Abstract
Population immunity depends on the dynamic levels of immunization coverage that countries achieve over time and any transmission of viruses that occur within the population that induce immunity. In the context of developing a dynamic transmission model for measles and rubella to support analyses of future immunization policy options, we assessed the model inputs required to reproduce past behavior and to provide some confidence about model performance at the national level. We reviewed the data available from the World Health Organization (WHO) and existing measles and rubella literature for evidence of historical reported routine and supplemental immunization activities and reported cases and outbreaks. We constructed model input profiles for 180 WHO member states and three other areas to support disease transmission model development and calibration. The profiles demonstrate the significant variability in immunization strategies used historically by regions and member states and the epidemiological implications of these historical choices. The profiles provide a historical perspective on measles and rubella immunization globally at the national level, and they may help immunization program managers identify existing immunity and/or knowledge gaps.
Keywords: Measles, routine immunization, rubella, supplemental immunization activities (SIAs)
1. INTRODUCTION
Individual immunity derives from infection and/or vaccination, which individuals, physicians, and health systems can track by recording cases of disease and receipt of vaccine. Population immunity represents the aggregation of all individual immunity in the population, and it changes with time.(1) Population immunity drives the dynamics of the transmission of infectious agents (e.g., sustained transmission, disease die-out, or episodic transmission following reintroduction), and it determines the burden of disease.(2) Research priorities for global measles and rubella control and eradication identified by a group of experts in 2012 included the need for disease modeling to better understand the levels of population immunity required for elimination in various settings.(3) Using models to characterize population immunity(1) represents a significant opportunity to improve efforts to manage vaccine-preventable diseases prospectively. For example, models can help policymakers evaluate the economic and health tradeoffs of potential management strategies, estimate the impact of immunization and burden of disease,(4–6) and visualize the benefits of interventions that otherwise may not get counted because surveillance systems do not detect prevented cases.(7,8) More importantly, as countries and regions make progress toward (and achieve) elimination goals by increasing population immunity, the number of cases declines significantly and approaches (or reaches) zero.
Modeling population immunity requires integrating data about population demographics, immunization, and exposure history, along with assumptions about the transmissibility of the virus, mixing, and other factors. Prior efforts to assess national-level population immunity led to the development of a simple measles strategic planning (MSP) tool intended for use by national program managers to approximate potential population immunity based on various vaccination strategies.(6) However, the MSP tool did not include a dynamic disease transmission model and a similar tool for rubella does not exist. Developing dynamic transmission models requires that they use time-varying inputs based on the best available evidence, recognizing that significant limitations may exist. Models should rely on transparent and well-founded assumptions and provide estimates consistent with historical programmatic experience, reported cases, and available serological study results.(9)
The Global Vaccine Action Plan(10) established by the World Health Organization (WHO) and its partners and approved by the World Health Assembly in 2012 set a target for measles and rubella elimination in at least five WHO regions by 2020.(10) The Measles and Rubella Initiative developed a strategic plan that indicates the need for additional global, national, and regional commitments to achieve and maintain “high levels of population immunity by providing high vaccination coverage with two doses of measles- and rubella-containing vaccines” through routine immunization (RI) and supplemental immunization activities (SIAs) to stop measles and rubella virus transmission and achieve elimination goals.(11) RI provides doses of vaccine to children as they reach the age(s) indicated by the national immunization schedule, which spreads these doses out throughout the calendar year. In contrast, SIAs involve campaigns that occur over a relatively short period of time and typically target a broad age range. RI and SIAs lead to very different epidemiological consequences, with RI providing steady inflow of immunization and SIAs boosting population immunity in pulses.
To support efforts to make an investment case for global management of measles and rubella,(12) we recognize the need to develop a dynamic model of measles and rubella viral transmission and population immunity. Although we focus on the global or regional scale, data reporting and policy interventions occur at the national level, and getting the global-level estimates correct implies the need to aggregate up from the national level. Application of the model to specific countries requires synthesis of the data used as model inputs, which led us to construct national model input profiles. The next section describes the methods we used to review the available evidence and to develop the immunization assumptions for the profiles. This work complements a separate review of the literature that characterizes the available serological data.(9)
2. METHODS
We obtained historical immunization data available from the WHO and UNICEF(13–15) and demographic data from the U.N. Population Division (UNPD)(16) to support the characterization of historical and forecasted population dynamics. We excluded from further analysis the following 14 relatively small WHO member states (out of 194 current WHO member states): Andorra, Antigua and Barbuda, Cook Islands, Dominica, Kiribati, The Marshall Islands, Monaco, Nauru, Niue, Palau, Saint Kitts and Nevis, San Marino, Seychelles, and Tuvalu. Our analysis also excludes WHO nonmember states, except that we included three population areas associated with two other large member states: Hong Kong and Macao (China) and Puerto Rico (United States) given the availability of population and immunization data for these areas. The left columns of Table I list the 180 member state profiles and three other geographic areas we characterized by their ISO code and World Bank Income Level(17) organized according to WHO region. The profiles provide data for the states as they existed in 2013, with retrospective population estimates from the UNPD.(16) The profiles account for some major changes that occurred for some states (e.g., creation of new states through independence as well as the dissolution of the Union of Soviet Socialist Republics and Yugoslavia).
Table I.
Characteristics of Modeled Areas in 2013, Including World Bank Income Level (WBIL),(17) Population,(16) Year of Routine Immunization (RI) Introduction of a Measles-Containing Vaccine First Dose (MCV1), Second Dose (MCV2), and Rubella-Containing Vaccine (RCV) in the Primary Series and/or Given Selectively to Female Adolescents; We Include Potential Future Introductions (Note a) and List the References Used to Characterize RI
| Region and Member State | ISO | WBIL | Population (Millions) | MCV1 | MCV2 | RCV Primary | RCV Female Adolescents | References(13–15,27) |
|---|---|---|---|---|---|---|---|---|
| Africa (AFR) | 28–33 | |||||||
| Algeria | DZA | UMI | 39.2 | 1985 | 1997 | 2015a | ||
| Angola | AGO | UMI | 21.5 | 1983 | 2017a | |||
| Benin | BEN | LOW | 10.3 | 1979 | 2017a | 34 | ||
| Botswana | BWA | UMI | 2.0 | 1976 | 2011 | 2016a | 35 | |
| Burkina Faso | BFA | LOW | 16.9 | 1980 | 2015a | 2015a | 36–38 | |
| Burundi | BDI | LOW | 10.2 | 1981 | 2013 | 2016a | 39 | |
| Cameroon | CMR | LMI | 22.3 | 1966 | 2015a | 40–45 | ||
| Cape Verde | CPV | LMI | 0.5 | 1985 | 2010 | 2010 | 46 | |
| Central African Republic | CAF | LOW | 4.6 | 1967 | 2017a | 47,48 | ||
| Chad | TCD | LOW | 12.8 | 1967 | 2018a | 49–53 | ||
| Comoros | COM | LOW | 0.7 | 1984 | 2016a | 2016a | ||
| Congo, Democratic Republic | COD | LOW | 67.5 | 1967 | 2017a | 54–57 | ||
| Congo, Republic | COG | LMI | 4.4 | 1967 | 2016a | |||
| Cote d’Ivoire | CIV | LMI | 20.3 | 1984 | 2017a | 58 | ||
| Equatorial Guinea | GNQ | UMIb | 0.8 | 1985 | 2018a | |||
| Eritrea | ERI | LOW | 6.3 | 1980 | 2012 | 2018a | ||
| Ethiopia | ETH | LOW | 94.1 | 1980 | 2016a | 2017a | 59–64 | |
| Gabon | GAB | UMI | 1.7 | 1985 | 2019a | |||
| Gambia, The | GMB | LOW | 1.8 | 1967 | 2012 | 2015a | 65–71 | |
| Ghana | GHA | LMI | 26.0 | 1978 | 2012 | 2013 | 72–74 | |
| Guinea | GIN | LOW | 11.7 | 1974 | 2018a | |||
| Guinea-Bissau | GNB | LOW | 1.7 | 1983 | 2018a | 75–77 | ||
| Kenya | KEN | LOW | 44.4 | 1978 | 2013 | 2015a | 78–85 | |
| Lesotho | LSO | LMI | 2.1 | 1981 | 1992 | 2016a | ||
| Liberia | LBR | LOW | 4.3 | 1974 | 2017a | 86 | ||
| Madagascar | MDG | LOW | 22.9 | 1984 | 2015a | 2016a | 87 | |
| Malawi | MWI | LOW | 16.4 | 1980 | 2015a | 2016a | 35,88,89 | |
| Mali | MLI | LOW | 15.3 | 1974 | 2017a | 90 | ||
| Mauritania | MRT | LMI | 3.9 | 1974 | 2017a | 91 | ||
| Mauritius | MUS | UMI | 1.2 | 1982 | 2003 | 2019a | ||
| Mozambique | MOZ | LOW | 25.8 | 1981 | 2015a | 2017a | 88,92–99 | |
| Namibia | NAM | UMI | 2.3 | 1975 | 2015a | 2015a | 100 | |
| Niger | NER | LOW | 17.8 | 1974 | 2018a | 101,102 | ||
| Nigeria | NGA | LMI | 173.6 | 1974 | 2017a | 103–110 | ||
| Rwanda | RWA | LOW | 11.8 | 1981 | 2014a | 2014 | 111 | |
| Sao Tome and Principe | STP | LMI | 0.2 | 1981 | 2013 | 2017a | ||
| Senegal | SEN | LMI | 14.1 | 1986 | 2014a | 2013 | 112 | |
| Sierra Leone | SLE | LOW | 6.1 | 1974 | 2015a | 2016a | 113 | |
| South Africa | ZAF | UMI | 52.8 | 1975 | 1994 | 2019a | 100,114 | |
| South Sudan | SSD | LOW | 11.3 | 1981 | 2017a | |||
| Swaziland | SWZ | LMI | 1.2 | 1981 | 1995 | 2014 | ||
| Togo | TGO | LOW | 6.8 | 1967 | 2016a | |||
| Uganda | UGA | LOW | 37.8 | 1981 | 2018a | |||
| United Republic of Tanzania | TZA | LOW | 49.3 | 1970 | 2014 | 115 | ||
| Zambia | ZMB | LMI | 14.5 | 1983 | 2016a | |||
| Zimbabwe | ZWE | LOW | 14.1 | 1981 | 2015 | |||
| Americas (AMR) | 116–119 | |||||||
| Argentina | ARG | UMI | 41.4 | 1978 | 1998 | 1997 | 120,121 | |
| Bahamas, The | BHS | HIGH | 0.4 | 1978 | 2001 | 1991 | ||
| Barbados | BRB | HIGH | 0.3 | 1977 | 1997 | 1977 | 122 | |
| Belize | BLZ | UMI | 0.3 | 1980 | 2005 | 1996 | ||
| Bolivia | BOL | LMI | 10.7 | 1979 | (2011)c | 2000 | 123 | |
| Brazil | BRA | UMI | 200.4 | 1973 | 1992 | 1992d | 124–126 | |
| Canada | CAN | HIGH | 35.2 | 1963 | 1997 | 1969 | 1971–1982 | 127 |
| Chile | CHL | HIGH | 17.6 | 1964 | 1992 | 1990 | 128–130 | |
| Colombia | COL | UMI | 48.3 | 1979 | 1997 | 1995 | 131 | |
| Costa Rica | CRI | UMI | 4.9 | 1967 | 1992 | 1974 | 132–134 | |
| Cuba | CUB | UMI | 11.3 | 1971 | 2004 | 1988 | 135,136 | |
| Dominican Republic | DOM | UMI | 10.4 | 1974 | (2004)c 2009 | 2004 | 137 | |
| Ecuador | ECU | UMI | 15.7 | 1979 | 2008 | 1997 | 138 | |
| El Salvador | SLV | LMI | 6.3 | 1979 | 2000 | 1997 | 138 | |
| Grenada | GRD | UMI | 0.1 | 1982 | 2000 | 1993 | ||
| Guatemala | GTM | LMI | 15.5 | 1980a | 2001 | 139 | ||
| Guyana | GUY | LMI | 0.8 | 1982 | 2001 | 1995 | ||
| Haiti | HTI | LOW | 10.3 | 1982 | 2008 | 140–142 | ||
| Honduras | HND | LMI | 8.1 | 1979 | 1997 | 143 | ||
| Jamaica | JAM | UMI | 2.8 | 1978 | 2002 | 1993 | 1978–2001 | 144,145 |
| Mexico | MEX | UMI | 122.3 | 1973 | 1991 | 1998 | 146,147 | |
| Nicaragua | NIC | LMI | 6.1 | 1979 | 1998 | |||
| Panama | PAN | UMI | 3.9 | 1979 | 1992 | 1992 | 148,149 | |
| Paraguay | PRY | LMI | 6.8 | 1980 | (2002)c 2004 | 2000 | 147 | |
| Peru | PER | UMI | 30.4 | 1979 | 2007 | 2003 | ||
| St. Lucia | LCA | UMI | 0.2 | 1982 | 1991 | 1986 | 150 | |
| St. Vincent and the Grenadines | VCT | UMI | 0.1 | 1982 | 1997 | 1991 | ||
| Suriname | SUR | UMI | 0.5 | 1979 | 2005 | 1993 | ||
| Trinidad and Tobago | TTO | HIGH | 1.3 | 1984 | 2001 | 1984 | 1982–2000 | 151,152 |
| United States | USA | HIGH | 320.1 | 1963 | 1989 | 1969 | 153–158 | |
| Puerto Ricoe | PRI | HIGH | 3.7 | 1963 | 1989 | 1969 | ||
| Uruguay | URY | HIGH | 3.4 | 1979 | 1992 | 1982 | ||
| Venezuela, RB | VEN | UMI | 30.4 | 1980 | 2009 | 1998 | 131,159,160 | |
| Eastern Mediterranean (EMR) | 161–163 | |||||||
| Afghanistan | AFG | LOW | 30.6 | 1978 | 2004 | 2018a | 164 | |
| Bahrain | BHR | HIGH | 1.3 | 1974 | 1985 | 1985 | 165,166 | |
| Djibouti | DJI | LMI | 0.9 | 1982 | 2011 | 2019a | ||
| Egypt, Arab Rep. | EGY | LMI | 82.1 | 1977 | 1999 | 1999 | 167,168 | |
| Iran, Islamic Rep. | IRN | UMI | 77.4 | 1967 | 1984 | 2004 | 169,170 | |
| Iraq | IRQ | UMI | 33.8 | 1980 | 1989 | 1989 | 1995–2004 | 171 |
| Jordan | JOR | UMI | 7.3 | 1979 | 1995 | 2000 | 172 | |
| Kuwait | KWT | HIGH | 3.4 | 1980 | 1985 | 1985 | 1975–2013 | 173 |
| Lebanon | LBN | UMI | 4.8 | 1982 | 1995 | 1995 | ||
| Libya | LBY | UMI | 6.2 | 1980 | 2001 | 2001 | ||
| Morocco | MAR | LMI | 33.0 | 1982 | 2003 | 1986d | 174 | |
| Oman | OMN | HIGH | 3.6 | 1980 | 1994 | 1994 | 1996–2013 | 175–177 |
| Pakistan | PAK | LMI | 182.1 | 1980 | 2009 | 2016a | ||
| Qatar | QAT | HIGH | 2.2 | 1980 | 1996 | 1992 | ||
| Saudi Arabia | SAU | HIGH | 28.8 | 1974 | 1991 | 1991 | 1978–1991 | 178 |
| Somalia | SOM | LOW | 10.5 | 1979 | 2016a | 179 | ||
| Sudan | SDN | LMI | 38.0 | 1981 | 2012 | 2015a | ||
| Syrian Arab Republic | SYR | LMI | 21.9 | 1980 | 1993 | 1999 | ||
| Tunisia | TUN | UMI | 11.0 | 1979 | 1981 | 2004 | 180,181 | |
| United Arab Emirates | ARE | HIGH | 9.3 | 1980 | 1984 | 1984 | 1998–2013 | 182 |
| Yemen, Rep. | YEM | LMI | 24.4 | 1980 | 2004 | 2014 | ||
| Europe (EUR) | 183–188 | |||||||
| Albania | ALB | UMI | 3.2 | 1990f | 2001 | 2001 | 189 | |
| Armenia | ARM | LMI | 3.0 | 1967 | 1986 | 2002 | 190 | |
| Austria | AUT | HIGH | 8.5 | 1974 | 1994 | 1994 | 1984–1994 | 191–193 |
| Azerbaijan | AZE | UMI | 9.4 | 1967 | 2001 | 2003 | 190 | |
| Belarus | BLR | UMI | 9.4 | 1967 | 1987 | 1996 | 190,194 | |
| Belgium | BEL | HIGH | 11.1 | 1975 | 1994 | 1985 | 1973–1994 | 195 |
| Bosnia and Herzegovina | BIH | UMI | 3.8 | 1969 | 1975 | 1975d | 1977–2008 | 196,197 |
| Bulgaria | BGR | UMI | 7.2 | 1969 | 1982 | 1993 | 1988–2000 | 198 |
| Croatia | HRV | HIGH | 4.3 | 1968 | 1968g | 1975 | 1975–2003 | 199,200 |
| Cyprus | CYP | HIGH | 1.1 | 1974 | (2004)c 2006 | 1989 | 1974–1989 | |
| Czech Republic | CZE | HIGH | 10.7 | 1969 | 1975 | 1986 | 1982–1997 | 201 |
| Denmark | DNK | HIGH | 5.6 | 1987 | 1987g | 1987 | 202 | |
| Estonia | EST | HIGH | 1.3 | 1967 | 1986 | 1992 | 190 | |
| Finland | FIN | HIGH | 5.4 | 1975 | 1982 | 1982 | 1975–1988 | 203–205 |
| France | FRA | HIGH | 64.3 | 1983 | 1996 | 1983 | 1970–1996 | 206–208 |
| Georgia | GEO | LMI | 4.3 | 1966 | (1988, 1989, 1993)c 1997 | 2004 | 209 | |
| Germany | DEU | HIGH | 82.7 | 1970 | 1986 | 1980 | 1975–1997 | 210–212 |
| Greece | GRC | HIGH | 11.1 | 1975 | 1991 | 1989 | 1977–1989 | 213,214 |
| Hungary | HUN | UMI | 10.0 | 1974 | 1989 | 1989 | 215,216 | |
| Iceland | ISL | HIGH | 0.3 | 1976 | 2003 | 1989 | 1977–2002 | 217 |
| Ireland | IRL | HIGH | 4.6 | 1983 | 1992 | 1988 | 1971–1988 | 218,219 |
| Israel | ISR | HIGH | 7.7 | 1967 | 1994 | 1988 | 1973–1979 | 220,221 |
| Italy | ITA | HIGH | 61.0 | 1976 | 2004 | 1990 | 1972–2008 | 222,223 |
| Kazakhstan | KAZ | UMI | 16.4 | 1967 | 1986 | 2005 | 190,224 | |
| Kyrgyz Republic | KGZ | LOW | 5.5 | 1967 | 1986 | 2002 | 190,225,226 | |
| Latvia | LVA | HIGH | 2.1 | 1967 | 1986 | 1993 | 1993–2002 | 190 |
| Lithuania | LTU | HIGH | 3.0 | 1967 | 1986 | 1992 | 1992–1996 | 190,227 |
| Luxembourg | LUX | HIGH | 0.5 | 1983 | 1994 | 1994 | 228 | |
| Macedonia, FYR | MKD | UMI | 2.1 | 1969 | 1997 | 1983 | 1975–2012 | 196,229 |
| Malta | MLT | HIGH | 0.4 | 1983 | 1991 | 1989d | 1976–1992 | 230 |
| Moldova | MDA | LMI | 3.5 | 1967 | 1986 | 2002 | 190 | |
| Montenegro | MNE | UMI | 0.6 | 1972 | 1995 | 1995 | 1977–1992 | 196,197,231 |
| Norway | NOR | HIGH | 5.0 | 1969 | 1983g | 1983 | 1978–1982 | 232 |
| Poland | POL | HIGH | 38.2 | 1974 | 1991 | 1988 | 1989–2005 | 233 |
| Portugal | PRT | HIGH | 10.6 | 1980 | 1990 | 1984 | 1984–1990 | 234,235 |
| Romania | ROU | UMI | 21.7 | 1998a | 1998 | 2002 | 236,237 | |
| Russian Federation | RUS | HIGH | 142.8 | 1967 | 1986 | 2000 | 190 | |
| Serbia | SRB | UMI | 9.5 | 1971 | 1993 | 1993 | 238 | |
| Slovak Republic | SVK | HIGH | 5.5 | 1980 | 1975 | 1986 | 1985–1992 | 201 |
| Slovenia | SVN | HIGH | 2.1 | 1968 | 1978 | 1990 | 1973–1990 | 239 |
| Spain | ESP | HIGH | 47.0 | 1978 | 1996 | 1981 | 1979–1994 | 240,241 |
| Sweden | SWE | HIGH | 9.6 | 1971 | 1982g | 1982 | 1972–1982 | 242 |
| Switzerland | CHE | HIGH | 8.1 | 1971 | 1996 | 1971 | 1974–1986 | 243–245 |
| Tajikistan | TJK | LOW | 8.2 | 1967 | 1986 | 2009 | 190 | |
| The Netherlands | NLD | HIGH | 16.8 | 1976 | 1987 | 1987 | 1974–1987 | 246–248 |
| Turkey | TUR | UMI | 74.9 | 1987 | 1997 | 2006 | 249–251 | |
| Turkmenistan | TKM | UMI | 5.2 | 1967 | 1988 | 2007 | 190 | |
| Ukraine | UKR | LMI | 45.2 | 1967 | 1986 | 2001 | 190,252 | |
| United Kingdom | GBR | HIGH | 63.1 | 1968 | 1996 | 1988 | 1970–1988 | 253–256 |
| Uzbekistan | UZB | LMI | 28.9 | 1967 | 1986 | 2006 | 190 | |
| Southeast Asia (SEAR) | 257,258 | |||||||
| Bangladesh | BGD | LOW | 156.6 | 1980 | 2012 | 2012 | 259 | |
| Bhutan | BTN | LMI | 0.8 | 1979 | 2006 | 2006 | 260 | |
| India | IND | LMI | 1252.1 | 1985 | 2010 | 2015a | 261,262 | |
| Indonesia | IDN | LMI | 249.9 | 1983 | 2003 | 2018a | 263 | |
| Korea, Dem. Rep. | PRK | LOW | 24.9 | 1980 | 2008 | 2015a | ||
| Maldives | MDV | UMI | 0.3 | 1981 | 2007 | 2007 | ||
| Myanmar | MMR | LOW | 53.3 | 1986 | 2008 | 2015a | 264 | |
| Nepal | NPL | LOW | 27.8 | 1981 | 2014a | 2014 | ||
| Sri Lanka | LKA | LMI | 21.3 | 1984 | 2001 | 1996 | 1996–2010 | 265 |
| Thailand | THA | UMI | 67.0 | 1984 | 1996 | 1996 | 1986–1998 | 266–269 |
| Timor-Leste | TLS | LMI | 1.1 | 1983 | 2015a | |||
| Western Pacific (WPR) | 270,271 | |||||||
| Australia | AUS | HIGH | 23.3 | 1975 | 1994 | 1993 | 1971–1993 | 272,273 |
| Brunei Darussalam | BRN | HIGH | 0.4 | 1970 | 1997 | 1984 | ||
| Cambodia | KHM | LOW | 15.1 | 1984 | 2012 | 2013 | ||
| China | CHN | UMI | 1385.6 | 1965 | 1965 | 2008 | 274–276 | |
| Hong Kong SAR, Chinae | HKG | HIGH | 7.2 | 1983 | 2001 | 1990 | 277–279 | |
| Macao SAR, Chinae | MAC | HIGH | 0.6 | 1983 | 2001 | 1985 | ||
| Fiji | FJI | UMI | 0.9 | 1980 | 2003 | 2003 | 1975–1994 | 280,281 |
| Japan | JPN | HIGH | 127.1 | 1966 | 2008 | 1989h | 1977–1994 | 282–284 |
| Korea, Rep. | KOR | HIGH | 49.3 | 1965 | 1997 | 1980 | 285,286 | |
| Lao PDR | LAO | LMI | 6.8 | 1981 | 2012 | 2013 | 287,288 | |
| Malaysia | MYS | UMI | 29.7 | 1983 | 2002 | 2002 | 1987–2008 | 289–293 |
| Micronesia, Fed. Sts. | FSM | LMI | 0.1 | 1987 | 1995 | 1970d | 294 | |
| Mongolia | MNG | LMI | 2.8 | 1967 | 1987 | 2009 | ||
| New Zealand | NZL | HIGH | 4.5 | 1969 | 1992 | 1970 | 1979–1991 | 295,296 |
| Papua New Guinea | PNG | LMI | 7.3 | 1983 | 1991i | 2015a | 297–299 | |
| Philippines | PHL | LMI | 98.4 | 1982 | 2009 | 2009 | ||
| Samoa | WSM | LMI | 0.2 | 1982 | (2002)c 2006 | 2004 | ||
| Singapore | SGP | HIGH | 5.4 | 1976 | 1990 | 1990 | 1976–1981a | 300,301 |
| Solomon Islands | SLB | LMI | 0.6 | 1986 | 2012 | 2012 | ||
| Tonga | TON | UMI | 0.1 | 1981 | 2002 | 2002 | ||
| Vanuatu | VUT | LMI | 0.3 | 1982 | 2014 | |||
| Vietnam | VNM | LMI | 91.7 | 1982 | 2006 | 2015a |
Assumption about future vaccine adoption.
High-income country grouped with UMI countries given vaccine use.
Schedule included second dose in year(s) in parentheses prior to the start of the continuous two-dose schedule that started in the year not in parentheses (if MCV2 introduced).
RCV phased in starting in year indicated (fully implemented in 2000 for Bolivia, 1978 for Federated States of Micronesia, 2003–2008 then 2014 for Morocco, 1981 in Bosnia and Herzegovina, 1992 in Malta).
Nonmember state.
Campaigns used to deliver vaccine during prior years.
Introduction of two-dose schedule in the indicated year led to actual delivery of a second dose to children in 1973 for Croatia, 1999 for Denmark, 1993 for Norway, and 1994 for Sweden (i.e., second dose introduced at an earlier age in the schedule).
MMR suspended due to mumps component of vaccine in 1993, which impacted delivery of rubella component.
Dose at six months counted such that dose at nine months counted as a second dose.
For each profile, we reconstructed the immunization history for measles and rubella for both RI and SIAs by year since measles vaccine became available for use in 1963. We used the data available from WHO as a base for reconstruction of national immunization histories, and we relied on papers we identified in our literature review to provide historical and other supplemental information, particularly related to immunization schedules. We started with the RI data available from the WHO-UNICEF estimates, which characterize historical vaccine coverage since 1980.(13–15) We also included data reported by some member states to WHO that extended back to the beginning of the Expanded Programme on Immunization (EPI) in 1974 (Marta Gacic-Dobo, Personal Communication, 2013). For countries established after 1980, UNPD provides retrospective population estimates. For the immunization assumptions, we assume the immunization strategy used by the parent prior member state as reported to WHO from 1974 up until the time of independence and we apportion historical estimates of cases based on the relative population sizes.
Vaccine schedules vary significantly across member states at any single point in time(18,19) and they change for individual member states over time. Consistent with current practice, we assume that vaccine options include continued use of measles- and rubella-containing vaccines (MRCVs) in high-income member states and continued use of measles with or without rubella-containing vaccines (M(R)CVs) in member states of all other income levels.(15,19) Given our focus on measles and rubella only, we characterize the historical vaccine use (i.e., schedule and coverage) according to the measles first dose (MCV1), measles second dose (MCV2), and rubella dose (RCV) given in the primary RI series or selectively to adolescent and adult females. We report the timing of vaccine introduction for MCV1, MCV2, and RCV (primary and/or selective for adolescent and adult females). Thus, although the WHO rubella vaccine position paper revised in 2011 recommends vaccination of both sexes, because unvaccinated males contribute to sustained rubella virus transmission(20) we sought to capture all historical practices.
To supplement the information available from WHO, we searched the literature indexed in PubMed and the Science Citation Index (ISI Web of Knowledge) up through June 10, 2014 for papers in English to identify additional information about national immunization histories. We searched using the key words “measles” or “rubella” and “(schedule or routine or EPI or supplement*)” and the names of continents, regions, and individual member states. We screened the records for relevance to national or regional immunization practices using indexed information or the full text of articles with insufficient information in the index. For each member state, we capture the RI vaccine used as M, R, or MR to indicate the use of a single antigen or use of both antigens of interest in a combination vaccine and ignoring any additional components (e.g., mumps in MMR).
In 2014, WHO-UNICEF provided estimates of MCV2 coverage for countries that reported MCV2 national coverage estimates for the years 2000–2013. In general, we used the WHO-UNICEF coverage estimates, although in a few instances we modified these based on national data. For example, the WHO-UNICEF MCV1 coverage estimates for the United States for 1980–1993 implied significantly higher coverage nationally than reported in the preceding or following years, and we could not identify the source for the estimates. Based on information from national immunization program experts, who noted the importance of the school immunization requirements and provided unpublished estimates of vaccine procurement data, we used judgment for the values in the profile. We interpolated for missing data and provided estimates for all second doses for those member states that do not report MCV2 coverage to WHO (i.e., the Czech Republic, Finland, Ireland, Italy, and the United States). For historical selective rubella immunization, which primarily occurred in Europe and Australia, we estimated coverage using a single value for the duration based on any national information that we found, although these uncertain values may require adjustment depending on behavior of the transmission model.
We identified some overlap in activities between the RI and SIA data we extracted for some doses, which we reconciled as either delivered through RI or as an SIA. For example, some member states implemented booster doses for children that included delivery in schools (e.g., Cyprus, Japan). If these occurred throughout a school year, we included these in RI, but if they occurred during a shorter period of time then we treated them as SIAs. In the absence of WHO-UNICEF estimates for RCVs, we generally assume the same coverage for measles and rubella for those doses in the RI schedule that include both vaccines. However, for some areas that phased in rubella immunization, we assumed different coverage levels for M and R during the phase-in period (e.g., during the first year or first few years), which leads to separate points (or series) in the RI profile for M and R until the time of complete phase-in. We also characterized the historical SIA activities, starting with a database maintained by the WHO for measles SIAs(21) and then adding information obtained from WHO(18) related to SIAs prior to 2000 and SIAs using rubella-containing vaccine and using information we found in the literature. For each SIA, we sought to characterize the timing (i.e., start and end date), target population (i.e., age, sex, and/or risk groups), antigens included in the vaccine used (i.e., M, R, or MR, ignoring any other antigens like mumps), estimated coverage, and other characteristics that might impact the interpretation of the data relevant for use in transmission modeling. We classified SIAs as national (n) or subnational (s) to ensure appropriate adjustment of SIA coverage estimates to account for the fraction of the national population targeted by the SIA, similar to the methods used in models to account for subnational polio SIAs.(2)
The information available for comparison to transmission model output comes in two primary forms: reported case series of associated disease and deaths (considering the age distribution when available), which we characterize here, and the results of serological surveys that provide a snapshot of the dynamic population immunity at the point in time of data collection, which we characterize separately.(9) The WHO summarizes reported annual cases for measles and rubella,(15) with data available for some member states back to 1974 for measles and back to 1998 for rubella.(18) We synthesized the reported health outcome data and supplemented them with data from the literature that summarized reported cases for earlier dates when available, and we particularly searched for information about the timing of large outbreaks that occurred prior to case reporting to WHO for the two different diseases.(15) Surveillance systems typically miss cases, and consequently reported cases most likely underestimate actual cases. Relatively recent modeling efforts provided retrospective estimates for measles,(5) which we considered for purposes of comparison.
3. RESULTS
Table I summarizes the evidence that we identified for the different member states organized by region. Since not all member states currently include rubella immunization in their existing national immunization schedules,(19) we include our current assumptions (noted with an a) about when member states yet to introduce rubella vaccine might do so in response to the current Gavi funding opportunity and/or regional goals to control or eliminate rubella.(22) For the full profile, we characterize the RI schedule, antigens included in the vaccine (i.e., M, R, or MR), and estimated coverage by year historically for each member state. Table I summarizes the evidence base we used as a basis for our assessments.
Table II summarizes the historical SIAs as national (n) or subnational (s) according to the year in which the SIAs started for the data we identified. Blank lines in Table II generally indicate no SIAs performed, particularly for high-income countries, but the data remain limited, probably miss many (most) SIAs conducted for outbreak response, and may include planned SIAs that did not actually occur or exclude SIAs that occurred but we did not find recorded. For areas with more than one SIA during a year (i.e., multiple subnational SIAs), we indicated the total number of separate SIAs. In some cases, we found information in the literature that indicated an SIA strategy instead of RI for the delivery of child immunization during the early part of the immunization program (e.g., Albania, Romania). In such cases, we used the WHO-UNICEF estimated coverage for SIAs during the affected years and we began RI once this approach ended. This change led to later implied dates of RI starting for MCV1 in Table I for some member states than implied by assuming that the WHO-UNICEF coverage estimates all reflect delivery in RI.
Table II.
Assumptions for Supplemental Immunization Activities (SIAs) for Areas with Any Historical SIAs and Associated References Characterized by the Antigen(s) Used (i.e., M, R, or MR, the Latter Two Bolded for Visibility), the SIA Start Year [Number of SIAs Started that Year if Greater than 1], and as National (n) or Subnational (s)
| Region and Member State | ISO | SIA Antigen Used: Year[Number Started that Year if Greater than 1] and Scope (n or s) | Refs.(21) |
|---|---|---|---|
| Africa (AFR) | 30,31,33,302,303 | ||
| Algeria | DZA | M:1996n, 2003n, 2007n, 2011s | |
| Angola | AGO | M: 1997s, 1999s, 2003n, 2006n, 2009n, 2011n, 2014n; MR: 2017na, 2020na | |
| Benin | BEN | M: 1967s, 1997s, 1998s, 1999s, 2001s, 2003s, 2005n, 2008n, 2011n, 2014n; MR: 2017na, 2020na | 28 |
| Botswana | BWA | M: 1997s, 1998s, 2001n, 2005n, 2009n, 2013n; MR: 2016na, 2019na | 35 |
| Burkina Faso | BFA | M: 1967s, 1998s, 1999n, 2001n, 2004n, 2007n, 2009s, 2011s, 2012[2]s; MR: 2014n 2018na, 2020na | 28,304–306 |
| Burundi | BDI | M: 1999s, 2001s, 2002s, 2003s, 2004n, 2005n, 2006n, 2009n, 2010[2]s, 2011s, 2012n; 2013n; MR: 2015na, 2019na | |
| Cameroon | CMR | M: 1969s, 1999s, 2001[2]s, 2002n, 2006s, 2007s, 2009n, 2010[2]s, 2011s, 2012n; MR: 2015na, 2018na | 28,40–45,307,308 |
| Cape Verde | CPV | M: 1998n, 2005n, 2009n; MR: 2013n, 2017na | |
| Central African Republic | CAF | M: 1967s, 1998s, 2005s, 2006s, 2008n, 2011n, 2013[4]s; MR: 2017na, 2019na | 28,47,48 |
| Chad | TCD | M: 1967s, 1997s, 1999s, 2005[2]s, 2006s, 2008n, 2009[3]s, 2011s, 2012n, 2014[2]n, 2016na; MR: 2018na, 2020na | 28,49–51,309 |
| Comoros | COM | M: 2003s, 2005s, 2006s, 2007n, 2010s, 2013n; MR: 2016na, 2019na | |
| Congo, Democratic Republic | COD | M: 1996s, 1997s, 1999s, 2000s, 2002s, 2003s, 2004s, 2005s, 2006[2]s, 2007n, 2008s, 2009s, 2010s, 2011[5]s, 2012[15]s, 2013[3]s, 2014[5]s; MR: 2016na, 2019na | 58 |
| Congo, Republic | COG | M: 1967s, 1998n, 2004[2]s, 2007n, 2010n, 2011[2]s, 2012n, 2013n, 2015na, 2016na; MR: 2017na, 2018na, 2019na, 2020na | 28,54,56,57 |
| Cote d’Ivoire | CIV | M: 1967s, 2003[2]s, 2005n, 2008n, 2011n, 2014n; MR: 2017na, 2020na | 28 |
| Equatorial Guinea | GNQ | M: 2003[2]s, 2005n, 2009n, 2011n, 2012n; MR: 2016na, 2018na, 2020na | |
| Eritrea | ERI | M: 1997s, 1998s, 1999s, 2000[2]s, 2001s, 2003n, 2004s, 2006s, 2009n, 2012s; MR: 2015na, 2019na | |
| Ethiopia | ETH | M: 1998s, 1999s, 2000[2]s, 2001s, 2002s, 2003[2]s, 2004s, 2005[2]s, 2006s, 2007s, 2008s, 2009[4]s, 2010[2]s, 2011[2]s, 2013n; MR: 2016na, 2019na | 59,60,63,64 |
| Gabon | GAB | M: 1967s, 2004n, 2007n, 2012n, 2013[2]s; MR: 2016na, 2018na, 2020na | 28 |
| Gambia, The | GMB | M: 1966s, 1967s, 1968s, 2003n, 2007n, 2011n; MR: 2015na, 2019na | 28,65–71 |
| Ghana | GHA | M: 1967s, 2001s, 2002s, 2006n, 2010n; MR: 2013n, 2017na | 28,72–74,310 |
| Guinea | GIN | M: 1967s, 2002s, 2003s, 2006n, 2009n, 2012n, 2015na; MR: 2018na | 28 |
| Guinea-Bissau | GNB | M: 1999s, 2003s, 2006n, 2009n, 2012n, 2015na; MR: 2018na | 75–77 |
| Kenya | KEN | M: 1994s, 1999s, 2000s, 2002n, 2004s, 2005s, 2006[2]s, 2009n, 2012n; MR: 2015na, 2018na | 78–85 |
| Lesotho | LSO | M: 1999s, 2000n, 2003n, 2007n, 2010n, 2013n; MR: 2016na, 2019na | |
| Liberia | LBR | M: 1967s, 2004s, 2007n, 2008s, 2010n, 2011n; MR: 2017na, 2020na | 28,86 |
| Madagascar | MDG | M: 1998[2]s, 2004n, 2007n, 2010n, 2013n; MR: 2016na, 2019na | 87 |
| Malawi | MWI | M: 1998n, 1999s, 2002n, 2005n, 2008n, 2010n, 2013n; MR: 2016na, 2019na | 35,88,89 |
| Mali | MLI | M: 1967s, 1998s, 1999s, 2001n, 2004n, 2007n, 2011n, 2012[2]s; MR: 2017na, 2020na | 28,90 |
| Mauritania | MRT | M: 1967s, 1995n, 1997n, 1998[2]s, 1999n, 2000n, 2004n, 2008s, 2011[2]s, 2012s, 2014n; MR: 2017na, 2020na | 28,91 |
| Mauritius | MUS | M: 1998s; MR: 2003s | |
| Mozambique | MOZ | M: 1997s, 1998s, 1999s, 2003[2]s, 2005n, 2008n, 2011n, 2013n; MR: 2016na, 2019na | 88,92–99 |
| Namibia | NAM | M: 1997[2]s, 1998n, 2000n, 2003n, 2006s, 2009n, 2012n; MR: 2015na, 2018na | 100 |
| Niger | NER | M: 1967s, 1997s, 1998s, 1999s, 2004s, 2005s, 2008n, 2010s, 2012n, 2015na; MR: 2018na | 28,101,102 |
| Nigeria | NGA | M: 1967s, 1999s, 2005s, 2006s, 2007[2]s, 2008n, 2011n, 2013[3]s, 2014s, 2015na; MR: 2017na, 2019na | 28,103–110,311 |
| Rwanda | RWA | M: 1999s, 2003n, 2006n, 2009n; MR: 2013n, 2017na | 111 |
| Sao Tome and Principe | STP | M: 1999n, 2007n, 2012n; MR: 2015n, 2018na | |
| Senegal | SEN | M: 1967s, 2003n, 2006n, 2010[2]n; MR: 2013na, 2017na | 28,112,312 |
| Sierra Leone | SLE | M: 1967s, 2003n, 2006n, 2009n, 2012n; MR: 2015na, 2019na | 28,113 |
| South Africa | ZAF | M: 1996[2]s, 1997[2]s, 2000s, 2004n, 2005s, 2007n, 2009[3]s, 2010n, 2013n; MR: 2016na, 2019na | 100,114 |
| South Sudan | SSD | M: 1998s, 1999s, 2003s, 2004[4]s, 2005[3]s, 2006[2]s, 2007[2]s, 2008[3]s, 2010[2]s, 2011[2]n, 2012n, 2014n, 2014s, 2016na; MR: 2018na | |
| Swaziland | SWZ | M: 1998n, 1999n, 2002n, 2006n, 2009n, 2010n, 2013n; MR: 2016na, 2019na | |
| Togo | TGO | M: 1967s, 2001n, 2004n, 2008n, 2010n, 2013n; MR: 2016na, 2019na | 28 |
| Uganda | UGA | M: 1997s, 1998[2]s, 1999s, 2000[2]s, 2001s, 2003n, 2005s, 2006n, 2009n, 2012n, 2015na; MR: 2018na | |
| United Republic of Tanzania | TZA | M: 1999[2]s, 2000s, 2001s, 2002s, 2005n, 2006n, 2008s, 2011n; MR: 2014n, 2018na | 115 |
| Zambia | ZMB | M: 1999s, 2002s, 2003s, 2007n, 2010n, 2012n 2015na; MR: 2018na, 2019na | |
| Zimbabwe | ZWE | M: 1997s, 1998n, 2002n, 2003s, 2006n, 2009n, 2010n, 2012n; MR: 2015na, 2018na | |
| Americas (AMR) | 116–119 | ||
| Argentina | ARG | M: 1993n, 1998n; MR: 2002n, 2005n, 2006n, 2008n, 2009n 2014n | 120,121 |
| Bahamas, The | BHS | M: 1991n; MR: 1997[2]s, 2003n, 2006n, 2007n | |
| Barbados | BRB | M: 1991n, 1996n; MR: 2004n, 2007n | 122 |
| Belize | BLZ | M: 1991n, 1993n, 1995n; MR: 2000n, 2004n, 2008n, 2010n | |
| Bolivia | BOL | M: 1994n, 1998n, 1999s, 2000s, 2002s; MR: 2003n, 2004[2]s, 2005s, 2006n, 2007s; 2015na | 123 |
| Brazil | BRA | M: 1992n, 1995n, 1997[2]s, 1998s, 2000n; R: 2000s; MR: 2001s, 2002s, 2004n, 2008n, 2011n, 2014n | 124–126 |
| Canada | CAN | M: 1996n | 127 |
| Chile | CHL | M: 1992n, 1996n, 1999n, 2001n; MR: 2005n, 2007n | 128–130 |
| Colombia | COL | M: 1993n, 1995n, 2002n, 2003s; MR: 2005s, 2006[2]s, 2010n, 2011s | 131 |
| Costa Rica | CRI | M: 1967n; MR: 1993n, 1998n, 1999s, 2001n, 2002n | 132–134 |
| Cuba | CUB | M: 1993[2]s, 2002n; R: 1982n; MR: 1986n, 2007n | 135,136 |
| Dominican Republic | DOM | M: 1993n, 1998n, 1999[2]s, 2001n, 2003n; MR: 2004[2]n, 2006n, 2010n | 137,139 |
| Ecuador | ECU | M: 1994n, 1998n, 2002n; MR: 2003n, 2004n, 2008n, 2009n, 2011n, 2012n | 138 |
| El Salvador | SLV | M: 1993n, 1996n, 1997s, 2001n, MR: 2004n, 2005n, 2006n, 2007n, 2008n, 2012[2]s | 138 |
| Grenada | GRD | M: 1991n, 1996n, 2001n | |
| Guatemala | GTM | M: 1972–1979bn, 1993n, 1996n; MR: 2002n, 2003n, 2007n, 2008n, 2013n | 139 |
| Guyana | GUY | M: 1991n, 1996n, 2003n; MR: 2000n, 2007n | 140–142 |
| Haiti | HTI | M: 1994n, 1999n, 2010s; MR: 2001n, 2002n, 2003n, 2004n, 2006n, 2007s, 2008s, 2012n, 2013[3]s, 2015na, 2018na | 142 |
| Honduras | HND | M: 1993n, 1996n, 2000n; MR: 2002n, 2003n, 2004n, 2008n, 2012[2]s | 143 |
| Jamaica | JAM | M: 1991n; MR: 1995n, 2000n, 2003s, 2004n, 2005n, 2006n, 2007n | 144,145 |
| Mexico | MEX | M: 1993n, 1998n; MR: 2002[2]s, 2004n, 2005n, 2006[3]s, 2008[2]s, 2010n, 2011n, 2012n, 2013n | 146,147 |
| Nicaragua | NIC | M: 1993n, 1996n, 1998n; MR: 2000n, 2002n, 2003n, 2004n, 2005[2]s, 2006n, 2008n, 2011n, 2012n | |
| Panama | PAN | M: 1993n, 1996n, 2000n; MR: 2003n, 2006n, 2008n, 2010[2]s | 148,149 |
| Paraguay | PRY | M: 1995n, 1998n; MR: 2003n, 2005n, 2009[2]s, 2013s, 2014n | 147 |
| Peru | PER | M: 1992n, 1995n, 1997n, 2001n; MR: 2003[2]s, 2004n, 2006n, 2011n | |
| St. Lucia | LCA | M: 1991n, 1996n | 150 |
| St. Vincent and the Grenadines | VCT | M: 1991n, 1995n | |
| Suriname | SUR | M: 1991n, 1997n; MR: 2005n, 2007n, 2010n | |
| Trinidad and Tobago | TTO | M: 1991n, 1997n | 151,152 |
| United States | USA | R:1970n | |
| Puerto Ricoc | |||
| Uruguay | URY | M: 1994n, 1998[3]s; MR: 2003n, 2008n | |
| Venezuela, RB | VEN | M: 1994n, 1998n; MR: 2001[2]s, 2002n, 2003[3]s, 2004s, 2005n, 2006n, 2007n, 2009[2]s, 2013n, 2014n | 131,159,160 |
| Eastern Mediterranean (EMR) | 161–163 | ||
| Afghanistan | AFG | M: 1994s, 1995[2]s, 1996n, 1999s, 2002n, 2003n, 2006[2]s, 2007s, 2009n, 2011[3]s, 2012[2]s, 2013n, 2014[2]s, 2015na; MR: 2018na | 164 |
| Bahrain | BHR | MR: 1998n, 1999n | 165,166 |
| Djibouti | DJI | M: 2002s, 2003s, 2004s, 2005n, 2007s, 2008n, 2011n, 2012[2]s; MR: 2015na, 2019na | |
| Egypt, Arab Rep. | EGY | M: 1998s, 2000s, 2002n; MR: 2008s, 2009s | 167,168 |
| Iran, Islamic Rep. | IRN | M: 1996s, 1999s; MR: 2003n, 2007[2]s, 2010s, 2012s | 169,170 |
| Iraq | IRQ | 1995n, 2002n, 2009[5]s, 2010[2]s, 2011s, 2012s, 2013; MR: 2004[3]s, 2005[2]s, 2006s, 2007n, 2008[5]s | 171 |
| Jordan | JOR | M: 1997n, 1999n, 2003s; MR: 2004s, 2005n, 2012s, 2013[2]s | 172 |
| Kuwait | KWT | M: 1994n; MR: 1998n, 2010s | 173 |
| Lebanon | LBN | M: 1978n, 1990n, 1994n; MR: 2000n, 2008s, 2013s | |
| Libya | LBY | MR: 2005n, 2008[2]s, 2009[2]s | |
| Morocco | MAR | MR: 2008[2]s, 2013n, 2016na | 174 |
| Oman | OMN | MR: 1994n | 175–177 |
| Pakistan | PAK | M: 2005s, 2007[4]s, 2008s, 2010[3]s, 2011[5]s, 2012n, 2013[2]s, 2014[3]s; MR: 2017na, 2020na | |
| Qatar | QAT | MR: 2000n, 2005n, 2007n | |
| Saudi Arabia | SAU | MR: 1998n, 2000s, 2004n, 2007n, 2011[2]n | 178 |
| Somalia | SOM | M: 2002s, 2005s, 2006s, 2007s, 2008[2]s, 2009[3]s, 2010n, 2011[8]s, 2012[3]s, 2013s, 2014s; MR: 2016na, 2018na, 2020na | 179 |
| Sudan | SDN | M: 1998s, 1999s, 2003s, 2004[4]s, 2005[3]s, 2006[2]s, 2007[2]s, 2008[3]s, 2010[2]s, 2011[5]s, 2012s, 2013s; MR: 2016na, 2019na | |
| Syrian Arab Republic | SYR | M: 2003s, MR: 1998n, 2004s, 2005s, 2007[2]s, 2008n, 2009s, 2012n, 2013[2]s, 2014n | |
| Tunisia | TUN | M: 1998n, 2001n, 2002n; MR: 2005 | 180,181 |
| United Arab Emirates | ARE | MR: 1998n, 1999n, 2001n | 182 |
| Yemen, Rep. | YEM | M: 2001s, 2004s, 2005s, 2006s, 2007s, 2009[2]s, 2010s, 2011s, 2012n, 2013s; MR: 2014n, 2017na, 2020na | |
| Europe (EUR) | 183–188,313,314 | ||
| Albania | ALB | M: 1970–1989bn; MR: 2000n, 2002n, 2003 | 189 |
| Armenia | ARM | M: 2008n; MR: 2007 | 190 |
| Austria | AUT | ||
| Azerbaijan | AZE | M: 2004n; MR: 2006[2]s, 2014n | 190 |
| Belarus | BLR | M: 2012n; R: 2005s, 2006n | 190,194 |
| Belgium | BEL | ||
| Bosnia and Herzegovina | BIH | ||
| Bulgaria | BGR | MR: 2009n, 2010s | 198 |
| Croatia | HRV | ||
| Cyprus | CYP | ||
| Czech Republic | CZE | ||
| Denmark | DNK | MR: 2012n | 202 |
| Estonia | EST | ||
| Finland | FIN | ||
| France | FRA | ||
| Georgia | GEO | M: 2004s; MR: M: 2004[4]s, 2005n, 2008[2]s, 2013n | 209 |
| Germany | DEU | ||
| Greece | GRC | ||
| Hungary | HUN | M: 1969n, 1970n, 1971n, 1973[2] n, 1974[2]n | 216 |
| Iceland | ISL | ||
| Ireland | IRL | MR: 2009n | 218,219 |
| Israel | ISR | ||
| Italy | ITA | MR: 2007s | 222,223 |
| Kazakhstan | KAZ | MR: 2005n | 190,224 |
| Kyrgyz Republic | KGZ | MR: 2001n, 2002s | 190,225,226 |
| Latvia | LVA | ||
| Lithuania | LTU | ||
| Luxembourg | LUX | ||
| Macedonia, FYR | MKD | MR: 2009n | 196,229 |
| Malta | MLT | ||
| Moldova | MDA | MR: 2002[2]s | 190 |
| Montenegro | MNE | ||
| Netherlands | NLD | ||
| Norway | NOR | ||
| Poland | POL | ||
| Portugal | PRT | ||
| Romania | ROU | M: 1979–1989dn; MR: 1998n, 2010n, 2011[2]s | 236,237 |
| Russian Federation | RUS | M: 2004s, 2005n, 2008n, 2010s | 190 |
| Serbia | SRB | MR: 2003s, 2004[2]s, 2005s | 238 |
| Slovak Republic | SVK | M: 2002n; MR: 2003s | 201 |
| Slovenia | SVN | ||
| Spain | ESP | ||
| Sweden | SWE | ||
| Switzerland | CHE | ||
| Tajikistan | TJK | M: 2004n; MR: 2009n | 190 |
| Turkey | TUR | M: 1985n, 2003[2]s, 2004s, 2005s | 249–251 |
| Turkmenistan | TKM | MR: 2007n | 190 |
| Ukraine | UKR | MR: 2008n | 190,252 |
| United Kingdom | GBR | M: 1994n; MR:1994n, 2004s | 253–256 |
| Uzbekistan | UZB | MR: 2006s, 2007s, 2011n | 257,258 |
| Southeast Asia (SEAR) | SEAR | ||
| Bangladesh | BGD | M: 1995s, 1998s, 1999s, 2001s, 2005s, 2006s, 2010n; MR: 2014n, 2018na | 259 |
| Bhutan | BTN | M: 1995n, 1996s, 2000s; MR: 2006n | 260 |
| India | IND | M: 1995s, 1996s, 1997s, 1999s, 2000s, 2001s, 2010s, 2011[2]s, 2012[2]s, 2013n | 261,262 |
| Indonesia | IDN | M: 1997s, 2000[2]s, 2002[2]s, 2003s, 2004s, 2005[2]s, 2006[3]s, 2007[4]s, 2008s, 2009[3]s, 2010s, 2011s; MR: 2016na, 2017na, 2018na | 263 |
| Korea, Dem. Rep. | PRK | M: 1995s, 1999n, 2007n | |
| Maldives | MDV | M: 1995n, 1996n, 1997s; MR: 2005n, 2006n, 2007n | |
| Myanmar | MMR | M: 1995s, 1996s, 1997[2]s 2002s, 2003s, 2004s, 2007n, 2012n; MR: 2015na | 264 |
| Nepal | NPL | M: 1995n, 2004n, 2005[2]s, 2008[3]s; MR: 2012[3]s, 2015na, 2018na | |
| Sri Lanka | LKA | M: 2003s, 2013s; MR: 2004n | 265 |
| Thailand | THA | ||
| Timor-Leste | TLS | M: 2003n, 2006n, 2009n, 2011n; MR: 2015na, 2020na | |
| Western Pacific (WPR) | 270,271 | ||
| Australia | AUS | M: 1998n | 272,273 |
| Brunei Darussalam | BRN | ||
| Cambodia | KHM | M: 2000n, 2001[2]s, 2002n, 2003n, 2004n, 2005n, 2007n, 2011[2]s; MR: 2013n, 2017na | |
| China | CHN | M: 2003s, 2004s, 2005[4]s, 2006s, 2007s, 2008[2]s, 2009[2]s, 2010n; MR: 1998s, 1999s | 274–276 |
| Hong Kong SAR, Chinac | HKG | M: 1997s; MR: 2002n | 315 |
| Macao SAR, Chinac | MAC | MR: 20011n | |
| Fiji | FJI | M: 1997n, 2001n; MR: 2006n, 2011 | 280,281 |
| Japan | JPN | ||
| Korea, Rep. | KOR | M: 2001n, 2004n, 2005n; MR: 2006n, 2007n, 2008n, 2009n | 285,286 |
| Lao PDR | LAO | M: 1998s, 2000s, 2001s, 2007n; MR: 2011s, 2012s, 2014n, 2017na, 2020na | 287,288 |
| Malaysia | MYS | M: 2004n, 2005n; R: 1989n; MR: 2012[3]n, 2012[3]s | 289–293 |
| Micronesia, Fed. Sts. | FSM | MR: 2004s, 2010s: 2011s, 2013s, 2014[3]s | 294 |
| Mongolia | MNG | M: 1994n, 1996n, 2000n, 2001s, 2007n; R: 2009s; MR: 2012n | |
| New Zealand | NZL | MR: 1997n, 1998n | 295,296 |
| Papua New Guinea | PNG | M: 1997n, 2003s, 2004s, 2005s, 2009s, 2010n, 2012n; MR: 2015na, 2018na | 297–299 |
| Philippines | PHL | M: 1998n, 2002s, 2004n, 2007n, 2009s, 2010s, 2013[2]s, 2014s; MR: 2011n, 2014na, 2018na | |
| Samoa | WSM | M: 1998n, 2001n; MR: 2003n, 2008n, 2009n | |
| Singapore | SGP | ||
| Solomon Islands | SLB | M: 1997n, 1998n, 2001n, 2006n, 2009n; MR: 2012n, 2014, 2018na | |
| Tonga | TON | M: 1998n, 2001n | |
| Vanuatu | VUT | M: 1998n, 2001n, 2006n, 2009n; MR: 2013n, 2014n | |
| Vietnam | VNM | M: 1993n, 1994[2]s, 1995[2]s, 1996[2]s, 1997[2]s, 1998[2]s, 1999s, 2001s, 2002s, 2003s, 2004s, 2005s, 2007s, 2008s, 2010n, 2013s; MR: 2014[2]n |
Assumption about future SIAs.
Annually for all of the years listed (between and including the years listed).
WHO nonmember.
Two times per year for all of the years listed (between and including the years listed), SIAs for years 1994–1998 included booster dose given to school-age children during one of the two SIAs.
Figs. 1–8 show the full profiles for the United States, the Netherlands, Japan, Oman, Vietnam, Kenya, Ethiopia, and Haiti, respectively, as examples. Part (a) in each profile shows the historical RI coverage estimates by year and schedule. The RI schedule includes the age and the antigens in the vaccine (i.e., M for measles vaccine, R for rubella vaccine, and MR for any combination vaccine including both antigens). We use colors (visible in the on-line version) to show different vaccines and/or schedules. If introduction of the vaccine occurred after January in a year, then we estimated the coverage for the full year adjusting for the fraction of the year with introduction. Thus, when the country switched from a MCV to a MRCV after January of a year, we showed the M and R coverage for the year separately, which makes the R value appear as a single point (e.g., Fig. 2a or 4a). The SIAs for each country appear in Table II. Parts (b) and (c) of the profile show the reported measles and rubella cases, respectively, and prior model estimates (if available) for comparison for relevant time periods.(5) We provide access to the full profiles for all of the 183 modeled areas on the Kid Risk website.(23)
Fig. 1.
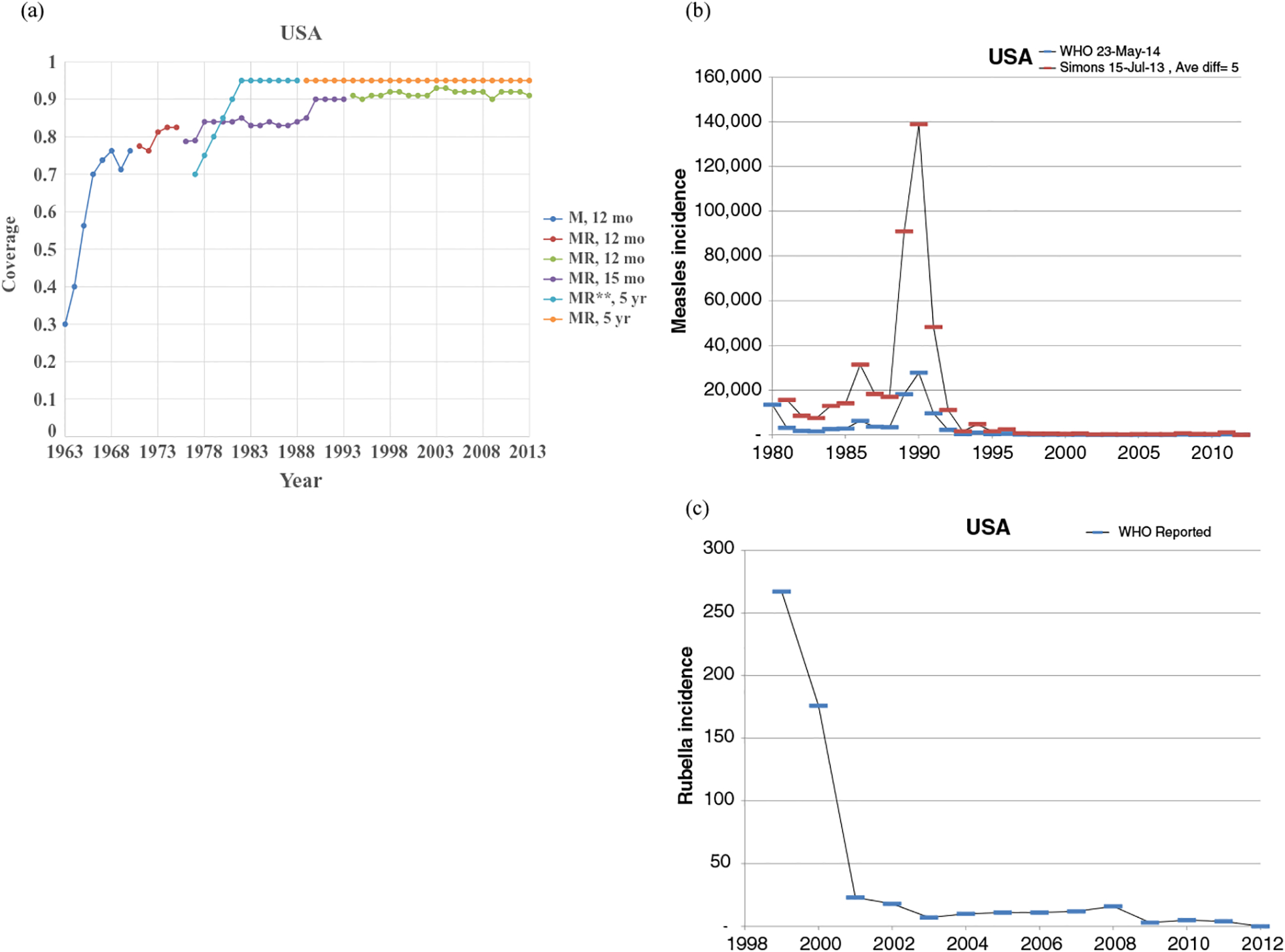
Profile for the United States.
Fig. 8.
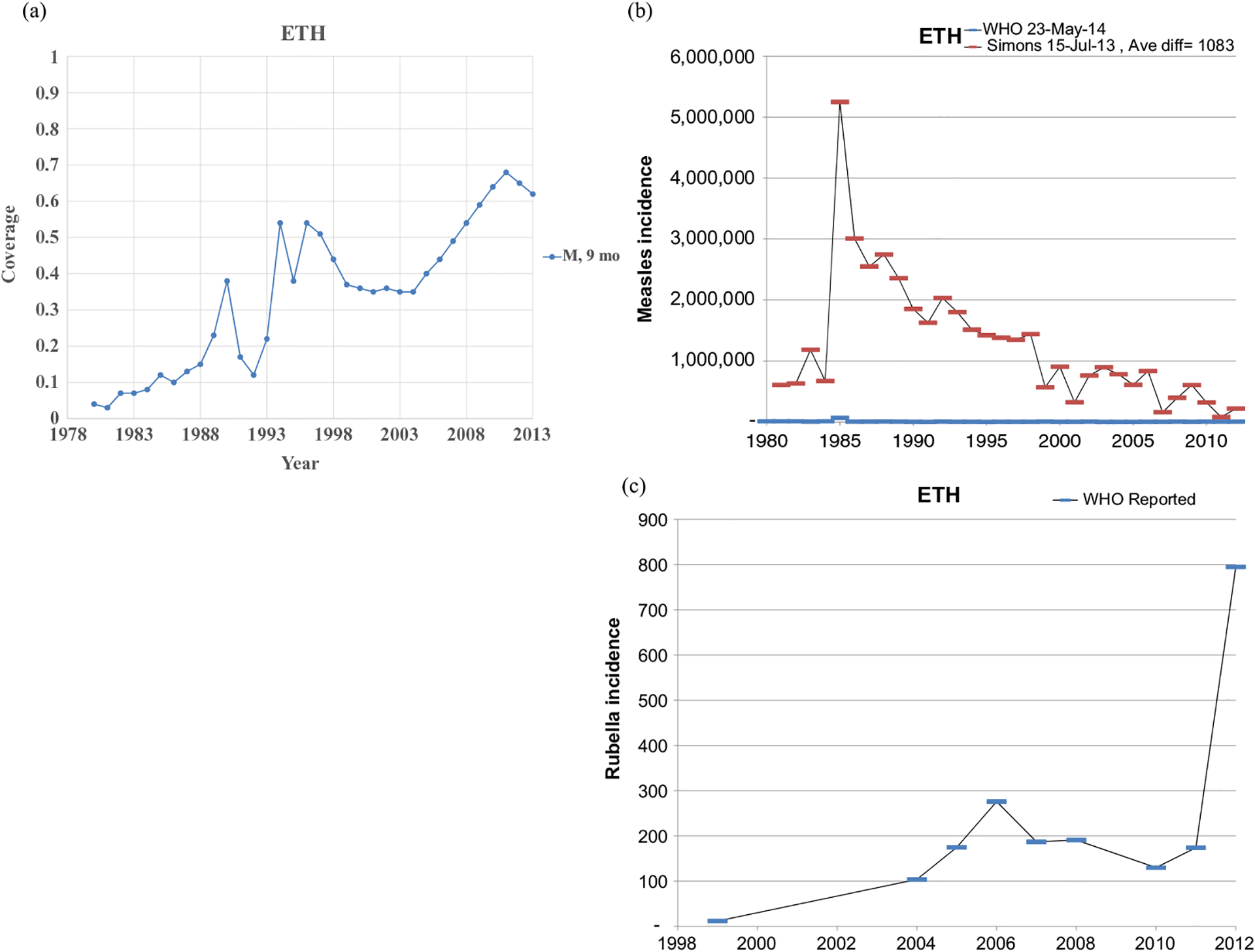
Profile for Ethiopia.
Fig. 2.
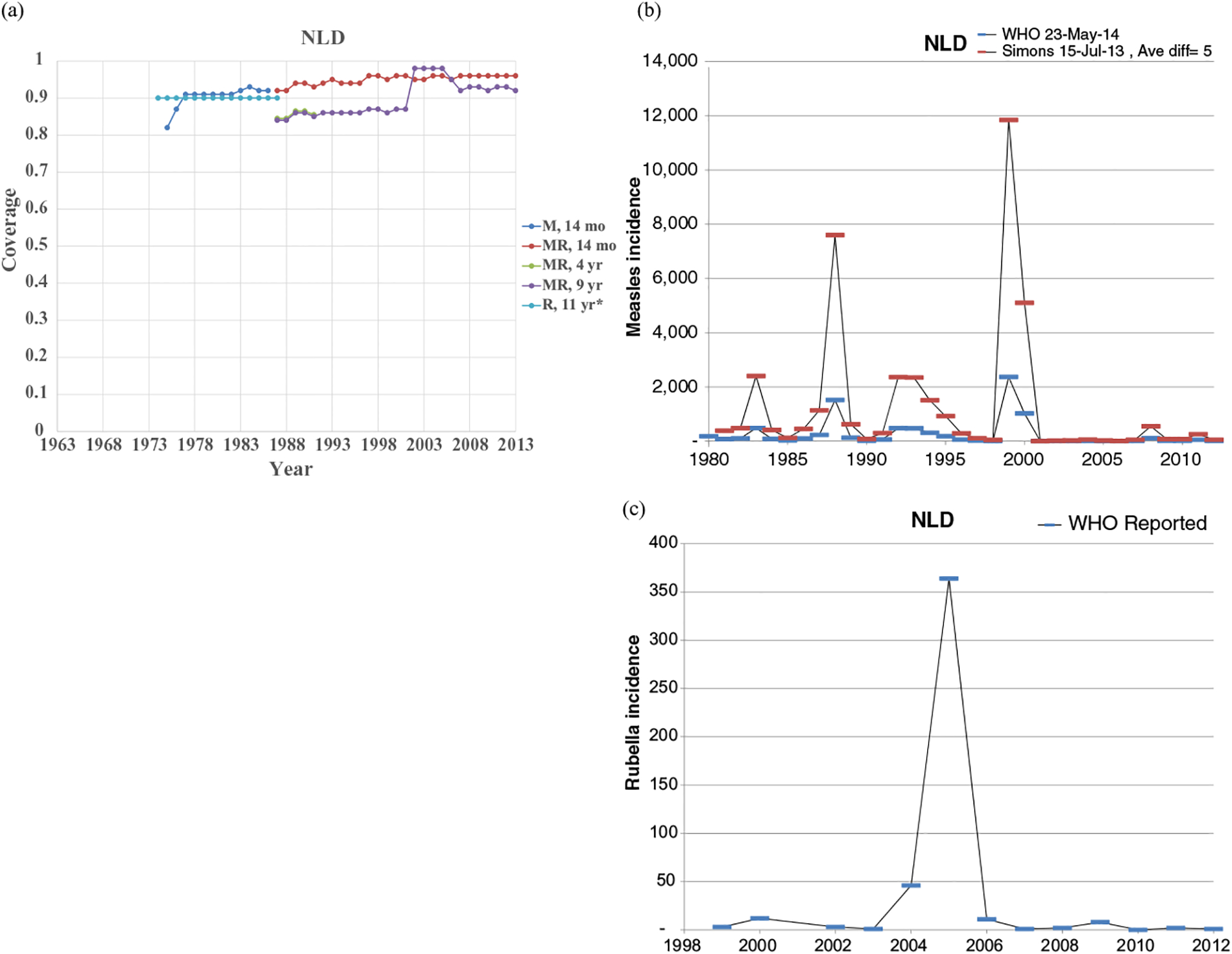
Profile for the Netherlands.
Fig. 4.
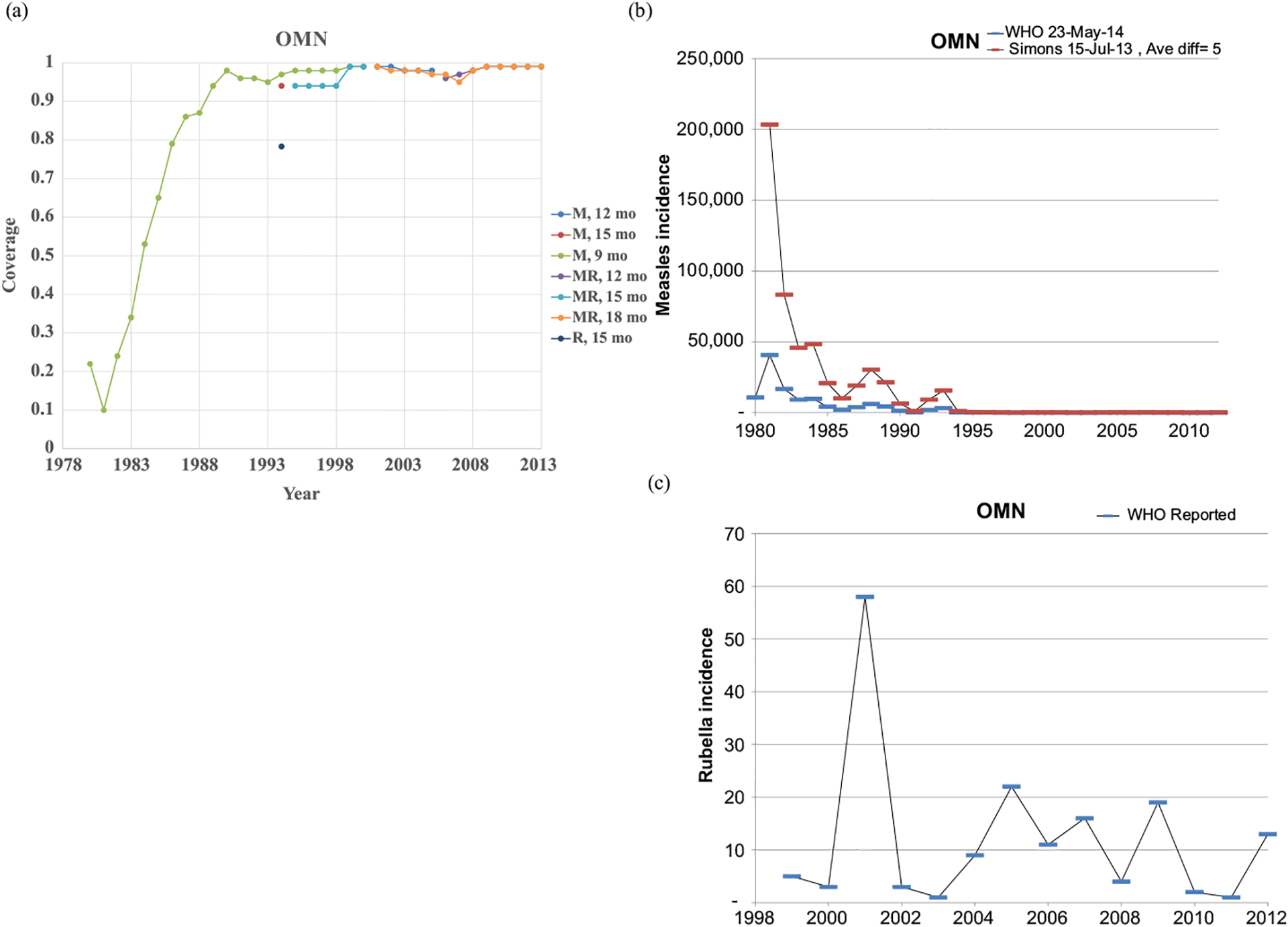
Profile for Oman.
The profiles show a wide range of immunization schedules and coverage that evolved over time, and highly variable epidemiological experience with measles and rubella cases. Comparing the figures for coverage levels and incidence overall suggests that increased immunization coverage significantly decreases incidence. However, as coverage increases to high levels, as occurred in the United States, the Netherlands, Japan, and Oman, the incidence data do not show complete disappearance of cases due to importations. In the United States, heterogeneity in immunization coverage continues to lead to outbreaks following the importation of measles,(24,25) although these cases appear barely visible in Fig. 1b compared to historical incidence. In the United States, aggressive outbreak response efforts control outbreaks relatively quickly. Starting the x-axis scale in 1998 in Fig. 1c for rubella for the United States misses the impact of heterogeneity and the outbreaks of rubella that occurred in the Amish in the early 1990s,(26) but uses a y-axis scale large enough that the relatively small number of annual importation-related cases appear barely visible. Fig. 4c for the much smaller total population in Oman shows the relatively small number of cases primarily from importations it reports annually. In contrast, Figs. 2b and 2c show episodic outbreaks in the Netherlands, which reflect the significant impacts of clustering of its under-vaccinated religious subpopulation. In Japan, rubella immunization initially targeted adolescent girls only, which did not eliminate rubella transmission in the general population. Difficulties due to the mumps antigen following the introduction of MMR around 1990 in Japan led to heterogeneity in coverage of M and R in RI, and the buildup of susceptible individuals, who supported outbreaks in the early 2000s and 2010s. Figs. 5b and 5c for Haiti show the impact of the aggressive efforts by the WHO Region of the Americas (i.e., PAHO) to use wide age range SIAs to eliminate measles and rubella, which in contrast to> Fig 6b shows the decreasing measles incidence with improved coverage and late introduction of MCV2 into RI in Vietnam. Figs 6c, 7c, and 8c show reported incidence of rubella, although this probably reflects underreporting given the absence of RCV use.
Fig. 5.
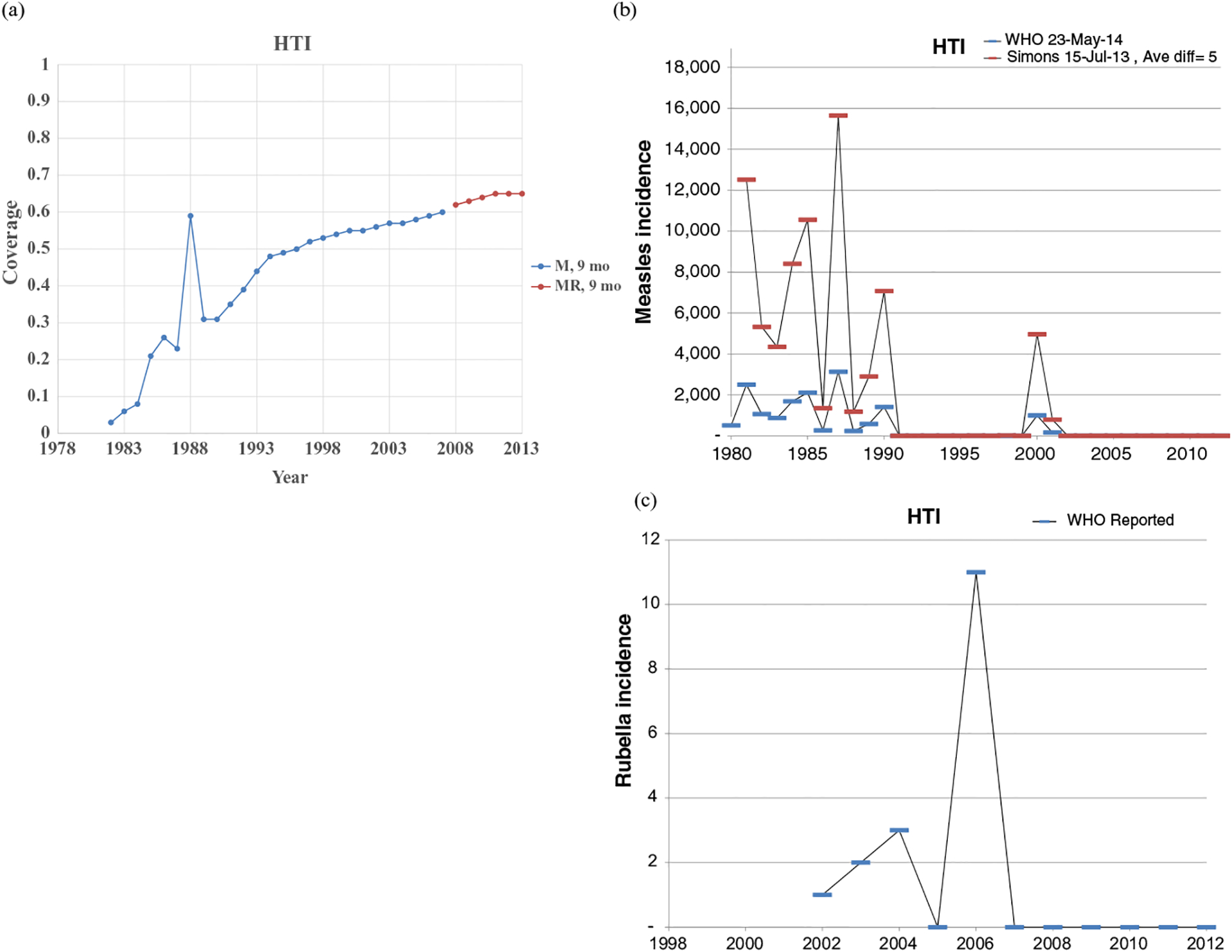
Profile for Haiti.
Fig. 6.

Profile for Vietnam.
Fig. 7.
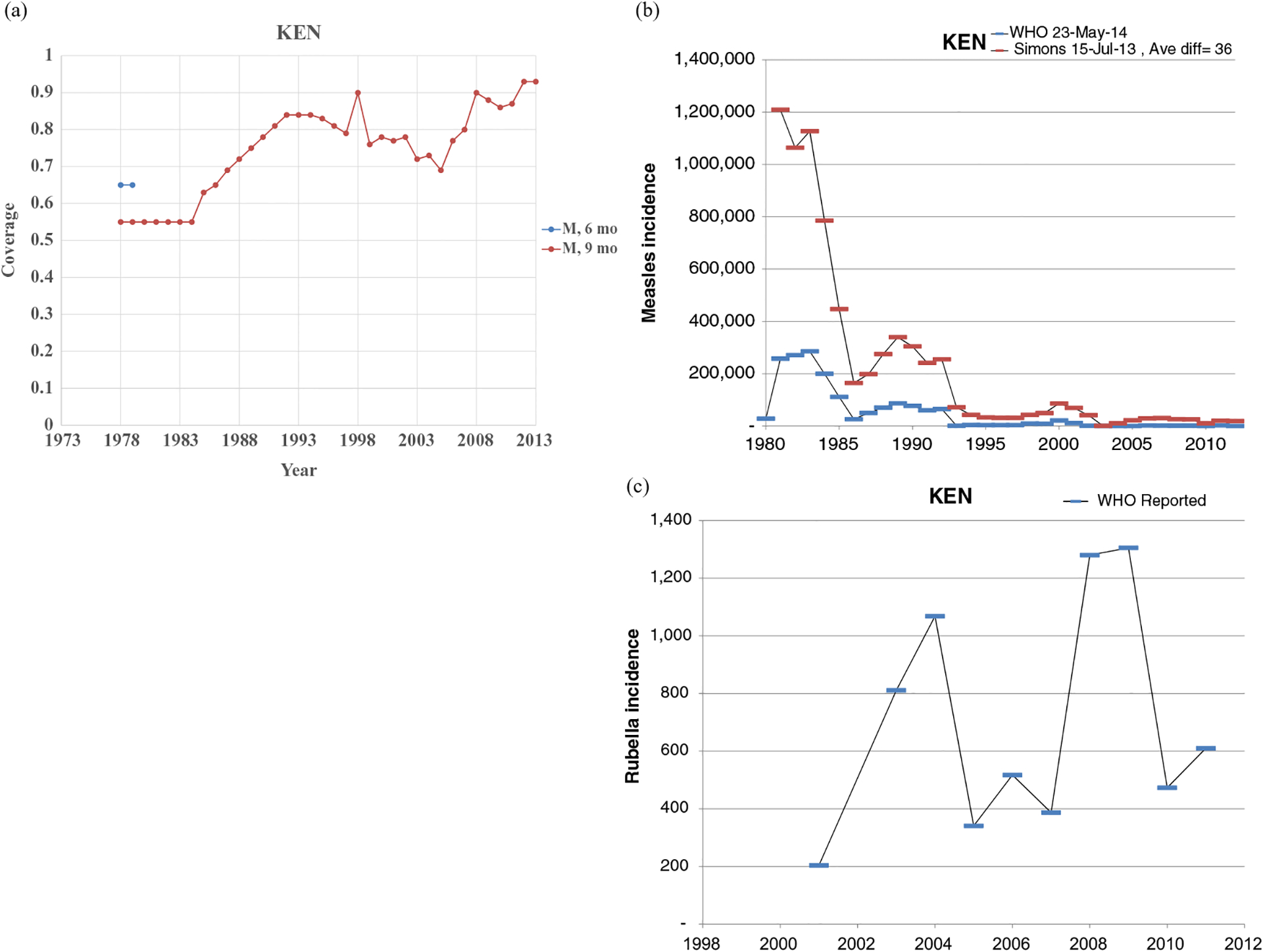
Profile for Kenya.
4. DISCUSSION
Our efforts to synthesize the available immunization and epidemiological data for measles and rubella at the national level provide a foundation for modeling measles and rubella transmission globally. Significant variability in the profiles suggests the need to model transmission of measles and rubella at the national level for larger-scale analyses and then aggregate the results to the regional or global level. We expect that our analysis should facilitate such modeling. The syntheses reveal potential immunity gaps, with low vaccination coverage in some years translating into relatively large proportions of accumulating susceptible individuals in the absence of SIAs to catch up unimmunized individuals. In some cases, outbreaks probably led to immunity in some fraction of the unimmunized individuals. Immunization program managers must manage population immunity to stop transmission, and these profiles may serve as a reminder about potentially accumulating susceptible individuals due to relatively low coverage in the past. Social disruptions (e.g., natural disasters, conflict) negatively impact national immunization programs, and catching up individuals missed due to such disruptions should represent a priority for national efforts to close immunity gaps.
The comparisons between the reported cases and cases estimated by prior models demonstrate the absence of prior model estimates for some countries. We identified historical reported cases before 1974 for only a relatively small number of high-income member states (e.g., the United States, the United Kingdom). We encountered the most difficulty finding information about rubella cases, particularly in Africa, and we suspect that member states most likely reported some of the historical rubella cases as measles cases due to the similarity of the clinical presentation. Increased use and expansion of laboratory methods that characterize both viruses promise to provide better information in the future.
Despite our extensive efforts, several data gaps remain. We could not find complete information for most of the 183 areas. We found conflicting information for some areas, and we did our best to resolve this using the available literature. Our efforts to reconstruct historical experiences did not benefit from review by national experts. In addition to concerns and limitations associated with underreported incidence, incorrect reporting of coverage also represents a concern. Underreporting of coverage, perhaps due to vaccine delivered in the private sector, may suggest immunity gaps that do not exist. A much larger concern, however, comes from overreporting of coverage, which may provide a false sense of security about the absence of immunity gaps. The use of the data in this synthesis comes with many limitations due to unknown and potentially poor data quality. We hope that this effort will motivate national experts to provide corrections to our assumptions such that modeling efforts can benefit from the best available information.
This synthesis of the available data should provide a useful starting point for measles and rubella transmission models and help to make assumptions about immunization inputs more transparent.
Fig. 3.
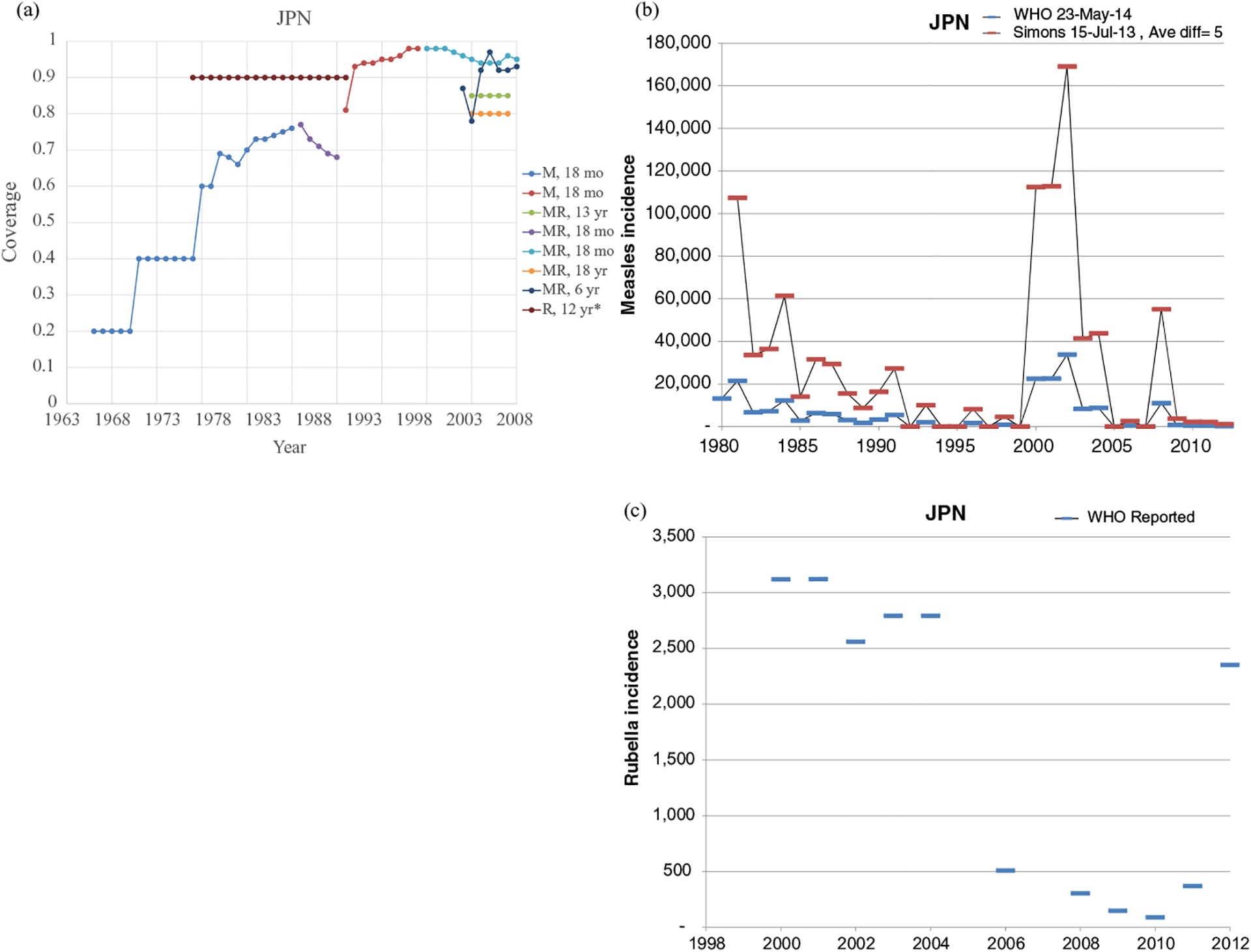
Profile for Japan.
ACKNOWLEDGMENTS
The first two authors acknowledge support for this work from the U.S. Centers for Disease Control and Prevention (CDC) for supporting this work under Cooperative Agreement U66IP000519. We thank Marta Gacic-Dobo and Tony Burton for helpful input. The contents of this article remain solely the responsibility of the authors and do not represent the official views of the U.S. Centers for Disease Control and Prevention or the World Health Organization.
REFERENCES
- 1.Thompson KM, Pallansch MA, Tebbens RJ, Wassilak SG, Cochi SL. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Analysis, 2013; 33(4):647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis, 2013; 33(4):703–749. [DOI] [PubMed] [Google Scholar]
- 3.Goodson JL, Chu SY, Rota PA, Moss WJ, Featherstone DA, Vijayaraghavan M, Thompson KM, Martin R. Research priorities for global measles and rubella control and eradication. Journal of Infectious Diseases, 2012; 30(32):4709–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein CE, Birmingham M, Kurian M, Duclos P, Strebel P. The global burden of measles in the year 2000—A model that uses country-specific indicators. Journal of Infectious Diseases, 2003; 187: S8–S14. [DOI] [PubMed] [Google Scholar]
- 5.Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, Strebel P. Assessment of the 2010 global measles mortality reduction goal: Results from a model of surveillance data. Lancet, 2012; 379(9832):2173–2178. [DOI] [PubMed] [Google Scholar]
- 6.Simons E, Mort M, Dabbagh A, Strebel P, Wolfson L. Strategic planning for measles control: Using data to inform optimal vaccination strategies. Journal of Infectious Diseases, 2011; 204 Suppl 1:S28–S34. [DOI] [PubMed] [Google Scholar]
- 7.Thompson KM, Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, Cochi SL. Polio eradicators use analytical models to make better decisions. Interfaces, 2015; 45(1): 5–25. [Google Scholar]
- 8.Thompson KM. Modeling poliovirus risks and the legacy of polio eradication. Risk Analysis, 2013; 33(4):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson KM, Odahowski CL. Systematic review of measles and rubella serology studies. Risk Analysis, 2015. 10.1111/risa.12430. [DOI] [PubMed] [Google Scholar]
- 10.Decade of Vaccines. Global Vaccine Action Plan, 2015. Available at: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/, Accessed April 12, 2015. [Google Scholar]
- 11.World Health Organization. Global Measles and Rubella Strategic Plan, 2012–2020. Available at: http://www.who.int/immunization/newsroom/Measles_Rubella_StrategicPlan_2012_2020.pdf. Accessed May 11, 2014.
- 12.Thompson KM, Duintjer Tebbens RJ. Development of investment cases for globally-coordinated management of infectious diseases. Journal of Vaccines and Vaccination, 2012; 3:164. doi: 10.4172/2157-7560.1000164. [DOI] [Google Scholar]
- 13.World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System, 2013. Global Summary Year of Introduction of Selected Vaccines Database. Available at: http://www.who.int/entity/immunization/monitoring_surveillance/data/year_vaccine_introduction.xls?ua=1, Accessed May 11, 2014. [Google Scholar]
- 14.World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System, 2014. Global Summary Time Series of Coverage. Available at: http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls?ua=1, Accessed July 15, 2014. [Google Scholar]
- 15.World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2014. Global Summary Schedules. Available at: http://www.who.int/entity/immunization/monitoring_surveillance/data/schedule_data.xls?ua=1, Accessed July 15, 2014. [Google Scholar]
- 16.United Nations Population Division. World Population Prospects: The 2012 Revision. Available at: http://esa.un.org/unpd/wpp/index.htm, Accessed September 11, 2013.
- 17.World Bank. New Country Classifications, World Bank Income Levels, 2013. Available at: http://data.worldbank.org/news/new-country-classifications, Accessed March 3, 2014.
- 18.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Pre-eradication national vaccine policy options for poliovirus infection and disease control. Risk Analysis 2013; 33(4):516–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson KM, Dabbagh A, Strebel PM, Perry R, Gacic-Dobo M, Cochi SL, Cairns L, Reef S. National and global options for managing the risks of measles and rubella. Journal of Vaccines and Vaccination, 2012; 3:165. doi: 10.4172/2157-7560.1000165. [DOI] [Google Scholar]
- 20.World Health Organization. Rubella vaccines. WHO position paper. Weekly Epidemiological Record, 2011; 86(29): 301–316. [PubMed] [Google Scholar]
- 21.World Health Organization. Retrospective Measles Data on Supplementary Immunization Activities, 2014. Available at: http://www.who.int/entity/immunization/monitoring_surveillance/data/Summary_Measles_SIAs_2000_2014_Nov2014.xls?ua=1, Accessed April 21, 2015.
- 22.Gavi: The Vaccine Alliance 2014. Available at: http://www.gavi.org/Library/Documents/GAVIdocuments/Guidelines-and-forms/Gavi-measles-second-dose-and-measles-rubella-vaccine-support-guidelines-2015/, Accessed 11 July 2015.
- 23.Measles and Rubella Investment Case Model Input Profiles, 2014. Available at: http://www.kidrisk.org, Accessed April 25, 2015.
- 24.Thompson KM, Logan GE, Research Team from Florida SHOTS™. Characterization of heterogeneity in childhood immunization coverage in central Florida using immunization registry data. Risk Analysis, 2015. 10.1111/risa.12424. [DOI] [PubMed] [Google Scholar]
- 25.Thompson KM, Kisjes KH. Modeling measles transmission in the North America Amish and options for outbreak response. Risk Analysis, 2015. 10.1111/risa.12440. [DOI] [PubMed] [Google Scholar]
- 26.Outbreaks of rubella among the Amish–United States, 1991. MMWR Morb Mortal Wkly Rep 1991; 40(16):264–265. [PubMed] [Google Scholar]
- 27.Rosenthal SR, Clements CJ. Two-dose measles vaccination schedules. Bulletin of the World Health Organization, 1993; 71(3–4):421–428. [PMC free article] [PubMed] [Google Scholar]
- 28.Millar JD, Foege WH. Status of eradication of smallpox (and control of measles) in west and central Africa. Journal of Infectious Diseases, 1969; 120(6):725–732. [DOI] [PubMed] [Google Scholar]
- 29.Henderson RH, Davis H, Eddins DL, Foege WH. Assessment of vaccination coverage, vaccination scar rates, and smallpox scarring in five areas of west Africa. Bulletin of the World Health Organization, 1973; 48(2):183–194. [PMC free article] [PubMed] [Google Scholar]
- 30.Ofosu-Amaah S. The control of measles in tropical Africa: A review of past and present efforts. Reviews of Infectious Diseases 1983; 5(3):546–553. [DOI] [PubMed] [Google Scholar]
- 31.Expanded Programme on Immunization (EPI). Measles control in the WHO African region. Weekly Epidemiological Record, 1996; 71(26):201–203. [PubMed] [Google Scholar]
- 32.Expanded Programme on Immunization (EPI). Immunization schedules in the WHO African region, 1995. Weekly Epidemiological Record, 1996; 71(12):90–94. [PubMed] [Google Scholar]
- 33.Masresha BG, Fall A, Eshetu M, Sosler S, Alleman M, Goodson JL, Katsande R, Nshimirimana D. Measles mortality reduction and pre-elimination in the African region, 2001–2009. Journal of Infectious Diseases, 2011; 204 Suppl 1: S198–S204. [DOI] [PubMed] [Google Scholar]
- 34.Velema JP, Alihonou EM, Gandaho T, Hounye FH. Childhood mortality among users and non-users of primary health care in a rural west African community. International Journal of Epidemiology, 1991; 20(2):474–479. [DOI] [PubMed] [Google Scholar]
- 35.Progress Toward Measles Elimination—Southern Africa, 1996–1998. Morbidity and Mortality Weekly Report 1999; 48(27):585–589. [PubMed] [Google Scholar]
- 36.Kidd S, Ouedraogo B, Kambire C, Kambou JL, McLean H, Kutty PK, Ndiaye S, Fall A, Alleman M, Wannemuehler K, Masresha B, Goodson JL, Uzicanin A. Measles outbreak in Burkina Faso, 2009: A case-control study to determine risk factors and estimate vaccine effectiveness. Vaccine, 2012; 30(33):5000–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black FL. Changing patterns in measles epidemiology. 1. Journal of the Egyptian Public Health Association, 1968; 43 Suppl:175–188. [PubMed] [Google Scholar]
- 38.Tall F, Nacro B, Nagalo K, Bonkoungou PS, Traore H, Traore H, Roisin A. Outbreak of measles and meningitis at Bobo-Dioulasso (Burkina Faso), during the first semester of 1996: Pediatric hospital data. Médecine et Maladies Infectieuses, 1997; 27:513–516. [Google Scholar]
- 39.Chen RT, Weierbach R, Bisoffi Z, Cutts F, Rhodes P, Ramaroson S, Ntembagara C, Bizimana F. A “post-honeymoon period” measles outbreak in Muyinga sector, Burundi. International Journal of Epidemiology, 1994; 23(1):185–193. [DOI] [PubMed] [Google Scholar]
- 40.Jato MN, Jato JG. Measles vaccination coverage in rural areas: A study of seven villages in Cameroon. International Nursing Review, 1981; 28(6):183–185. [PubMed] [Google Scholar]
- 41.Guyer B, McBean AM. The epidemiology and control of measles in Yaounde, Cameroun, 1968–1975. International Journal of Epidemiology, 1981; 10(3):263–269. [DOI] [PubMed] [Google Scholar]
- 42.Brown J, Djogdom P, Murphy K, Kesseng G, Heymann D. Identifying the reasons for low immunization coverage. A case study of Yaounde (United Republic of Cameroon). Revue d’Epidemiologie et de Sante Publique, 1982; 30(1):35–47. [PubMed] [Google Scholar]
- 43.Cummings DAT, Moss WJ, Long K, Wiysonge CS, Muluh TJ, Kollo B, Nomo E, Wolfe ND, Burke DS. Improved measles surveillance in Cameroon reveals two major dynamic patterns of incidence. International Journal of Infectious Diseases, 2006; 10(2):148–155. [DOI] [PubMed] [Google Scholar]
- 44.Goodson JL, Sosler S, Pasi O, Johnson T, Kobella M, Monono ME, Uzicanin A. Impact of a measles outbreak response immunization campaign: Maroua, Cameroon, 2009. Journal of Infectious Diseases, 2011; 204 Suppl 1: S252–259. [DOI] [PubMed] [Google Scholar]
- 45.Sume GE, Fouda AA, Kobela M, Nguele S, Emah I, Atem P, Mbida D, Njock K. Epidemiology and clinical characteristics of the measles outbreak in the Nylon Health District, Douala Cameroon: A retrospective descriptive cross sectional study. Pan African Medical Journal, 2012; 13:66. [PMC free article] [PubMed] [Google Scholar]
- 46.Sixl W, Sixl-Voigt B. Serological screenings of various infectious diseases on the Cape Verde islands (west Africa). Journal of Hygiene, Epidemiology, Microbiology, and Immunology, 1987; 31 Suppl 4:469–471. [PubMed] [Google Scholar]
- 47.Kahn JG, Mokdad AH, Deming MS, Roungou JB, Boby AM, Excler JL, Waldman RJ. Avoiding missed opportunities for immunization in the Central African Republic: Potential impact on vaccination coverage. Bulletin of the World Health Organization, 1995; 73(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- 48.Gouandjika-Vasilache I, Waku-Kouomou D, Menard D, Beyrand C, Guye F, Ngoay-Kossy JC, Selekon B, Wild TF. Cocirculation of measles virus genotype B2 and B3.1 in Central African Republic during the 2000 measles epidemic. Journal of Medical Virology, 2006; 78(7):964–970. [DOI] [PubMed] [Google Scholar]
- 49.Luthi JC, Kessler W, Boelaert M. Vaccine efficacy in the city of Bongor (Chad) and its operational consequences for the immunization programme. Bulletin of the World Health Organization, 1997; 75(5):427–433. [PMC free article] [PubMed] [Google Scholar]
- 50.Ndikuyeze A, Cook A, Cutts FT, Bennett S. Priorities in global measles control: Report of an outbreak in N’Djamena, Chad. Epidemiology and Infection, 1995; 115(2):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Expanded Programme on Immunization (EPI). Measles outbreak in N’Djamena. Weekly Epidemiological Record, 1995; 70(5):31–35. [PubMed] [Google Scholar]
- 52.Grais RF, Dubray C, Gerstl S, Guthmann JP, Djibo A, Nargaye KD, Coker J, Alberti KP, Cochet A, Ihekweazu C, Nathan N, Payne L, Porten K, Sauvageot D, Schimmer B, Fermon F, Burny ME, Hersh BS, Guerin PJ. Unacceptably high mortality related to measles epidemics in Niger, Nigeria, and Chad. PLoS Med, 2007; 4(1):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choto R, Chadambuka A, Shambira G, Gombe N, Tshimanga M, Midzi S, Mberikunashe J. Trends in performance of the national measles case-based surveillance system, Ministry of Health and Child Welfare, Zimbabwe (1999–2008). Pan African Medical Journal, 2012; 11:2. [PMC free article] [PubMed] [Google Scholar]
- 54.Wood PB, Soheranda KS, Bracken PM, Houser NE. Measles vaccination in Zaire—When and how? Transactions of the Royal Society of Tropical Medicine and Hygiene, 1980; 74(3):381–382. [DOI] [PubMed] [Google Scholar]
- 55.Cooke FJ, Shapiro DS. Measles in Democratic Republic of Congo. International Journal of Infectious Diseases, 2004; 8(3):138–138. [Google Scholar]
- 56.Alberti KP, King LA, Burny ME, Ilunga BK, Grais RF. Reactive vaccination as an effective tool for measles outbreak control in measles mortality reduction settings, Democratic Republic of Congo, 2005–2006. International Health, 2010; 2(1):65–68. [DOI] [PubMed] [Google Scholar]
- 57.Grout L, Minetti A, Hurtado N, Francois G, Fermon F, Chatelain A, Harczi G, Ngoie JDI, N’Goran A, Luquero FJ, Grais RF, Porten K. Measles in Democratic Republic of Congo: An outbreak description from Katanga, 2010–2011. BMC Infectious Diseases, 2013; 13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kouadio IK, Koffi AK, Attoh-Toure H, Kamigaki T, Oshitani H. Outbreak of measles and rubella in refugee transit camps. Epidemiology and Infection, 2009; 137(11):1593–1601. [DOI] [PubMed] [Google Scholar]
- 59.Freij L, Krishnaswamy MS, Sterky G. Measles in Addis Ababa. Ethiopian Medical Journal, 1972; 10(2):61–70. [PubMed] [Google Scholar]
- 60.Gedlu E, Tesemma T. Immunization coverage and identification of problems associated with vaccination delivery in Gondar, north west Ethiopia. East African Medical Journal, 1997; 74(4):239–241. [PubMed] [Google Scholar]
- 61.Berhane Y, Schluter WW, Oyewole F, Babaniyi OA. Age at first dose of measles vaccination in Ethiopia. East African Medical Journal, 2009; 86(3):115–119. [DOI] [PubMed] [Google Scholar]
- 62.Mitiku K, Bedada T, Masresha B, Kegne W, Nafo-Traore F, Tesfaye N, Beyene B. The epidemiology of rubella disease in Ethiopia: Data from the measles case-based surveillance system. Journal of Infectious Diseases, 2011; 204 Suppl 1:S239–S242. [DOI] [PubMed] [Google Scholar]
- 63.Mitiku K, Bedada T, Masresha BG, Kegne W, Nafo-Traore F, Tesfaye N, Yigzaw A. Progress in measles mortality reduction in Ethiopia, 2002–2009. Journal of Infectious Diseases, 2011; 204 Suppl 1:S232–S238. [DOI] [PubMed] [Google Scholar]
- 64.Measles—Horn of Africa, 2010–2011. Morbidity and Mortality Weekly Report. 2012; 61(34):678–684. [PubMed] [Google Scholar]
- 65.McGregor IA. Measles and child mortality in the Gambia. West African Medical Journal, 1964; 13:251–257. [PubMed] [Google Scholar]
- 66.Williams PJ, Hull HF. Status of measles in the Gambia, 1981. Reviews of Infectious Diseases, 1983; 5(3):391–394. [DOI] [PubMed] [Google Scholar]
- 67.Assaad F Measles: Summary of worldwide impact. Reviews of Infectious Diseases, 1983; 5(3):452–459. [DOI] [PubMed] [Google Scholar]
- 68.Whittle HC, Rowland MG, Mann GF, Lamb WH, Lewis RA. Immunisation of 4–6 month old Gambian infants with Edmonston-Zagreb measles vaccine. Lancet, 1984; 2(8407):834–837. [DOI] [PubMed] [Google Scholar]
- 69.Lamb WH. Epidemic measles in a highly immunized rural west African (Gambian) village. Reviews of Infectious Diseases, 1988; 10(2):457–462. [DOI] [PubMed] [Google Scholar]
- 70.Fortuin M, Maine N, Mendy M, Hall A, George M, Whittle H. Measles, polio and tetanus toxoid antibody levels in Gambian children aged 3 to 4 years following routine vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1995; 89(3):326–329. [DOI] [PubMed] [Google Scholar]
- 71.Njie-Jobe J, Nyamweya S, Miles DJC, vander Sande M, Zaman S, Touray E, Hossin S, Adetifa J, Palmero M, Burl S, Jeffries D, Rowland-Jones S, Flanagan K, Jaye A, Whittle H. Immunological impact of an additional early measles vaccine in Gambian children: Responses to a boost at 3 years. Vaccine, 2012; 30(15):2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosu WK, Essel-Ahun M, Adjei S, Strebel P. Progress in the control of measles in Ghana, 1980–2000. Journal of Infectious Diseases, 2003; 187:S44–S50. [DOI] [PubMed] [Google Scholar]
- 73.Blankson JM. Measles and its problems as seen in Ghana. Journal of Tropical Pediatrics and Environmental Child Health, 1975; 21(1-b):51–54. [PubMed] [Google Scholar]
- 74.Commey JO, Dekyem P. Measles in southern Ghana: 1985–1993. West African Journal of Medicine, 1994; 13(4):223–226. [PubMed] [Google Scholar]
- 75.Aaby P, Bukh J, Lisse IM, Smits AJ. Introduction of measles into a highly immunised west African community: The role of health care institutions. Journal of Epidemiology and Community Health, 1985; 39(2):113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garly ML, Martins CL, Bale C, da Costa F, Dias F, Whittle H, Aaby P. Early two-dose measles vaccination schedule in Guinea-Bissau: Good protection and coverage in infancy. International Journal of Epidemiology, 1999; 28(2):347–352. [DOI] [PubMed] [Google Scholar]
- 77.Hornshoj L, Benn CS, Fernandes M, Rodrigues A, Aaby P, Fisker AB. Vaccination coverage and out-of-sequence vaccinations in rural Guinea-Bissau: An observational cohort study. British Medical Journal Open, 2012; 2(6):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lema JT. Three years of measles vaccination in Mombasa district—Kenya. East African Medical Journal, 1975; 52(2):70–76. [PubMed] [Google Scholar]
- 79.Collaborative Study by the Ministry of Health of Kenya and the World Health Organization. Measles immunity in the first year after birth and the optimum age for vaccination in Kenyan children. Bulletin of the World Health Organization, 1977; 55(1):21–31. [PMC free article] [PubMed] [Google Scholar]
- 80.Muller AS, Voorhoeve AM, Mannetje W, Schulpen TW. The impact of measles in a rural area of Kenya. East African Medical Journal, 1977; 54(7):364–372. [PubMed] [Google Scholar]
- 81.Bell TM, Tukei PM, Ademba GR, Mbugua FM, Gathara GW, Magana JM, Kinyanjui P, Muli J, Hazlett DT, Alwar JE, et al. Investigation of the effectiveness of measles vaccination in children in Kenya. Journal of Hygiene, 1985; 95(3):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burstrom B, Aaby P, Mutie DM, Kimani G, Bjerregaard P. Severe measles outbreak in western Kenya. East African Medical Journal, 1992; 69(8):419–423. [PubMed] [Google Scholar]
- 83.Burstrom B, Aaby P, Mutie DM. Measles in infants: A review of studies on incidence, vaccine efficacy and mortality in east Africa. East African Medical Journal, 1995; 72(3): 155–161. [PubMed] [Google Scholar]
- 84.Arya SC, Agarwal N. Rural Kenya: Measles during pregnancy and early infancy. Journal of Infectious Diseases, 2006; 193(1):165–166. [DOI] [PubMed] [Google Scholar]
- 85.Maina LC, Karanja S, Kombich J. Immunization coverage and its determinants among children aged 12–23 months in a peri-urban area of Kenya. Pan African Medical Journal, 2013; 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weeks RM. A perspective on controlling vaccine-preventable diseases among children in Liberia. Infection Control, 1984; 5(11):538–541. [DOI] [PubMed] [Google Scholar]
- 87.Goodson JL, Kulkarni MA, Vanden Eng JL, Wannemuehler KA, Cotte AH, Desrochers RE, Randriamanalina B, Luman ET. Improved equity in measles vaccination from integrating insecticide-treated bednets in a vaccination campaign, Madagascar. Tropical Medicine and International Health, 2012; 17(4):430–437. [DOI] [PubMed] [Google Scholar]
- 88.Porter JD, Gastellu-Etchegorry M, Navarre I, Lungu G, Moren A. Measles outbreaks in the Mozambican refugee camps in Malawi: The continued need for an effective vaccine. International Journal of Epidemiology, 1990; 19(4):1072–1077. [DOI] [PubMed] [Google Scholar]
- 89.Minetti A, Kagoli M, Katsulukuta A, Huerga H, Featherstone A, Chiotcha H, Noel D, Bopp C, Sury L, Fricke R, Iscla M, Hurtado N, Ducomble T, Nicholas S, Kabuluzi S, Grais RF, Luquero FJ. Lessons and challenges for measles control from unexpected large outbreak, Malawi. Emerging Infectious Diseases, 2013; 19(2):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kertesz DA, Toure K, Berthe A, Konate Y, Bougoudogo F. Evaluation of urban measles mass campaigns for children aged 9–59 months in Mali. Journal of Infectious Diseases, 2003; 187:S69–S73. [DOI] [PubMed] [Google Scholar]
- 91.Expanded Programme on Immunization (EPI). Cost-effectiveness study. Mauritania. Weekly Epidemiological Record, 1987; 62(14):93–100. [Google Scholar]
- 92.Cutts FT, Smith PG, Colombo S, Mann G, Ascherio A, Soares AC. Field evaluation of measles vaccine efficacy in Mozambique. American Journal of Epidemiology, 1990; 131(2):349–355. [DOI] [PubMed] [Google Scholar]
- 93.Cutts F, Soares A, Jecque AV, Cliff J, Kortbeek S, Colombo S. The use of evaluation to improve the Expanded Programme on Immunization in Mozambique. Bulletin of the World Health Organization, 1990; 68(2):199–208. [PMC free article] [PubMed] [Google Scholar]
- 94.Mortality among newly arrived Mozambican refugees—Zimbabwe and Malawi, 1992. Morbidity and Mortality Weekly Report 1993; 42(24):468–469, 475–477. [PubMed] [Google Scholar]
- 95.Jani JV, Jani IV, Araujo C, Sahay S, Barreto J, Bjune G. Assessment of routine surveillance data as a tool to investigate measles outbreaks in Mozambique. BMC Infectious Diseases, 2006; 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jani JV, Holm-Hansen C, Mussa T, Zango A, Manhica I, Bjune G, Jani IV. Assessment of measles immunity among infants in Maputo City, Mozambique. BMC Public Health, 2008; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cliff J, Simango A, Augusto O, Vander Paal L, Biellik R. Failure of targeted urban supplemental measles vaccination campaigns (1997–1999) to prevent measles epidemics in Mozambique (1998–2001). Journal of Infectious Diseases, 2003; 187:S51–S57. [DOI] [PubMed] [Google Scholar]
- 98.Mandomando I, Naniche D, Pasetti MF, Cuberos L, Sanz S, Valles X, Sigauque B, Macete E, Nhalungo D, Kotloff KL, Levine MM, Alonso PL. Assessment of the epidemiology and burden of measles in southern Mozambique. American Journal of Tropical Medicine and Hygiene, 2011; 85(1):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muloliwa AM, Camacho LAB, Verani JFS, Simoes TC, Dgedge MD. Impact of vaccination on the incidence of measles in Mozambique in the period 2000 to 2011. Cadernos De Saude Publica, 2013; 29(2):258–270. [DOI] [PubMed] [Google Scholar]
- 100.McMorrow ML, Gebremedhin G, vanden Heever J, Kezaala R, Harris BN, Nandy R, Strebel P, Jack A, Cairns KL. Measles outbreak in South Africa, 2003–2005. South African Medical Journal, 2009; 99(5):314–319. [PMC free article] [PubMed] [Google Scholar]
- 101.Malfait P, Jataou IM, Jollet MC, Margot A, DeBenoist AC, Moren A. Measles epidemic in the urban community of Niamey: Transmission patterns, vaccine efficacy and immunization strategies, Niger, 1990 to 1991. Pediatric Infectious Disease Journal, 1994; 13(1):38–45. [DOI] [PubMed] [Google Scholar]
- 102.Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, Strebel P, Cairns L. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clinical Infectious Diseases, 2006; 42(3):322–328. [DOI] [PubMed] [Google Scholar]
- 103.Collard P, Hendrickse RG, Montefiore D, Sherman P, Van-Der Wall HM, Morley D, Goffe AP, Laurence GD, Pollock TM. Vaccination against measles. II. Clinical trial in Nigerian children. British Medical Journal, 1961; 2(5262):1246–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morley DC. Measles in Nigeria. American Journal of Diseases of Children, 1962; 103:230–233. [DOI] [PubMed] [Google Scholar]
- 105.Hendrickse RG, Montefiore D, Sherman PM, Vanderwall HM. Studies on measles vaccination in Nigerian children. British Medical Journal, 1964; 1(5381):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hendrickse RG, Montefiore D, Sherman PM, Sofoluwe GO. A further study on measles vaccination in Nigerian children. Bulletin of the World Health Organization, 1965; 32(6):803–808. [PMC free article] [PubMed] [Google Scholar]
- 107.Whittle HC, Bradley-Moore A, Fleming A, Greenwood BM. Effects of measles on immune-response of Nigerian children. Archives of Disease in Childhood, 1973; 48(10):753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harry TO, Ogunmekan DA. Optimal age for vaccinating Nigerian children against measles. I. Neonatal antibody profile and subsequent susceptibility to measles. Tropical and Geographical Medicine, 1981; 33(4):375–378. [PubMed] [Google Scholar]
- 109.Adu FD, Akinwolere OAO, Tomori O, Uche LN. Low seroconversion rates to measles-vaccine among children in Nigeria. Bulletin of the World Health Organization, 1992; 70(4):457–460. [PMC free article] [PubMed] [Google Scholar]
- 110.Ushie BA, Fayehun OA, Ugal DB. Trends and patterns of under-5 vaccination in Nigeria, 1990–2008: What manner of progress? Child: Care, Health and Development, 2014; 40(2):267–274. [DOI] [PubMed] [Google Scholar]
- 111.Lepage P, deMol P. Measles mortality in Rwanda. Lancet, 1979; 2(8152):1133–1134. [DOI] [PubMed] [Google Scholar]
- 112.Garenne M, Aaby P. Pattern of exposure and measles mortality in Senegal. Journal of Infectious Diseases, 1990; 161(6):1088–1094. [DOI] [PubMed] [Google Scholar]
- 113.Sugerman DE, Fall A, Guigui MT, N’Dolie M, Balogun T, Wurie A, Goodson JL. Preplanned national measles vaccination campaign at the beginning of a measles outbreak—Sierra Leone, 2009–2010. Journal of Infectious Diseases, 2011; 204 Suppl 1:S260–269. [DOI] [PubMed] [Google Scholar]
- 114.Gear JH. Measles in South Africa. American Journal of Diseases of Children, 1962; 103:261–264. [DOI] [PubMed] [Google Scholar]
- 115.Mandara MP, Remme J. Current measles control in Tanzania. Reviews of Infectious Diseases, 1983; 5(3):554–557. [DOI] [PubMed] [Google Scholar]
- 116.Vaccination schedules in PAHO member countries. Expanded Program on Immunization in the Americas, 1979; 1(1):7. [PubMed] [Google Scholar]
- 117.Immunization schedule in PAHO member countries. Expanded Program on Immunization in the Americas, 1994; 16(3):6. [PubMed] [Google Scholar]
- 118.deQuadros CA, Izurieta H, Venczel L, Carrasco P. Measles eradication in the Americas: Progress to date. Journal of Infectious Diseases, 2004; 189:S227–S235. [DOI] [PubMed] [Google Scholar]
- 119.Castillo-Solorzano C, Marsigli C, Bravo-Alcantara P, Flannery B, Matus CR, Tambini G, Gross-Galiano S, Andrus JK. Elimination of rubella and congenital rubella syndrome in the Americas. Journal of Infectious Diseases, 2011; 204:S571–S578. [DOI] [PubMed] [Google Scholar]
- 120.Bilkis MD, Barrero PR, Mistchenko AS. Measles resurgence in Argentina: 1997–8 outbreak. Epidemiology and Infection, 2000; 124(2):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arguelles MH, Orellana ML, Castello AA, Villegas GA, Masini M, Belizan AL, Ayala SG, Vera OD, Glikmann G. Measles virus-specific antibody levels in individuals in Argentina who received a one-dose vaccine. Journal of Clinical Microbiology, 2006; 44(8):2733–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.St John MA, Benjamin S. An epidemic of congenital rubella in Barbados. Annals of Tropical Paediatrics, 2000; 20(3):231–235. [DOI] [PubMed] [Google Scholar]
- 123.Quiroga R, Barrezueta O, Venczel L, Halkyer P, Gil F, Machicao E, Landaverde M, Quinonez A, Izurieta H. Interruption of indigenous measles transmission in Bolivia since October 2000. Journal of Infectious Diseases, 2003; 187:S121–S126. [DOI] [PubMed] [Google Scholar]
- 124.Risi JB, Jr. Control of measles in Brazil. Reviews of Infectious Diseases, 1983; 5(3):583–587. [DOI] [PubMed] [Google Scholar]
- 125.Zanetta RAC, Amaku M, Azevedo RS, Zanetta DMT, Burattini MN, Massad E. Optimal age for vaccination against measles in the state of Sao Paulo, Brazil, taking into account the mother’s serostatus. Vaccine, 2001; 20(1–2):226–234. [DOI] [PubMed] [Google Scholar]
- 126.Prevots DR, Parise MS, Segatto TCV, Siqueira MM, dos Santos ED, Ganter B, Perreira M, Domingues CA, Lanzieri T, da Silva JB. Interruption of measles transmission in Brazil, 2000–2001. Journal of Infectious Diseases, 2003; 187:S111–S120. [DOI] [PubMed] [Google Scholar]
- 127.Duclos P, Redd SC, Varughese P, Hersh BS. Measles in adults in Canada and the United States: Implications for measles elimination and eradication. International Journal of Epidemiology, 1999; 28(1):141–146. [DOI] [PubMed] [Google Scholar]
- 128.Chile averts measles outbreak. Expanded Program on Immunization in the Americas, 1994; 16(3):8. [PubMed] [Google Scholar]
- 129.Gallegos D, Olea A, Sotomayor V, Gonzalez C, Munoz JC, Ramos M, Espinoza MC, Mendoza G, Torres G, Espineira E, Andrade W, Fernandez J, Fasce R. Rubella outbreaks following virus importations: The experience of Chile. Journal of Infectious Diseases, 2011; 204 Suppl 2:S669–S674. [DOI] [PubMed] [Google Scholar]
- 130.Villagra E, Delgado LV, Olea A. Enhanced surveillance for congenital rubella syndrome following mass rubella vaccination of girls and reproductive-aged women. Journal of Infectious Diseases, 2011; 204 Suppl 2:S642–S646. [DOI] [PubMed] [Google Scholar]
- 131.Outbreak of measles—Venezuela and Colombia, 2001–2002. Morbidity and Mortality Weekly Report 2002; 51(34):757–760. [PubMed] [Google Scholar]
- 132.Leon de Coto EM. Evolution of the measles vaccination program in Costa Rica. Reviews of Infectious Diseases, 1983; 5(3):588–591. [DOI] [PubMed] [Google Scholar]
- 133.Morice A, Carvajal X, Leon M, Machado V, Badilla X, Reef S, Lievano F, Depetris A, Castillo-Solorzano C. Accelerated rubella control and congenital rubella syndrome prevention strengthen measles eradication: The Costa Rican experience. Journal of Infectious Diseases, 2003; 187:S158–S163. [DOI] [PubMed] [Google Scholar]
- 134.Morice A, Ulloa-Gutierrez R, Avila-Aguero ML. Congenital rubella syndrome: Progress and future challenges. Expert Review of Vaccines, 2009; 8(3):323–331. [DOI] [PubMed] [Google Scholar]
- 135.Hinman AR, Hersh BS, deQuadros CA. Rational use of rubella vaccine for prevention of congenital rubella syndrome in the Americas. Pan American Journal of Public Health, 1998; 4(3):156–160. [DOI] [PubMed] [Google Scholar]
- 136.Galindo MA, Santin M, Resik S, Ribas MA, Guzman M, Mas Lago P, Strassburg M, Hersh BS, deQuadros CA. Eradication of measles in Cuba. Pan American Journal of Public Health, 1998;4(3):171–177. [DOI] [PubMed] [Google Scholar]
- 137.Ehrenkranz NJ, Ventura AK, Medler EM, Jackson JE, Kenny MT. Clinical evaluation of a new measles-mumps-rubella combined live virus vaccine in the Dominican Republic. Bulletin of the World Health Organization, 1975; 52(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- 138.Gloyd S, Suarez Torres J, Mercer MA. Immunization campaigns and political agendas: Retrospective from Ecuador and El Salvador. International Journal of Health Services, 2003; 33(1):113–128. [DOI] [PubMed] [Google Scholar]
- 139.CDC. Measles mortality—Guatemala. Morbidity and Mortality Weekly Report, 1979; 28(29):582. [Google Scholar]
- 140.Narod S Measles vaccination in Haiti. New England Journal of Medicine, 1986; 314(9):581–582. [DOI] [PubMed] [Google Scholar]
- 141.Venczel L, Dobbins J, Andre J, Laender F, Izurieta H, Delorme P, Voltaire HC. Measles eradication in the Americas: Experience in Haiti. Journal of Infectious Diseases, 2003; 187:S127–S132. [DOI] [PubMed] [Google Scholar]
- 142.Rainey JJ, Danovaro-Holliday MC, Magloire R, Kananda G, Lee CE, Chamouillet H, Lacapere F, Mung K, Luman ET. Haiti 2007–2008 national measles-rubella vaccination campaign: Implications for rubella elimination. Journal of Infectious Diseases, 2011; 204 Suppl 2:S616–S621. [DOI] [PubMed] [Google Scholar]
- 143.Molina IB, Mendoza LO, Palma MA. Congenital rubella syndrome surveillance in Honduras. Journal of Infectious Diseases, 2011; 204 Suppl 2:S637–S641. [DOI] [PubMed] [Google Scholar]
- 144.Christie CD, Lee-Hirsh J, Rogall B, Merrill S, Ramlal AA, Karian V, Black FL. Durability of passive measles antibody in Jamaican children. International Journal of Epidemiology, 1990; 19(3):698–702. [DOI] [PubMed] [Google Scholar]
- 145.Baxter DN. Control of the congenital rubella syndrome in Jamaica. West Indian Medical Journal, 1986; 35(1):50–54. [PubMed] [Google Scholar]
- 146.Decastro JF. Measles in Mexico. Review of Infectious Diseases, 1983; 5(3):422–426. [DOI] [PubMed] [Google Scholar]
- 147.Golubjatnikov R, Elsea WR, Leppla L. Measles and rubella hemagglutination-inhibition antibody patterns in Mexican and Paraguayan children. American Journal of Tropical Medicine and Hygiene, 1971; 20(6):958–963. [DOI] [PubMed] [Google Scholar]
- 148.Kenny MT, Jackson JE, Medler EM, Miller SA, Osborn R. Age-related immunity to measles, mumps and rubella in middle American and United States children. American Journal of Epidemiology, 1976; 103(2):174–180. [DOI] [PubMed] [Google Scholar]
- 149.Owens CS, Espino RT. Rubella in Panama—Still a problem. Pediatric Infectious Disease Journal, 1989; 8(2):110–115. [PubMed] [Google Scholar]
- 150.Evans AS, Cook JA, Kapikian AZ, Nankervis G, Smith AL, West B. A serological survey of St Lucia. International Journal of Epidemiology, 1979; 8(4):327–332. [DOI] [PubMed] [Google Scholar]
- 151.Ali Z, Hull B, Lewis M. Neonatal manifestation of congenital rubella following an outbreak in Trinidad. Journal of Tropical Pediatrics, 1986; 32(2):79–82. [DOI] [PubMed] [Google Scholar]
- 152.Bisno AL, Spence LP, Stewart JA, Casey HL. Rubella in Trinidad: Sero-epidemiologic studies of an institutional outbreak. American Journal of Epidemiology, 1969; 89(1):74–81. [DOI] [PubMed] [Google Scholar]
- 153.Orenstein WA, Hinman AR. The immunization system in the United States—The role of school immunization laws. Vaccine, 1999; 17:S19–S24. [DOI] [PubMed] [Google Scholar]
- 154.Simpson DM, Ezzati-Rice TM, Zell ER. Forty years and four surveys: How does our measuring measure up? American Journal of Preventive Medicine, 2001; 20(4):6–14. [DOI] [PubMed] [Google Scholar]
- 155.Roush SW, Murphy TV, Vaccine Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. Journal of the American Medical Association, 2007; 298(18):2155–2163. [DOI] [PubMed] [Google Scholar]
- 156.Reef SE, Plotkin SA. Rubella vaccine. Pp. 688–717 in Plotkin SA, Orenstein WA, Offit PA (eds). Vaccines: Expert Consult, 6th ed, Philadelphia, PA: Elsevier Saunders, 2012. [Google Scholar]
- 157.Strebel PM, Papania MJ, Fiebelkorn AP, Halsey NA. Measles vaccine. Pp. 352–387 in Plotkin SA, Orenstein WA, Offit PA (eds). Vaccines: Expert Consult, 6th ed, Philadelphia, PA: Elsevier Saunders, 2012. [Google Scholar]
- 158.Hinman AR, Orenstein WA, Papania MJ. Evolution of measles elimination strategies in the United States. Journal of Infectious Diseases, 2004; 189:S17–S22. [DOI] [PubMed] [Google Scholar]
- 159.Measles update. Expanded Program on Immunization in the Americas, 1998; 20(4):1. [Google Scholar]
- 160.Sarmiento H, Cobo OB, Morice A, Zapata R, Benitez MV, Castillo-Solorzano C. Measles outbreak in Venezuela: A new challenge to postelimination surveillance and control? Journal of Infectious Diseases, 2011; 204 Suppl 2:S675–S682. [DOI] [PubMed] [Google Scholar]
- 161.Expanded Programme on Immunization (EPI). Immunization schedules in the WHO Eastern Mediterranean region, 1995. Weekly Epidemiological Record, 1996; 71(23):173–176. [PubMed] [Google Scholar]
- 162.Gaafar T, Moshni E, Lievano F. The challenge of achieving measles elimination in the eastern Mediterranean region by 2010. Journal of Infectious Diseases, 2003; 187:S164–S171. [DOI] [PubMed] [Google Scholar]
- 163.Naouri B, Ahmed H, Bekhit R, Teleb N, Mohsni E, Alexander JP Jr. Progress toward measles elimination in the eastern Mediterranean region. Journal of Infectious Diseases, 2011; 204 Suppl 1:S289–S298. [DOI] [PubMed] [Google Scholar]
- 164.Islamic Republic of Afghanistan Ministry of Public Health. Expanded Program on Immunization Financial Sustainability Plan 2004–2009. General Directorate of Health Care and Promotion National EPI Office, February 2005. Available at: http://moph.gov.af/Content/Media/Documents/Monitoring-Evaluation-Policy-Strategy2912201016288921.pdf, Accessed June 24, 2014. [Google Scholar]
- 165.Jawad JS, Al-Sayyad AS, Sataih F, Naouri B, Alexander JP Jr. Toward measles elimination in Bahrain—A Middle East country experience. Journal of Infectious Diseases, 2011; 204 Suppl 1:S299–S304. [DOI] [PubMed] [Google Scholar]
- 166.Dutta SR, Atrash HK, Mathew L, Mathew PP, Mahmood RA. Seroepidemiology of rubella in Bahrain. International Journal of Epidemiology, 1985; 14(4):618–623. [DOI] [PubMed] [Google Scholar]
- 167.El Sayed N, Kandeel N, Barakat I, Moussa I, Alexander JP Jr., Naouri B, Reef SE. Progress toward measles and rubella elimination in Egypt. Journal of Infectious Diseases, 2011; 204 Suppl 1:S318–S324. [DOI] [PubMed] [Google Scholar]
- 168.Abbassy AA, Barakat SS, Abd El Fattah MM, Said ZN, El Metwally HA. Could the MMR vaccine replace the measles vaccine at one year of age in Egypt? Eastern Mediterranean Health Journal, 2009; 15(1):85–93. [PubMed] [Google Scholar]
- 169.Zahraei SM, Gouya MM, Azad TM, Soltanshahi R, Sabouri A, Naouri B, Alexander JP Jr. Successful control and impending elimination of measles in the Islamic Republic of Iran. Journal of Infectious Diseases, 2011; 204 Suppl 1:S305–S311. [DOI] [PubMed] [Google Scholar]
- 170.Esteghamati A, Gouya MM, Zahraei SM, Dadras MN, Rashidi A, Mahoney F. Progress in measles and rubella elimination in Iran. Pediatric Infectious Disease Journal, 2007; 26(12):1137–1141. [DOI] [PubMed] [Google Scholar]
- 171.al-Sheikh OG, al-Samarrai JI, al-Sumaidaie MM, Mohammad SA, al-Dujaily AA. Immunization coverage among children born between 1989 and 1994 in Saladdin Governorate, Iraq. Eastern Mediterranean Health Journal, 1999; 5(5):933–940. [PubMed] [Google Scholar]
- 172.Bdour S, Batayneh N. Present anti-measles immunity in Jordan. Vaccine, 2001; 19(28–29):3865–3869. [DOI] [PubMed] [Google Scholar]
- 173.Makhseed M, Moussa MAA, Ahmed MA, Abdulla N. The status of rubella immunity among pregnant women in Kuwait: Screening in childbearing age should be reintroduced. Acta Tropica, 2001; 78(1):35–40. [DOI] [PubMed] [Google Scholar]
- 174.Caidi H, Bloom S, Azilmaat M, Benjouad A, Reef S, El Aouad R. Rubella seroprevalence among women aged 15–39 years in Morocco. Eastern Mediterranean Health Journal, 2009; 15(3):526–531. [PubMed] [Google Scholar]
- 175.Kohler KA, Suleiman AJM, Robertson SE, Malankar P, Al-Khusaiby S, Helfand RF, Brown D, Bellini WJ, Sutter RW. Immunogenicity of measles and rubella vaccines in Oman: A prospective clinical trial. Journal of Infectious Diseases, 2003; 187:S177–S185. [DOI] [PubMed] [Google Scholar]
- 176.Al-Awaidy S, Griffiths UK, Nwar HM, Bawikar S, Al-Aisiri MS, Khandekar R, Mohammad AJ, Robertson SE. Costs of congenital rubella syndrome (CRS) in Oman: Evidence based on long-term follow-up of 43 children. Vaccine, 2006; 24(40–41):6437–6445. [DOI] [PubMed] [Google Scholar]
- 177.Patel PK, Al-Awaidy ST, Bawikar S, Al-Busaidy S, Al-Mahrooqi S. Measles epidemiology and its implications for a vaccination programme in Oman. Eastern Mediterranean Health Journal, 2008; 14(3):579–589. [PubMed] [Google Scholar]
- 178.Khalil MK, Al-Mazrou YY, Al-Jeffri M, Al-Ghamdy YS. Measles immunization in Saudi Arabia: The need for change. Eastern Mediterranean Health Journal, 2001; 7(4–5):829–834. [PubMed] [Google Scholar]
- 179.Kamadjeu R, Assegid K, Naouri B, Mirza IR, Hirsi A, Mohammed A, Omer M, Dualle AH, Mulugeta A. Measles control and elimination in Somalia: The good, the bad, and the ugly. Journal of Infectious Diseases, 2011; 204 Suppl 1: S312–S317. [DOI] [PubMed] [Google Scholar]
- 180.Garbouj M, Slim A, Ben Ghorbal M, Khamassi S, Gaafar T, Moshni E, Lievano F. Progress toward interruption of endemic measles transmission in Tunisia. Journal of Infectious Diseases, 2003; 187:S172–S176. [DOI] [PubMed] [Google Scholar]
- 181.Bahri O, Ben Halima MB, Ben Ghorbal M, Dali K, Arrouji Z, Khammassi S, Triki H, Slim A. Measles surveillance and control in Tunisia: 1979–2000. Vaccine, 2003; 21(5–6):440–445. [DOI] [PubMed] [Google Scholar]
- 182.Sheek-Hussein M, Hashmey R, Alsuwaidi AR, Al Maskari F, Amiri L, Souid AK. Seroprevalence of measles, mumps, rubella, varicella-zoster and hepatitis A-C in Emirati medical students. BMC Public Health, 2012; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Expanded Programme on Immunization. Immunization schedules in the European region, 1995. Weekly Epidemiological Record, 1995; 70(31):221–228. [PubMed] [Google Scholar]
- 184.deMelker H, Pebody RG, Edmunds WJ, Levy-Bruhl D, Valle M, Rota MC, Salmaso S, vanden Hof S, Berbers G, Saliou P, Conyn-van Spaendonck M, Crovari P, Davidkin I, Gabutti G, Hesketh L, Morgan-Capner P, Plesner AM, Raux M, Tischer A, Miller E. The seroepidemiology of measles in western Europe. Epidemiology and Infection, 2001; 126(2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Spika JS, Wassilak S, Pebody R, Lipskaya G, Deshevoi S, Guris D, Emiroglu N. Measles and rubella in the World Health Organization European region: Diversity creates challenges. Journal of Infectious Diseases, 2003; 187:S191–S197. [DOI] [PubMed] [Google Scholar]
- 186.Spika JS, Hanon FX, Wassilak S, Pebody R, Emiroglu N. Preventing congenital rubella infection in the European region of WHO: 2010 target. Euro Surveillance, 2004; 9(4):4–5. [PubMed] [Google Scholar]
- 187.Nardone A, Tischer A, Andrews N, Backhouse J, Theeten H, Gatcheva N, Zarvou M, Kriz B, Pebody RG, Bartha K, O’Flanagan D, Cohen D, Duks A, Griskevicius A, Mossong J, Barbara C, Pistol A, Slacikova M, Prosenc K, Johansen K, Miller E. Comparison of rubella seroepidemiology in 17 countries: Progress towards international disease control targets. Bulletin of the World Health Organization, 2008; 86(2):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andrews N, Tischer A, Siedler A, Pebody RG, Barbara C, Cotter S, Duks A, Gacheva N, Bohumir K, Johansen K, Mossong J, deOry F, Prosenc K, Slacikovaa M, Theeten H, Zarvou M, Pistol A, Bartha K, Cohen D, Backhouse J, Griskevicius A, Nardone A. Towards elimination: Measles susceptibility in Australia and 17 European countries. Bulletin of the World Health Organization, 2008; 86(3):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bino S, Kakarriqi E, Xibinaku M, Ion-Nedelcu N, Bukli M, Emiroglu N, Uzicanin A. Measles-rubella mass immunization campaign in Albania, November 2000. Journal of Infectious Diseases, 2003; 187:S223–S229. [DOI] [PubMed] [Google Scholar]
- 190.Onishchenko G, Ezhlova E, Gerasimova A, Tsvirkun O, Shulga S, Lipskaya G, Mamayeva T, Aleshkin V, Tikhonova N. Progress toward measles elimination in the Russian Federation, 2003–2009. Journal of Infectious Diseases, 2011; 204 Suppl 1:S366–S372. [DOI] [PubMed] [Google Scholar]
- 191.Schmid D, Holzmann H, Abele S, Kasper S, Konig S, Meusburger S, Hrabcik H, Luckner-Hornischer A, Bechter E, DeMartin A, Stirling J, Heissenhuber A, Siedler A, Bernard H, Pfaff G, Schorr D, Ludwig MS, Zimmerman HP, Lovoll O, Aavitsland P, Allerberger F. An ongoing multi-state outbreak of measles linked to nonimmune anthroposophic communities in Austria, Germany, and Norway, March-April 2008. Euro Surveillance, 2008; 13(16):pii=18838. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18838. [PubMed] [Google Scholar]
- 192.Schmid D, Kasper S, Kuo HW, Aberle S, Holzmann H, Daghofer E, Wassermann-Neuhold M, Feenstra O, Krischka C, Allerberger F. Ongoing rubella outbreak in Austria, 2008–2009. Euro Surveillance, 2009; 14(16):pii=19184. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19184. [PubMed] [Google Scholar]
- 193.Schmid D, Holzmann H, Schwarz K, Kasper S, Kuo HW, Aberle SW, Redlberger-Fritz M, Hautmann W, Santibanez S, Mankertz A, Konig C, Magnet E, Reichart S, Meusburger S, Luckner-Hornischer A, DeMartin A, Bechter E, Stirling J, Allerberger F. Measles outbreak linked to a minority group in Austria, 2008. Epidemiology and Infection, 2010; 138(3):415–425. [DOI] [PubMed] [Google Scholar]
- 194.Samoilovich EO, Yermalovich MA, Semeiko GV, Svirchevskaya EI, Rimzha MI, Titov LP. Outbreak of measles in Belarus, January–June 2006. Euro Surveillance, 2006; 11(7):E060727.3. [DOI] [PubMed] [Google Scholar]
- 195.Belgium. Measles Elimination Plan, 2004. Available at: http://ecdc.europa.eu/en/activities/surveillance/euvac/Documents/Measles-Belgium-NationalPlan-2004.pdf, Accessed July 24, 2014.
- 196.Ikic DM. Edmonston-Zagreb strain of measles vaccine: Epidemiologic evaluation in Yugoslavia. Reviews of Infectious Diseases, 1983; 5(3):558–563. [PubMed] [Google Scholar]
- 197.Hukic M, Hubschen JM, Seremet M, Salimovic-Besic I, Mulaomerovic M, Mehinovic N, Karakas S, Charpentier E, Muller CP. An outbreak of rubella in the Federation of Bosnia and Herzegovina between December 2009 and May 2010 indicates failure to vaccinate during wartime (1992–1995). Epidemiology and Infection, 2012; 140(3): 447–453. [DOI] [PubMed] [Google Scholar]
- 198.Bojinova VS, Dimova PS, Belopitova LD, Mihailov AS, Gatcheva NL, Mihneva ZG, Hristova MT. Clinical and epidemiological characteristics of subacute sclerosing panencephalitis in Bulgaria during the past 25 years (1978–2002). European Journal of Paediatric Neurology, 2004; 8(2):89–94. [DOI] [PubMed] [Google Scholar]
- 199.Borcic B, Mazuran R, Kaic B. Immunity to measles in the Croatian population. European Journal of Epidemiology, 2003; 18(11):1079–1083. [DOI] [PubMed] [Google Scholar]
- 200.Kaic B, Gjenero-Margan I, Kurecic-Filipovic S, Muscat M. A measles outbreak in Croatia, 2008. Euro Surveillance, 2009; 14(1):pii=19083. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19083. [PubMed] [Google Scholar]
- 201.Sejda J Control of measles in Czechoslovakia (CSSR). Reviews of Infectious Diseases, 1983; 5(3):564–567. [DOI] [PubMed] [Google Scholar]
- 202.Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, Thorsen P, Olsen J, Melbye M. A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine, 2002; 347(19):1477–1482. [DOI] [PubMed] [Google Scholar]
- 203.Peltola H Stopping measles with a second vaccine dose. Archives De Pediatrie, 1998; 5(6):599–601. [DOI] [PubMed] [Google Scholar]
- 204.Peltola H, Davidkin I, Paunio M, Valle M, Leinikki P, Heinonen OP. Mumps and rubella eliminated from Finland. Journal of the American Medical Association, 2000; 284(20):2643–2647. [DOI] [PubMed] [Google Scholar]
- 205.Davidkin I, Kontio M, Paunio M, Peltola H. MMR vaccination and disease elimination: The Finnish experience. Expert Review of Vaccines, 2010; 9(9):1045–1053. [DOI] [PubMed] [Google Scholar]
- 206.Levy-Bruhl D, Six C, Parent I. Rubella control in France. Euro Surveillance, 2004; 9(4):15–16. [PubMed] [Google Scholar]
- 207.Parent du Chatelet I, Floret D, Antona D, Levy-Bruhl D. Measles resurgence in France in 2008, a preliminary report. Euro Surveillance, 2009; 14(6):pii = 19118. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19118. [PubMed] [Google Scholar]
- 208.Antona D, Levy-Bruhl D, Baudon C, Freymuth F, Lamy M, Maine C, Floret D, Parent du Chatelet I. Measles elimination efforts and 2008–2011 outbreak, France. Emerging Infectious Diseases, 2013; 19(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Doshi S, Khetsuriani N, Zakhashvili K, Baidoshvili L, Imnadze P, Uzicanin A. Ongoing measles and rubella transmission in Georgia, 2004–05: Implications for the national and regional elimination efforts. International Journal of Epidemiology, 2009; 38(1):182–191. [DOI] [PubMed] [Google Scholar]
- 210.Siedler A, Mankertz A, Feil F, Ahlemeyer G, Hornig A, Kirchner M, Beyrer K, Dreesman J, Scharkus S, Marcic A, Reiter S, Matysiak-Klose D, Santibanez S, Krause G, Wichmann O. Closer to the goal: Efforts in measles elimination in Germany 2010. Journal of Infectious Diseases, 2011; 204 Suppl 1:S373–S380. [DOI] [PubMed] [Google Scholar]
- 211.Poethko-Muller C, Mankertz A. Sero-epidemiology of measles-specific IgG antibodies and predictive factors for low or missing titres in a German population-based cross-sectional study in children and adolescents (KiGGS). Vaccine, 2011; 29(45):7949–7959. [DOI] [PubMed] [Google Scholar]
- 212.Poethko-Muller C, Mankertz A. Seroprevalence of measles-, mumps- and rubella-specific IgG antibodies in German children and adolescents and predictors for seronegativity. PLoS One, 2012; 7(8):e42867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gioula G, Diza-Mataftsi E, Alexiou-Daniel S, Kyriazopoulou-Dalaina V. Seroepidemiology of rubella in northern Greece. European Journal of Clinical Microbiology and Infectious Diseases, 2004; 23(8):631–633. [DOI] [PubMed] [Google Scholar]
- 214.Gioula G, Fylaktou A, Exindari M, Atmatzidis G, Chatzidimitriou D, Melidou A, Kyriazopoulou-Dalaina V. Rubella immunity and vaccination coverage of the population of northern Greece in 2006. Euro Surveillance, 2007; 12(11):E9–E10. [DOI] [PubMed] [Google Scholar]
- 215.Measles—Hungary. Morbidity and Mortality Weekly Report 1989; 38(39):665–668. [PubMed] [Google Scholar]
- 216.Agocs MM, Markowitz LE, Straub I, Domok I. The 1988–1989 measles epidemic in Hungary: Assessment of vaccine failure. International Journal of Epidemiology, 1992; 21(5):1007–1013. [DOI] [PubMed] [Google Scholar]
- 217.Gudmundsdottir S, Antonsdottir A, Gudnadottir S, Elefsen S, Einarsdottir B, Olafsson O, Gudnadottir M. Prevention of congenital rubella in Iceland by antibody screening and immunization of seronegative females. Bulletin of the World Health Organization, 1985; 63(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 218.Johnson H, Hillary IB, McQuoid G, Gilmer BA. MMR vaccination, measles epidemiology and sero-surveillance in the Republic of Ireland. Vaccine, 1995; 13(6):533–537. [DOI] [PubMed] [Google Scholar]
- 219.Coughlan S, Connell J, Cohen B, Jin L, Hall WW. Suboptimal measles-mumps-rubella vaccination coverage facilitates an imported measles outbreak in Ireland. Clinical Infectious Diseases, 2002; 35(1):84–86. [DOI] [PubMed] [Google Scholar]
- 220.Ginsberg GM, Tulchinsky TH. Costs and benefits of a second measles inoculation of children in Israel, the West Bank, and Gaza. Journal of Epidemiology and Community Health, 1990; 44(4):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tulchinsky TH, Abed Y, Ginsberg G, Shaheen S, Friedman JB, Schoenbaum ML, Slater PE. Measles in Israel, the West Bank, and Gaza: Continuing incidence and the case for a new eradication strategy. Reviews of Infectious Diseases, 1990; 12(5):951–958. [DOI] [PubMed] [Google Scholar]
- 222.Rota MC, Bella A, Gabutti G, Giambi C, Filia A, Guido M, DeDonno A, Crovari P, Ciofi Degli Atti ML. Rubella seroprofile of the Italian population: An 8-year comparison. Epidemiology and Infection, 2007; 135(4):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Filia A, Bella A, Rota MC, Tavilla A, Magurano F, Baggieri M, Nicoletti L, Iannazzo S, Pompa MG, Declich S. Analysis of national measles surveillance data in Italy from October 2010 to December 2011 and priorities for reaching the 2015 measles elimination goal. Euro Surveillance, 2013; 18(20):18–24. [PubMed] [Google Scholar]
- 224.Akmatov MK, Kretzschmar M, Kramer A, Mikolajczyk RT. Determinants of childhood vaccination coverage in Kazakhstan in a period of societal change: Implications for vaccination policies. Vaccine, 2007; 25(10):1756–1763 [DOI] [PubMed] [Google Scholar]
- 225.Dayan GH, Zimmerman L, Shteinke L, Kasymbekova K, Uzicanin Z, Strebel P, Reef S. Investigation of a rubella outbreak in Kyrgyzstan in 2001: Implications for an integrated approach to measles elimination and prevention of congenital rubella syndrome. Journal of Infectious Diseases, 2003; 187:S235–S240. [DOI] [PubMed] [Google Scholar]
- 226.Malakmadze N, Zimmerman LA, Uzicanin A, Shteinke L, Caceres VM, Kasymbekova K, Sozina I, Glasser JW, Joldubaeva M, Aidyralieva C, Icenogle JP, Strebel PM, Reef SE. Development of a rubella vaccination strategy: Contribution of a rubella susceptibility study of women of childbearing age in Kyrgyzstan, 2001. Clinical Infectious Diseases, 2004; 38(12):1780–1783. [DOI] [PubMed] [Google Scholar]
- 227.Rix BA, Zhobakas A, Wachmann CH, Bakasenas V, Ronne T. Immunity from diphtheria, tetanus, poliomyelitis, measles, mumps and rubella among adults in Lithuania. Scandinavian Journal of Infectious Diseases, 1994; 26(4):459–467. [DOI] [PubMed] [Google Scholar]
- 228.Mossong J, Putz L, Schneider F. Seroprevalence of measles, mumps and rubella antibodies in Luxembourg: Results from a national cross-sectional study. Epidemiology and Infection, 2004; 132(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kuzmanovska G, Polozhani A, Mikik V, Stavridis K, Aleksoski B, Cvetanovska Z, Binnendijk R, Bosevska G. Mumps outbreak in the former Yugoslav Republic of Macedonia, January 2008–June 2009: Epidemiology and control measures. Euro Surveillance, 2010; 15(23):13–21. [PubMed] [Google Scholar]
- 230.Spiteri G, Fenech Magrin AM, Muscat M. A cluster of rubella in Malta, December 2007–January 2008. Euro Surveillance, 2008; 13(16):pii=18840. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18840. [PubMed] [Google Scholar]
- 231.Laušević D, Tiodorović B, Rakočević B, Medenica V, Beatović V, Hadžifejzović A. Timeliness and level of primary immunization coverage against measles and rubella in Montenegro. Acta Medica Medianae, 2009; 48(3):9–14. [Google Scholar]
- 232.Noah ND. Immunisation before school entry: Should there be a law? British Medical Journal (Clinical Research Edition), 1987; 294(6582):1270–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zimmerman L, Rogalska J, Wannemuehler KA, Haponiuk M, Kosek A, Pauch E, Plonska E, Veltze D, Czarkowski MP, Buddh N, Reef S, Stefanoff P. Toward rubella elimination in Poland: Need for supplemental immunization activities, enhanced surveillance, and further integration with measles elimination efforts. Journal of Infectious Diseases, 2011; 204 Suppl 1:S389–S395. [DOI] [PubMed] [Google Scholar]
- 234.Goncalves G, Nascimento MSJ, Reu C, Cutts FT. Levels of rubella antibody among vaccinated and unvaccinated Portuguese mothers and their newborns. Vaccine, 2006; 24(49–50):7142–7147. [DOI] [PubMed] [Google Scholar]
- 235.Giria M, Rebelo-de-Andrade H, Fernandes T, Pedro S, Freitas G. Report on the measles situation in Portugal. Euro Surveillance, 2008; 13(42):pii=19010. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19010. [PubMed] [Google Scholar]
- 236.Hennessey KA, Ion-Nedelcu N, Craciun MD, Toma F, Wattigney W, Strebel PM. Measles epidemic in Romania, 1996–1998: Assessment of vaccine effectiveness by case-control and cohort studies. American Journal of Epidemiology, 1999; 150(11):1250–1257. [DOI] [PubMed] [Google Scholar]
- 237.Pistol A, Hennessey K, Pitigoi D, Ion-Nedelcu N, Lupulescu E, Walls L, Bellini W, Strebel P. Progress toward measles elimination in Romania after a mass vaccination campaign and implementation of enhanced measles surveillance. Journal of Infectious Diseases, 2003; 187:S217–S222. [DOI] [PubMed] [Google Scholar]
- 238.Seguliev Z, Duric P, Petrovic V, Stefanovic S, Cosic G, Hrnjakovic IC, Milosevic V, Karagiannis I, Boxall N, Jankovic D. Current measles outbreak in Serbia: A preliminary report. Euro Surveillance, 2007; 12(3):E070315.2. [DOI] [PubMed] [Google Scholar]
- 239.Grgic-Vitek M, Frelih T, Ucakar V, Prosenc K, Tomazic J, Petrovec M, Kraigher A. Spotlight on measles 2010: A cluster of measles in a hospital setting in Slovenia, March 2010. Euro Surveillance, 2010; 15(20):5. [PubMed] [Google Scholar]
- 240.Amela C, Pachon I, de Ory F. Evaluation of the measles, mumps and rubella immunisation programme in Spain by using a sero-epidemiological survey. European Journal of Epidemiology, 2003; 18(1):71–79. [DOI] [PubMed] [Google Scholar]
- 241.Lemos C, Ramirez R, Ordobas M, Guibert DH, Sanz JC, Garcia L, Martinez Navarro JF. New features of rubella in Spain: The evidence of an outbreak. Euro Surveillance, 2004; 9(4):9–11. [PubMed] [Google Scholar]
- 242.Bottiger M, Christenson B, Romanus V, Taranger J, Strandell A. Swedish experience of two dose vaccination programme aiming at eliminating measles, mumps, and rubella. British Medical Journal (Clinical Research Edition), 1987; 295(6608):1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Matter HC, Cloetta J, Zimmermann H. Measles, mumps, and rubella: Monitoring in Switzerland through a sentinel network, 1986–94. Journal of Epidemiology and Community Health, 1995; 49 Suppl 1:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matter L, Bally F, Germann D, Schopfer K. The incidence of rubella virus infections in Switzerland after the introduction of the MMR mass vaccination programme. European Journal of Epidemiology, 1995; 11(3):305–310. [DOI] [PubMed] [Google Scholar]
- 245.Matter L, Germann D, Bally F, Schopfer K. Age-stratified seroprevalence of measles, mumps and rubella (MMR) virus infections in Switzerland after the introduction of MMR mass vaccination. European Journal of Epidemiology, 1997; 13(1):61–66. [DOI] [PubMed] [Google Scholar]
- 246.de Haas R, van den Hof S, Berbers GA, deMelker HE, Conyn-van Spaendonck MA. Prevalence of antibodies against rubella virus in the Netherlands 9 years after changing from selective to mass vaccination. Epidemiology and Infection, 1999; 123(2):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.van den Hof S, Berbers GAM, de Melker HE, Conyn-van Spaendonck MAE. Sero-epidemiology of measles antibodies in the Netherlands, a cross-sectional study in a national sample and in communities with low vaccine coverage. Vaccine, 1999; 18(9–10):931–940. [DOI] [PubMed] [Google Scholar]
- 248.van den Hof S, Conyn-van Spaendonck C, de Melker HE, Geubbels ELPE, Suijkerbuijk AW, Talsma E, Plantinga AD, Rumke HC. The Effects of Vaccination, the Incidence of the Target Diseases, 1998. Bilthoven, The Nethelands: RIVM National Institute of Public Health and the Environment, Available at: http://www.rivm.nl/bibliotheek/rapporten/213676008.html, Accessed September 10, 2014. [Google Scholar]
- 249.Kuyucu N, Dogru U, Akar N. Antibody response to measles vaccination in Turkish children. Infection, 1996; 24(2):156–158. [DOI] [PubMed] [Google Scholar]
- 250.Guris D, Bayazit Y, Ozdemirer U, Buyurgan V, Yalniz C, Toprak I, Aycan S. Measles epidemiology and elimination strategies in Turkey. Journal of Infectious Diseases, 2003; 187:S230–S234. [DOI] [PubMed] [Google Scholar]
- 251.Bozkurt AI, Bostanci M, Cevahir N, Ergin A, Sancak I, Yilmaz B, Catak B. Evaluation of a mass measles vaccination campaign among school children aged 7–14 years old in Denizli, Turkey. Indian Journal of Pediatrics, 2010; 77(8):879–883. [DOI] [PubMed] [Google Scholar]
- 252.Velicko I, Muller LL, Pebody R, Gergonne B, Aidyralieva C, Kostiuchenko N, Spika JS. Nationwide measles epidemic in Ukraine: The effect of low vaccine effectiveness. Vaccine 2008; 26(52):6980–6985. [DOI] [PubMed] [Google Scholar]
- 253.Miller CL. Current impact of measles in the United Kingdom. Reviews of Infectious Diseases, 1983; 5(3):427–432. [DOI] [PubMed] [Google Scholar]
- 254.Tobin JO, Sheppard S, Smithells RW, Milton A, Noah N, Reid D. Rubella in the United Kingdom, 1970–1983. Reviews of Infectious Diseases, 1985; 7 Suppl 1:S47–S52. [PubMed] [Google Scholar]
- 255.Miller E Rubella in the United Kingdom. Epidemiology and Infection, 1991; 107(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Parkman PD. Making vaccination policy: The experience with rubella. Clinical Infectious Diseases, 1999; 28:S140–S146. [DOI] [PubMed] [Google Scholar]
- 257.Expanded Programme on Immunization (EPI). Immunization schedules in the WHO South-East Asia region, 1995. Weekly Epidemiological Record, 1996; 71(13):100–102. [PubMed] [Google Scholar]
- 258.O’Connor PM, Liyanage JB, Mach O, Anand A, Ramamurty N, Balakrishnan MR, Singh S. South-East Asia regional update on measles mortality reduction and elimination, 2003–2008. Journal of Infectious Diseases, 2011; 204 Suppl 1:S396–402. [DOI] [PubMed] [Google Scholar]
- 259.Wiesen E, Wannemuehler K, Goodson JL, Anand A, Mach O, Thapa A, O’Connor P, Linayage J, Diorditsa S, Hasan AS, Uzzaman S, Jalil Mondal MD. Stability of the age distribution of measles cases over time during outbreaks in Bangladesh, 2004–2006. Journal of Infectious Diseases, 2011; 204 Suppl 1:S414–S420. [DOI] [PubMed] [Google Scholar]
- 260.Giri BR, Namgyal P, Tshering K, Sharma K, Dorji T, Tamang C. Mass measles rubella immunization campaign: Bhutan experience. Indian Journal of Community Medicine, 2011; 36(2):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ramamurty N, Raja D, Gunasekaran P, Varalakshmi E, Mohana S, Jin L. Investigation of measles and rubella outbreaks in Tamil Nadu, India—2003. Journal of Medical Virology, 2006; 78(4):508–513. [DOI] [PubMed] [Google Scholar]
- 262.Awofeso N, Rammohan A, Iqbal K. Age-appropriate vaccination against measles and DPT-3 in India—Closing the gaps. BMC Public Health, 2013; 13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fernandez R, Rammohan A, Awofeso N. Correlates of first dose of measles vaccination delivery and uptake in Indonesia. Asian Pacific Journal of Tropical Medicine, 2011; 4(2):140–145. [DOI] [PubMed] [Google Scholar]
- 264.Chin J, Thaung UM. The unchanging epidemiology and toll of measles in Burma. Bulletin of the World Health Organization, 1985; 63(3):551–558. [PMC free article] [PubMed] [Google Scholar]
- 265.Palihawadana P, Wickremasinghe AR, Perera J. Seroprevalence of rubella antibodies among pregnant females in Sri Lanka. Southeast Asian Journal of Tropical Medicine and Public Health, 2003; 34(2):398–404. [PubMed] [Google Scholar]
- 266.Ueda S, Okuno Y, Sangkawibha N, Jayavasu J, Tuchinda P. Studies on measles in Thailand. 1. Seroepidemiological examination. Biken Journal, 1967; 10(3):129–133. [PubMed] [Google Scholar]
- 267.Puthavathana P, Wasi C, Kositanont U, Lamkom R, Thongcharoen P. Rubella outbreak in Thailand 1983–1984: A study at Siriraj hospital. Southeast Asian Journal of Tropical Medicine and Public Health, 1985; 16(2):207–213. [PubMed] [Google Scholar]
- 268.Tharmaphornpilas P, Yoocharean P, Rasdjarmrearnsook A, Theamboonlers A, Poovorawan Y. Seroprevalence of antibodies to measles, mumps, and rubella among Thai population: Evaluation of measles/MMR immunization programme. Journal of Health, Population and Nutrition, 2009; 27(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lumbiganon P, Sookpranee T, Tattawasart U, Sukprasert S, Paholpak S. Measles immunisation in Thai children aged nine to 14 months. Asia-Pacific Journal of Public Health, 1988; 2(4):241–244. [DOI] [PubMed] [Google Scholar]
- 270.Expanded Programme on Immunization (EPI). Immunization schedules in the WHO Western Pacific region, 1995. Weekly Epidemiological Record 1996; 71(18):133–137. [PubMed] [Google Scholar]
- 271.Sniadack DH, Mendoza-Aldana J, Jee Y, Bayutas B, Lorenzo-Mariano KM. Progress and challenges for measles elimination by 2012 in the Western Pacific region. Journal of Infectious Diseases, 2011; 204 Suppl 1:S439–S446. [DOI] [PubMed] [Google Scholar]
- 272.Menser MA, Hudson JR, Murphy AM, Upfold LJ. Epidemiology of congenital rubella and results of rubella vaccination in Australia. Reviews of Infectious Diseases, 1985; 7 Suppl 1:S37–S41. [DOI] [PubMed] [Google Scholar]
- 273.Gilbert GL, Escott RG, Gidding HF, Turnbull FM, Heath TC, McIntyre PB, Burgess MA. Impact of the Australian measles control campaign on immunity to measles and rubella. Epidemiology and Infection, 2001; 127(2):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Han YR, Zhao K, Gong YX, Hao SL, Wang SZ, Wang CT. Rubella vaccine in the People’s Republic of China. Reviews of Infectious Diseases, 1985; 7 Suppl:1:S79. [PubMed] [Google Scholar]
- 275.Ma YJ, Bo F, Sun ZD, Huang H, Gao SR, An ZJ. Epidemiological investigation of measles in sera of healthy people in Heilongjiang province, China. Japanese Journal of Infectious Diseases, 2011; 64(3):208–210. [PubMed] [Google Scholar]
- 276.Li X, Kang D, Zhang Y, Wei G, Liu W, Fang L, Yang H, Cao W. Epidemic trend of measles in Shandong province, China, 1963–2005. Public Health, 2012; 126(12):1017–1023. [DOI] [PubMed] [Google Scholar]
- 277.Shortridge KF, Osmund IF. Seroepidemiology of rubella infection in Chinese and Caucasians in Hong Kong. Journal of Hygiene, 1979; 83(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lau YL, Chow CB, Leung TH. Changing epidemiology of measles in Hong Kong from 1961 to 1990—Impact of a measles vaccination program. Journal of Infectious Diseases, 1992; 165(6):1111–1115. [DOI] [PubMed] [Google Scholar]
- 279.Chuang SK, Lau YL, Lim WL, Chow CB, Tsang T, Tse LY. Mass measles immunization campaign: Experience in the Hong Kong special administrative region of China. Bulletin of the World Health Organization, 2002; 80(7):585–591. [PMC free article] [PubMed] [Google Scholar]
- 280.Measles outbreak and response—Fiji, February—May 2006. Morbidity and Mortality Weekly Report 2006; 55(35):963–966. [PubMed] [Google Scholar]
- 281.Singh S, Bingwor F, Tayler-Smith K, Manzi M, Marks GB. Congenital rubella syndrome in Fiji, 1995–2010. Journal of Tropical Medicine, 2013; 2013:956234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hirayama M Measles vaccines used in Japan. Reviews of Infectious Diseases, 1983; 5(3):495–503. [DOI] [PubMed] [Google Scholar]
- 283.Terada K. Rubella and congenital rubella syndrome in Japan: Epidemiological problems. Japanese Journal of Infectious Diseases, 2003; 56(3):81–87. [PubMed] [Google Scholar]
- 284.Nishiura C, Hashimoto H. Screening for measles vaccination in young Japanese non-healthcare workers through self-reported history. Journal of Occupational Health, 2012; 54(2):154–157. [DOI] [PubMed] [Google Scholar]
- 285.Elimination of measles—South Korea, 2001–2006. Morbidity and Mortality Weekly Report 2007; 56(13):304–307. [PubMed] [Google Scholar]
- 286.Choe YJ, Bae GR. Current status of measles in the Republic of Korea: An overview of case-based and seroepidemiological surveillance scheme. Korean Journal of Pediatrics, 2012; 55(12):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Phimmasane M, Douangmala S, Koffi P, Reinharz D, Buisson Y. Factors affecting compliance with measles vaccination in Lao PDR. Vaccine, 2010; 28(41):6723–6729. [DOI] [PubMed] [Google Scholar]
- 288.Phengxay M, Hayakawa Y, Phan TG, Uneno-Yamamoto K, Tanaka-Taya K, Vongphrachanh P, Komase K, Ushijima H. Seroprevalence of rubella and measles antibodies in Lao PDR. Clinical Laboratory, 2011; 57(3–4):237–244. [PubMed] [Google Scholar]
- 289.Lo EK, AliHussein N. Measles in Malaysia: A brief report of the 1981 anti-measles pilot study. Reviews of Infectious Diseases, 1983; 5(3):405. [DOI] [PubMed] [Google Scholar]
- 290.Chen ST, Lam SK. Optimum age for measles immunization in Malaysia. Medical Journal of Malaysia, 1985; 40(4):281–288. [PubMed] [Google Scholar]
- 291.Tan DS. Congenital rubella syndrome in Malaysia. Medical Journal of Malaysia, 1985; 40(1):11–14. [PubMed] [Google Scholar]
- 292.Narimah A Rubella immunization in Malaysia—20 years on, and the challenges ahead. Medical Journal of Malaysia, 2005; 60(3):267–268. [PubMed] [Google Scholar]
- 293.Sekawi Z, Muizatul WM, Marlyn M, Jamil MA, Ilina I. Rubella vaccination programme in Malaysia: Analysis of a seroprevalence study in an antenatal clinic. Medical Journal of Malaysia, 2005; 60(3):345–348. [PubMed] [Google Scholar]
- 294.McIntyre RC, Preblud SR, Polloi A, Korean M. Measles and measles vaccine efficacy in a remote island population. Bulletin of the World Health Organization, 1982; 60(5):767–775. [PMC free article] [PubMed] [Google Scholar]
- 295.Riseley RC, Smith AH, Laugesen M, Chapman CJ. Computer assessment of alternative rubella vaccination strategies in New Zealand. New Zealand Medical Journal, 1983; 96(729):235–238. [PubMed] [Google Scholar]
- 296.Roberts MGT MI. Predicting and preventing measles epidemics in New Zealand: Application of a mathematical model. Epidemiology and Infection, 2000; 124(2):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Senn N, Riddell M, Omena M, Siba P, Reeder JC, Clements CJ, Morgan C. Measles in Papua New Guinea: An age-specific serological survey. Vaccine, 2010; 28(7):1819–1823. [DOI] [PubMed] [Google Scholar]
- 298.Manning L, Laman M, Edoni H, Mueller I, Karunajeewa HA, Smith D, Hwaiwhanje I, Siba PM, Davis TM. Subacute sclerosing panencephalitis in Papua New Guinean children: The cost of continuing inadequate measles vaccine coverage. PLoS Neglected Tropical Diseases, 2011; 5(1):e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Riddell M, Senn N, Clements CJ, Hobday L, Cowie B, Kurubi J, Kevin A, Siba P, Reeder JC, Morgan C. Rubella control in Papua New Guinea: Age-specific immunity informs strategies for introduction of rubella vaccine. Vaccine, 2012; 30(52):7506–7512. [DOI] [PubMed] [Google Scholar]
- 300.Ong G, Hoon HB, Ong A, Chua LT, Kai CS, Tai GK. A 24-year review on the epidemiology and control of measles in Singapore, 1981–2004. Southeast Asian Journal of Tropical Medicine and Public Health, 2006; 37(1):96–101. [PubMed] [Google Scholar]
- 301.Ang LW, Lai FY, Tey SH, Cutter J, James L, Goh KT. Prevalence of antibodies against measles, mumps and rubella in the childhood population in Singapore, 2008–2010. Epidemiology and Infection, 2013; 141(8):1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Otten M, Kezaala R, Fall A, Masresha B, Martin R, Cairns L, Eggers R, Biellik R, Grabowsky M, Strebel P, Okwo-Bele JM, Nshimirimana D. Public-health impact of accelerated measles control in the WHO African region 2000–03. Lancet, 2005; 366(9488):832–839. [DOI] [PubMed] [Google Scholar]
- 303.Biellik RJ, Brown DWG. Measles mortality reduction in Africa. Lancet, 2009; 373(9668):984–985. [DOI] [PubMed] [Google Scholar]
- 304.Zuber PLF, Conombo KSG, Traore AD, Millogo JD, Ouattara A, Ouedraogo IB, Valian A. Mass measles vaccination in urban Burkina Faso, 1998. Bulletin of the World Health Organization, 2001; 79(4):296–300. [PMC free article] [PubMed] [Google Scholar]
- 305.Kambire C, Konde MK, Yameogo A, Tiendrebeogo SRM, Ouedraogo RT, Otten MW, Cairns KL, Zuber PLF. Measles incidence before and after mass vaccination campaigns in Burkina Faso. Journal of Infectious Diseases, 2003; 187:S80–S85. [DOI] [PubMed] [Google Scholar]
- 306.Yameogo KR, Perry RT, Yameogo A, Kambire C, Konde MK, Nshimirimana D, Kezaala R, Hersh BS, Cairns KL, Strebel P. Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. International Journal of Epidemiology, 2005; 34(3):556–564. [DOI] [PubMed] [Google Scholar]
- 307.McBean AM, Foster SO, Herrmann KL, Gateff C. Evaluation of a mass measles immunization campaign in Yaounde, Cameroun. Transactions of the Royal Society of Tropical Medicine and Hygiene, 1976; 70(3):206–212. [DOI] [PubMed] [Google Scholar]
- 308.Luquero FJ, Pham-Orsetti H, Cummings DA, Ngaunji PE, Nimpa M, Fermon F, Ngoe N, Sosler S, Strebel P, Grais RF. A long-lasting measles epidemic in Maroua, Cameroon 2008–2009: Mass vaccination as response to the epidemic. Journal of Infectious Diseases, 2011; 204 Suppl 1:S243–S251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Guerrier G, Guerra J, Fermon F, Talkibing WB, Sekkenes J, Grais RF. Outbreak response immunisation: The experience of Chad during recurrent measles epidemics in 2005 and 2010. International Health, 2011; 3(4):226–230. [DOI] [PubMed] [Google Scholar]
- 310.Belcher DW, Nicholas DD, Ofosu-Amaah S, Wurapa FK. A mass immunization campaign in rural Ghana. Factors affecting participation. Public Health Reports, 1978; 93(2):170–176. [PMC free article] [PubMed] [Google Scholar]
- 311.Harry TO. Anti-measles IgM in healthy adult Nigerians. Journal of Tropical Medicine and Hygiene, 1981; 84(4):171–173. [PubMed] [Google Scholar]
- 312.Essombam P Serological research of rubella in Cameroon and Senegal by hemagglutination inhibition test. Médecine et Maladies Infectieuses, 1973; 3:501–502. [Google Scholar]
- 313.Khetsuriani N, Deshevoi S, Goel A, Spika J, Martin R, Emiroglu N. Supplementary immunization activities to achieve measles elimination: Experience of the European region. Journal of Infectious Diseases, 2011; 204 Suppl 1:S343–S352. [DOI] [PubMed] [Google Scholar]
- 314.Pebody RG, Edmunds WJ, Conyn-van Spaendonck M, Olin P, Berbers G, Rebiere I, Lecoeur H, Crovari P, Davidkin I, Gabutti G, Gerike E, Giordano C, Hesketh L, Plesner AM, Raux M, Rota MC, Salmaso S, Tischer A, Valle M, Miller E. The seroepidemiology of rubella in western Europe. Epidemiology and Infection, 2000; 125(2):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Progress towards the 2012 measles elimination goal in WHO’s Western Pacific region, 1990–2008. Weekly Epidemiological Record 2009; 84(27):271–279. [PubMed] [Google Scholar]


