Abstract
Transport in and out of the endolysosomal compartment represents a key step in the regulation of cellular cholesterol homeostasis. Despite important recent advances, how LDL-derived, free cholesterol is exported from the lumen of endolysosomes to other organelles is still a matter of debate. We recently devised a CRISPR/Cas9 genome-scale strategy to uncover genes involved in the regulation of endolysosomal cholesterol homeostasis and the functionally linked phospholipid, bis(monoacylglycerol)-phosphate. This approach confirmed known genes and pathways involved in this process, and more importantly revealed previously unrecognized roles for new players, such as Sorting Nexin-13 (SNX13). Here we discuss the unexpected regulatory role of SNX13 in endolysosomal cholesterol export.
Keywords: endolysosomes, cholesterol transport, endoplasmic reticulum, membrane contact sites, niemann-pick disease
The importance of cholesterol in cell regulation is widely appreciated, and cells have evolved an elaborate repertoire of regulatory mechanisms to finely tune cholesterol homeostasis. Maintenance of cellular cholesterol content is governed by two main processes: Low density lipoprotein (LDL) uptake and de novo cholesterol biosynthesis. LDL uptake is controlled by multiple genes that drive LDL receptor (LDLR) endocytosis, recycling and ubiquitylation (Lu et al., 2022). Once within the endolysosomal compartment, cholesterol esters derived from LDL particles are cleaved by lysosomal acid lipase, and free cholesterol intracellular transport begins. First, cholesterol is extracted from intralumenal vesicles (ILVs) by Niemann-Pick C2 (NPC2) protein, a process enhanced by binding to bis(monoacylglycerol)phosphate (BMP; McCauiff et al., 2019). BMP is a unique glycerophospholipid exclusively enriched in ILVs (Gruenberg, 2020). Next, NPC2 transfers cholesterol to membrane-bound Niemann-Pick C1 (NPC1) that facilitates its transfer to the outer leaflet of the endolysosomal membrane (Pfeffer, 2019). Loss-of-function mutations in NPC1/2 genes cause Niemann-Pick type C (NPC) disease, a fatal lysosomal storage disorder characterized by cholesterol accumulation in endolysosomes at the cellular level. NPC patients suffer devastating neurodegenerative symptoms.
Previous structural work has begun to elucidate how cholesterol is translocated by NPC1 from the lumenal side, through the dense glycocalyx, to the cytosolic surface of endolysosomes (Li and Pfeffer, 2016; Li et al., 2016a; Li et al., 2016b; Winkler et al., 2019; Long et al., 2020; Qian et al., 2020). However, it is still unclear what happens next to transfer cholesterol to the endoplasmic reticulum (ER), a central cholesterol homeostatic sensor. Immediately after its exit from endolysosomes, free cholesterol is first transported to the plasma membrane (PM) before reaching the ER (Das et al., 2014; Trinh et al., 2020; Trinh et al., 2022). In addition, endolysosomal-ER membrane contacts sites (MCS) have been described as an alternative direct route, bypassing the PM (Höglinger et al., 2019; Meneses-Salas et al., 2020). Once in the ER, cholesterol is redistributed to other membranes or, when in excess, is re-esterified and stored in lipid droplets.
BMP is essential for proper cholesterol export from endolysosomes. BMP also functions as a co-factor for lysosomal sphingolipid hydrolases. Consequently, antibody-mediated disruption of BMP activities profoundly alters endolysosomal homeostasis (Gruenberg, 2020). Indeed, intracellular BMP levels are secondarily elevated in numerous lysosomal storage disorders, including NPC (Gruenberg, 2020). Nevertheless, how BMP is synthesized and degraded in cells is still a mystery. The fact that phosphatidylglycerol (PG), a structural isomer and precursor of BMP, is synthesized and confined to mitochondria has led to speculation that mitophagy may provide a source of PG for BMP biosynthesis in endolysosomes (Gruenberg, 2020).
We recently set out to uncover novel homeostatic regulators of cholesterol and its functional partner, BMP (Lu et al., 2022). To do this, genome-wide CRISPR-edited cells were stained for cholesterol using fluorescent, Perfringolysin-O* protein or for BMP using a specific anti-BMP antibody. Stained cells were then sorted by flow cytometry according to high or low lipid levels. By quantifying sgRNA enrichment in each sorted cell population, genes that upon deletion increased or decreased either lipid were uncovered. Screens were performed under control conditions and conditions where NPC1 was pharmacologically inhibited with U18666A. This complementary approach allowed us to recapitulate well-established genes and pathways involved in cholesterol metabolism and intracellular transport, and enabled the discovery of new players involved in these processes.
Many genes whose deletion increased or decreased cholesterol, yielded equivalent phenotypes for BMP, supporting their functional connection (Gruenberg, 2020). Furthermore, the majority of hits revealed in the screens were negative regulators: their absence increased lipid levels, even under NPC1 inhibition conditions. These findings suggest that cells have naturally evolved more mechanisms to prevent accumulation of free cholesterol, potentially harmful for the cell. Conversely, fewer genes were able to lower overall cellular cholesterol or to rescue the NPC phenotype when knocked out. Such genes deserve particular attention, since they represent potential therapeutic targets that could benefit NPC patients.
Interestingly, loss of either SNX13 and SNX14, two paralogs of the poorly characterized sorting nexin (SNX)-RGS family of SNXs (Hariri and Henne, 2022), rescued the lipid accumulation phenotype seen upon inhibition of NPC1. Remarkably, SNX13-depleted cells exhibited clear PM redistribution of cholesterol without altering total cholesterol content, suggesting that SNX13 functions as a negative regulator of an NPC1 bypass pathway towards the PM. Knockdown of SNX13 also increased the amount of lipid droplets (LDs). Moreover, these cells displayed altered levels of triglycerides (TAGs), free fatty acids and BMP isomers. Previous work showed that knockout of the yeast ortholog MDM1 also leads to increased LD numbers, altered levels of neutral lipids (including TAGs) and susceptibility to fatty acid-induced lipotoxicity (Henne et al., 2015; Hariri et al., 2019). Consistent with the latter phenotype, SNX13 silencing or overexpression is toxic for mammalian cells (our unpublished observations), which may explain its very low abundance in cells (1,812 copies of SNX13, 947,857 copies of LAMP1 or 29,193 copies of NPC1 per HeLa cell (Itzhak et al., 2016)).
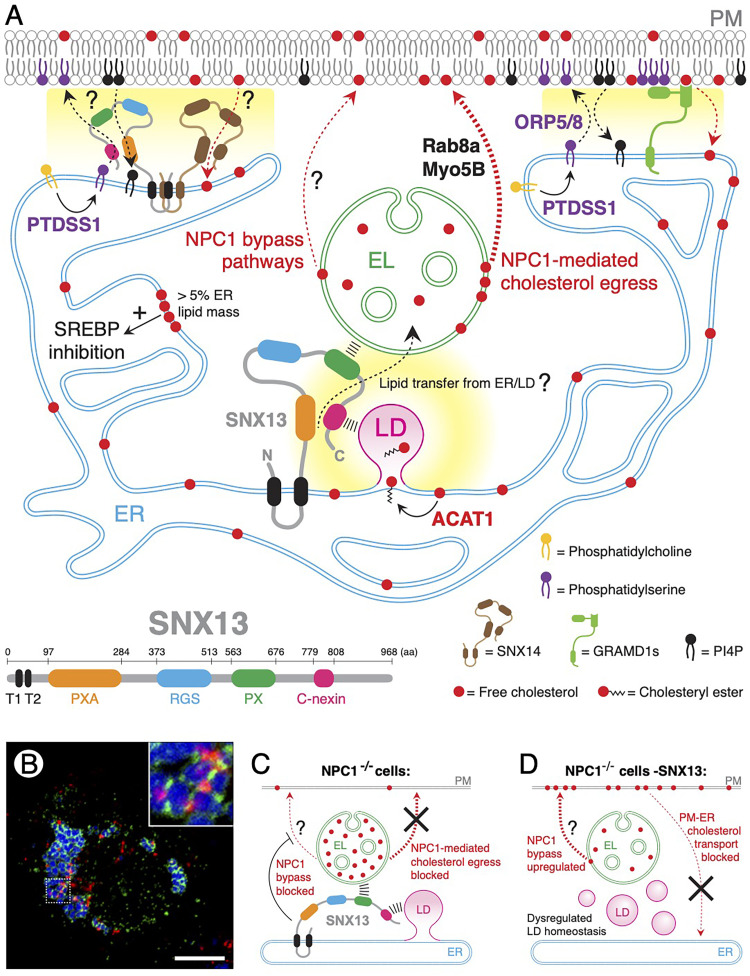
SNX13 and the family of human SNX-RGS proteins are ER-resident transmembrane proteins characterized by a multidomain-containing structure (Figure 1A). Structural analysis revealed that their N-terminal PXA domain together with their C-terminal PXC domain (also known as C-nexin domain; see Figure 1A) are predicted to fold into a unique structure harboring a hydrophobic pocket that is likely to serve for mobilizing lipids (Paul et al., 2022), suggesting that this protein family may represent a novel class of lipid transfer proteins. In addition to this, all SNX-RGS proteins contain a PX domain that, in most cases, binds to phosphoinositides (PIs) and other lipids. While SNX13's PX domain binds endosomal phosphatidylinositol 3-phosphate (PI3P) and PI(3,5)P2 or PM-enriched PI4P, SNX14 does not seem to bind any PI species (Zheng et al., 2001; Chandra et al., 2019). PI binding enables SNX-RGS proteins to function as molecular tethers between the ER and endosomal compartments (Hariri and Henne, 2022). Our study revealed that SNX13 also displays triorganelle tethering activity between ER domains, LDs and BMP-positive endolysosomes (Figure 1B). Moreover, overexpression of SNX13 resulted in increased numbers and stability of ER-endolysosome contacts, further suggesting a role for this protein as an interorganelle tethering factor (Lu et al., 2022).
Figure 1.
Proposed roles for SNX13 in cholesterol transport regulation. (A) Bottom left, SNX13 multidomain structure is shown. SNX13 is an endoplasmic reticulum (ER)-localized transmembrane protein that mediates ER-endolysosome (EL) and ER-lipid droplet (LD) membrane contacts through which lipids may be transferred. Under normal conditions, free cholesterol export from ELs is mainly mediated by NPC1. Other NPC1 bypass pathways may co-exist. EL-to-plasma membrane (PM) transport step can be regulated by Rab8 and Myosin5b (Myo5b), as previously described (Kanerva et al., 2013). In the ER, phosphatidylcholine is converted into phosphatidylserine (PS) by PTDSS1. Top right, PS is exchanged for PI4P at the PM by means of membrane contact sites (MCS, represented in yellow background) established by ORP5/8 (Chung et al., 2015). PS at the inner leaflet of the PM, recruits ER-resident GRAMD1 proteins that transfer accessible-cholesterol to the ER. When ER cholesterol content exceeds 5% of the organelle's lipid mass, SREPB is inhibited, and cholesterol is esterified by ACAT1 and stored in LDs. Alternatively, an interplay between SNX13 and SNX14 may regulate exchange of PS for cholesterol at PM-ER MCS. Question marks denote unknown pathways; red dotted arrows indicate cholesterol transport routes. (B) Confocal microscopy image of a U2OS cell treated with oleic acid, showing ring-like SNX13-positive ER domains (green) making contacts with both LDs (blue) and ELs (red); see enlarged inset. Bar is 10 μm. (C) In the absence of NPC1, cholesterol accumulates in ELs and SNX13 suppresses cholesterol egress by alternative NPC1-independent mechanisms. (D) In cells lacking both NPC1 and SNX13, upregulation of an unknown NPC1 bypass pathway allows cholesterol export from ELs towards the PM. Under these conditions, mobilization of PM-accessible cholesterol to the ER is blocked and LD homeostasis is dysregulated.
How is cholesterol transported to the PM in the absence of SNX13 and NPC1 function? One possibility is that, analogous to SNX19-mediated control of early endosome motility (Saric et al., 2021), SNX13 could restrict endolysosome positioning to the perinuclear region. Supporting this idea, yeast MDM1 avoids cortical ER and uniquely localizes at the nucleus-vacuole junction, perinuclear ER in close contact with the vacuole (Henne et al., 2015). In this scenario, loss of SNX13 would allow cholesterol-laden endolysosomes to establish MCS with the PM through which cholesterol could be transferred directly. Alternatively, depletion of SNX13 may enhance endolysosomal exocytosis perhaps via a Rab8A/Myosin5B-dependent mechanism (Figure 1A; Kanerva et al., 2013). However, RAB8 was not detected as a hit in our screen. We cannot discard the possibility that other cell types may utilize a different subset of Rabs to control cholesterol transport. Finally, another possibility is the role that increased BMP levels, observed in SNX13-depleted cells (Lu et al., 2022), may play in cholesterol egress. Indeed, upregulation of BMP biosynthesis has been shown to reduce cholesterol overload in NPC1 mutant cells (Moreau et al., 2019; Inytska et al., 2021).
SNX13/NPC1-negative cells display striking cholesterol PM re-distribution and increased LD biogenesis that is independent of cholesterol esterification (Figure 1C and D). These data suggest these cells are unable to mobilize PM-accessible cholesterol en route to the ER (Das et al., 2014). Recently, Trinh et al. discovered a role for PTDSS1, a phosphatidylserine (PS) synthase, in transport of cholesterol from the PM to the ER (Trinh et al., 2020). Deletion of PTDSS1, resulted in increased PM-accessible cholesterol, decreased total PS content and concomitant failure to inhibit the sterol regulatory element-binding protein (SREBP) pathway. This shows that cholesterol was unable to reach the ER. Remarkably, SNX13 appeared as a top candidate hit in their study, and vice versa, PTDSS1 was also identified in ours. Trinh et al. also reported that ER-localized GRAMD1 proteins are responsible for transporting PM-cholesterol in a PS-dependent manner (Trinh et al., 2022), by means of ER-PM MCS (Sandhu et al., 2018). Nevertheless, their data also indicated that additional PS-dependent transport pathways may exist; perhaps regulated by SNX13.
While human SNX13 and the Drosophila ortholog, Snazarus bind PS (Chandra et al., 2019; Ugrankar et al., 2019), they do not contain a putative cholesterol binding domain like GRAMD1s (Hulce et al., 2013; Sandhu et al., 2018). On the other hand, the paralog SNX14 is a cholesterol binding protein (Hulce et al., 2013) that, when mutated, causes autosomal recessive spinocerebellar ataxia 20 (SCAR20) with endolysosomal cholesterol accumulation as in NPC (Bryant et al., 2018). Since SNX13 and SNX14 form heterodimers (Huttlin et al., 2021; M. Henne personal communication), they may operate in parallel to GRAMD1s in exchanging cholesterol for PS at the PM (Figure 1A). Future work will explain the dual roles of SNX13 as a coordinator of interorganelle association, and as a possible lipid transfer protein at ER-endolysosomal and PM MCS. In summary, our study, together and work from other teams provide exciting new insights into the intricate itinerary taken by LDL-derived cholesterol, and remind us that more surprises are surely expected.
Abbreviations
- BMP
bis(monoacyglycero)phosphate
- EL
endolysosome
- ER
endoplasmic reticulum
- LD
lipid droplet
- MCS
membrane contact sites
- NPC1
Niemann-Pick C1
- NPC2
Niemann-Pick C2
- PG
phosphatidylglycerol
- PM
plasma membrane
- SNX
sorting nexin
- SREBP
sterol regulatory element-binding protein
- TAG
triglyceride
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Michael J. Fox Foundation for Parkinson's Research, (grant number MJFF-019043).
ORCID iD: Albert Lu https://orcid.org/0000-0002-7507-3330
References
- Bryant D., Liu Y., Datta S., Hariri H., Seda M., Anderson G., Peskett E., Demetriou C., Sousa S., Jenkins D., Clayton P., Bitner-Glindzicz M., Moore G. E., Henne W. M., Stanier P. (2018). SNX14 Mutations affect endoplasmic reticulum-associated neutral lipid metabolism in autosomal recessive spinocerebellar ataxia 20. Human Molecular Genetics, 27(11), 1927–1940. 10.1093/hmg/ddy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M., Chin Y. K., Mas C., Feathers J. R., Paul B., Datta S., Chen K. E., Jia X., Yang Z., Norwood S. J., Mohanty B., Bugarcic A., Teasdale R. D., Henne W. M., Mobli M., Collins B. M. (2019). Classification of the human phox homology (PX) domains based on their phosphoinositide binding specificities. Nature Communications, 10(1), 1528. 10.1038/s41467-019-09355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Torta F., Masai K., Lucast L., Czapla H., Tanner L. B., Narayanaswamy P., Wenk M. R., Nakatsu F., De Camilli P. (2015). INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science (New York, N.Y.), 349(6246), 428–432. 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Brown M. S., Anderson D. D., Goldstein J. L., Radhakrishnan A. (2014). Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife, 3, e02882. 10.7554/eLife.02882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J. (2020). Life in the lumen: the multivesicular endosome. Traffic (Copenhagen, Denmark), 21(1), 76–93. 10.1111/tra.12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri H., Henne W. M. (2022). Filling in the gaps: SNX-RGS proteins as multiorganelle tethers. The Journal of Cell Biology, 221(5), e202203061. 10.1083/jcb.202203061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri H., Speer N., Bowerman J., Rogers S., Fu G., Reetz E., Datta S., Feathers J. R., Ugrankar R., Nicastro D., Henne W. M. (2019). Mdm1 maintains endoplasmic reticulum homeostasis by spatially regulating lipid droplet biogenesis. The Journal Of Cell Biology, 218(4), 1319–1334. 10.1083/jcb.201808119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W. M., Zhu L., Balogi Z., Stefan C., Pleiss J. A., Emr S. D. (2015). Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. The Journal Of Cell Biology, 210(4), 541–551. 10.1083/jcb.201503088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger D., Burgoyne T., Sanchez-Heras E., Hartwig P., Colaco A., Newton J., Futter C. E., Spiegel S., Platt F. M., Eden E. R. (2019). NPC1 Regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nature Communications, 10(1), 4276. 10.1038/s41467-019-12152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulce J. J., Cognetta A. B., Niphakis M. J., Tully S. E., Cravatt B. F. (2013). Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nature Methods, 10(3), 259–264. 10.1038/nmeth.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin E. L., Bruckner R. J., Navarrete-Perea J., Cannon J. R., Baltier K., Gebreab F., Gygi M. P., Thornock A., Zarraga G., Tam S., Szpyt J., Gassaway B. M., Panov A., Parzen H., Fu S., Golbazi A., Maenpaa E., Stricker K., Guha Thakurta S., … Gygi S. P. (2021). Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell, 184(11), 3022–3040.e28. 10.1016/j.cell.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilnytska O., Lai K., Gorshkov K., Schultz M. L., Tran B. N., Jeziorek M., Kunkel T. J., Azaria R. D., McLoughlin H. S., Waghalter M., Xu Y., Schlame M., Altan-Bonnet N., Zheng W., Lieberman A. P., Dobrowolski R., Storch J. (2021). Enrichment of NPC1-deficient cells with the lipid LBPA stimulates autophagy, improves lysosomal function, and reduces cholesterol storage. The Journal Of Biological Chemistry, 297(1), 100813. 10.1016/j.jbc.2021.100813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak D. N., Tyanova S., Cox J., Borner G. H. (2016). Global, quantitative and dynamic mapping of protein subcellular localization. eLife, 5, e16950. 10.7554/eLife.16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva K., Uronen R. L., Blom T., Li S., Bittman R., Lappalainen P., Peränen J., Raposo G., Ikonen E. (2013). LDL Cholesterol recycles to the plasma membrane via a Rab8a-Myosin5b-actin-dependent membrane transport route. Developmental Cell, 27(3), 249–262. 10.1016/j.devcel.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Li J., Pfeffer S. R. (2016). Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. eLife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Saha P., Li J., Blobel G., Pfeffer S. R. (2016b). Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proceedings of the National Academy of Sciences of the United States of America, 113(36), 10079–10084. 10.1073/pnas.1611956113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang J., Coutavas E., Shi H., Hao Q., Blobel G. (2016a). Structure of human niemann-pick C1 protein. Proceedings of the National Academy of Sciences of the United States of America, 113(29), 8212–8217. 10.1073/pnas.1607795113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T., Qi X., Hassan A., Liang Q., De Brabander J. K., Li X. (2020). Structural basis for itraconazole-mediated NPC1 inhibition. Nature Communications, 11(1), 152. 10.1038/s41467-019-13917-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A., Hsieh F., Sharma B. R., Vaughn S. R., Enrich C., Pfeffer S. R. (2022). CRISPR Screens for lipid regulators reveal a role for ER-bound SNX13 in lysosomal cholesterol export. The Journal Of Cell Biology, 221(2), e202105060. 10.1083/jcb.202105060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccauliff L. A., Langan A., Li R., Ilnytska O., Bose D., Waghalter M., Lai K., Kahn P. C., Storch J. (2019). Intracellular cholesterol trafficking is dependent upon NPC2 interaction with lysobisphosphatidic acid. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses-Salas E., García-Melero A., Kanerva K., Blanco-Muñoz P., Morales-Paytuvi F., Bonjoch J., Casas J., Egert A., Beevi S. S., Jose J., Llorente-Cortés V., Rye K. A., Heeren J., Lu A., Pol A., Tebar F., Ikonen E., Grewal T., Enrich C. & Rentero, C. (2020). Annexin A6 modulates TBC1D15/Rab7/StARD3 axis to control endosomal cholesterol export in NPC1 cells. Cellular And Molecular Life Sciences : CMLS, 77(14), 2839–2857. 10.1007/s00018-019-03330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau D., Vacca F., Vossio S., Scott C., Colaco A., Paz Montoya J., Ferguson C., Damme M., Moniatte M., Parton R. G., Platt F. M., Gruenberg J. (2019). Drug-induced increase in lysobisphosphatidic acid reduces the cholesterol overload in niemann-pick type C cells and mice. EMBO Reports, 20(7), e47055. 10.15252/embr.201847055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Weeratunga S., Tillu V. A., Hariri H., Henne W. M., Collins B. M. (2022). Structural predictions of the SNX-RGS proteins suggest they belong to a new class of lipid transfer proteins. Frontiers In Cell And Developmental Biology, 10, 826688. 10.3389/fcell.2022.826688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. (2019). NPC Intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. The Journal Of Biological Chemistry, 294(5), 1706–1709. 10.1074/jbc.TM118.004165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Wu X., Du X., Yao X., Zhao X., Lee J., Yang H., Yan N. (2020). Structural basis of low-pH-dependent lysosomal cholesterol egress by NPC1 and NPC2. Cell, 182(1), 98–111.e18. 10.1016/j.cell.2020.05.020 [DOI] [PubMed] [Google Scholar]
- Sandhu J., Li S., Fairall L., Pfisterer S. G., Gurnett J. E., Xiao X., Weston T. A., Vashi D., Ferrari A., Orozco J. L., Hartman C. L., Strugatsky D., Lee S. D., He C., Hong C., Jiang H., Bentolila L. A., Gatta A. T., Levine T. P., … Tontonoz P. (2018). Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell, 175(2), 514–529.e20. 10.1016/j.cell.2018.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saric A., Freeman S. A., Williamson C. D., Jarnik M., Guardia C. M., Fernandopulle M. S., Gershlick D. C., Bonifacino J. S. (2021). SNX19 Restricts endolysosome motility through contacts with the endoplasmic reticulum. Nature Communications, 12(1), 4552. 10.1038/s41467-021-24709-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh M. N., Brown M. S., Goldstein J. L., Han J., Vale G., McDonald J. G., Seemann J., Mendell J. T., Lu F. (2020). Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proceedings of the National Academy of Sciences of the United States of America, 117(31), 18521–18529. 10.1073/pnas.2010682117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh M. N., Brown M. S., Seemann J., Vale G., McDonald J. G., Goldstein J. L., Lu F. (2022). Interplay between asters/GRAMD1s and phosphatidylserine in intermembrane transport of LDL cholesterol. Proceedings of the National Academy of Sciences of the United States of America, 119(2), e2120411119. 10.1073/pnas.2120411119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrankar R., Bowerman J., Hariri H., Chandra M., Chen K., Bossanyi M. F., Datta S., Rogers S., Eckert K. M., Vale G., Victoria A., Fresquez J., McDonald J. G., Jean S., Collins B. M., Henne W. M. (2019). Drosophila snazarus regulates a lipid droplet population at plasma membrane-droplet contacts in adipocytes. Developmental Cell, 50(5), 557–572.e5. 10.1016/j.devcel.2019.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M., Kidmose R. T., Szomek M., Thaysen K., Rawson S., Muench S. P., Wüstner D., Pedersen B. P. (2019). Structural insight into eukaryotic sterol transport through niemann-pick type C proteins. Cell, 179(2), 485–497.e18. 10.1016/j.cell.2019.08.038 [DOI] [PubMed] [Google Scholar]
- Zheng B., Ma Y. C., Ostrom R. S., Lavoie C., Gill G. N., Insel P. A., Huang X. Y., Farquhar M. G. (2001). RGS-PX1, a GAP for GalphaS and sorting nexin in vesicular trafficking. Science (New York, N.Y.), 294(5548), 1939–1942. 10.1126/science.1064757 [DOI] [PubMed] [Google Scholar]