Abstract
Three new bulky cycloalkyl α,β-dehydroamino acids (ΔAAs) have been designed and synthesized. Each residue enhances the rigidity of model peptides and their stability to proteolysis, with larger ring sizes exhibiting greater effects. Peptides containing bulky cycloalkyl ΔAAs are inert to conjugate addition by a nucleophilic thiol. The results suggest that these residues will be effective tools for improving the proteolytic stability of bioactive peptides.
Graphical Abstract

The vast medicinal potential of peptides1 has fueled efforts aimed at increasing their short half-lives,2–12 which are a consequence of their rapid cleavage by proteases and facile renal clearance. Approximately 40 years ago, Stammer and coworkers discovered the positive impact of α,β-dehydroamino acids (ΔAAs) on proteolytic stability. They demonstrated that incorporating small ΔAAs such as ΔAla or medium-sized ΔAAs such as Z-ΔPhe or Z-ΔLeu into enkephalin analogs increased the resistance of these peptides to degradation by proteases.13 The A1,3 strain inherent in the trisubstituted alkene moiety of medium-sized ΔAAs presumably limits their low-energy conformations. This increases rigidity and gives peptides containing these residues a higher propensity to adopt folded states, which are more proteolytically stable than random coil conformations.14
Our interest in ΔAAs15 was prompted by our synthetic studies of yaku’amide A and analogs.16 This anticancer peptide natural product contains ΔVal and ΔIle, which are bulky ΔAAs by virtue of their tetrasubstituted alkenes. We reasoned that these residues might be even more effective than medium-sized ΔAAs at enhancing proteolytic stability due to their elevated levels of A1,3-strain. We inserted ΔVal and its bis-homologated analog dehydroethylnorvaline (ΔEnv) into the turn region of a model β-hairpin,17 and we observed a substantial (ca. 6–7-fold) increase in proteolytic stability for the ΔAA-modified peptides relative to the original peptide.18 We subsequently demonstrated that incipient 310-helical peptides19 and β-sheet-like peptides containing ΔVal are inert to conjugate addition by the reactive thiol cysteamine.20 This result suggests that bioactive peptides containing bulky ΔAAs could be employed as therapeutic agents without degradation caused by attack from biological nucleophiles.
Spectroscopic and computational studies of the structures of our bulky ΔAA-containing β-hairpins indicated that the larger ΔEnv residue imparted less rigidity to the peptides than the smaller ΔVal residue.18 We tentatively attributed this phenomenon to the intrinsic flexibility of the ethyl groups present in ΔEnv (Figure 1). We reasoned that cycloalkyl dehydroamino acids would exhibit a substantial rigidifying effect on peptides due to their reduced flexibility relative to ΔEnv and their larger size relative to ΔVal. Based on the documented correlation between a higher degree of rigidity or folding and greater resistance to degradation by proteases,14 we posited that cyclic ΔAAs might enhance proteolytic stability more than the previously studied acyclic ΔAAs. We also recognized the value of developing a suite of cycloalkyl ΔAAs of varying ring sizes with the ability to fill different-sized binding pockets on the surface of protein targets of interest.21 A handful of cycloalkyl ΔAAs are known, but they have primarily been used as substrates for enantioselective hydrogenations.22 We are only aware of two reports describing their incorporation into peptides.23
Figure 1.
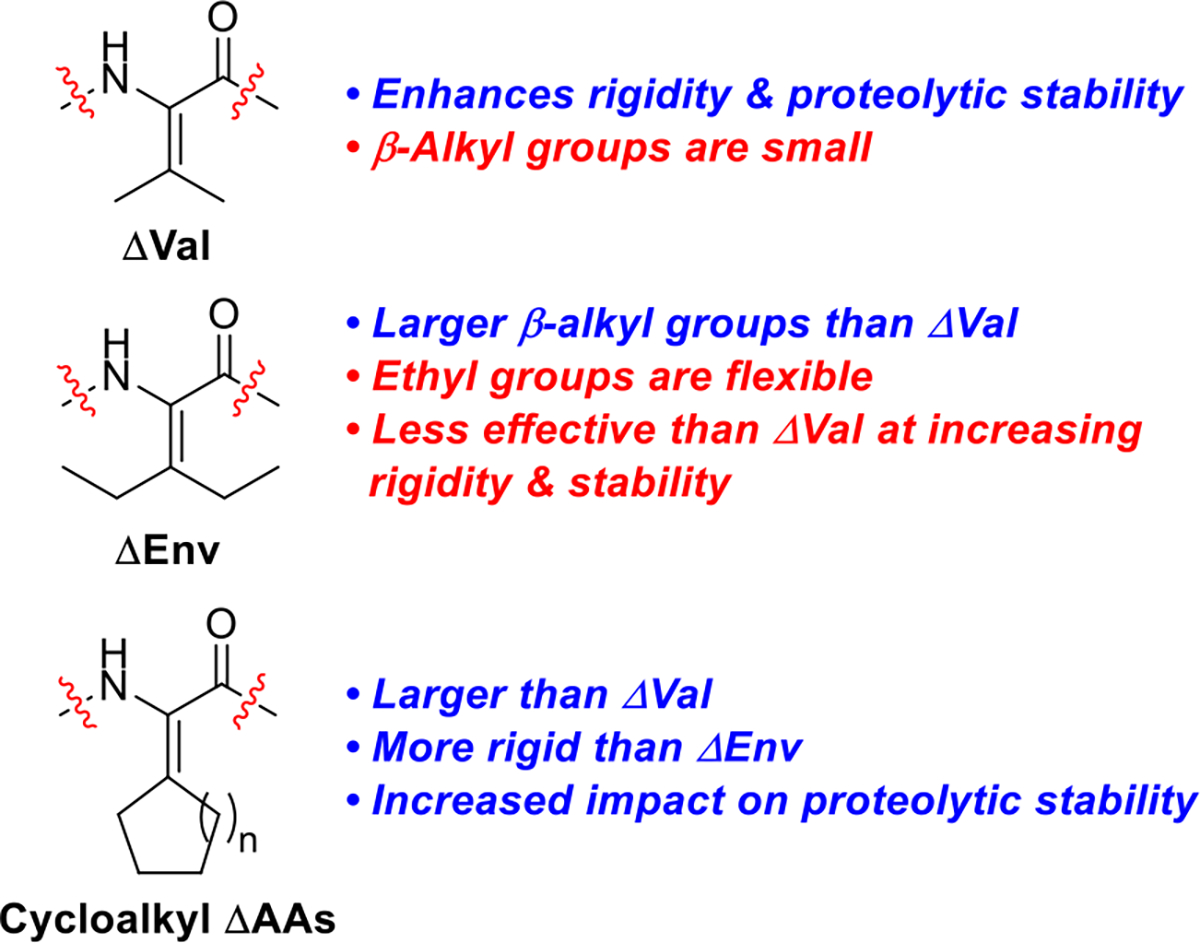
Comparison of acyclic and cyclic ΔAAs.
Herein we disclose the synthesis of derivatives of dehydrocyclobutylglycine (ΔCbg), dehydrocyclopentylglycine (ΔCpg), and dehydrocyclohexylglycine (ΔChg) that can be readily installed in peptides. Studies of their effect on the structure and stability of β-sheet-like peptides revealed a positive impact on proteolytic stability exceeding that of ΔVal. The ability of cycloalkyl ΔAAs to protect these peptides from proteolysis exhibited a positive correlation with increasing ring size. Importantly, peptides containing cycloalkyl ΔAAs were inert to conjugate additions by thiols. Our results suggest that these residues can be incorporated into peptide drugs and drug candidates to generate agents resistant to proteolysis that will not undergo in vivo degradation caused by biological nucleophiles.
As in our prior studies, we planned to access the targeted dehydroamino acids via dehydration chemistry. The requisite cyclic β-hydroxy amino acids 2a–c were constructed via OsO4-catalyzed base-free aminohydroxylation24 as shown in Scheme 1. The regioselectivity and brevity of this route compensate for the moderate yields, which are presumably a consequence of the sterically hindered enoate substrates 1a–c.
Scheme 1. Synthesis of Cyclic β-Hydroxy Amino Acids.

Our previous work used Balaram’s incipient 310 helical peptide19 (6, Scheme 2) as a system for evaluating the impact of various ΔAAs on peptide structure and stability. We demonstrated that replacement of the embedded Aib residue of 6 (shown in red in Scheme 2) with ΔVal yielded a β-sheet-like peptide with enhanced resistance to proteolysis.20 Accordingly, we decided to construct variants of 6 possessing cycloalkyl ΔAAs in place of the embedded Aib to compare the stabilizing effect of these residues with that of ΔVal. The requisite peptides were synthesized as outlined in Scheme 2. Hydrogenolysis of the Cbz groups of cyclic β-hydroxy amino acids 2a–c was followed by coupling to Cbz-Aib-Pro (3). The resulting tripeptides 4a–c were obtained as 1:1 mixtures of diastereomers due to the racemic nature of 2a–c. Efforts to improve the yields by changing the coupling agent or solvent were unsuccessful. Saponification of the ester moieties of 4a–c furnished crude carboxylic acids, which were treated with NaOAc and Ac2O to promote dehydration of the tertiary alcohols and azlactone formation. Addition of Ala-OMe•HCl, DMAP, and NMM to the crude azlactones triggered ring opening and amidation,16a,20 delivering the desired tetrapeptides 5a–c. The low yields in the peptide couplings and azlactone ring-openings can at least partially be attributed to the hindered nature of both the β-hydroxy amino acids and the dehydroamino acids. However, we are unsure why the yields in the cyclobutyl series were measurably lower than those in the cyclopentyl and cyclohexyl series. We also attempted to access 5a–c via dehydrations of tetrapeptides containing β-hydroxy amino acids, but the various dehydration protocols examined either failed or gave poorer yields than the azlactone ring-openings shown in Scheme 2. Fortunately, the concise route to 5a–c mitigates the impact of the low yields.
Scheme 2. Synthesis of Peptides Containing Cycloalkyl ΔAAs.
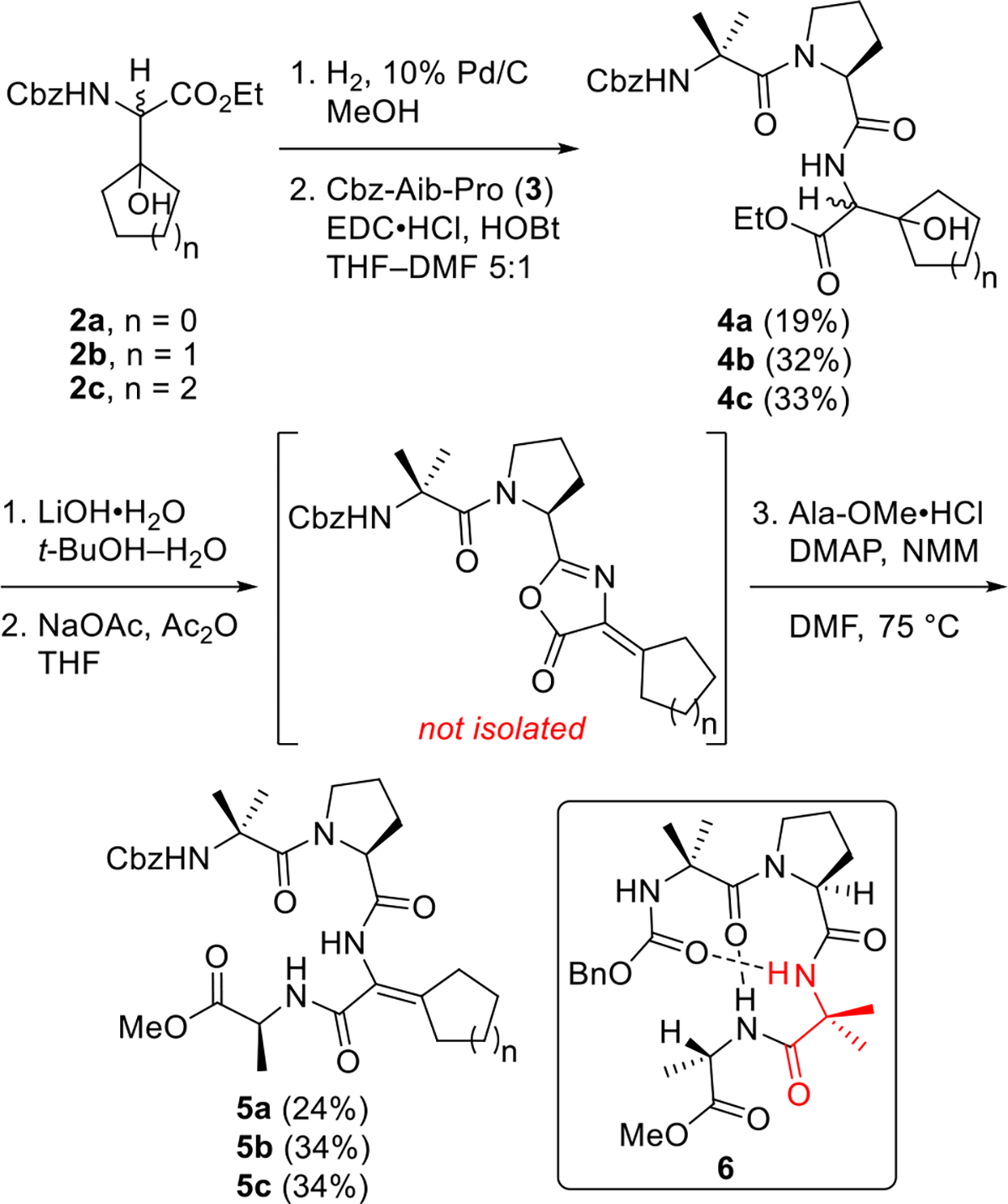
1H NMR studies conducted in CDCl3 saturated with D2O indicated that cycloalkyl ΔAA-containing peptides 5a–c adopt β-sheet-like structures resembling the conformation of the corresponding ΔVal-containing peptide that we synthesized previously. In each peptide, the amide hydrogen belonging to the ΔAA residue underwent relatively rapid H–D exchange. In contrast, the amide hydrogens belonging to the Aib and Ala residues exchanged slowly with deuterium (Figure 2). Variable-temperature 1H NMR experiments performed in DMSO-d6 revealed that the polar solvent disrupts both intramolecular hydrogen bonds present in 5c but only the hydrogen bond involving the Aib carbamate hydrogen in 5b. The analogous hydrogen bond in 5a is also disrupted by DMSO-d6; however, overlapping signals in the 1H NMR spectrum of this compound prevented us from determining the fate of the other intramolecular hydrogen bond involving the Ala amide hydrogen. These experiments suggest that the intramolecular hydrogen bonds present in 5a–c are of varying strengths.
Figure 2.
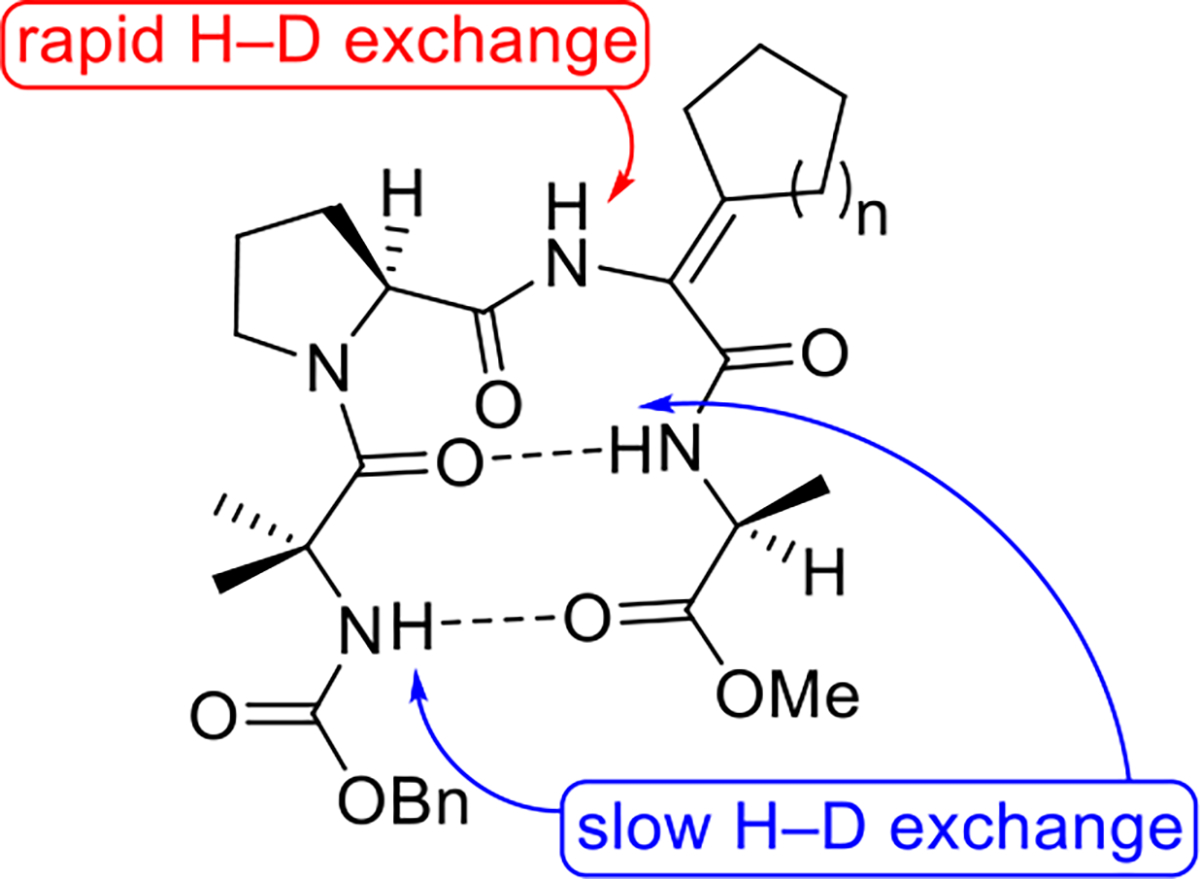
β-Sheet-like conformations of 5a–c.
The conformations of peptides 5a–c were investigated using NOE-restrained structural calculations. ROESY spectra were acquired for each peptide, and the observed correlations were employed as upper-distance limits for simulated annealing calculations performed by CYANA 2.1.25 This program has been used to generate solution-phase structures of peptides from NMR data.18,20 The intramolecular hydrogen bonds detected by the H–D exchange experiments were also included in these calculations. The ten lowest-energy structures of each peptide that satisfied the experimentally determined restraints were superimposed to create ensembles that are depicted in Figure 3 along with the minimized structures of 5a–c. These calculated structures provide further evidence for the incipient β-sheet-like conformation described above and are similar to the calculated structure of the previously studied ΔVal congener.20 Interestingly, the RMSD values calculated for each ensemble indicated that the rigidity of the peptides increases with the size of the carbocyclic ring. Thus, ΔCbg-containing peptide 5a exhibited the largest RMSD value (0.71 Å), followed by ΔCpg-containing peptide 5b (0.66 Å) and ΔChg-containing peptide 5c (0.45 Å). For comparison, application of the same NMR experiments and structural calculations to Balaram’s peptide 6 yielded an RMSD value of 1.27 Å.
Figure 3.
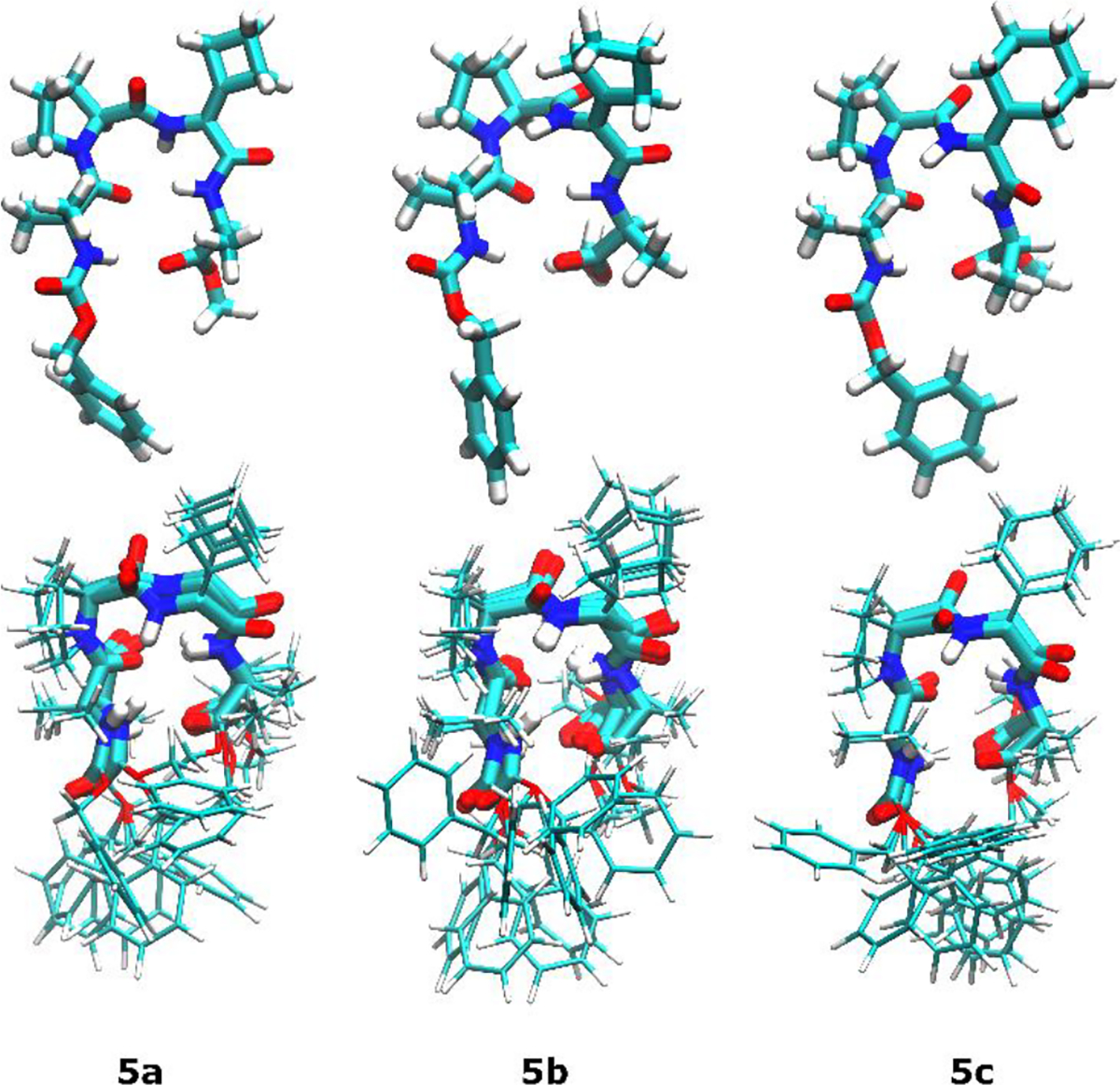
Lowest-energy solution-phase structures (top) and structural ensembles (bottom) of 5a–c as calculated using hydrogen bonding and NOE distance restraints.
The proteolytic stability of cycloalkyl ΔAA-containing peptides 5a–c was measured by exposing them to Pronase, a cocktail of proteases from Streptomyces griseus that promotes nonspecific peptide cleavage.26 Balaram’s peptide 6 was included as a control in this assay. Each peptide exhibited some resistance to proteolysis; accordingly, high concentrations of enzymes were required to observe degradation within a useful timeframe. Protease self-cleavage can occur at these concentrations, and this precluded the measurement of peptide degradation rates or half-lives. Fortunately, we found that comparing the amounts of each peptide remaining after 30 min of incubation with Pronase provided a reliable method of determining their relative stabilities to proteolysis.27 These data are contained in Table 1 and demonstrate a correlation between enhanced resistance to cleavage and larger ring sizes. Additionally, the relative stabilities of 5a–c were consistent with their relative rigidity as determined by the NOE-restrained structural calculations described above. Importantly, all three cycloalkyl ΔAA-containing peptides were more resistant to proteolysis than Balaram’s peptide, demonstrating that these residues exert a stronger stabilizing effect than the Aib residue in 6 that they replaced. This is noteworthy, since Aib is known to impart substantial proteolytic resistance to peptides that contain it.28
Table I.
Proteolysis of Cycloalkyl ΔAA-Containing Peptidesa
| peptide | amount remaining after 30 min (%)b |
|---|---|
|
| |
| 6 | 33 |
| 5a | 66 |
| 5b | 74 |
| 5c | 84 |
Solutions of peptides (0.5 mM, 500 μL) in 1X PBS buffer (10 mM sodium phosphate, 137 mM NaCl, 2.7 mM KCl buffer, pH 7.4) were each treated with a solution of Pronase (0.88 mg/mL, 200 μL). Degradation of peptides was monitored by HPLC.
The assays were conducted in triplicate, and values represent the average of the three runs.
Pronase assays conducted previously on the ΔVal analog of 5a–c also used Balaram’s peptide as a control and yielded a very similar result (i.e., 31% of 6 remaining after 30 min of incubation20 vs 33% in the current assay). Thus, it is possible to directly compare the results of the prior and current assays. This reveals that the proteolytic stabilizing ability of ΔVal (61% remaining after 30 min of incubation)20 is comparable to that of ΔCbg. While it is unclear if the results obtained from these small model peptides can be generalized to more complex systems, we are encouraged by the fact that both ΔCpg and ΔChg impart enhanced proteolytic stability relative to ΔVal.
Exposure of 5a–c to the potent nucleophile cysteamine (HSCH2CH2NH2) demonstrated that these cycloalkyl ΔAA-containing peptides are inert to conjugate addition at temperatures up to 100 °C, at which point the thiol decomposed. This result is consistent with our previous studies of the ΔVal-containing analog of 5a–c. Presumably, the substantial steric hindrance of the β-carbon prevents the α,β-unsaturated carbonyl system from participating as an electrophile in conjugate additions.
In conclusion, we have designed and synthesized three new bulky cycloalkyl dehydroamino acids: dehydrocyclobutylglycine, dehydrocyclopentylglycine, and dehydrocyclohexylglycine. The brevity of the synthetic route compensates for the modest yields that are a consequence of the steric hindrance and A1,3 strain inherent to these residues. All three cycloalkyl ΔAAs substantially enhance the rigidity and proteolytic stability of model peptides, and both effects are increased with larger ring sizes. Importantly, none of the peptides containing these cycloalkyl ΔAAs were susceptible to conjugate addition by a highly nucleophilic thiol. Together, these observations suggest that cycloalkyl ΔAAs can be employed to increase the resistance of bioactive peptides to proteolysis without rendering them susceptible to degradation by conjugate addition. We envision that these residues are likely to prove especially valuable in situations where a ΔAA is required to enhance proteolytic stability and a large nonpolar side chain is needed to fill a hydrophobic pocket on a protein binding partner. Moreover, the fact that bulky ΔAA-containing peptides are stable to the acids and bases that are typically employed in SPPS29 bodes well for the incorporation of cycloalkyl ΔAAs into complex peptides with long sequences. Studies involving the insertion of these residues into bioactive peptides are underway in our laboratory and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (R15GM114789-02) and Brigham Young University (Roland K. Robins Fellowship to D.J.; Undergraduate Research Awards to S.A.M., J.C.C., A.K.L, and T.A.B.) for financial support.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data, data from proteolysis assays and peptide HPLC traces, tabulated restraints and parameters used in structural calculations, structures of peptides calculated using NMR data, ROESY spectrum snapshots, and copies of 1H and 13C NMR spectra for all new compounds. (PDF)
REFERENCES
- (1).(a) Muttenthaler M; King GF; Adams DJ; Alewood PF Trends in Peptide Drug Discovery. Nat. Rev. Drug. Discov. 2021, 20, 309–325. [DOI] [PubMed] [Google Scholar]; (b) Henninot A; Collins JC; Nuss JM The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [DOI] [PubMed] [Google Scholar]; (c) Tsomaia N Peptide Therapeutics: Targeting the Undruggable Space. Eur. J. Med. Chem. 2015, 94, 459–470. [DOI] [PubMed] [Google Scholar]; (d) Fosgerau K; Hoffmann T Peptide Therapeutics: Current Status and Future Directions. Drug Discovery Today 2015, 20, 122–128. [DOI] [PubMed] [Google Scholar]; (e) Kaspar AA; Reichert JM Future Directions for Peptide Therapeutics Development. Drug Discovery Today 2013, 18, 807–817. [DOI] [PubMed] [Google Scholar]; (f) Craik DJ; Fairlie DP; Liras S; Price D The Future of Peptide-Based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [DOI] [PubMed] [Google Scholar]
- (2).Peptoids: Webster AM; Cobb SL Recent Advances in the Synthesis of Peptoid Macrocycles. Chem. - Eur. J. 2018, 24, 7560–7573.Sun J; Zuckermann RN Peptoid Polymers: A Highly Designable Bioinspired Material. ACS Nano 2013, 7, 4715–4732.Yoo B; Shin SBY; Huang ML; Kirshenbaum K Peptoid Macrocycles: Making the Rounds with Peptidomimetic Oligomers. Chem. - Eur. J. 2010, 16, 5528–5537.Fowler SA; Blackwell HE Structure–Function Relationships in Peptoids: Recent Advances Toward Deciphering the Structural Requirements for Biological Function. Org. Biomol. Chem. 2009, 7, 1508–1524.
- (3).β- and α/β-peptides: Cabrele C; Martinek TA; Reiser O; Berlicki Ł Peptides Containing β-Amino Acid Patterns: Challenges and Successes in Medicinal Chemistry. J. Med. Chem. 2014, 57, 9718–9739.Horne WS; Gellman SH Foldamers with Heterogeneous Backbones. Acc. Chem. Res. 2008, 41, 1399–1408.Seebach D; Gardiner J β-Peptidic Peptidomimetics. Acc. Chem. Res. 2008, 41, 1366–1375.Cheng RP; Gellman SH; DeGrado WF β-Peptides: From Structure to Function. Chem. Rev. 2001, 101, 3219–3252.
- (4).Stapled peptides: Li X; Chen S; Zhang W-D; Hu H-G Stapled Helical Peptides Bearing Different Anchoring Residues. Chem. Rev. 2020, 120, 10079–10144.Reguera L; Rivera DG Multicomponent Reaction Toolbox for Peptide Macrocyclization and Stapling. Chem. Rev. 2019, 119, 9836–9860.Lau YH; de Andrade P; Wu Y; Spring DR Peptide Stapling Techniques Based on Different Macrocyclisation Chemistries. Chem. Soc. Rev. 2015, 44, 91–102.Cromm PM; Spiegel J; Grossmann TN Hydrocarbon Stapled Peptides as Modulators of Biological Function. ACS Chem. Biol. 2015, 10, 1362–1375.Walensky LD; Bird GH Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288.
- (5).Hydrogen bond surrogates: Patgiri A; Jochim AL; Arora PS A Hydrogen Bond Surrogate Approach for Stabilization of Short Peptide Sequences in α-Helical Conformation. Acc. Chem. Res. 2008, 41, 1289–1300.
- (6).γAA-peptides: Sang P; Shi Y; Huang B; Xue S; Odom T; Cai J Sulfono-γ-AApeptides as Helical Mimetics: Crystal Structures and Applications. Acc. Chem. Res. 2020, 53, 2425–2442.Teng P; Shi Y; Sang P; Cai J γ-AAPeptides as a New Class of Peptidomimetics. Chem. - Eur. J. 2016, 22, 5458–5466.Shi Y; Teng P; Sang P; She F; Wei L; Cai J γ-AAPeptides: Design, Structure, and Applications. Acc. Chem. Res. 2016, 49, 428–441.
- (7).D-Peptides: Weinstock MT; Francis JN; Redman JS; Kay MS Protease-Resistant Peptide Design—Empowering Nature’s Fragile Warriors Against HIV. Biopolymers (Pept. Sci.) 2012, 98, 431–442.
- (8).α,α-Disubstituted amino acids: Toniolo C; Formaggio F; Kaptein B; Broxterman QB You Are Sitting on a Gold Mine! Synlett 2006, 1295–1310.
- (9).N-Amino peptides: Sarnowski MP; Kang CW; Elbatrawi YM; Wojtas L; Del Valle JR Peptide N-Amination Supports β-Sheet Conformations. Angew. Chem., Int. Ed. 2017, 56, 2083–2086.
- (10).β-Turn mimics: Lubell WD; Khashper A Design, Synthesis, Conformational Analysis and Application of Indolizidin-2-one Dipeptide Mimics. Org. Biomol. Chem. 2014, 12, 5052–5070.MacDonald M; Aubé J Approaches to Cyclic Peptide β-Turn Mimics. Curr. Org. Chem. 2001, 5, 417–438.
- (11).(a) Nischan N; Hackenberger CPR Site-specific PEGylation of Proteins: Recent Developments. J. Org. Chem. 2014, 79, 10727–10733. PEGylation: [DOI] [PubMed] [Google Scholar]; (b) Jevševar S; Kunstelj M; Porekar VG PEGylation of Therapeutic Proteins. Biotechnol J. 2010, 5, 113–128. [DOI] [PubMed] [Google Scholar]; (c) Harris JM; Chess RB Effect of PEGylation on Pharmaceuticals. Nat. Rev. Drug Discovery 2003, 2, 214–221. [DOI] [PubMed] [Google Scholar]
- (12).Conjugation to albumin or antibody fragments: Liu Z; Chen X Simple Bioconjugate Chemistry Serves Great Clinical Advances: Albumin as a Versatile Platform for Diagnosis and Precision Therapy. Chem. Soc. Rev. 2016, 45, 1432–1456.Elsadek B; Kratz F Impact of Albumin on Drug Delivery – New Applications on the Horizon. J. Controlled Release 2012, 157, 4–28.Pollaro L; Heinis C Strategies to Prolong the Plasma Residence Time of Peptide Drugs. Med. Chem. Commun. 2010, 1, 319–324.
- (13).(a) English ML; Stammer CH The Enzyme Stability of Dehydropeptides. Biochem. Biophys. Res. Commun. 1978, 83, 1464–1467. [DOI] [PubMed] [Google Scholar]; (b) English ML; Stammer CH D-Ala2, ΔZPhe4-Methionine Enkephalin Amide, a Dehydropeptide Hormone. Biochem. Biophys. Res. Commun. 1978, 85, 780–782. [DOI] [PubMed] [Google Scholar]; (c) Shimohigashi Y; Chen H-C; Stammer CH The Enzyme Stability of Dehydro-Enkephalins. Peptides 1982, 3, 985–987. [DOI] [PubMed] [Google Scholar]; (d) Shimohigashi Y; Stammer CH Dehydro-enkephalins. Part 7. A Potent Dehydroleucine-enkephalin Resistant to Carboxypeptidase. J. Chem. Soc., Perkin Trans. 1 1983, 803–808. [Google Scholar]
- (14).(a) Daniel RM; Cowan DA; Morgan HW; Curran MP A Correlation Between Protein Thermostability and Resistance to Proteolysis. Biochem. J. 1982, 207, 641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Imoto T; Yamada H; Ueda T Unfolding Rates of Globular Proteins Determined by Kinetics of Proteolysis. J. Mol. Biol. 1986, 190, 647–649. [DOI] [PubMed] [Google Scholar]; (c) Parsell DA; Sauer RT The Structural Stability of a Protein Is an Important Determinant of Its Proteolytic Susceptibility in Escherichia coli. J. Biol. Chem. 1989, 264, 7590–7595. [PubMed] [Google Scholar]; (d) Klink TA; Raines RT Conformational Stability Is a Determinant of Ribonuclease A Cytotoxicity. J. Biol. Chem. 2000, 275, 17463–17467. [DOI] [PubMed] [Google Scholar]; (e) Ahmad S; Kumar V; Ramanand KB; Rao NM Probing Protein Stability and Proteolytic Resistance by Loop Scanning: A Comprehensive Mutational Analysis. Protein Sci. 2012, 21, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).For a review, see: Jiang J; Ma Z; Castle SL Bulky α,β-Dehydroamino Acids: Their Occurrence in Nature, Synthesis, and Applications. Tetrahedron 2015, 71, 5431–5451.
- (16).(a) Lo CCL; Joaquin D; Moyá DA; Ramos A; Kastner DW; White SM; Christensen BL; Naglich JG; Degnen WJ; Castle SL Synthesis and Evaluation of Potent Yaku’amide A Analogs. Chem. Sci. 2022, 13, 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cai Y; Ma Z; Jiang J; Lo CCL; Luo S; Jalan A; Cardon JM; Ramos A; Moyá DA; Joaquin D; Castle SL Convergent Total Synthesis of Yaku’amide A. Angew. Chem. Int. Ed. 2021, 60, 5162–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ma Z; Jiang J; Luo S; Cai Y; Cardon JM; Kay BM; Ess DH; Castle SL Selective Access to E- and Z-ΔIle-Containing Peptides via a Stereospecific E2 Dehydration and an O → N Acyl Transfer. Org. Lett. 2014, 16, 4044–4047. [DOI] [PubMed] [Google Scholar]
- (17).Tatko CD; Waters ML Comparison of C–H···π and Hydrophobic Interactions in a β-Hairpin Peptide: Impact on Stability and Specificity. J. Am. Chem. Soc. 2004, 126, 2028–2034. [DOI] [PubMed] [Google Scholar]
- (18).Jalan A; Kastner DW; Webber KGI; Smith MS; Price JL; Castle SL Bulky Dehydroamino Acids Enhance Proteolytic Stability and Folding in β-Hairpin Peptides. Org. Lett. 2017, 19, 5190–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Nagaraj R; Shamala N; Balaram P Stereochemically Constrained Linear Peptides. Conformations of Peptides Containing α-Aminoisobutyric Acid. J. Am. Chem. Soc. 1979, 101, 16–20. [Google Scholar]
- (20).Joaquin D; Lee MA; Kastner DW; Singh J; Morrill ST; Damstedt G; Castle SL Impact of Dehydroamino Acids on the Structure and Stability of Incipient 310-Helical Peptides. J. Org. Chem. 2020, 85, 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Rooklin D; Modell AE; Li H; Berdan V; Arora PS; Zhang Y Targeting Unoccupied Surfaces on Protein–Protein Interfaces. J. Am. Chem. Soc. 2017, 139, 15560–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Modell AE; Marrone F III; Panigrahi NR; Zhang Y; Arora PS Peptide Tethering: Pocket-Directed Fragment Screening for Peptidomimetic Inhibitor Discovery. J. Am. Chem. Soc. 2022, 144, 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Gridnev ID; Yamanoi Y; Higashi N; Tsuruta H; Yasutake M; Imamoto T “Asymmetric Hydrogenation Catalyzed by (S,S)-R-BisP*-Rh and (R,R)-R-MiniPHOS Complexes: Scope, Limitations, and Mechanism.” Adv. Synth. Catal. 2001, 343, 118–136. [Google Scholar]
- (23).(a) Cativiela C; Díaz-de-Villegas MD; Gálvez JA; Su G “Synthesis and Conformational Properties of Model Dipeptides Containing Novel Axially Chiral α,β-Didehydroamino Acids at the (i + 1) Position of a β-Turn Conformation.” Tetrahedron 2004, 60, 11923–11932. [Google Scholar]; (b) Coghlan PA; Easton CJ β-Nitro-α-amino Acids as Latent α,β-Dehydro-α-amino Acid Residues in Solid-Phase Peptide Synthesis. ARKIVOC 2004, (10), 101–108.18490966 [Google Scholar]
- (24).(a) Ma Z; Naylor BC; Loertscher BM; Hafen DD; Li JM; Castle SL Regioselective Base-Free Intermolecular Aminohydroxylations of Hindered and Functionalized Alkenes. J. Org. Chem. 2012, 77, 1208–1214. [DOI] [PubMed] [Google Scholar]; (b) Masuri; Willis AC; McLeod MD Osmium-Catalyzed Vicinal Oxyamination of Alkenes by N-(4-Toluenesulfonyloxy)carbamates. J. Org. Chem. 2012, 77, 8480–8491. [DOI] [PubMed] [Google Scholar]; (c) Harris L; Mee SPH; Furneaux RH; Gainsford GJ; Luxenburger A Alkyl 4-Chlorobenzoyloxycarbamates as Highly Effective Nitrogen Source Reagents for the Base-Free, Intermolecular Aminohydroxylation Reaction. J. Org. Chem. 2011, 76, 358–372. [DOI] [PubMed] [Google Scholar]
- (25).(a) Güntert P; Mumenthaler C; Wüthrich K Torsion Angle Dynamics for NMR Structure Calculation with the New Program DYANA. J. Mol. Biol. 1997, 273, 283–298. [DOI] [PubMed] [Google Scholar]; (b) Yilmaz EM; Güntert P NMR Structure Calculation for All Small Molecule Ligands and Non-Standard Residues from the PDB Chemical Component Dictionary. J. Biomol. NMR 2015, 63, 21–37. [DOI] [PubMed] [Google Scholar]
- (26).Cline LL; Waters ML The Structure of Well-Folded β-Hairpin Peptides Promotes Resistance to Peptidase Degradation. Biopolymers (Pept. Sci.) 2009, 92, 502–507. [DOI] [PubMed] [Google Scholar]
- (27).The amount of peptide remaining was also measured at several other time points. Please see the Supporting Information for the full data set from the proteolysis assays.
- (28).Yamaguchi H; Kodama H; Osada S; Kato F; Jelokhani-Niaraki M; Kondo M Effect of α,α-Dialkyl Amino Acids on the Protease Resistance of Peptides. Biosci., Biotechnol., Biochem. 2003, 67, 2269–2272. [DOI] [PubMed] [Google Scholar]
- (29).(a) Itoh H; Miura K; Kamiya K; Yamashita T; Inoue M Solid-Phase Total Synthesis of Yaku’amide B Enabled by Traceless Staudinger Ligation. Angew. Chem. Int. Ed. 2020, 59, 4564–4571. [DOI] [PubMed] [Google Scholar]; (b) Kamiya K; Miura K; Itoh H; Inoue M Divergent Solid-Phase Synthesis and Biological Evaluation of Yaku’amide B and Its Seven E/Z Isomers. Chem. - Eur. J. 2021, 27, 1088–1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


