Abstract
We sought to assess the feasibility of virtually assisted personalized tracheostomy tube (vapTT) implementation for patients with congenital airway anomalies (CAAs) and persistent tracheostomy tube (TT)–related respiratory failure at a tertiary pediatric hospital. Three patients (0–18 years) with CAAs and recurrent TT-related respiratory complications were managed with vapTT over 5 years. Patients underwent airway computed tomography acquisition with 3-dimensional reconstruction and TT virtual modeling for shape customization. Models were transferred to Bivona for fabrication based on industry-standard materials and processes. Clinical information and tracheoscopies assessing position, obstruction, and granulation were reviewed. Patients demonstrated resolution of visualized TT-related obstruction, granulation, or ulceration and de-escalation of respiratory support. Clinical events requiring urgent tracheoscopy decreased in all 3 patients. Sufficient relief of critical airway obstruction allowed progression of medical care and/or discharge. VapTTs are feasible for patients with CAA. This new frontier in personalized devices may serve uniquely challenging patient populations for whom standard treatments have failed.
Keywords: virtually assisted modeling, personalized medicine, tracheostomy, airway malformation, 3D printing, customized tracheostomy, virtual planning, airway management, 3D modeling
Pediatric congenital airway anomalies (CAAs) are rare, challenging entities with significant morbidity and mortality. CAAs comprise anatomic variants that significantly deviate in morphology (ie, tracheal cartilaginous sleeve, severe airway tortuosity). CAAs are more prevalent in populations with craniofacial disorders or skeletal dysplasia, who often experience multilevel airway obstruction.1
Tracheostomy tubes (TTs) among patients with CAA can result in TT-related complications. TTs are designed for patients with normal orientation of the tracheobronchial tree. Intrinsic tissue differences and airway tortuosity in CAAs can lead to tube malposition, obstruction, and chronic airway injury. Restriction of oxygenation and/or ventilation can be fatal if not recognized and treated expeditiously.2
Virtual 3-dimensional modeling can address challenging clinical problems.3,4 We designed virtually assisted personalized TTs (vapTTs) for medically complex cases with CAAs. The impact on respiratory status and patient outcomes is discussed.
Methods
Patients treated with vapTTs were reviewed after institutional review board approval (Seattle Children’s Hospital, 2016–2020). Patients with CAAs experiencing recurrent acute hypoxic or hypercapnic respiratory failure due to ongoing TT obstruction following exhaustion of commercially available standard and customized options (defined as inability to resolve TT-related events despite iterative use of multiple TTs) were considered for vapTTs and included. Success or failure of each TT was assessed through serial tracheoscopy and intensive care unit surveillance. Patients tolerating industry-standard, conventionally customized TTs or cases prophylactically considered for vapTTs were excluded. VapTT success or failure was assessed by tracheoscopy and respiratory monitoring.
VapTT Design
Consensus on clinical candidacy was reached by multidisciplinary review (otolaryngology, radiology, pulmonology, critical care, respiratory therapy, and bioengineering). Caregivers provided verbal informed consent for vapTT design, manufacturing, and placement. Standard Bivona (Smith’s Medical) TT template files served as reference for personalization. High-resolution neck/chest computed tomography (CT) scans were obtained (0.5-mm slice thickness; Siemens Force scanner) with the TTs in situ to evaluate airway configuration and position when cannulated.
CT images were exported to Mimics 19 software (Materialise). Anatomic structures were modeled (lumen, bone, skin, esophagus, in-dwelling TTs). Clinical, endoscopic, and radiologic data were incorporated for accurate structure annotation (eg, to avoid granulation tissue on endoscopy being mislabeled as tracheal wall). Objects were exported to 3- matic software (Materialise) for modeling new TTs by using known dimensions and verifying optimal skin flange interface to ensure adequate trajectory and minimize outer dimension wall contact. Often, varying TT models were drawn (curvature, length, and diameter) to assess fit and ease of placement/removal. Models defined as optimal by the clinical team were returned to the DICOM imaging space and reviewed for overlap to the raw DICOM images. Detailed discussion with Bivona was critically helpful to ensure that functional patient details were incorporated (eg, mobility, postural bias, stoma site integrity). The patient’s body section encompassing the TTs (above stoma to past carina) and the model tubes were printed in a mixture of Agilus, Tissue Matrix, and Gel Matrix on a J750 Digital Anatomy Printer for final review. These models were used to demonstrate the plan and facilitate discussion with the patient’s medical team and family. Final in situ virtual product images were exported to standard tessellation language files with templates completed per the ordering work at Bivona. VapTTs were manufactured by Bivona using industry-quality standards and specifications. VapTT placement occurred in the intensive care unit or operating room with immediate and surveillance tracheoscopies to confirm position and monitor ulceration/granulation. Patients continued ongoing monitoring for resolution of events for which vapTT was indicated.
Results
Three patients underwent vapTT design, fabrication, and utilization. Case summaries, airway models, vapTT indication, and outcomes are presented (Table 1). Previously trialed TTs are described (Table 2). Comparison of airway models is presented (Figure 1). Obstructive clinical events concerning for TT obstruction requiring urgent tracheoscopy decreased in all patients after vapTT placement. All patients had resolution of TT-related airway obstruction and de-escalation of respiratory support following vapTT implementation (patient 1 in Figure 2). Sufficient relief of critical airway obstruction allowed progression of medical care and/or discharge.
Table 1.
Patient-Specific Airway Abnormalities, Personal Tracheostomy Tube Indications and Modifications, and Posttreatment Outcomes.
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Congenital airway anomaly | Tracheobronchial stenosis, TCS, tracheal tortuosity | TCS | Thoracic cavity constriction causing vertical airway buckling and high ventilatory pressures. Progressive tracheal tortuosity with focal tracheomegaly |
| Primary diagnosis | Apert syndrome | Pfeiffer syndrome | Metatropic dysplasia |
| Indication for vapTTa | UAO; RLD; recurrent hypoxic, hypercapnic respiratory failure. Cartilage exposure, ulceration, and granulation | UAO; RLD; recurrent hypoxic, hypercapnic respiratory failure | UAO; RLD; recurrent hypoxic, hypercapnic respiratory failure. Cartilage exposure, granulation formation, and thoracic vertebral erosion and remodeling |
| Triplanar customizationsb | 45° in the axial plane and 20° anterior angulation in the sagittal plane at 1 cm from the distal tip of the tube | 22.5° anterior angulation in the sagittal plane 5 mm from the distal tip of the tube | 22° lateral bend in the coronal plane 1 cm from the tip of the tube. Mirrored S-shaped bend, custom length, custom length and position of the Aire-Cuf. |
| Outcome | Resolution of obstructive granulation tissue and ulceration causing recurrent acute respiratory failure. Stabilization allowed discharge on home-appropriate ventilator after a 3-y hospitalization | Resolution of obstructive granulation tissue causing recurrent acute respiratory failure | Resolution of obstructive granulation tissue, ulceration, and hypercarbia. Stabilization allowed discharge on home-appropriate ventilator. |
All patients had chronic respiratory failure with long-term ventilatory requirements necessitating tracheostomy tube dependence.
Triplanar angulations define the point-defining angles for the custom tracheostomy tube angulations. All bends were contoured beyond the descriptions provided in a layered model of the airway to create a 3-dimensional product.
Table 2.
Standard and Conventionally Customized TTs Used Prior to Consideration of Virtually Assisted Personalized TTs.
| Patient | Standard | Standard Flextend | Hyperflex Flextend custom length | Custom bend and/or length | Total |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 4 | 17 | 22 |
| 2 | 0 | 1 | 0 | 1 | 2 |
| 3 | 0 | 1 | 0 | 3 | 4 |
Abbreviation: TT, tracheostomy tube.
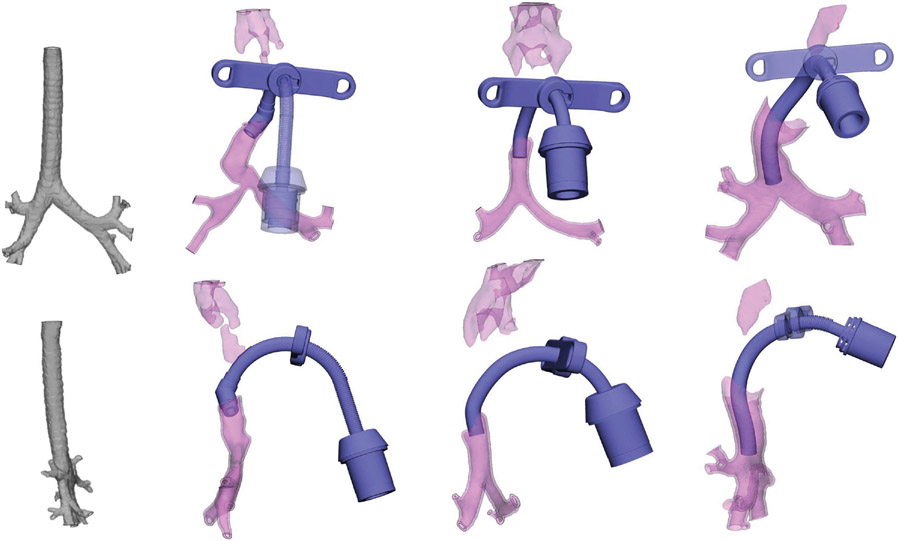
Figure 1.
Three-dimensional airway modeling for study patients: coronal and sagittal modeling of patients 1-3 (left to right) with vapTT in situ. Normal airway model (far left panel) for comparison. vapTT, virtually assisted personalized tracheostomy tube.
Figure 2.
Patient 1: tracheoscopy before (A-C) and after (D-E) vapTT placement: (A) Ulceration/granulation from tracheostomy tube–wall contact. (B) Midlesion granulation/ulceration. (C) Luminal narrowing (yellow outline). (D) Remucosalization. (E) Improved airway patency. Star, tracheostomy tube tip. vapTT, virtually assisted personalized tracheostomy tube.
Discussion
CAAs are complex anatomic variants where commercially available TTs can be insufficient for adequate oxygenation and ventilation or result in chronic airway trauma. Conventional TT customizations can alter the shaft length, diameter, connector, neck flange, cuff, or options (ie, hyperflex) but cannot accommodate tortuous anatomy.5 We describe 3 tracheostomy-dependent patients with CAAs and recurrent life-threatening obstructive events despite exhaustion of commercially available custom TTs. Patients offered vapTTs had severe craniofacial differences or skeletal dysplasia with multilevel airway obstruction and tortuosity of the tracheobronchial tree. VapTTs resolved these episodes and achieved sufficient relief of critical airway obstruction, permitting progression of medical care and in some instances discharge after prolonged hospitalizations (3 years). Despite being a small retrospective series, personalized medical engineering’s utility on these patients cannot be undervalued.
There are limitations to consider. Software limitations in visualizing certain tissue interfaces (eg, cartilage or granulation tissue) require endoscopic and clinical correlation. Another potential gap lies in the information used to model the vapTT. While CT provides outstanding anatomic information, it is a single view in time, taken in a position that may not be the child’s common habitus. Integrating other factors, such as the thickness of stoma padding desired, the child’s activity level, or default biases in positional range, may weigh heavily on the final design. Last, truly objective parameters are limited in demonstrating the profound clinical implications that vapTTs had on our patients. Further studies to explore the safety, efficacy, and long-term outcomes of these novel vapTTs will be instrumental in extending this work.
Acknowledgments
We greatly appreciate the expertise and collaboration of Jerry Cabrera, RRT, and colleagues at Smiths-Medical for their remarkable support in operationalizing these customized tracheostomy solutions and their contributions to improving the quality of life for these patients. We also thank Joshua Wilcox and the respiratory therapy discharge team at Seattle Children’s for the outstanding service that they provide to patients with tracheostomies and to Vanessa Masco for her exceptional graphic support services.
Funding source:
This study was funded by the US National Institutes of Health under the National Institute on Deafness and Other Communication Disorders, University of Washington Otolaryngology Research Training Program (2T32000018 for C.R.); the Seattle Children’s Research Institute, Excellence in Research New Investigator Award (J.B.-V.); and the Research Integration Hub (R.A.B.).
Footnotes
Competing interests: Randall Bly is cofounder and holds a financial interest of ownership equity with Edus Health, Inc and Eigen-Health, Inc. He is coinventor and consultant for Spiway, LLC. There are no conflicts of interest with this article.
This article was presented at the 2021 AAO-HNSF Annual Meeting & OTO Experience; October 5, 2021; Los Angeles, California.
References
- 1.Cielo CM, Montalva FM, Taylor JA. Craniofacial disorders associated with airway obstruction in the neonate. Semin Fetal Neonatal Med. 2016;21(4):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alapati D, Shaffer TH. Skeletal dysplasia: respiratory management during infancy. Respir Med. 2017. Oct 1; 131:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoang D, Perrault D, Stevanovic M. Surgical applications of three-dimensional printing: a review of the current literature and how to get started. Ann Transl Med. 2016;4(23):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology—head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045–2052. [DOI] [PubMed] [Google Scholar]
- 5.Smiths-Medical. Bivona customization services. Published 2021. https://www.smiths-medical.com/products/tracheostomy/silicone-tracheostomy-tubes/customized-tracheostomy-tube-service/bivona-customization-services