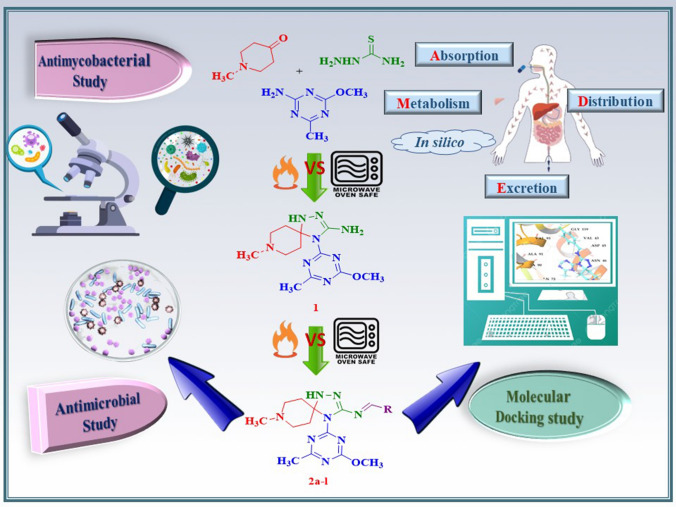
Abstract
Compound 1 is formed by a microwave-assisted multicomponent reaction of 1-methylpiperidin-4-one, 2-amino-4-methoxy-6-methyl-1,3,5-triazine, and thiosemicarbazide, followed by the synthesis of Schiff base 2a–l with a variety of aldehydes. A comparison was made between the conventional and microwave methods, and the microwave approach was shown to be considerably superior to the classical method since it takes less time and produces higher yields. Several spectral investigations, including 1H NMR, 13C NMR, Mass, and IR spectroscopy, are used to characterize the complete series. In vitro antibacterial testing suggests that compounds 2c, 2f, and 2g are promising antibacterial agents, although compounds 2d, 2e, and 2l are effective antimycobacterial agents when compared to the conventional medicine Rifampicin. The docking score from docking studies is considerable, which validates the results of the biological examination. Molecular docking was performed on Escherichia coli DNA gyrase. According to the in silico ADME analysis, each drug molecule is ideal for use in terms of drug solubility, hydrogen bonding, and cell permeability.
Graphical abstract
Keywords: ADME study, Antimicrobial, Antitubercular, Microwave synthesis, Molecular docking
Introduction
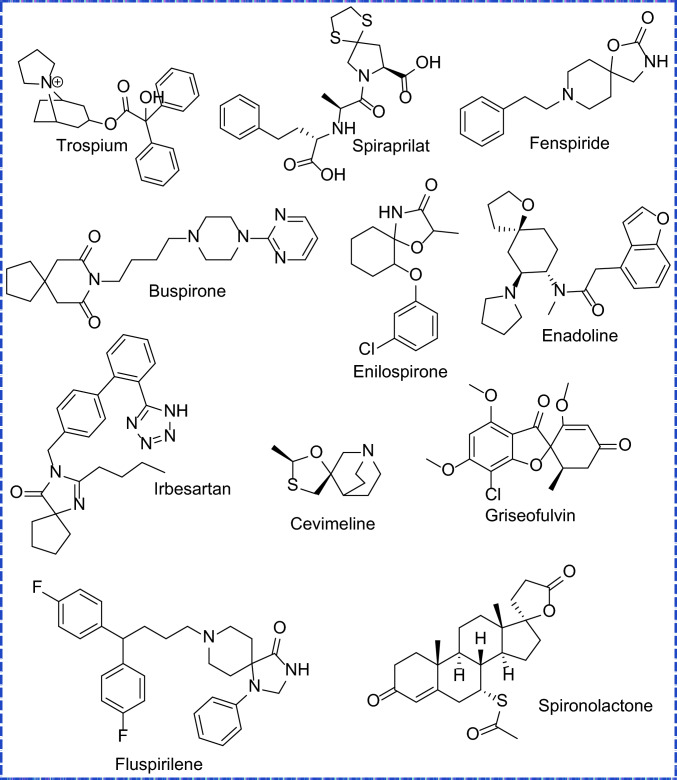
Spiro compounds have recently gained attention in the field of medicinal chemistry due to their diverse biological activity with tiny and rigid structure. It’s important to have a drug molecule with fewer side effects and more pharmacological effects as the molecule should be designed tiny. Many spiro compounds are universally used as standard drug molecules [1]. Spiro compounds have special structural properties and reported that it possesses diverse biological activities and pharmaceutical properties such as anticonvulsant [2], antiepileptic [3], anticancer [4], antitubercular [5], antioxidant [6], antimalarial [7], antiprotozoal [8], antibacterial [9], anti-anxiolytic [10]. The present work contains tiny spiro cycles with lots of nitrogen atoms in their cycle as well as in the triazine substituent. Nitrogen containing spiro compounds have been found highly active against cancer cell lines [11, 12] as well as antitubercular strains [13, 14]. Generally, spiro compounds are prepared using one pot multicomponent reactions such as Ugi reaction [15, 16] and Biginelli reaction [17, 18]. The present work carries the synthesis of novel 4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-amine derivatives as nitrogen is most common atom for the potency in the field of medicinal chemistry. Recently, Apaydın et al. [19] synthesize 1-thia-4-azaspiro[4.5]decane derivatives which are found potent against coronavirus. Mane et al. [20] introduced novel 2H-chromen-8-azaspiro[4.5]decane-7,9-dione conjugates and they are well inhibitors of tubercular strains and possess a significant amount of anti-proliferative activity. Amirani Poor et al. [21] prepared gabapentin based spiro moieties via multicomponent Ugi reaction which active drug molecule during in silico studies. The presence of the triazine ring as well as the triazole ring imparts a huge pharmacological effect in the molecule. Previously triazole mixed with pyrazole derivatives exerts high antitubercular as well as antidiabetic effects [22]. Each synthetic route is followed by the microwave method and compared with the conventional route as the microwave method has a huge advantage over the conventional method [23]. Microwave method has been considered as green method as it reduces time of reaction in high proportion, utilize less amount of solvent, and energy saving technique [24, 25]. Demirci et al. [26] synthesized hybrid structure via conventional and microwave synthesis and reveals the importance of microwave method in synthesis of hybrid molecules as current study involved the synthesis of spiro compounds (Fig. 1).
Fig. 1.
Standard marketed drugs containing spiro structure
According to the CDC’s National Tuberculosis Surveillance System (NTSS), 7860 cases of tuberculosis (TB) have been reported by the 50 United States and the District of Columbia (DC) in 2021, which is more than in 2020 (7173 cases) [27]. According to the Government of India’s official TB Nikshay portal, during the COVID-19 pandemic, TB patients suffer too many challenges due to the lockdown [28]. On average, 10 million people worldwide suffer from tuberculosis each year [29]. So, the present work aims to establish potent antitubercular and antibacterial agents in terms of less toxicity. For that moieties are checked in vitro antimicrobial and antitubercular potency as well as in silico study. In silico studies comprise molecular docking analysis and ADME study [30].
Result and discussion
Chemistry
The initial phase in this multicomponent reaction is a reaction between 1-methylpiperidin-4-one and thiosemicarbazide, followed by a Schiff base reaction on the –NHNH2 group, which is cyclized with 2-amino-4-methoxy-6-methy-1,3,5-triazine to produce intermediate compound 1 (Fig. 2), and then compound 1 is treated with various aldehydes to give Schiff bases 2a–l. Figure 3 is the reaction mechanism of the previous reaction which briefly describes the reaction approach by molecules. The intermediate formed during the process is imine which gives cycloaddition reaction with 2-amino-4-methoxy-6-methy-1,3,5-triazine. IR, 1H NMR, 13C NMR, and mass spectroscopy are employed to evaluate synthesized molecules. An IR band around 3650 cm−1 confirms the presence of a –NH2 group in compound 1. Compound 1 also features an extra –NH– stretching band at roughly 2950 cm−1. Except for the –NH2 group area, compounds 2a and 2e have similar frequencies. The C=N stretching area in compounds 2a and 2e is approximately 1700 cm−1. Compound 1 has a significant increase in the free –NH2 group at 6.10 ppm and 4.91 ppm for the secondary amine. Both series compounds 2a and 2e give a critical peak of hydrogen present on doubly bonded carbon at roughly 8–9 ppm. All compounds provide a significant spiro compound peak at around 73 ppm in the 13C NMR spectrum. Mass spectroscopy is used to investigate the molecular weights of compounds in terms of m/z numbers. To synthesize all compounds, both conventional and microwave methods are used, with the microwave technique winning out in terms of time and product yield percentages as conventional method required 6–7 h to complete whole reaction while microwave process concluded within 6 min. Further, it utilizes less energy being generated from the core of the reaction mixture, so it’s environment friendly. Product obtained by microwave provides greater yield than the conventional heating process by 20–30% of yield (Table 1).
Fig. 2.
Reaction to synthesize compounds 2a–l
Fig. 3.
Mechanism to synthesize compounds 2a–l
Table 1.
Comparison of conventional and microwave route
| No. | Compound | Conventional | Microwave | ||
|---|---|---|---|---|---|
| Yield % | Time (h) | Yield % | Time (min) | ||
| 1 | 2a | 68 | 6 | 90 | 6 |
| 2 | 2b | 70 | 6 | 92 | 6 |
| 3 | 2c | 72 | 6 | 94 | 6 |
| 4 | 2d | 65 | 6 | 89 | 6 |
| 5 | 2e | 73 | 8 | 93 | 6 |
| 6 | 2f | 69 | 7 | 90 | 6 |
| 7 | 2g | 63 | 7 | 88 | 6 |
| 8 | 2h | 66 | 6 | 86 | 6 |
| 9 | 2i | 58 | 6 | 84 | 6 |
| 10 | 2j | 68 | 6 | 92 | 6 |
| 11 | 2k | 64 | 6 | 88 | 6 |
| 12 | 2l | 70 | 8 | 94 | 6 |
Biology
In vitro antibacterial activity
In vitro testing was conducted on compounds 2a–l against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes. Compounds 2f and 2g have the greatest antibacterial efficacy against all four strains. These compounds were compared to the standard drugs Ampicillin and Chloramphenicol, and both showed comparative activity with Chloramphenicol and significant activity with Ampicillin. We can see that both compounds 2f and 2g have fluorine substitutions, which are accountable for their more intense activities. Furthermore, every substitution with an electron donating group has comparative action in other compounds. Compound 2c, for example, has no replacement and thus has the lowest activities. Compounds with halogen in their structures possess enhanced activities due to their high electronegative atoms, which can readily form H-bonds. Because there are no electron donating groups on compounds 2j and 2k with heterocyclic substitution, their activities are reduced. A comparison of compound activities reveals that the complete series is more effective against gram negative antibacterial organisms E. coli (Table 2).
Table 2.
In vitro antimicrobial evaluation of synthesized compounds 2a–l
| Sr no. | Code no. | Antibacterial data MIC [µg/ml] | Antifungal data MIC [µg/ml] | |||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | S. pyogenus | C. albicans | A. niger | A. clavatus | ||
| MTCC 443 | MTCC 1688 | MTCC 96 | MTCC 442 | MTCC 227 | MTCC 282 | MTCC 1323 | ||
| 1 | 2a | 125 | 125 | 100 | 100 | 500 | 500 | 1000 |
| 2 | 2b | 100 | 100 | 125 | 125 | 500 | 1000 | 500 |
| 3 | 2c | 250 | 100 | 100 | 100 | 500 | 500 | 500 |
| 4 | 2d | 125 | 125 | 125 | 100 | 250 | 1000 | 1000 |
| 5 | 2e | 125 | 100 | 250 | 125 | 500 | 1000 | 1000 |
| 6 | 2f | 50 | 62.5 | 62.5 | 62.5 | 100 | 500 | 250 |
| 7 | 2g | 62.5 | 62.5 | 50 | 62.5 | 250 | 500 | 500 |
| 8 | 2h | 125 | 125 | 125 | 125 | 500 | 500 | 500 |
| 9 | 2i | 100 | 100 | 100 | 100 | 500 | 1000 | 1000 |
| 10 | 2j | 125 | 125 | 250 | 125 | 500 | 1000 | 500 |
| 11 | 2k | 125 | 250 | 125 | 125 | 250 | 500 | 1000 |
| 12 | 2l | 100 | 125 | 125 | 125 | 250 | 500 | 500 |
| 13 | Ampicillin | 100 | 100 | 250 | 100 | – | – | – |
| 14 | Chloram-phenicol | 50 | 50 | 50 | 50 | – | – | – |
| 15 | Nystatin | – | – | – | – | 100 | 100 | 100 |
| 16 | Griseofulvin | – | – | – | – | 500 | 100 | 100 |
In vitro antifungal activity
In vitro antifungal activities of compounds 2a–l have been evaluated against C. albicans, A. niger, and A. clavatus. Compound 2f has comparative activity with standard drugs Nystatin and Griseofulvin. Other compounds with halogen substitution found mild activities against all strains. All derivatives are more potent against C. albicans compared to the other antifungal strains (Table 2).
In vitro antimycobacterial activity
As shown in Table 3, compounds 2f and 2g have the lowest MIC values of 0.45 µg/ml and 0.56 µg/ml, respectively, with the greatest activities. The antitubercular strain M. tuberculosis H37Rv had been examined in vitro and compared to the usual antitubercular drugs isoniazid and rifampicin. Compound 2c has the highest MIC value in the sequence, 1.32 µg/ml, and thus the lowest active moiety. Compound 2c lacks a single group on a substituted benzene ring, whereas the compound with halogen atoms substituted develops finer antimycobacterial activities than non-substituted compounds in the following series. Compound 2d has some extra biological effect concerning compounds 2a and 2b so, electron donating nature enhances the activities of parent moieties. So as the presence of heterocyclic substitutions, with MIC values of around 0.72 µg/ml and 0.69 µg/ml, exert a higher antitubercular effect compared to the standard drug Rifampicin. Compounds 2e and 2l are also near active to compound 2d with MIC values of 0.58 µg/ml and 0.50 µg/ml, which is due to the presence of methoxy and bromo substitutions on it, respectively. All newly synthesized derivatives are very promising antitubercular agents with respect to Rifampicin (Table 3).
Table 3.
In vitro antimycobacterial activity of compound 2a–l
| Antimycobacterial activity | ||
|---|---|---|
| Sr. no. | Code no. | MIC µg/ml |
| 1 | 2a | 1.01 |
| 2 | 2b | 0.90 |
| 3 | 2c | 1.32 |
| 4 | 2d | 0.83 |
| 5 | 2e | 0.73 |
| 6 | 2f | 0.45 |
| 7 | 2g | 0.56 |
| 8 | 2h | 0.76 |
| 9 | 2i | 0.80 |
| 10 | 2j | 0.72 |
| 11 | 2k | 0.69 |
| 12 | 2l | 0.74 |
| 13 | Isoniazid | 0.2 |
| 14 | Rifampicin | 40 |
Computational study
In silico molecular docking analysis
Molecular docking studies of the synthesized compounds were performed to determine binding modes and binding affinity with the target protein. E. coli DNA gyrase was selected for docking studies because bacterial DNA gyrase, a topoisomerase type II enzyme that catalyses DNA structure by rupturing and re-joining double-stranded DNA, is a well-validated target for the development of antibacterial medicines [31]. When compared to the reference co-crystal ligand (− 6.310), all synthesized compounds had significant docking scores in the range of − 7.75 to − 6.295 kcal/mol. Among the synthesized compounds, compounds 2b (− 7.75 kcal/mol), 2g (− 7.073 kcal/mol), 2j (− 6.933 kcal/mol), 2h (− 6.925 kcal/mol), 2l (− 6.861 kcal/mol), 2a (− 6.714 kcal/mol), 2c (− 6.564 kcal/mol), 2i (− 6.555 kcal/mol), and 2f (− 6.371 kcal/mol) have a significantly higher docking score than the co-crystal ligand (Table 4). Compound 2f shows a significant docking score as well as a significant MIC value of 50 μM against the MTCC 443 E. coli strains. Maestro’s ligand-interaction tool was used to generate 2D and 3D graphical depictions of the ligand–protein interactions of representative compounds seen in Fig. 1. According to a binding mode analysis, the Vander Wall interaction accounted for the majority of the interactions between the three compounds in the DNA GyrB binding cavity. At a distance of 2.50 Å, the protonated nitrogen group of 4-fluorobenzylidene compound 2f forms a hydrogen bond with the amino acid Asn46 (Fig. 4). While the nitrogen of 1-methylpiperidine forms a bidentate interaction with the charged negative residue Glu50 through hydrogen bonding and the salt bridge.
Table 4.
Glide docking score of synthesized derivatives (2a–l) in the active of E. coli DNA GyrB (PDB ID: 5L3J)
| Compd. | Docking score |
|---|---|
| 2a | − 6.714 |
| 2b | − 7.75 |
| 2c | − 6.564 |
| 2d | − 6.295 |
| 2e | − 5.847 |
| 2f | − 6.371 |
| 2g | − 7.073 |
| 2h | − 6.925 |
| 2i | − 6.555 |
| 2j | − 6.933 |
| 2k | − 6.295 |
| 2l | − 6.861 |
| Co-crystal ligand | − 6.310 |
Fig. 4.
2D and 3D binding interaction of compound 2f (A) and 2g (B) in the active site of E. coli DNA GyrB (PDB ID: 5L3J)
In silico ADME study
To determine how these synthesized drug molecules will behave in our bodies, ADME analysis has been brought in. This study helps to investigate drug likeness. Versatile pharmacodynamic and pharmacokinetic properties have been evaluated in silico during this analysis. A number of rotatable bonds (nROTB), topological polar surface area (TPSA), log of aqueous solubility (logS), log of octanol/water partition (logP), synthetic accessibility score (SAscore), and Caco2 cell permeability (PCaco2) are examined in this study. Table 5 describes all properties in detail for all synthesized compounds. Every compound carries 7–8 H-Acceptor sites and 1–2 H-Donor sites. Compounds 2a, 2b, 2e, 2j, and 2k have large polar surface areas. All compounds hold logS values between the range of − 3 to − 4, which leads to higher aqueous solubility. All compounds have promising SA scores for the synthesis of entire series compounds. PCaco2 value of all compounds is in the range of − 6 to − 7 which explains the absorption of the drug.
Table 5.
In silico ADME predictions of targeted compounds (2a–l)
| Comp. code | MWa | nHAb | nHDc | nROTBd | TPSAe | logSf | logPg | SAscoreh | PCaco2i |
|---|---|---|---|---|---|---|---|---|---|
| 2a | 396.45 | 8 | 2 | 4 | 111.36 | − 3.86 | 2.96 | 4.41 | − 7.00 |
| 2b | 396.45 | 8 | 2 | 4 | 111.36 | − 3.86 | 3.05 | 4.43 | − 7.00 |
| 2c | 380.45 | 7 | 1 | 4 | 91.13 | − 4.00 | 3.27 | 4.41 | − 6.65 |
| 2d | 414.89 | 7 | 1 | 4 | 91.13 | − 4.60 | 3.48 | 4.39 | − 6.41 |
| 2e | 410.47 | 8 | 1 | 5 | 100.36 | − 4.08 | 3.81 | 4.49 | − 6.85 |
| 2f | 398.44 | 8 | 1 | 4 | 91.13 | − 4.17 | 3.40 | 4.40 | − 6.69 |
| 2g | 398.44 | 8 | 1 | 4 | 91.13 | − 4.17 | 3.41 | 4.41 | − 6.69 |
| 2h | 414.89 | 7 | 1 | 4 | 91.13 | − 4.60 | 3.60 | 4.42 | − 6.41 |
| 2i | 414.89 | 7 | 1 | 4 | 91.13 | − 4.60 | 3.40 | 4.40 | − 6.41 |
| 2j | 370.41 | 8 | 1 | 4 | 104.27 | − 3.55 | 3.20 | 4.53 | − 7.01 |
| 2k | 386.47 | 7 | 1 | 4 | 119.37 | − 4.03 | 3.12 | 4.48 | − 6.68 |
| 2l | 459.34 | 7 | 1 | 4 | 91.13 | − 4.92 | 3.42 | 4.41 | − 6.64 |
a Molecular weight; b Number of H-bond acceptors; c Number of H-bond donors; d Number of rotatable bonds; e Topological polar surface area; f Log of the aqueous solubility; g Log of the octanol/water partition; h Synthetic accessibility score; i Caco2 cell Permeability
Conclusion
Comparison between conventional and microwave methods of synthesis leads to a vast number of advantages of microwave method over conventional, as it increases yield percentages up to 20–30% compared to conventional process and reduces reaction time up to 98% over conventional heating method. Further, it utilizes less amount of solvent, which is considered as an eco-friendly reaction. Each compound from the entire series gives respective spectrums during all 1H NMR, 13C NMR, IR, and Mass spectral analyses. In vitro antibacterial analysis comes out with extraordinary outcomes with compounds 2f and 2g carrying comparative potencies concerning to the standard drug Ampicillin and Chloramphenicol. Further, compound 2f possesses a significant antifungal effect against C. albicans strain. Compound 2f was established to be a most potent antitubercular agent with 0.45 µg/ml MIC value, which is much more effective than the standard drug Rifampicin and nearer active with respect to Isoniazid. In vitro activities are supported by the in silico analysis as both compounds 2f and 2g are having high docking scores against E. coli DNA gyrase. ADME analysis gives an idea about the ideal behaviour of synthesized drug molecules with respect to water solubility, total polar surface area, skin permeability, and synthetic accessibility score.
Experimental
Materials and methods
The chemical used during synthetic work had been arranged from Sigma Aldrich and Fisher Scientific Ltd. Compounds are purified by recrystallization method and purity is checked using TLC plates and column chromatography (silica gel G). The melting point of obtained compounds is carried out using the open capillary method. Microwave synthesizer CATA R by Catalyst company has been used for microwave synthesis with maximum capacity of 850 W. Characterization is done by several spectral techniques such as IR, 1H NMR, 13C NMR, and mass spectroscopy. FT-IR spectroscopy was used to derive IR spectrums from potassium bromide pellets using a Perkin Elmer RZX infrared spectrophotometer and an Agilent resolution Pro FT-IR spectrometer; frequencies are given in cm−1. The 1H-NMR and 13C-NMR spectra were analysed in dimethyl sulfoxide (DMSO-d6) using a Bruker Advance II 400 spectrometer (500 MHz FT-NMR) and tetramethyl silane as the internal standard (Chemical shifts in ppm). The Q–T micro mass spectra of WATERS were obtained (Electrospray ionization-MS). On silica gel 60, Merck column chromatography (0.043–0.06 mm) was conducted. In vitro antimicrobial and antitubercular activities are carried against various gram positive and Gram-negative antibacterial strains. E. coli, P. aeruginosa, S. aureus, and S. pyogenus are used as antibacterial agents, C. albicans, A. niger and A. clavatus are used as antifungal agents. Mycobacterium tuberculosis H37Rv is used as an antitubercular strain. In vitro analysis is carried out via the Broth dilution method on Lj medium. For docking analysis, the 2D structures of synthesized compounds were generated using Chem-BioDraw Ultra and treated with Schrodinger’s LigPrep. It allows for ligand shape optimization and the generation of low energy 3D structures with appropriate chirality. Synthesized compounds were docked onto the active site of E. coli DNA gyrase B using Schrödinger glide (PDB ID: 5L3J) [32, 33]. Protein structure was downloaded from the PDB repository, hydrogens were added, bond orders were assigned, metal bonds were created in zero order, Prime was used to fill in any missing side chains and loops, water beyond 5 Å was removed from heteroatoms, and the Epik module was used to create het states in the protein 3D structure [34, 35]. Optimal potentials for liquid simulation 3e (OPLS3e) were used until the non-hydrogen atoms’ mean root mean square deviation (RMSD) converged at 0.30 Å. The co-crystallized ligand was used to identify the centroid active site using the grid generation tool. Overall, using the standard precision (SP) technique, the docking process was completed in glide. In silico ADME study is done using online software, Swiss ADME.
Synthesis of 4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-amine (1) and its derivatives (2a–l)
Conventional method
1-Methylpiperidin-4-one (0.02 mol), 2-amino-4-methoxy-6-methyl-1,3,5-triazine (0.02 mol), and thiosemicarbazide (0.02 mol) were subjected to multicomponent reaction in glacial acetic acid. The reaction is carried out at a refluxed temperature of 120 °C for 6–7 h. After completion of the reaction, entire bulk is kept overnight to generate product 1. Product 1 is purified by the recrystallization method in EtOH. The ultimate product was prepared by reaction of compound 1 (0.007 mol) with numerous aromatic aldehydes (0.007 mol) in EtOH at 100 °C for 8 h. via the addition of a catalytic amount of acetic acid. After completion of the reaction, the mixture is kept overnight to acquire final products 2a–l. final compounds were purified using recrystallization in EtOH. Both steps of these reactions are observed via TLC using ethyl acetate: n-hexane (2.5:7.5) as the mobile phase. All final products 2a–l were also purified using column chromatography using ethyl acetate: n-hexane (2.5:7.5) as the mobile phase.
Microwave method
1-Methylpiperidin-4-one (0.02 mol), 2-amino-4-methoxy-6-methyl-1,3,5-triazine (0.02 mol), and thiosemicarbazide (0.02 mol) were besides for microwave synthesis in microwave oven and proceed for 4 min at 340 Watts. Temperature is set to 120 °C. This reaction is also monitored via TLC using ethyl acetate: n-hexane (2.5:7.5) as the mobile phase. Final product 1 was obtained after holding it for a night. The solid product is filtered and recrystallized with EtOH. Compound 1 and different aldehydes were reacted in microwave conditions for 6 min at 340 watts at 100 °C. After completion of reaction solid product 2a–l is obtained. Final products were purified using recrystallization in ethanol and column chromatography (n-hexane: ethyl acetate = 2.5:7.5).
4-(4-Methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-amine (1)
Dark yellow; m.p.: 210 °C; C12H20N8O; IR (KBr, cm−1): 3650 (N–H, str., Primary amine), 3200 (NH, str., secondary amine); 1H NMR: 2.09–2.22 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.69–2.73 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 4.91 (s, 1H, NH), 6.10–6.16 (dd, 2H, –NH2); 13C NMR: 25.17, 33.55, 46.19, 49.83, 54.91, 73.72, 147.35, 156.07, 171.88, 172.57; m/z (%): 292.7273 (M+).
(E)-4-(((4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)imino)methyl)phenol (2a)
Dark brown; m.p.: 244 °C; C19H24N8O2; IR (KBr, cm−1): 3600–3560 (O–H, str.), 3350 (N–H, str.), 1700 (C=N, str.), 1350 (C–O, str.); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 6.87–6.90 (dd, 2H, Ar–CH), 7.31 (s, 1H, –OH), 7.54–7.56 (dd, 2H, Ar–CH), 8.85 (s, 1H, –CH=); 13C NMR: 25.17, 33.55, 46.19, 49.83, 54.91, 74.95, 116.33, 123.67, 131.06, 147.57, 156.86, 157.22, 159.58, 171.90, 172.19; m/z (%): 397.3725 (M+).
(E)-2-(((4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)imino)methyl)phenol (2b)
Yellow; m.p.: 234 °C; C19H24N8O2; IR (KBr, cm−1): 3600 (–OH str.), 3450 (N–H str.), 1650 (–C=C–), 1370 (C–O); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.64 (s, 1H, NH), 6.88–6.89 (m, 1H, Ar–CH), 6.90–6.92 (m, 1H, Ar–CH), 7.28–7.31 (m, 1H, Ar–CH), 7.53 (m, 1H, Ar–CH), 7.55 (s, 1H, –OH), 8.77 (s, 1H, –CH=); 13C NMR: 25.17, 33.55, 46.19, 49.83, 54.91, 74.95, 114.56, 117.72, 120.16, 131.93, 132.99, 147.21, 156.86, 159.65, 159.65, 160.71, 171.90, 172.19; m/z (%): 397.1695 (M+).
(E)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)-1-phenylmethanimine (2c)
Pale yellow; m.p.: 220 °C; C19H24N8O; IR (KBr, cm−1): 3400 (N–H str.), 1590 (–C=C–), 1350 (C–O); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 7.34–7.42 (m, 3H, Ar–CH), 7.77–7.79 (m, 2H, Ar–CH), 8.79 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 128.86, 129.03, 130.67, 131.55, 147.57, 156.86, 159.42, 171.90, 172.19; m/z (%): 381.2256 (M+).
(E)-1-(4-chlorophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2d)
Brown; m.p.: 222 °C; C19H23ClN8O; IR (KBr, cm−1): 3450 (N–H str.), 1650 (–C=C–), 1350 (C–O), 650 (C–Cl); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.69–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 7.62–7.64 (m, 2H, Ar–CH), 7.66–7.68 (m, 2H, Ar–CH), 8.79 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 129.19, 129.41, 130.14, 136.84, 147.57, 156.86, 159.37, 171.90, 172.19; m/z (%): 417.0657 (M+).
(E)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)-1-(4-methoxyphenyl)methanimine (2e)
Brown; m.p.: 254 °C; C20H26N8O2; IR (KBr, cm−1): 3390 (N–H str.), 1600 (–C=C–)1400 (C–O); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.69–2.75 (m, 4H, 2CH2), 3.81 (s, 3H, –OCH3), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 6.94–6.97 (m, 2H, Ar–CH), 7.61–7.63 (m, 2H, Ar–CH), 8.85 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 115.12, 125.17, 130.81, 147.57, 156.86, 159.48, 162.44, 171.90, 172.19; m/z (%): 410.2328 (M+).
(E)-1-(4-fluorophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2f)
Dark yellow; m.p.: 248 °C; C19H23FN8O; IR (KBr, cm−1): 3360 (N–H str.), 1600 (–C=C–), 1390 (C–O), 640 (C–F); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.69–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 7.11–7.15 (m, 2H, Ar–CH), 7.74–7.77 (m, 2H, Ar–CH), 8.86 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 116.14, 116.30, 127.60, 127.62, 130.67, 130.73, 147.57, 156.86, 159.52, 161.46, 163.47, 171.90, 172.19; m/z (%): 399.1342 (M+).
(E)-1-(2-fluorophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2g)
Yellow; m.p.: 224 °C; C19H23FN8O; IR (KBr, cm−1): 3450 (N–H str.), 1590 (–C=C–), 1360 (C–O), 650 (C–F); 1H NMR: 2.09–2.21 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.76 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.64 (s, 1H, NH), 7.12–7.16 (m, 1H, Ar–CH), 7.25–7.29 (m, 1H, Ar–CH), 7.47–7.50 (m, 1H, Ar–CH), 7.72–7.75 (m, 1H, Ar–CH), 8.85 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 115.92, 116.08, 122.25, 122.41, 126.16, 126.18, 129.32, 129.38, 132.78, 132.85, 147.03, 153.78, 153.85, 156.86, 161.13, 163.15, 171.90, 172.19; m/z (%): 292.72 (M+).
(E)-1-(2-chlorophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2h)
Yellow; m.p.: 236 °C; C19H23ClN8O; IR (KBr, cm−1): 3400 (N–H str.), 1650 (–C=C–), 1400 (C–O), 760 (C–Cl); 1H NMR: 2.09–2.13 (m, 4H, 2CH2), 2.20 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.76 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.64 (s, 1H, NH), 7.34–7.35 (m, 1H, Ar–CH), 7.36–7.37 (m, 1H, Ar–CH), 7.38–7.39 (m, 1H, Ar–CH), 7.65–7.69 (m, 1H, Ar–CH), 8.81 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 128.71, 129.49, 129.78, 131.45, 132.31, 135.92, 146.65, 156.37, 156.86, 171.90, 172.19; m/z (%): 418.0910 (M+).
(E)-1-(3-chlorophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2i)
Dark brown; m.p.: 238 °C; C19H23ClN8O; IR (KBr, cm−1): 3380 (N–H str.), 1560 (–C=C–), 1350 (C–O), 800 (C–Cl); 1H NMR: 2.09–2.24 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.69–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 7.41–7.42 (m, 1H, Ar–CH), 7.43–7.46 (m, 1H, Ar–CH), 7.66–7.67 (m, 1H, Ar–CH), 7.68–7.71 (m, 1H, Ar–CH), 8.83 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 126.95, 128.52, 129.21, 130.94, 133.43, 134.88, 147.51, 156.86, 158.16, 171.90, 172.19; m/z (%): 418.6158 (M+).
(E)-1-(furan-2-yl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2j)
Dark brown; m.p.: 256 °C; C17H22N8O2; IR (KBr, cm−1): 3400 (N–H str.), 1600 (–C=C–), 1360 (C–O); 1H NMR: 2.09–2.13 (m, 4H, 2CH2), 2.20 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.75 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.69 (s, 1H, NH), 6.70–6.71 (m, 1H, Ar–CH), 6.90–6.91 (m, 1H, Ar–CH), 7.71 (m, 1H, Ar–CH), 8.40 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.96, 112.77, 116.46, 142.41, 146.67, 147.81, 149.97, 156.81, 171.90, 172.19; m/z (%): 372.9650 (M+).
(E)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)-1-(thiophen-2-yl)methanimine (2k)
Dark brown; m.p.: 264 °C; C17H22N8OS; IR (KBr, cm−1): 3450 (N–H str.), 1620 (–C=C–), 1420 (C–O); 1H NMR: 2.09–2.21 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.76 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.80 (s, 1H, NH), 7.12–7.14 (m, 1H, Ar–CH), 7.39–7.40 (m, 1H, Ar–CH), 7.61–7.62 (m, 1H, Ar–CH), 8.57 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.96, 127.90, 129.93, 130.71, 137.69, 147.45, 154.19, 156.86, 171.90, 172.19; m/z (%): 387.2645 (M+).
(E)-1-(3-bromophenyl)-N-(4-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-yl)methanimine (2l)
Brown; m.p.: 234 °C; C19H23ClN8O; IR (KBr, cm−1): 3350 (N–H str.), 1600 (–C=C–), 1390 (C–O), 650 (C–Br); 1H NMR: 2.09–2.21 (m, 4H, 2CH2), 2.28 (s, 3H, –NCH3), 2.38 (s, 3H, CH3), 2.68–2.76 (m, 4H, 2CH2), 3.90 (s, 3H, –OCH3), 6.67 (s, 1H, NH), 7.37–7.39 (m, 1H, Ar–CH), 7.54–7.57 (m, 1H, Ar–CH), 7.69–7.72 (m, 1H, Ar–CH), 7.83 (m, 1H, Ar–CH), 8.80 (s, 1H, –CH=); 13C NMR: 25.18, 33.54, 46.19, 49.83, 54.91, 74.95, 123.26, 127.50, 129.73, 131.16, 131.39, 134.06, 147.51, 156.86, 157.59, 171.90, 172.19; m/z (%): 456.3508 (M+).
Acknowledgements
The authors would like to express their gratitude to the Head of the Department of Chemistry at Veer Narmad South Gujarat University in Surat for providing the resources for this study. We are also grateful to the UGC-BSR Faculty Fellow [No. F.4-5(11)2019(BSR)] for their financial support. The spectrum data provided by the Director, CIL, and SAIF; Panjab University is quite valuable. The authors also gave thanks to the MicroCare laboratory for biological research.
Author contributions
PPP wrote the manuscript. NBP provides proper guidelines for current work. MST helped with the spectral analysis. VMP designed the scheme and prepared figures. IA and HP carried out in silico molecular docking study.
Declarations
Conflict of interest
Authors declare that they had no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Parth P. Patel, Email: patelparth917@gmail.com
Navin B. Patel, Email: drnavinbpatel@gmail.com
Manesh S. Tople, Email: tople143@gmail.com
Vatsal M. Patel, Email: vatsalpatel1904@gmail.com
Iqrar Ahmed, Email: ansariiqrar50@gmail.com.
Harun Patel, Email: hpatel_38@yahoo.com.
References
- 1.Zheng Y, Tice CM, Singh SB. The use of spirocyclic scaffolds in drug discovery. Bioorg Med Chem Lett. 2014;24(16):3673–3682. doi: 10.1016/j.bmcl.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 2.Receveur J-M, Bryans JS, Field MJ, Singh L, Horwell DC. Synthesis and biological evaluation of conformationally restricted gabapentin analogues. Bioorg Med Chem Lett. 1999;9(16):2329–2334. doi: 10.1016/S0960-894X(99)00383-2. [DOI] [PubMed] [Google Scholar]
- 3.Potschka H, Feuerstein TJ, Löscher W. Gabapentin-lactam, a close analogue of the anticonvulsant gabapentin, exerts convulsant activity in amygdala kindled rats. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:200–205. doi: 10.1007/s002109900174. [DOI] [PubMed] [Google Scholar]
- 4.Yu B, Yu D-Q, Liu H-M. Spirooxindoles: promising scaffolds for anticancer agents. Eur J Med Chem. 2015;97:673–698. doi: 10.1016/j.ejmech.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 5.Badiola KA, Quan DH, Triccas JA, Todd MH. Efficient synthesis and anti-tubercular activity of a series of spirocycles: an exercise in open. Science. 2014 doi: 10.1371/journal.pone.0111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssef MM, Amin MA. Microwave assisted synthesis of some new heterocyclic spiro-derivatives with potential antimicrobial and antioxidant activity. Molecules. 2010;5(12):8827–8840. doi: 10.3390/molecules15128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarrahpour A, Ebrahimi E, De Clercq E, Sinou V, Latour C, Djouhri BL, Brunel JM. Synthesis of mono-, bis-spiro- and dispiro-β-lactams and evaluation of their antimalarial activities. Tetrahedron. 2011;67(45):8699–8704. doi: 10.1016/j.tet.2011.09.041. [DOI] [Google Scholar]
- 8.Heilmann J, Mayr S, Brun R, Rali T, Sticher O. Antiprotozoal activity and cytotoxicity of novel 1,7-dioxadispiro[5.1.5.2]pentadeca-9,12-dien-11-one derivatives from Amomum aculeatum. Helv Chim Acta. 2000;83(11):2939–2945. doi: 10.1002/1522-2675(20001108)83. [DOI] [Google Scholar]
- 9.El-Hashash M, Rizk S, Atta-Allah S. Synthesis and regioselective reaction of some unsymmetrical heterocyclic chalcone derivatives and spiro heterocyclic compounds as antibacterial agents. Molecules. 2015;20(12):22069–22083. doi: 10.3390/molecules201219827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anikina L, Vikharev YB, Rozhkova YS, Shklyaev YV. Synthesis and biological activity of 3-spiro[adamantane-2,3′-isoquinolines] Pharm Chem J. 2013;46(12):707–710. doi: 10.1007/11094-013-0874-9. [DOI] [Google Scholar]
- 11.Ameen MA, Ahmed EK, Ramadan M, Abd El-Naby HA, Abdel-Haseeb AA. Synthesis and evaluation of anticancer and PDE 5 inhibitory activity of spiro-substituted quinazolin-4-ones. Monatsh Chem. 2017;148(8):1513–1523. doi: 10.1007/s00706-017-1961-5. [DOI] [Google Scholar]
- 12.Barakat A, Islam MS, Ghawas HM, Al-Majid AM, El-Senduny FF, Badria FA, Elshaier YAM, Ghabbour HA. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018;8(26):14335–14346. doi: 10.1039/C8RA02358A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapnakumari M, Narayana B, Shashidhara KS, Sarojini BK. Multicomponent synthesis, biological evaluation and molecular docking of new spiro-oxindole derivatives. J Taibah Univ Sci. 2017;11(6):1008–1018. doi: 10.1016/j.jtusci.2017.04.002. [DOI] [Google Scholar]
- 14.Pogaku V, Krishna VS, Sriram D, Rangan K, Basavoju S. Ultrasonication-ionic liquid synergy for the synthesis of new potent anti-tuberculosis 1,2,4-triazol-1-yl-pyrazole based spirooxindolopyrrolizidines. Bioorg Med Chem Lett. 2019;29(13):1682–1687. doi: 10.1016/j.bmcl.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Santra S, Andreana PR. A rapid, one-pot, microwave-influenced synthesis of spiro-2,5-diketopiperazines via a cascade Ugi/6-exo-TrigAza-Michael reaction. J Org Chem. 2011;76(7):2261–2264. doi: 10.1021/jo102305q. [DOI] [PubMed] [Google Scholar]
- 16.Sharma N, Li Z, Sharma UK, Van der Eycken EV. Facile access to functionalized spiro[indoline-3,2′-pyrrole]-2,5′-diones via post-Ugi Domino Buchwald–Hartwig/Michael reaction. Org Lett. 2014;16(15):3884–3887. doi: 10.1021/ol5019079. [DOI] [PubMed] [Google Scholar]
- 17.Byk G, Kabha E. Anomalous regioselective four-member multicomponent Biginelli reaction II: one-pot parallel synthesis of spiro heterobicyclic aliphatic rings. J Comb Chem. 2004;6(4):596–603. doi: 10.1021/cc049962i. [DOI] [PubMed] [Google Scholar]
- 18.Shaabani A, Bazgir A, Bijanzadeh HR. A reexamination of Biginelli-like multicomponent condensation reaction: one-pot regioselective synthesis of spiro heterobicyclic rings. Mol Divers. 2004;8(2):141–145. doi: 10.1023/b:modi.0000025613.35304.25. [DOI] [PubMed] [Google Scholar]
- 19.Apaydın ÇB, Cesur N, Stevaert A, Naesens L, Cesur Z. Synthesis and anti-coronavirus activity of a series of 1-thia-4-azaspiro[4.5]decan-3-one derivatives. Arch Pharm. 2019;352(6):1800330. doi: 10.1002/ardp.201800330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mane DSG, Reddy DS, Katagi KS, Kumar A, Munnolli RS, Kadam NS, Nagarajaiah H. Design, synthesis, molecular docking, anti-proliferative and anti-TB studies of 2H-chromen-8-azaspiro[4.5]decane-7,9-dione conjugates. J Mol Struct. 2020 doi: 10.1016/j.molstruc.2020.129530. [DOI] [Google Scholar]
- 21.Amirani Poor M, Darehkordi A, Anary-Abbasinejad M, Mohammadi M. Gabapentin-base synthesis and theoretical studies of biologically active compounds: N-cyclohexyl-3-oxo-2-(3-oxo-2-azaspiro[4.5] decan-2-yl)-3-arylpropanamides and N-(tert-butyl)-2-(3-oxo-2-azaspiro[4.5]decan-2-yl)-2-arylacetamide derivatives. J Mol Struct. 2018;1152:44–52. doi: 10.1016/j.molstruc.2017.09.061. [DOI] [Google Scholar]
- 22.Dinari M, Gharah F, Asadi P. Synthesis, spectroscopic characterization, antimicrobial evaluation and molecular docking study of novel triazine-quinazolinone based hybrids. J Mol Struct. 2018;1156:43–50. doi: 10.1016/j.molstruc.2017.11.087. [DOI] [Google Scholar]
- 23.Yang G, Park S-J. Conventional and microwave hydrothermal synthesis and application of functional materials: a review. Materials. 2019;12(7):1177. doi: 10.3390/ma12071177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mermer A, Demirbas N, Demirbas A, Colak N, Ayaz FA, Alagumuthu M, Arumugam S. Synthesis, biological activity and structure activity relationship studies of novel conazole analogues via conventional, microwave and ultrasound mediated techniques. Bioorg Chem. 2018;81:55–70. doi: 10.1016/j.bioorg.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Mermer A, Demirbas N, Colak A, Demir EA, Kulabas N, Demirbas A. One-pot, four-component green synthesis, carbonic anhydrase II inhibition and docking studies of 5-arylidenerhodanines. ChemistrySelect. 2018;3(43):12234–12242. doi: 10.1002/slct.201802677. [DOI] [Google Scholar]
- 26.Demirci S, Mermer A, Ak G, Aksakal F, Colak N, Demirbas A, Demirbas N. Conventional and microwave-assisted total synthesis, antioxidant capacity, biological activity, and molecular docking studies of new hybrid compounds. J Heterocycl Chem. 2016;54(3):1785–1805. doi: 10.1002/jhet.2760. [DOI] [Google Scholar]
- 27.Filardo TD, Feng PJ, Pratt RH, Price SF, Self JL. Tuberculosis—United States, 2021. MMWR Morb Mortal Wkly. 2022;71(12):441. doi: 10.15585/mmwr.mm7112a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain VK, Iyengar KP, Samy DA, Vaishya R. Tuberculosis in the era of COVID-19 in India. Diabetes Metab Syndr. 2020;14(5):1439–1443. doi: 10.1016/j.dsx.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suárez I, Fünger SM, Kroger S, Rademacher J, Fatkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. Dtsch Arztebl Int. 2019 doi: 10.3238/arztebl.2019.0729. [DOI] [PubMed] [Google Scholar]
- 30.Jia CY, Li JY, Hao GF, Yang GF. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. 2019;31(2):213–218. doi: 10.1016/j.drudis.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Desai NC, Jadeja DJ, Jethawa AM, Ahmad I, Patel H, Dave BP. Design and synthesis of some novel hybrid molecules based on 4-thiazolidinone bearing pyridine-pyrazole scaffolds: molecular docking and molecular dynamics simulations of its major constituent onto DNA gyrase inhibition. Mol Divers. 2023 doi: 10.1007/s11030-023-10612-y. [DOI] [PubMed] [Google Scholar]
- 32.Osmaniyea D, Ahmad I, Sağlık BN, Leventa S, Patel HM, Ozkay Y, Kaplancıkl ZA. Design, synthesis and molecular docking and ADME studies of novel hydrazone derivatives for AChE inhibitory, BBB permeability and antioxidant effects. J Biomol Struct Dyn. 2022 doi: 10.1080/07391102.2022.2139762. [DOI] [PubMed] [Google Scholar]
- 33.Halder SK, Ahmad I, Shathi JF, Mim MM, Hassan MR, Jewel MJI, Dey P, Islam MS, Patel H, Morshed MR, Shakil MS, Hossen MS. A comprehensive study to unleash the putative inhibitors of serotype2 of dengue virus: insights from an in silico structure-based drug discovery. Mol Biotechnol. 2022 doi: 10.1007/s12033-022-00582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsagaby SA, Iqbal D, Ahmad I, Patel H, Mir SA, Madkhali YA, Oyouni A, Hawsawi YM, Alhumaydhi FA, Alshehri B, Alturaiki W, Alanazi B, Mir MA, Al Abdulmonem W. In silico investigations identified butyl xanalterate to competently target CK2α (CSNK2A1) for therapy of chronic lymphocytic leukemia. Sci Rep. 2022;12(1):17648. doi: 10.1038/s41598-022-21546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayipo YO, Ahmad I, Alananzeh W, Lawal A, Patel H, Mordi MN. Computational modelling of potential Zn-sensitive non-β-lactam inhibitors of imipenemase-1 (IMP-1) J Biomol Struct Dyn. 2022 doi: 10.1080/07391102.2022.2153168. [DOI] [PubMed] [Google Scholar]