Lactones 30b and 31b possessing substitution at their β-position but with fully unsubstituted α-positions were isolated as the anti-diastereomers along with separable minor products δ-lactones 30a and 31a in ca. 2:1 ratio, with a total yield of 50-70%. Dicarboxylic acids with structures that would allow lactonization to occur on branched substituents but not on the parent alkyl chain were also investigated. Interestingly, the product 51b arising from α-benzyl succinic acid was produced only with the use of ligand L2 but not with ligand L1, suggesting a γ-C─H activation pathway was likely for its lactonization. This product was isolated in 70% yield after conversion to its benzyl ester. The product 52b, arising from β-benzyl glutaric acid, was isolated as its benzyl ester in 25% yield as a single anti-diastereomer. The low yield for 52b was due to low conversion. Substrates with endocyclic γ-methylene C─H bonds were also found to be viable for this lactonization protocol, as exemplified with products 25b and 53b, generating lactones with fused 5,5-bicyclic and fused 5,6-bicyclic systems in good yields as single diastereomers. Benzo-fused γ-lactones (32b-44b) were also forged in 40-75% total yields with predominant (γ:β > 8:1) site-selectivity at the γ-position (32, 33), except for products 36b, 39b, 41b, and 44b in which the site-selectivity was found to be lower. The collective synthesis of a series of γ-lactones (2b, 26b-28b, 30b-44b) and δ-lactones (2a, 26a-28a, 30a-44a) from the same substrates elegantly demonstrates the desired ligand-controlled switchable site-selectivity for carboxylic acid directed, β-methylene and γ-methylene C─H lactonizations (Fig. 3B). The scalability of this γ-lactonization reaction via γ-C─H activation was also investigated, in which the lactone 2b could be obtained in 63% isolated yield with 1.0 g of pimelic acid 2 as starting material (see Supplementary Materials for details on experimental set-up).
Fig. 3. Substrate scope of γ-lactones from γ-C─H activation, demonstration of switchable site-selectivity, and examples of silver-free lactonization reactions with MnO2 as the oxidant.
(A) γ-Lactonization via γ-C─H activation. Reaction conditions: Substrate (0.1 mmol), Pd(OAc)2 10 mol%, Ligand L2 12 mol%, Ag2CO3 (2.0 equiv.), p-xyloquinone (2.0 equiv.), K2HPO4 (0.35 equiv.), CsOAc (0.4 equiv.), HFIP (1.0 mL), 100 °C, 36h. Isolated yields are reported. *Reaction conducted with 1.0 g of pimelic acid 2. †Isolated as the corresponding benzyl ester. ‡Based on reactive diastereomer (see Supplementary Material p. S92-S93 for explanation). (B) Demonstration of switchable site-selectivity with 19 examples. (C) Examples of silver-free lactonization with MnO2 as the oxidant (22 examples). Reaction conditions: Substrate (0.1 mmol), Pd(OAc)2 10 mol%, Ligand L1 or L2 12 mol%, MnO2 (4.0 equiv.), p-xyloquinone (2.0 equiv.), K2HPO4:KH2PO4:CsOAc (1.0:1.5:1.0, 0.75 equiv. in total), HFIP (1.0 mL), 100 °C, 36h. §NMR yields.
With the scope of the lactonization established, silver-free conditions for the three modes of lactonization reactions were sought and MnO2 was found to be a viable replacement for Ag2CO3 as the oxidant. Upon further fine-tuning of the reaction conditions (see tables S2 and S11), the lactonization reactions could be rendered silver-free across a series of substrates (Fig. 3C).
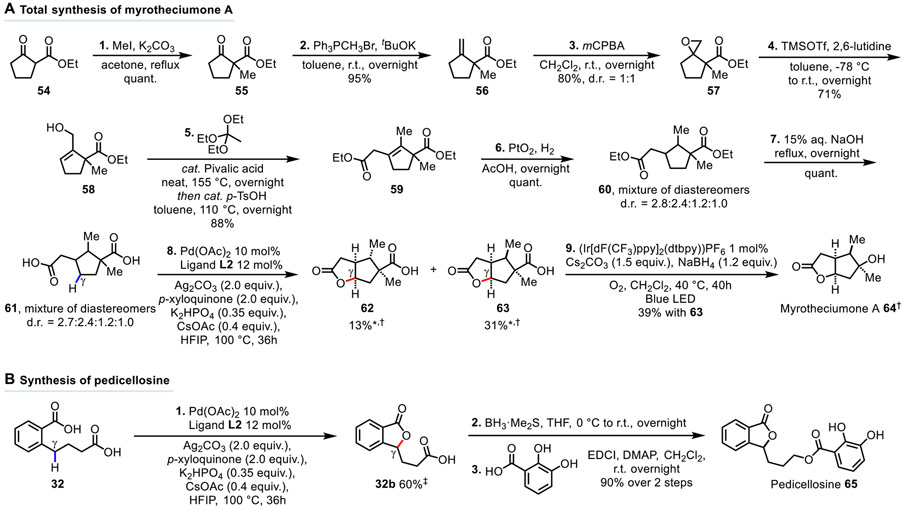
In light of the broad scope of lactones prepared by this methodology, we embarked on tackling two total syntheses as a means to demonstrate the synthetic utility offered by the fusion of dicarboxylic acids and C─H lactonization (Fig. 4). Myrotheciumone A 64 (34), a target recently completed by Begum and co-workers (35), is a bicyclic cytotoxic lactone isolated from the fungus Ajuga decumbens that possesses the bicyclic 5,5-fused scaffold as in lactone 25b (Fig. 3A). Retrosynthetic disconnection at the lactone C─O bond meant complex dicarboxylic acid 61 would be the synthetic intermediate for the key C─H lactonization step. This intermediate 61 was prepared efficiently, albeit as a diastereomeric mixture, from commercially available ethyl 2-oxocyclopentane-1-carboxylate 54. Specifically, ethyl 2-oxocyclopentane-1-carboxylate 54 was methylated with MeI in refluxing acetone to provide 55 in quantitative yield, followed by a Wittig reaction with Ph3PCH3Br to give terminal alkene 56 in 95% yield. The terminal alkene 56 was epoxidized with mCPBA to give an inconsequential 1:1 diastereomeric mixture of epoxides 57 in 80% yield, which were isomerized with TMSOTf and 2,6-lutidine in toluene (-78 °C to room temperature, overnight) to give allylic alcohol 58 in 71% yield. The allylic alcohol 58 underwent a Johnson-Claisen rearrangement, followed by olefin isomerization with p-TsOH in refluxing toluene to give the complex cyclopentene 59 in 88% yield. The cyclopentene 59 was hydrogenated with catalytic PtO2 in acetic acid under the H2 pressure of a 4-layered balloon overnight to provide the complex cyclopentane 60 in quantitative yield as a diastereomeric mixture. Subsequent basic ester hydrolysis of 60 with 15% aq. NaOH at reflux provided the dicarboxylic acid 61 in quantitative yield. The γ-lactonization of dicarboxylic acid 61 provided the desired lactone 63 in 31% yield, and its diastereomer 62 in 13% yield. The yields of the lactones 62 and 63 were calculated based on the reactive diastereomers of 61 (see Supplementary Material p. S118 for detailed analysis). The last step of the synthesis was a photocatalytic decarboxylative hydroxylation of 63 which provided the racemic natural product myrotheciumone A 64 in 39% yield (36). The total synthesis was completed in a total of 9 steps from ethyl 2-oxocyclopentane-1-carboxylate. Pedicellosine 65 (37), a phthalide isolated from the leaves of Gentiana pedicellate and previously synthesised by Li and co-workers (38), possesses the benzo-fused lactone structure as in lactone 32b (Fig. 3A). The synthesis began with the preparation of lactone 32b from the dicarboxylic acid 32 via γ-C─H lactonization, followed by the reduction of the carboxyl group of 32b to the alcohol using BH3•Me2S. The crude alcohol was esterified with 2,3-dihydroxybenzoic acid using EDCI as the coupling reagent, providing the simple natural product pedicellosine 65 in three steps from the dicarboxylic acid 32. These two representative syntheses demonstrate that the fusion of dicarboxylic acids and C─H lactonization constitutes a viable synthetic strategy amenable to the preparation of molecular targets of varying complexities at both late-stage and early-stage of the synthesis.
Fig. 4. Syntheses of natural products.
(A) Total synthesis of Myrotheciumone A. *Yields based on reactive diastereomers of 61 (see Supplementary Material p. S118 for detailed analysis). †Relative configurations are shown as the synthesis is racemic. (B) Synthesis of Pedicellosine. ‡Isolated as the carboxylic acid. mCPBA = meta-Chloroperoxybenzoic acid, TMSOTf = Trimethylsilyl trifluoromethanesulfonate, EDCI = 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide, DMAP = 4-Dimethylaminopyridine.
In conclusion, we have achieved site-selective β- and γ-methylene C─H activation of aliphatic acids with two quinoline-pyridone ligands L1 and L2. This led to the development of three modes of lactonization reaction for the syntheses of γ- and δ-lactones from a great variety of dicarboxylic acids. The utility of the methodology is demonstrated by the syntheses of two natural products. Further development of the current methodology for the syntheses of lactones of other ring sizes, and extension of the method towards the synthesis of other heterocycles are underway.
Supplementary Material
Acknowledgements:
We thank D. Strassfeld, M. Tomanik, and K. Wu for proofreading and providing helpful suggestions in preparing the manuscript. We thank N. Lam for helpful discussions. We thank Z. Wang for the preparation of ligands.
Funding:
We acknowledge The Scripps Research Institute, NIH (NIGMS, 2R01GM084019), and Croucher Foundation (Postdoctoral Fellowship to H. S. S. C) for financial support.
Footnotes
Competing interests: J.-Q. Y. and H. S. S. C. are inventors on a patent application related to this work (US Patent Application Pending) filed by The Scripps Research Institute. The authors declare no other competing interests.
Data and materials availability: All data are in the supplementary materials.
References:
- 1.Kao LC, Sen A, Platinum(II) Catalysed Selective Remote Oxidation of Unactivated C─H Bonds in Aliphatic Carboxylic Acids. J. Chem. Soc., Chem. Commun 1242–1243 (1991). [Google Scholar]
- 2.Dangel BD, Johnson JA, Sames D, Selective functionalization of amino acids in water: A synthetic method via catalytic C─H bond activation. J. Am. Chem. Soc 123, 8149–8150 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ, Palladium-catalyzed methylation and arylation of sp2 and sp3 C─H bonds in simple carboxylic acids. J. Am. Chem. Soc 129, 3510–3511 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Zhuang Z, Yu CB, Chen G, Wu QF, Hsiao Y, Joe CL, Qiao JX, Poss MA, Yu JQ, Ligand-Enabled β-C(sp3)─H Olefination of Free Carboxylic Acids. J. Am. Chem. Soc 140, 10363–10367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang Z, Herron AN, Fan Z, Yu JQ, Ligand-Enabled Monoselective β-C(sp3)─H Acyloxylation of Free Carboxylic Acids Using a Practical Oxidant. J. Am. Chem. Soc 142, 6769–6776 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang Z, Yu JQ, Lactonization as a general route to β-C(sp3)─H functionalization. Nature. 577, 656–659 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Z, Herron AN, Yu JQ, Synthesis of Cyclic Anhydrides via Ligand-Enabled C─H Carbonylation of Simple Aliphatic Acids. Angew. Chem. Int. Ed 60, 16382–16387 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh KK, van Gemmeren M, Pd-Catalyzed β-C(sp3)─H Arylation of Propionic Acid and Related Aliphatic Acids. Chemistry - A European Journal. 23, 17697–17700 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Ghosh KK, Uttry A, Koldemir A, Ong M, van Gemmeren M, Direct β-C(sp3)─H acetoxylation of aliphatic carboxylic acids. Org. Lett 21, 7154–7157 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Ghosh KK, Uttry A, Ghiringhelli F, Mondal A, van Gemmeren M, Ligand-Enabled γ-C(sp3)─H Olefination of Free Carboxylic Acids. Angew. Chem. Int. Ed 59, 12848–12852 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Uttry A, Ghosh KK, van Gemmeren M, Direct β-and γ-C(sp3)─H Alkynylation of Free Carboxylic Acids. Angew. Chem. Int. Ed 59, 23127–23131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uttry A, Mal S, van Gemmeren M, Late-Stage β-C(sp3)─H Deuteration of Carboxylic Acids. J. Am. Chem. Soc 143, 10895–10901 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Hu L, Chekshin N, Zhuang Z, Qian S, Qiao JX, Yu J-Q, Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C─H activation. Science. 374, 1281–1285 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MS, White MC, A Predictably Selective Aliphatic C─H Oxidation Reaction for Complex Molecule Synthesis. Science. 318, 783–787 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Vicens L, Bietti M, Costas M, General Access to Modified α-Amino Acids by Bioinspired Stereoselective γ-C─H Bond Lactonization. Angew. Chem. Int. Ed 60, 4740–4746 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Schwarz J, König B, Decarboxylative reactions with and without light-a comparison. Green Chemistry. 20, 323–361 (2018). [Google Scholar]
- 17.Sartori SK, Diaz MAN, Diaz-Muñoz G, Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron. 84, 132001–132039 (2021). [Google Scholar]
- 18.Seitz M, Reiser O, Synthetic approaches towards structurally diverse γ-butyrolactone natural-product-like compounds. Current Opinion in Chemical Biology. 9 (2005), pp. 285–292. [DOI] [PubMed] [Google Scholar]
- 19.Corey EJ, Kang MC, Desai MC, Ghosh AK, Houpis IN, Total synthesis of (.+-.)-ginkgolide B. J. Am. Chem. Soc 110, 649–651 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crimmins MT, Pace JM, Nantermet PG, Kim-Meade AS, Thomas JB, Watterson SH, Wagman AS, The total synthesis of (±)-ginkgolide B. J. Am. Chem. Soc 122, 8453–8463 (2000). [Google Scholar]
- 21.Corey EJ, Su WG, Total synthesis of a C15 ginkgolide, (.+-.)-bilobalide. J. Am. Chem. Soc 109, 7534–7536 (1987). [Google Scholar]
- 22.Baker MA, Demoret RM, Ohtawa M, Shenvi RA, Concise asymmetric synthesis of (−)-bilobalide. Nature. 575, 643–646 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corey EJ, Pearce HL, Total synthesis of picrotoxinin. J. Am. Chem. Soc 101, 5841–5843 (1979). [Google Scholar]
- 24.Trost BM, Krische MJ, General Strategy for the Asymmetric Synthesis of the Picrotoxanes. J. Am. Chem. Soc 118, 233–234 (1996). [Google Scholar]
- 25.Crossley SWM, Tong G, Lambrecht MJ, Burdge HE, Shenvi RA, Synthesis of (-)-Picrotoxinin by Late-Stage Strong Bond Activation. J. Am. Chem. Soc 142, 11376–11381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee E, Yoon CH, Sung Y, Kim YK, Yun M, Kim S, Total Synthesis of (+)-Cladantholide and (−)-Estafiatin: 5-Exo, 7-Endo Radical Cyclization Strategy for the Construction of Guaianolide Skeleton. J. Am. Chem. Soc 119, 8391–8392 (1997). [Google Scholar]
- 27.Shabir G, Saeed A, El-Seedi HR, Natural isocoumarins: Structural styles and biological activities, the revelations carry on. Phytochemistry. 181, 112568–112787 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Kinghorn AD, Falk H, Gibbons S, Kobayashi J, Eds., Progress in the Chemistry of Organic Natural Products (Springer Nature, 2017), vol. 104. [Google Scholar]
- 29.Zaragoza Dörwald F, Side Reactions in Organic Synthesis: A Guide to Successful Synthesis Design (Wiley, 2004). [Google Scholar]
- 30.Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ, Palladium-catalyzed alkylation of aryl C─H bonds with sp3 organotin reagents using benzoquinone as a crucial promoter. J. Am. Chem. Soc 128, 78–79 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Xia G, Weng J, Liu L, Verma P, Li Z, Yu JQ, Reversing conventional site-selectivity in C(sp3)─H bond activation. Nature Chemistry. 11, 571–577 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novák P, Correa A, Gallardo-Donaire J, Martin R, Synergistic palladium-catalyzed C(sp3)─H Activation/C(sp3)─O bond formation: A direct, step-economical route to benzolactones. Angew. Chem. Int. Ed 50, 12236–12239 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Lee JM, Chang S, Pt-Catalyzed sp3 C─H bond activation of o-alkyl substituted aromatic carboxylic acid derivatives for the formation of aryl lactones. Tetrahedron Letters. 47, 1375–1379 (2006). [Google Scholar]
- 34.Lin T, Wang G, Shan W, Zeng D, Ding R, Jiang X, Zhu D, Liu X, Yang S, Chen H, Myrotheciumones: Bicyclic cytotoxic lactones isolated from an endophytic fungus of Ajuga decumbens. Bioorganic and Medicinal Chemistry Letters. 24, 2504–2507 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Begum S, Chakraborty TK, Cp2TiCl-Mediated Reductive Cyclization: Total Synthesis of Pestalotiolactone A, Myrotheciumone A, and Scabrol A. J. Org. Chem 86, 11812–11821 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Khan SN, Zaman MK, Li R, Sun Z, A General Method for Photocatalytic Decarboxylative Hydroxylation of Carboxylic Acids. J. Org. Chem 85, 5019–5026 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Mpondo EM, Garcia J, Chulia AJ, New Phthalides from Gentiana pedicellata. Planta Medica. 53, 297–298 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Gao Q, Li SB, Synthesis of 3-(3-Hydroxypropyl)-phthalide and Pedicellosine. Chinese Chemical Letters. 10, 109–110 (1999). [Google Scholar]
- 39.Li Z, Wang Z, Chekshin N, Qian S, Qiao JX, Cheng PT, Yeung K-S, Ewing WR, Yu J-Q, A tautomeric ligand enables directed C─H hydroxylation with molecular oxygen. Science. 372, 1452–1457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shneider OS, Pisarevsky E, Fristrup P, Szpilman AM, Oxidative umpolung α-alkylation of ketones. Org. Lett 17, 282–285 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Azimioara M, Alper P, Cow C, Mutnick D, Nikulin V, Lelais G, Mecom J, McNeill M, Michellys PY, Wang Z, Reding E, Paliotti M, Li J, Bao D, Zoll J, Kim Y, Zimmerman M, Groessl T, Tuntland T, Joseph SB, McNamara P, Seidel HM, Epple R, Novel tricyclic pyrazolopyrimidines as potent and selective GPR119 agonists. Bioorganic and Medicinal Chemistry Letters. 24, 5478–5483 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Bume DD, Pitts CR, Jokhai RT, Lectka T, Direct, visible light-sensitized benzylic C─H fluorination of peptides using dibenzosuberenone: selectivity for phenylalanine-like residues. Tetrahedron. 72, 6031–6036 (2016). [Google Scholar]
- 43.Pinhey JT, Ritchie E, Taylor WC, The Chemical Constituents Of Himantandra (Galbulimima) Species. Australian Journal of Chemistry. 14, 106–134 (1961). [Google Scholar]
- 44.le Nôtre J, van Mele D, Frost CG, A new method for constructing quaternary carbon centres: Tandem rhodium-catalysed 1,4-addition/intramolecular cyclisation. Advanced Synthesis and Catalysis. 349, 432–440 (2007). [Google Scholar]
- 45.Bonati F, Minghetti G, Mercury(II) Derivatives Of Acetylacetone. Journal of Organometallic Chemistry. 22, 5–10 (1970). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.