Abstract
Attractive Targeted Sugar Baits (ATSB) have been demonstrated to result in significant reductions in malaria vector numbers in areas of scarce vegetation cover such as in Mali and Israel, but it is not clear whether such an effect can be replicated in environments where mosquitoes have a wide range of options for sugar resources. The current study evaluated the attractiveness of the predominant flowering plants of Asembo Siaya County, western Kenya in comparison to an ATSB developed by Westham Co. Sixteen of the most common flowering plants in the study area were selected and evaluated for relative attractiveness to malaria vectors in semi-field structures. Six of the most attractive flowers were compared to determine the most attractive to local Anopheles mosquitoes. The most attractive plant was then compared to different versions of ATSB. In total, 56,600 Anopheles mosquitoes were released in the semi-field structures. From these, 5150 mosquitoes (2621 males and 2529 females) of An. arabiensis, An. funestus and An. gambiae were recaptured on the attractancy traps. Mangifera indica was the most attractive sugar source for all three species while Hyptis suaveolens and Tephrosia vogelii were the least attractive plants to the mosquitoes. Overall, ATSB version 1.2 was significantly more attractive compared to both ATSB version 1.1 and Mangifera indica. Mosquitoes were differentially attracted to various natural plants in western Kenya and ATSB. The observation that ATSB v1.2 was more attractive to local Anopheles mosquitoes than the most attractive natural sugar source indicates that this product may be able to compete with natural sugar sources in western Kenya and suggests this product may have the potential to impact mosquito populations in the field.
Background
Current vector control tools, such as long lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) have contributed substantially to the reduction in the global malaria burden since the early 2000s [1,2]. These strategies and others currently under evaluation target known mosquito vulnerabilities such as indoor, late night blood feeding, and resting behaviors to control them [3]. However, other life history traits such as sugar feeding have remained largely understudied although sugar feeding has recently been identified as a potential vulnerability that could be exploited for vector control [4–7].
Mosquitoes of both sexes require sugar for energetic needs and survival [8,9] and their main natural sources are flowering plant nectaries, ripe-rotting fruits, or honeydew [10]. Mosquitoes are thought to identify sugar meals by volatiles emitted by floral blossoms which they eventually cue in to [11]. Attractive targeted sugar baits (ATSB) contain an attractant, sugar source as a feeding stimulant mixed with an oral toxicant to kill mosquitoes upon ingestion by exploiting the sugar feeding behavior of mosquitoes to reduce their populations [12]. For ATSBs to optimally impact mosquito populations, they would have to compete favorably with the available natural sugar resources. Several preferred sugar sources for mosquitoes have been described in the literature [10,13–15], some of which have been exploited for vector control and surveillance [16,17].
ATSB applications have been demonstrated to effectively reduce mosquito densities. In Mali, a 90% reduction in the density of An. gambiae sl. and a reduction in the longevity of female mosquitoes were observed following ATSB spot spraying of non-flowering vegetation around artificial ponds [4]. Similarly, the density of An. sergentii, one of the principal potential malaria vector in Israel, was reduced by >95% when the sugar-rich vegetation around oases was sprayed with ATSB solutions [5,7] resulting in significantly lowered biting pressures. Most recently in Mali, ATSB stations markedly reduced the density of An. gambiae, including a 90% reduction of older females who had survived three or more gonotrophic cycles and were therefore potentially infectious was reduced by 90% [18]. Importantly, ATSBs can be used to target a wide range of mosquito vector species and have been shown to be efficacious against resistant mosquito populations of Aedes aegypti, and An. arabiensis in semi-field and laboratory evaluations [19,20]. ATSBs are therefore a potentially effective complementary addition to the existing malaria vector control tools.
To date, most successful field evaluations of ATSB have been conducted in arid/semi-arid environments where the baits are likely to face little competition due to scarcity of natural sugar sources such as Mali [4,18] and Israel [5,7]. The efficacy of ATSB in more vegetated environments where mosquitoes may have a wide range of options for sugar sources remains largely unexplored. This study, therefore, aimed to compare the relative attractancy of mosquitoes to different versions of an ATSB manufactured by the Westham Co. against a variety of flower blossoms of plants found in western Kenya. These findings will be key to identifying the potentially competitive plants and estimating how they may affect the deployment of ATSBs during the epidemiological evaluations that are currently taking place in three countries in Africa including Kenya [21] and eventually during public health campaigns.
Methods
Study site
The attractancy experiments were conducted in three semi-field structures measuring 18m x 9m x 3m within the Kenya Medical Research Institute (KEMRI) located at the Kisian research station in Kisumu, Kenya, as described in [22]. Prior to the semi-field experiments, a team of entomologists and botanists conducted a botanical survey in Asembo (0.183694°S, 34.383694°E) Siaya County to characterize the common flora of the area. During the survey, the coverage of all flowering plants around the peridomestic spaces was estimated across ten clusters between May-August 2020. The clusters were made up of several adjacent villages with 100–400 households [21]. The most common flowering plants that were potential sugar sources for mosquitoes were identified by the botanist with the aid of a mobile app (PlantSnap® app, Eden Tech Labs; Sofia, Sofia-City; Bulgaria). Flowers tested in attractancy experiments were the most common flowering plant species collected fresh daily from Asembo where a large-scale, cluster-randomized trial of ATSBs is being conducted. The area receives seasonal rainfall with the heaviest long rains between March and May and short rains between October and December. The average annual rainfall is between 1000–1250 mm and the daily maximum temperature ranges between 17-35°C. It lies at a mean altitude of 1070 m above sea level. The area is malaria endemic with transmission occurring throughout the year. Asembo forms part of the KEMRI health and demographic surveillance system (HDSS) continuous malaria surveillance site where over 223,000 individuals have been monitored for more than a decade [23–25].
Collection and maintenance of mosquitoes for bioassays
Anopheles gambiae complex mosquitoes were collected as larvae, An. arabiensis from Ahero (0.16°S, 35.19°E) in Kisumu County and An. gambiae from Bumula (0.5742°N, 34.4420°E) in Bungoma County using larval dipping. Anopheles funestus were collected as adults from Alego-Usonga (0.0606193°N, 34.2859671°E) in Siaya County. The field-collected larvae were raised under laboratory conditions with temperature maintained between 27–30°C and relative humidity 80 ±10%. The larvae were fed on TetraMin fish food up to the pupal stage after which they were transferred to plastic cups and placed in 30x30x30cm cages. Adults were maintained on a 10% sugar solution soaked in cotton wool until they were to 3–5 days post-emergence for bioassays. Adult An. funestus were collected from the field using a combination of mechanical and mouth aspiration and transported back to the insectary in Kisumu, sorted by abdominal status so that only fed and gravid An. funestus were maintained in separate cages. The rest were discarded. The sorted cages were maintained on 10% sugar for at least 48 hours after which a laying pad was introduced into the cages with gravid mosquitoes. Eggs were collected the next day and hatched overnight. Anopheles funestus larvae were reared using the same conditions for An. gambiae described above. Previous studies have shown that An. gambiae are the predominant species in Bungoma, An. arabiensis is the main species in Ahero while An. funestus is the major malaria vector in Siaya [26–28]. Thus, mosquito identification was done morphologically. However, PCR results conducted as part of a separate study confirmed these species distributions Kosgei et al., [unpublished]”.
Field experiments
Initial experiments were conducted in an open field in the study area where the most common flowering plants were tested for attraction to malaria mosquitoes. The field site was centrally located in the community and served as a common grazing ground for livestock. This site was chosen for the experiments due to its proximity to mosquito larval sites and for the security of traps which was provided by the community members. Sixteen flowering plants and water as negative control were tested. A total of 17 glue-netted traps were positioned 10 meters apart in a straight line in the field with each enclosing either a test flower or water as a negative control. The glue-netted trapping method was adapted from Müller GC et al. [10] to test fruits, seedpods, and flowers for attraction to malaria mosquitoes. In brief, a black plastic netting with a mesh-size of 0.5cm x 0.5cm was cut into 70cm x 70cm pieces and folded into cylinders then fixed with plastic seals to form the traps. The top part of the cylinders was enclosed with the same plastic netting. The netted traps were supported with two 20 cm sticks firmly fixed into the ground on either side to prevent them from toppling during strong winds. To the outer surfaces of the cylinder netting, an even layer of a non-toxic, odorless Tangle foot® Tangle-Trap® Sticky Coating (The Scott Company, Marysville, OH, USA) was applied to trap any mosquitoes which cued into the floral scents to seek nectar. The glue was applied daily prior to the experiment to enhance its stickiness. The traps bearing flowering plants were rotated daily in the field to eliminate the effect of positioning bias. However, only 132 culicine mosquitoes were caught on the glue-netted traps in a period of 20 days but with no anopheline species, so the glue-netted trap experiment was transferred to the semi-field structures.
Semi-field experiments
In an 18m x 9m section of the semi-field structures, glue netted traps similar to those previously used in the field experiment (Fig 1) were used. The traps were placed at each of the four corners of the release area of the semi-field structure at a distance of 10m x 8m apart. Empty 500ml plastic jars were buried in the ground and filled with tap water. Wearing industrial hand gloves, fresh flowers of the flowering plants previously identified and tested in the ATSB study area were cut with a machete and about 0.5 kg (40cm-60cm) were placed in the cylinder netting each evening of the experiment with their stems inside the 500ml plastic jar with tap water to keep them fresh overnight. One netted trap with only tap water in the 500ml plastic jar was included in each semi-field structure as a negative control so that a total of 3 different flowering plants or ATSBs were tested each night.
Fig 1.
S1; a 500ml plastic jar with tap water for keeping the flowering plants fresh. S2; Black glue-netted trap and S3; shows the application of tangle foot glue on the netted tap using an 8cm handy brush applicator. S4 & S5; Fixing flowering plant in glue netted trap within the semi-field structure. On each evening of the experiment, 600 field collected An. gambiae, An. arabiensis or 200 F1 progeny of An. funestus adult mosquitoes with equal numbers of males and females were released at the center of the semifield structures between 1700–1800 HRS and left overnight. All mosquitoes were 3–5 days old and had been starved for 24 hours. The next morning, between 0600-0700HRS, all mosquitoes trapped with the tangle foot glue were picked with a pair of forceps from the glue-netted traps, counted, sexed then transferred to 1.5 ml Eppendorf tubes, and subsequently desiccated in silica gel for archiving (VWR International bvba Geldenaaksebaan 464-B-300I, Leuven, Belgium). In between the experiments, a time gap of at least two days was left for the semi-fields to clear of any remaining mosquitoes from the previous release by natural mortality.
Sixteen flowering plants in the study area were tested for attraction to An. arabiensis and An. gambiae mosquitoes in experiment 1. These plants were: Parthenium hysterophorous (Asteraceae), Mangifera indica (Anacardiaceae), Tithonia diversifolia (Asteraceae), Leonotis leonurus (Lamiaceae), Hyptis suaveolens (Lamiaceae), Markhamia lutea (Bignonaceae), Senna siamea (Fabaceae), Bougainvillea glabra (Nyctiginaceae), Ocimum gratissimum (Lamiaceae), Lantana camara (Verbenaceae), Senna bicupsularis (Fabaceae), Crotalaria pallida (Fabaceae), Senna dydimobotrya (Fabaceae), Tephrosia vogelii (Fabaceae), Caranthus roseous (Apocynaceae) and Rumex conglomeratus (Polygonaceae). It was not possible to raise sufficient numbers of An. funestus for inclusion in the first semifield experiment comparing the relative attractiveness of these sixteen floral species. Each night, test flowers were randomized for testing in each of the three semi-field structures. At least three randomly selected flowers and a water control were tested in each of the three semi-field structures with both An. arabiensis and An. gambiae. The test flowers were replicated twice per species and thereafter, the 10 least attractive flowering plants were excluded from subsequent studies.
The six remaining flowering plants that had attracted the most mosquitoes were selected for further tests against different versions of ATSB developed by Westham Co. (Hod-Hasharon, Israel) in the second stage of the experiments. The six most attractive flowers were randomized for the 3 semi-field structures on each experiment day. In each of the 3 semi-field structures, one ATSB version 1.1.1 (also referred to as v1.1) was compared to two different flowering plant species, and water as a negative control. The test materials were rotated each night within the semi-field structures to avoid positional bias. The test materials were replicated four times at this stage with each of the 3 species of mosquitoes. The most attractive flower out of the six was selected and further compared with two versions of ATSBs (versions 1.1 and 1.1.2) and water control in the third stage of the experiments. Nine replicates of these test materials were evaluated with each of the 3 mosquito species. Lastly, in experiment 4, ATSB v1.2 was evaluated against ATSB v1.1, the most attractive floral blossom out of the 6 flowers, and water as the negative control. In this stage of the experiment, 3 replicates of test materials were conducted with each of the 3 mosquito species. During these replications, the two ATSB versions, water control, and the most attractive flowering plant were rotated each night within the semifield structures to avoid positional bias. The ATSB versions tested in this study were versions 1.1, 1.1.2, and 1.2. Version 1.2 is currently under epidemiological trial in Kenya, Zambia, and Mali. All three ATSB versions were similar in bait formulation although v1.1 and v1.1.2 were manually produced, with V1.1.2 incorporating a rainproof layer to prevent leaking, particularly when rained on. In contrast, v1.2 was machine produced with improved sealing between the tray and bait station membrane. The two previous versions 1.1 and 1.1.2 despite having similarity in bait formulation to v1.2, were key to the improved features of the final version used in the main trials. These ATSBs contained a fruit syrup as an attractant, dinotefuran as the active ingredient which is an oral neonicotinoid insecticide, sugar as a feeding stimulant and Bittrex added to deter human consumption [21]. The dinotefuran insecticide has been indicated to be of low toxicity to humans and has a negligible effect on non-target organisms [29]. In this experiment, the baits were placed inside the sticky netted-traps hence mosquitoes were not expected to access or be able to feed on them.
Data analysis
Raw data was entered into MS excel workbook then transferred to R (version 3.6.3) for analysis. The mean proportion of mosquitoes recaptured was computed to evaluate the relative attraction of the 16 most dominant floral blossoms and the 6 most attractive flowers for experiments 1 & 2 respectively. For the comparison of the ATSB variants with the most attractive plant identified in experiment 1&2 and the water control, a binomial general linear mixed model (GLMM) was used with a fixed effect for the test material and a random effect for date of experiment and the screen house for each of the 3 species using the lme4 package. The significance of the test material variable was assessed using a log-likelihood ratio test against a null model using the lmtest package. Post-hoc multiple pairwise comparisons were carried out using a Tukey’s all-pair comparison using the ghlt package with a Holm-Bonferroni correction to account for multiple testing.
Ethical consideration
Attractancy experiment procedures were reviewed by the Kenya Medical Research Institute Scientific and Ethical Review Unit (SERU, Approval number: 3613). The study was also approved by the Liverpool School of Tropical Medicine Research Ethics Committee (Approval number LSTM 18–015) and the US Centers for Disease Control and Prevention (Approval number 7112) based on a reliance agreement with KEMRI SERU. All floral materials used in the experiments were picked from non-endangered plant species and with verbal consent from the local community. The individual whose image appears in this manuscript has given written informed consent to publish these case details.
Results
Field experiment
After a rigorous 20 days of field experiment within the study site, negligible collections of Anopheles mosquitoes and a total of 132 culicine mosquitoes (105 female and 27 males) were recaptured on glue-netted traps set. Thus, the experiment was shifted to semi-field structures where conducted artificial release of a known number, species and sex of mosquitoes.
Semi-field experiment
Number of Mosquitoes by species released and recaptured in the 4 sets of experiments. A total of 56,600 Anopheles mosquitoes were released in the 3 semi-field structures consisting of 28,300 males and 28,300 females in the whole experiment season. From this, a total of 2621 males and 2529 female mosquitoes were recaptured from the traps surrounding the test flowers and the different ATSB versions (Table 1).
Table 1. The total number of mosquitoes of different species released and recaptured in the 4 experiments.
| Experiment | Mosquito Species | Total No. Released |
Total No. Recaptured | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| 1 | An. arabiensis | 3600 | 3600 | 210 | 179 |
| An. gambiae | 3600 | 3600 | 195 | 341 | |
| 2 | An. gambiae | 3200 | 3200 | 206 | 231 |
| An. arabiensis | 3200 | 3200 | 227 | 164 | |
| An. funestus | 1200 | 1200 | 186 | 258 | |
| 3 | An. funestus | 1200 | 1200 | 189 | 170 |
| An. gambiae | 2700 | 2700 | 261 | 143 | |
| An. arabiensis | 2700 | 2700 | 177 | 149 | |
| 4 | An. arabiensis | 2700 | 2700 | 302 | 259 |
| An. funestus | 1500 | 1500 | 264 | 261 | |
| An. gambiae | 2700 | 2700 | 404 | 374 | |
Attractiveness test of An. gambiae and An. arabiensis to flowering plant blossoms
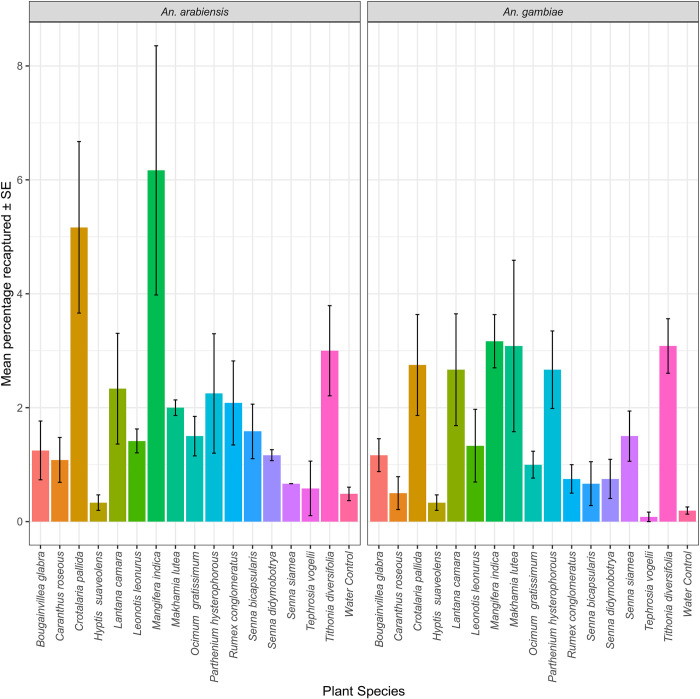
In the first experiment comparing the attractiveness of the 16 most common flowering plants to An. gambiae and An. arabiensis, the six floral species that had the highest recapture rates across both mosquito species and sex were M. indica, C. pallida, P. hysterophorous, M. lutea, L. camara, and T. diversifolia. Floral blossoms of T. vogelii and H. suaveolens were consistently less attractive to both An. arabiensis and An. gambiae. The mosquito recapture rates were much higher in An. arabiensis than An. gambiae but the overall relationship to the dominant floral species was similar (Fig 2). These six flowers with the highest recapture rates were further tested against ATSB v1.1 in the next phase of the experiment.
Fig 2. The mean proportion of mosquitoes recaptured from 16 flowering plants in a multiple choice semi-field assay including a water control.
Error bars represent the standard error of the mean.
Selection of the most attractive flowers for further testing
The second experiments evaluating the six most attractive flower species in comparison to ATSB v1.1, and water control found that Mangifera indica had the highest mean percent recapture rates and was the most preferred flowering plant across both sexes of the three mosquito species (Figs 3 and 4). The most attractive flower, Mangifera indica was further tested against ATSB v1.1 and v1.1.2 in the third stage of experiments.
Fig 3. The mean proportion of mosquitoes recaptured from the 6 most attractive flowering plants including ATSB v1.1, and water control in a multiple-choice semi-field assay.
Error bars represent the standard error of the mean.
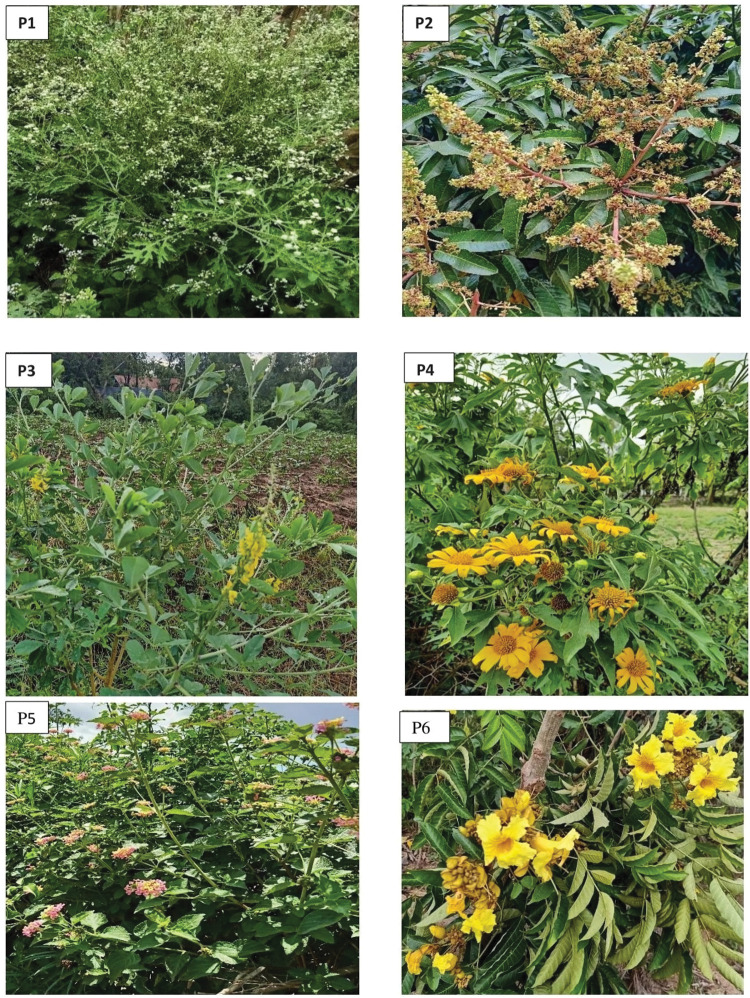
Fig 4.
The most attractive flowering plants as found in experiment 1: Parthenium hysterophorous (P1), Mangifera indica (P2), Crotalaria pallida (P3), Tithonia diversifolia (P4), Lantana camara (P5) and Makhamia lutea (P6).
Comparison of Mangifera indica flower to the ATSB v1.1 and 1.1.2
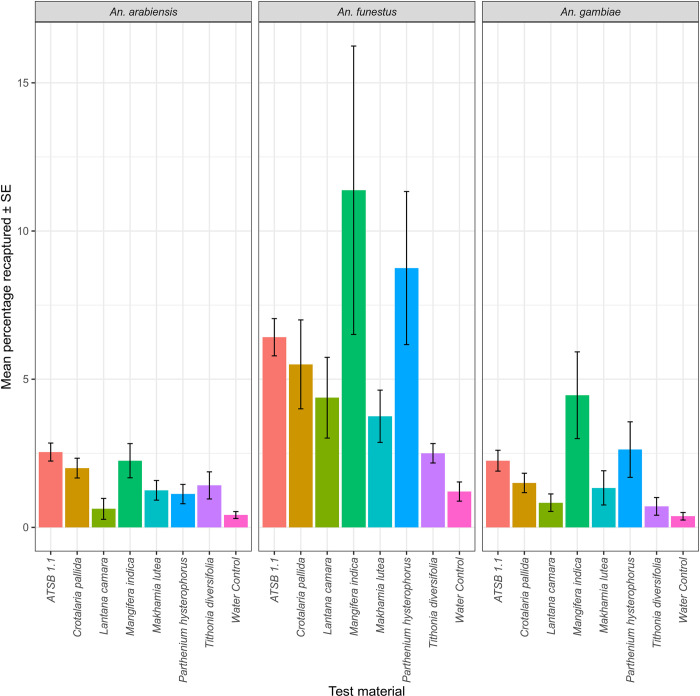
In experiment 3, the inclusion of the test material as a variable provided a significant improvement in model fit for An. gambiae (χ2 (3) = 58.37, p = <0.001), An. funestus (χ2 (3) = 54.32, p = <0.001), and An. arabiensis (χ2 (3) = 28.68, p = <0.001). In the subsequent pairwise comparisons, ATSB 1.1.2, tested against An. arabiensis (μ = 1.96%), was the only case where there was no significant difference compared to the water control (μ = 1.6%; Z = 1.61, p = 0.37) with ATSB 1.1 significantly outperforming it (μ = 2.95%; Z = -3.28, p = 0.006). The opposite relationship was found in An. funestus with ATSB 1.1.2 (μ = 7.94%) having a greater recapture rate than both ATSB 1.1 (μ = 6.56%; Z = 1.61, p = 0.368) and Mangifera indica (μ = 5.44%; Z = 2.99, p = 0.014). While there was a significant increase in attraction of An. funestus to ATSB 1.1.2 than the dominant sugar source Mangifera indica, across the three species the absolute difference in recapture rates was small overall and would not indicate a substantial increase in attraction to the ATSBs compared to an available natural source but rather show that in its previous iterations, the ATSBs were as competitive in attracting malaria mosquitoes in these semi-field conditions (Fig 5). Finally, we compared the attractiveness of the newest ATSB v1.2 and the ATSB v1.1 against Mangifera indica in the fourth stage of the experiments.
Fig 5. The mean proportion of mosquitoes recaptured from the experiment comparing ATSB versions 1.1 and 1.1.2 with the most attractive plant; Mangifera indica and a water control.
P-value significance from a post-hoc Tukey’s all-pair comparison indicated by ‘*’ if ≤0.05, ‘**’ if ≤0.01, and ‘***’ if ≤0.001; non-significant relationships not shown.
Comparison of Mangifera indica to ATSB v1.1 and 1.2
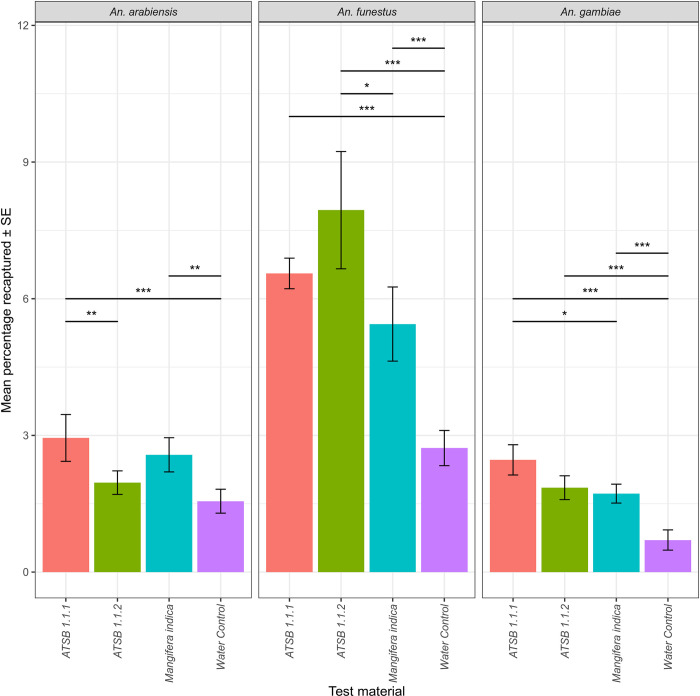
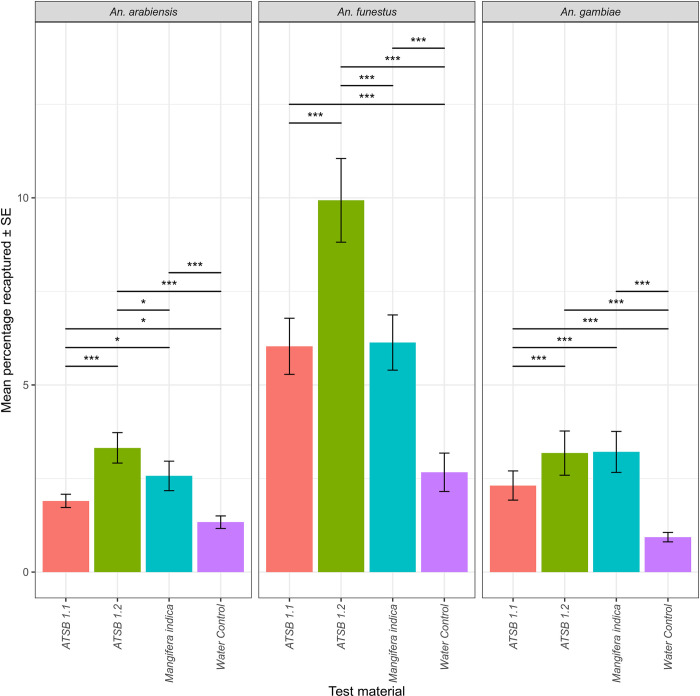
In experiment 4 comparing the most attractive flower Mangifera indica, with the ATSB versions 1.1 and 1.2 to Anopheles mosquitoes, the inclusion of test material variable led to a significant improvement in the model fit for all three mosquito species compared to the null model. All three test materials (ATSB 1.1, ATSB 1.2 Mangifera indica) displayed a significant increase in their recapture rate compared to the control for all 3 Anopheles spp. The ATSB 1.2 significantly outperformed the ATSB 1.1 against An. arabiensis, An. funestus, and An. gambiae. The largest difference was seen in An. funestus where ATSB 1.2 (μ = 9.93%) displayed a greater attraction than the earlier iteration of ATSB 1.1 (μ = 6.03%; Z = 5.54, p = <0.001) and Magnifera indica (μ = 6.1%; Z = 5.39, p = <0.001). Similarly, the ATSB 1.2 (μ = 3.3%) slightly outcompeted the Mangifera indica for An. arabiensis (μ = 2.57%; Z = 2.66, p = 0.039) but had comparable recapture rate for An. gambiae (Fig 6).
Fig 6. The mean proportion of mosquitoes recaptured from the experiment comparing ATSB versions 1.1 and 1.2 with the most attractive plant; Mangifera indica and a water control.
P-value significance from a post-hoc Tukey’s all-pair comparison indicated by ‘*’ if ≤0.05, ‘**’ if ≤0.01 and ‘***’ if ≤0.001; non-significant relationships not shown.
Discussion
This study established that different flowering plants in western Kenya have varying levels of attractancy to different local Anopheles species. The ATSB v1.2, which is the final version currently undergoing epidemiological evaluation, was significantly more attractive to all the three Anopheles species tested than the previous version 1.1. Additionally, the ATSB v1.2 was significantly more attractive than the most attractive local flowering plant (M. indica), tested to An. arabiensis, An. funestus and a comparable mean percent recaptures with An. gambiae. While this increase wasn’t substantial it has established that the ATSBs can compete as potential sugar source expanding the evidence base for their potential successful use in non-arid environments.
The field experiment testing local malaria vector attraction to floral blossoms didn’t yield the desired results after rigorous tests conducted at the study site. We attributed the failure to possibly a strong competition of volatiles released by the flora growing at the periphery of the study site, given that in our study we used only branches of the flowering plants with a few flowers intact. Thus, the phytochemicals released by the many flowers on the remaining natural vegetation might have outperformed and diverted mosquitoes away from the glue-netted traps, which informed further analysis in the semi-field structures. Sugar feeding is a critical aspect of mosquito behavior, providing energy for flight, mate seeking, swarming, and egg laying [8]. The energy gained from sugar meals is necessary to drive dispersal and mosquito survivorship [30]. Accordingly, shrubs like the highly invasive Prosopis juliflora that serve as regular sugar sources to An. gambiae in Mali have been shown to accelerate the mosquito vectorial capacity [31] suggesting that clearing of such vegetation would reduce mosquito density by reducing nectar sources available.
Flowers typically are the most readily available sugar sources for mosquitoes though they occasionally may utilize seedpods, honeydew, and ripe-rotting fruits [10,32]. Mosquitoes rely on visual and olfactory cues to locate sugar resources in the environment [33,34]. Visual cues are chiefly responsible for a short-range attraction while odors play the role of long-range attraction to vectors [35]. Like other pollinator insects, mosquitoes in the field have been shown to be attracted to strongly fragrant and light-colored flowers [36,37] but only certain flower odors are attractive and can elicit mosquito aggregation and feeding [38]. In this study, a strong attraction was observed towards inflorescences of M. indica, C. pallida, T. diversifolia, M. lutea, and P. hysterophorous, implying that mosquito orientation to, and location of the flowers may have been due to odor released by the individual flowers. Previous studies in western Kenya indicated that P. hysterophorous is highly attractive to An. gambiae [39,40], and the M. indica flower was a preferred nectar source by male An. gambiae [14] which is consistent with our findings suggesting that these plants are beneficial to Anopheles vectors as sources of sugar.
Light colored flowers are frequently visited due to their ability to reflect light at night enabling their recognition by the nocturnal sugar seeking mosquitoes [41]. In our study, M. lutea, C. pallida, and T. diversifolia which have bright yellow flowers and the bright white inflorescence of P. hysterophorous were frequently selected while the dull H. suaveolens and T. vogelii were the least attractive, suggesting that flower color may be important in the selection of sugar sources by the Anopheles mosquitoes in this experiment despite them being enclosed in a mesh. Multimodal floral cues including odor and floral color, have been demonstrated to work together to initiate mosquito response to host plant volatiles [33]. Earlier studies identified components of the floral scents that are attractive to malaria vectors including terpenes, phenols, aliphatic esters, and aldehydes [11,42,43]. However, the current study did not isolate these phytochemical compounds from the tested flowers. Additional studies screening the attractive volatiles in these flowers may identify compounds for potential use in mosquito surveillance traps or to optimize future versions of ATSBs. Further, future studies should also explore the potential role of colour in attracting the vectors to ATSBs.
The ATSB was attractive to malaria mosquitoes of both sexes despite the availability of M. indica in semi-field conditions. This suggests that the attractant currently included in the ATSB may be effective in natural settings. Furthermore, the consistently higher attraction of An. funestus to the ATSB is a promising sign for the trial being conducted in An. funestus dominant area. However, given the consistently higher recapture rates in An. funestus, even in the plant species comparison, this may point to a potential species-specific difference that would need further investigation. This approach to mosquito control has been successful in reducing mosquito populations in dry areas [4,5,7,16,44] through a topical spray of ATSB on vegetation and using ATSB bait stations deployed on the outside walls of housing structures in Mali [18]. Although the different versions of ATSB had similarities in bait formulation,their attractiveness to the local malaria vectors varied. The ATSB v1.2 stood were superior to the previous version 1.1 in the experiments. This disparity could have been caused by differences in the mode of production or membrane surfaces, which may have improved the bait solution retention. ATSB v1.2 was machine produced with a smooth membrane surface while v1.1 was manually produced with rough membranes prone to clogging with environmental dust. Additionally, a rapid depletion of the quantity of bait solution in the v1.1 membrane, might have led to the gradual reduction in the attractiveness of ATSB v1.1 compared to the v1.2 where the cases were rare. This is the first semi-field demonstration of the competitive advantage ATSBs have in luring mosquitoes when compared to natural sugar sources available in the local environment. The bait stations’ extended efficacy period, technological simplicity, and oral mode of insecticide delivery as opposed to the conventional contact mode on LLINs and IRS, and ease of deployment make them a promising tool to manage residual malaria transmission [18,29,45]. Additionally, the bait station design limits the chances of contact between ATSB and non-target organisms as opposed to the spraying method used in previous studies [6,7,46]. Therefore, ATSBs present a potential option for the outdoor malaria vector control where impacts on non-target organisms is a concern.
The current study had a number of limitations. First, we may not have exhausted all the potential sugar sources as we only selected 16 flowering plants for our test based on their abundance in the natural environment from a botanical survey conducted in this area Yalla et al., [Unpublished]”. It is possible that some highly attractive flowers were out of their season and were missed by this study. However, if they were able to outcompete the ATSBs, it would only be seasonally. Furthermore, confining mosquitoes in semi-field conditions may have altered their behaviors towards the flowers or maybe the fact that the plants were confined in the glue-netted traps made them not truly visible at the normal range for the mosquitoes. Further, this experiment was conducted with a fixed quantity of flowering plants against a single ATSB in a controlled semi-field structure. Future work will need to investigate the role varying the density of both natural sugar sources and ATSBs have on attractiveness and how this may change seasonally. Finally, while the ATSBs showed some degrees of attraction compared to natural sugar sources in the semi-field experiments, it is unclear how they will perform outside the semi-field setting where a greater quantity of these attractive plants are available. Further studies on deployment may be required in different settings to optimize their effectiveness.
Conclusion
Mosquitoes exhibit varying attractiveness to flowering plants in western Kenya. In a series of experiments, M. indica was the most attractive. The Westham ATSB v1.2 exhibited superior attractiveness in a semi-field setting indicating this product may be attractive to wild Anopheles mosquitoes despite the availability of natural sugar sources in the environment. This novel tool may prove suitable for use alongside existing vector control interventions but may require more field experiments to validate its attractiveness as well as an optimal deployment strategy.
Supporting information
(CSV)
(CSV)
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
We thank the KEMRI-Entomology section staff for their dedicated support, which made it possible to draft this manuscript.
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
This study was funded by the Innovative Vector Control Consortium (IVCC), UK, which is funded by grants from the Bill and Melinda Gates Foundation and other funding partners. AF and JE are consultants with IVCC and offered technical support in the design of this trial but had no role in the data collection and analysis of this manuscript. They reviewed and approved this manuscript.
References
- 1.WHO. Who global malaria programme: world malaria report: 2013. Who global malaria programme: world malaria report: 2013; 2013. p. 255. [Google Scholar]
- 2.Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526 (7572):207–11. doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan RC, Sorensen AE, Ladeau S. Citizen science as a tool for mosquito control. Journal of the American Mosquito Control Association. 2017;33 (3):241–5. doi: 10.2987/17-6644R.1 [DOI] [PubMed] [Google Scholar]
- 4.Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malaria journal. 2010;9(1):1–7. doi: 10.1186/1475-2875-9-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beier JC, Müller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malaria journal. 2012;11(1):1–7. doi: 10.1186/1475-2875-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naranjo DP, Qualls WA, Müller GC, Samson DM, Roque D, Alimi T, et al. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitology research. 2013;112(4):1583–7. doi: 10.1007/s00436-013-3312-8 [DOI] [PubMed] [Google Scholar]
- 7.Revay EE, Schlein Y, Tsabari O, Kravchenko V, Qualls W, De-Xue R, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta tropica. 2015;150:29–34. doi: 10.1016/j.actatropica.2015.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster WA. Mosquito sugar feeding and reproductive energetics. Annual review of entomology. 1995;40(1):443–74. doi: 10.1146/annurev.en.40.010195.002303 [DOI] [PubMed] [Google Scholar]
- 9.Yuval B. The other habit: sugar feeding by mosquitoes. Bulletin of the Society of Vector Ecologists. 1992;17(2):150–6. [Google Scholar]
- 10.Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malaria journal. 2010;9(1):1–11. doi: 10.1186/1475-2875-9-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyasembe VO, Torto B. Volatile phytochemicals as mosquito semiochemicals. Phytochemistry letters. 2014;8:196–201. doi: 10.1016/j.phytol.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan SA. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. Journal of Vector Ecology. 2011;36(1):59–67. doi: 10.1111/j.1948-7134.2011.00141.x [DOI] [PubMed] [Google Scholar]
- 13.Sissoko F, Junnila A, Traore MM, Traore SF, Doumbia S, Dembele SM, et al. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB). PloS one. 2019;14(6):e0214170. doi: 10.1371/journal.pone.0214170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouagna LC, Poueme RS, Dabiré KR, Ouédraogo JB, Fontenille D, Simard F. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). Journal of Vector Ecology. 2010;35(2):267–76. doi: 10.1111/j.1948-7134.2010.00082.x [DOI] [PubMed] [Google Scholar]
- 15.Manda H, Gouagna L-C, Nyandat E, Kabiru E, Jackson R, Foster W, et al. Discriminative feeding behaviour of Anopheles gambiae ss on endemic plants in western Kenya. Medical and veterinary entomology. 2007;21(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlein Y, Müller GC. An Approach to Mosquito Control: Using the Dominant Attraction of Flowering Tamarix jordanis Trees Against Culex pipiens. Journal of Medical Entomology. 2014;45[3]:384–90. [DOI] [PubMed] [Google Scholar]
- 17.Xue R-D, Muller GC, Kline DL, Barnard DR. Effect of application rate and persistence of boric acid baits applied topically to plants for control of Aedes albopictus. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malaria journal. 2020;19(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS One. 2013;8(12):e84168. doi: 10.1371/journal.pone.0084168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson MA, Blore K, Efstathion C, Aryaprema VS, Muller GC, Xue RD, et al. Evaluation of boric acid as toxic sugar bait against resistant Aedes aegypti mosquitoes. Journal of Vector Ecology. 2020;45(1):100–3. doi: 10.1111/jvec.12377 [DOI] [PubMed] [Google Scholar]
- 21.Eisele TP, Kleinschmidt I, Sarrassat S, TerKuile F, Miller J, Chanda J, et al. Attractive Targeted Sugar Bait Phase III Trial Group. Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials 23, 640 (2022). 10.1186/s13063-022-06555-8. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machani MG, Ochomo E, Amimo F, Mukabana WR, Githeko AK, Yan G, et al. Behavioral responses of pyrethroid resistant and susceptible Anopheles gambiae mosquitoes to insecticide treated bed net. PloS one. 2022;17(4):e0266420. doi: 10.1371/journal.pone.0266420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amek N, Vounatsou P, Obonyo B, Hamel M, Odhiambo F, Slutsker L, et al. Using health and demographic surveillance system (HDSS) data to analyze geographical distribution of socio-economic status; an experience from KEMRI/CDC HDSS. Acta tropica. 2015;144:24–30. doi: 10.1016/j.actatropica.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 24.Were V, Buff AM, Desai M, Kariuki S, Samuels A, Phillips-Howard P, et al. Trends in malaria prevalence and health related socioeconomic inequality in rural western Kenya: results from repeated household malaria cross-sectional surveys from 2006 to 2013. BMJ open. 2019;9(9):e033883. doi: 10.1136/bmjopen-2019-033883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ombok M, Odhiambo FO. The KEMRI/CDC Health and Demographic Surveillance System—Western Kenya. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degefa T, Yewhalaw D, Zhou G, Lee M-c, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malaria journal. 2017;16(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, et al. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerging infectious diseases. 2015;21(12):2178. doi: 10.3201/eid2112.150814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. The American journal of tropical medicine and hygiene. 2014;90(4):597. doi: 10.4269/ajtmh.13-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khallaayoune K, Qualls WA, Revay EE, Allan SA, Arheart KL, Kravchenko VD, et al. Attractive toxic sugar baits: control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environmental entomology. 2013;42(5):1040–5. doi: 10.1603/EN13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu W, Müller G, Schlein Y, Novak RJ, Beier JC. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PloS one. 2011;6(1):e15996. doi: 10.1371/journal.pone.0015996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller GC, Junnila A, Traore MM, Traore SF, Doumbia S, Sissoko F, et al. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: a habitat manipulation experiment. Malar J. 2017;16(1):237. doi: 10.1186/s12936-017-1878-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone CM, Foster WA. Plant-sugar feeding and vectorial capacity. Ecology of parasite-vector interactions: Springer; 2013. p. 35–79. [Google Scholar]
- 33.Peach DA, Gries R, Zhai H, Young N, Gries G. Multimodal floral cues guide mosquitoes to tansy inflorescences. Scientific reports. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasites & vectors. 2012;5(1):1–11. doi: 10.1186/1756-3305-5-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jepson P, Healy T. The location of floral nectar sources by mosquitoes: an advanced bioassay for volatile plant odours and initial studies with Aedes aegypti (L.)(Diptera: Culicidae). Bulletin of Entomological Research. 1988;78(4):641–50. [Google Scholar]
- 36.Sandholm HA, Price RD. Field observations on the nectar feeding habits of some Minnesota mosquitoes. Mosquito News. 1962;22(4):346–9. [Google Scholar]
- 37.Grimstad P, DeFoliart G. Nectar sources of Wisconsin mosquitoes. Journal of Medical Entomology. 1974;11(3):331–41. doi: 10.1093/jmedent/11.3.331 [DOI] [PubMed] [Google Scholar]
- 38.Thorsteinson A, Brust R. The influence of flower scents on aggregations of caged adult Aedes aegypti. Mosquito News. 1962;22(4). [Google Scholar]
- 39.Manda Gouagna LC, Foster WA Jackson RR, Beier JC Githure JI, et al. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malaria journal. 2007;6(1):1–11. doi: 10.1186/1475-2875-6-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyasembe VO, Teal PE, Sawa P, Tumlinson JH, Borgemeister C, Torto B. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Current Biology. 2014;24(2):217–21. doi: 10.1016/j.cub.2013.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Percival M, Morgan P. Observations on the floral biology of Digitalis species. New Phytologist. 1965;64(1):1–22. [Google Scholar]
- 42.Foster WA, Hancock R. Nectar-related olfactory and visual attractants for mosquitoes. Journal of the American Mosquito Control Association. 1994;10(2 Pt 2):288–96. [PubMed] [Google Scholar]
- 43.Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA. Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. Journal of chemical ecology. 2012;38(7):873–81. doi: 10.1007/s10886-012-0150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. Journal of the American Mosquito Control Association. 2008;24(1):147–9. doi: 10.2987/8756-971X(2008)24[147:DOASAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 45.Tenywa FC, Kambagha A, Saddler A. et al. Tenywa F.C., Kambagha A., Saddler A. et al. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. 2017. doi: 10.1186/s12936-017-1994-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qualls WA, Müller GC, Traore SF, Traore MM, Arheart KL, Doumbia S, et al. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malaria journal. 2015;14(1):1–8. doi: 10.1186/s12936-015-0819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]