Abstract
Purpose of Review:
The purpose of this article is to discuss the diagnosis and treatment of diseases that affect both the skin and the esophagus.
Recent Findings:
The diagnosis of dermatological conditions that affect the esophagus often requires endoscopy and biopsy with some conditions requiring further investigation with serology, immunofluorescence, manometry, or genetic testing. Many conditions that affect the skin and esophagus can be treated successfully with systemic steroids and immunosuppressants including pemphigus, pemphigoid, HIV, esophageal lichen planus, and Crohn’s disease. Many conditions are associated with esophageal strictures which are treated with endoscopic dilation. Furthermore, many of the diseases are pre-malignant and require vigilance and surveillance endoscopy.
Summary:
Diseases that affect the skin and esophagus can be grouped by their underlying etiology: autoimmune (scleroderma, dermatomyositis, pemphigus, pemphigoid), infectious (herpes simplex virus, cytomegalovirus, human immunodeficiency virus), inflammatory (lichen planus and Crohn’s disease), and genetic (epidermolysis bullosa, Cowden syndrome, focal dermal hypoplasia, and tylosis). It is important to consider primary skin conditions that affect the esophagus when patients present with dysphagia of unknown etiology and characteristic skin findings.
Introduction
The skin and the esophagus have distinct embryological origins; however, they are both lined by stratified squamous epithelium and therefore some diseases present with both skin and esophageal findings. As such, an understanding of the presentation, endoscopic and radiographic findings as well as management options for esophageal involvement is important for the practicing gastroenterologist. Esophageal involvement of dermatologic conditions may mimic the primary disease process such as in infectious or bullous diseases, or relate to an underlying pathophysiology that leads to esophageal pathology such as in scleroderma or dermatomyositis. Patients with a dermatological condition that affects the esophagus frequently present with dysphagia, therefore management of these conditions may be endoscopic, topical, or systemic.
Within this article we attempt to categorize diseases affecting the skin and the esophagus by their underlying etiology: autoimmune, infectious, inflammatory, and genetic. The purpose of this review article is to provide an in-depth discussion of these conditions, specifically with respect to clinical features of esophageal involvement, diagnostics relevant to gastroenterologists, and treatment options specifically directed towards esophageal involvement.
Autoimmune
Scleroderma
Scleroderma is an autoimmune, systemic connective tissue disorder categorized into two patterns, systemic sclerosis and limited cutaneous scleroderma. Scleroderma has many dermatological manifestations including Raynaud’s phenomenon, telangiectasias, sclerodactyly, calcinosis, digital scars, hyperpigmentation, and contractures [1••].
Scleroderma affects the esophagus in approximately 90% of patients, and resultant dysmotility may lead to pathologic gastroesophageal reflux disease (GERD) with downstream consequences including peptic strictures, erosive esophagitis, and Barrett’s esophagus [2]. In addition to these features, endoscopic examination of the patient with scleroderma may also show telangiectasias [3]. The presentation of esophageal involvement in patients with scleroderma mirrors the presentation of pathologic GERD and its consequences. Symptoms frequently include heartburn, dysphagia, chest pain, regurgitation, and weight loss [4]. Serology may be helpful in the diagnosis of scleroderma. Associated antibodies include anti-nuclear antibodies, anti-topoisomerase antibodies, anti-RNA polymerase III antibodies, and anti-centromere antibodies. Abnormal findings on high-resolution esophageal manometry (HREM) are common in patients with a diagnosis of scleroderma (75–80%). Based on the Chicago Classification of esophageal motor disorders, patients with scleroderma demonstrate absent contractility and Ineffective Esophageal Motility (IEM) [5]. Specific manometric findings include hypomotility of the smooth muscle esophagus (distal two-thirds) and a hypotonic lower esophageal sphincter [2]. Manometric findings, however, can be variable with classic scleroderma esophagus (absent contractility with increased esophagogastric pressures) only observed in 33% of patients. Absent contractility is the most common finding seen in 56% of patients, followed by normal manometry observed in 26% of patients, and IEM observed in 10% of patients [6•].
The treatment of scleroderma is individualized to the patient and is dependent upon the severity of symptoms. For the gastroenterologist, treatment of esophageal involvement centers on the underlying esophageal dysmotility and predisposition to reflux. Proton pump inhibitors (PPIs) are a mainstay of treatment [7,8]. High-dose PPIs are preferred given suboptimal response rates to conventionally dosed PPIs. Incomplete response to PPI therapy is frequent, and it is our practice to add alginates for breakthrough symptoms. Antireflux surgeries should be considered with caution in patients with scleroderma due to the frequent underlying esophageal dysmotility and predisposition to post-fundoplication dysphagia. For the symptom of dysphagia, endoscopy to assess for the presence of an esophageal stricture is warranted with endoscopic dilation performed if a stricture is encountered. There is no data suggesting one method of dilation over another. The use of prokinetic medications such as erythromycin, metoclopramide and domperidone for esophageal dysmotility is controversial with limited substantive data supporting this medical approach. Small studies have suggested that agents such as buspirone, bethanechol or pyridostigmine may augment esophageal function and as such it is our practice to trial these therapies in cases of severe dysphagia without an obvious esophageal stricture amenable to endoscopic dilation [9–11].
Dermatomyositis
Dermatomyositis is an autoimmune disorder thought to arise in genetically susceptible individuals after an unknown environmental insult [12]. Cutaneous changes occur in 30–40% of adults and 95% of children [12]. Gottron’s papules and Gottron’s sign are pathognomonic for dermatomyositis. Gottron’s papules are lichenoid erythematous papules over the knuckles, and Gottron’s sign are erythematous patches over the extensor surfaces. Patients with dermatomyositis frequently have abnormal esophageal motility, and approximately 15–50% of patients with dermatomyositis have dysphagia [12]. Other esophageal symptoms include heartburn, regurgitation, and odynophagia [13].
The diagnosis of dermatomyositis involves evaluation of the skin, muscle strength, and laboratory results. Characteristic serologic abnormalities include elevated enzymes (CK, LDH, AST, aldolase), Dermatomyositis specific autoantibodies (Mi2, TIF1, MDA5, NXP2, SAE), Antisynthetase autoantibodies (Jo-1, PL-7, PL-12, EJ, OJ), and other connective tissue disease antibodies (ANA, Ro/La, dsDNA, anti-SM, Scl-70) [14]. With respect to dysphagia, endoscopy, barium studies (esophagram or modified barium swallow) and HREM are reasonable diagnostics. HREM is frequently normal however it may display ineffective esophageal motility, absent contractility, or jackhammer esophagus [13].
The treatment for dermatomyositis depends on the severity of disease and organ systems involved. Patients with reflux symptoms are treated with PPIs [12]. Of importance to the gastroenterologist, patients with dermatomyositis have an increased risk of developing cancer, and the prevalence of malignancy is approximately 20% [14]. The risk is highest within a year after diagnosis and remains elevated 5 years after diagnosis. Patients should therefore receive age-appropriate screening and symptom-targeted diagnostics [14].
Pemphigus and Pemphigoid Diseases
Pemphigus is an autoimmune skin disorder characterized by antibodies that target desmosomes causing loss of cell-to-cell adhesion. Patients present with intraepithelial skin and mucous membrane vesiculobullous lesions and are frequently Nikolsky’s sign positive where mechanical contact with the skin results in exfoliation of the outermost layer [15]. Pemphigus vulgaris is the most common form of pemphigus and patients often display painful oropharyngeal lesions that precede cutaneous manifestations. Pemphigoid disorders similarly affect the skin and are caused by autoantibodies that target the basement membrane. Pemphigoid disorders are characterized by sub-epidermal bullae that are Nikolsky’s sign negative. The esophagus is frequently involved in patients with mucous membrane pemphigoid (MMP), however patients with MMP more frequently display oral and ocular lesions rather than skin manifestations [16].
There are many similarities in the clinical presentation of esophageal pemphigus and pemphigoid diseases. For both disorders, the esophagus may be involved without effecting the skin, and patients may complain of odynophagia, dysphagia, and weight loss [16–20]. Furthermore, the proximal esophagus is more likely to be affected than the distal esophagus, and the esophageal lining may easily detach during endoscopy (Nikolsky’s sign positive) [18–21]. The esophagus can be involved in 47–68% of patients with pemphigus vulgaris, compared to 4–11% of patients with MMP [17–20, 22, 23]. Endoscopic findings of pemphigus vulgaris include strictures, erosions, ulcers, blisters, vesiculobullous lesions, and furrows throughout the esophagus [17, 18, 24]. Endoscopic findings of MMP include esophageal webs, stenosis, and erosions [16,23]. Esophageal involvement may be difficult to diagnose in patients with MMP with one case series reporting 17–156 months delay until treatment was initiated [21].
As opposed to pemphigoid diseases, the histologic features of the esophagus in pemphigus are similar to the dermatological features. Diagnosis of pemphigus can be supported with pathology demonstrating acantholytic cells in the basal layer with a “tombstone” appearance and intraepithelial bullae [18, 24]. Immunofluorescence further supports the diagnosis with demonstration of autoantibody deposition of IgG, IgA, and C3 complement in a net-like pattern [24]. The diagnosis of pemphigoid diseases is made with clinical findings, dermal pathology, and immunofluorescence [16]. Histology of the skin demonstrates sub-epithelial blister formation, and immunofluorescence shows linear deposits of IgG at the basement membrane (anti-BP-180, antiBP-230). Esophageal biopsy infrequently displays the same immunofluorescent patterns and markers [25].
The treatment of esophageal involvement for both pemphigus and pemphigoid diseases consists of systemic steroids, immunotherapy, and esophageal dilation in patients with strictures. Specifically, regarding pemphigus diseases, patients requiring 3 or more treatment modalities are more likely to receive an esophagogastroduodenoscopy (EGD) and present with dysphagia [26•]. Several studies of pemphigus have shown good response to treatment with complete resolution of symptoms and pathology on EGD [26•]. In one study, 28 patients with oral pemphigus showed esophageal involvement in 68% of patients with subsequent resolution on endoscopy in 100% of patients after starting therapy with systemic corticosteroids [27]. Patients that do not respond to corticosteroids are often treated with more aggressive treatment modalities including rituximab and intravenous immune globulin resulting in better disease control in refractory cases [26•]. Treatment options for strictures include endoscopic dilation with balloons or bougies, stricturoplasty, and stenting [28]. One case report described successful treatment of recurrent strictures that failed multiple balloon dilations with intralesional steroid injection followed by topical steroids [28].
In patients with pemphigoid diseases, endoscopy should only be performed in symptomatic patients and preferably by experienced providers. The esophageal mucosa is fragile, and the endoscope may cause longitudinal erosions, subepithelial hemorrhages, and bullae formation [16, 25]. Additionally, patients are at higher risk for esophageal perforation after dilation [29••]. It is important to distinguish between active and cicatricial (scarred) lesions because active lesions require medical therapy [25]. Patients can be treated with systemic steroids and immunosuppressants (rituximab, mycophenolate, or cyclophosphamide), and strictures may require esophageal dilation [29••]. One case series composed of 12 patients with MMP describes the successful treatment of esophageal stricture with balloon dilation, however this is only recommended after the disease is adequately controlled with medical therapy. Within the case series, balloon dilation was less likely to be successful in patients who had severe dysphagia with uncontrolled disease [29••].
Infectious
HSV/CMV/HIV Esophagitis
There are several infections that affect the skin and the esophagus. The most notable are herpes simplex virus (HSV), cytomegalovirus (CMV), and human immunodeficiency virus (HIV). A viral esophagitis typically presents with odynophagia and dysphagia in an immunosuppressed individual; HSV, however, can occur within immunocompetent individuals [1••]. Endoscopically, HSV esophagitis typically displays multiple small, painful, discrete ulcers, whereas HIV and CMV are classically characterized by fewer, larger ulcers. HSV typically presents in the distal esophagus with punched-out ulcers with a yellow-rim and vesicles [30,31]. Histologically, HSV esophagitis displays intranuclear eosinophilic inclusions (Cowdry A bodies). HSV esophagitis is treated with acyclovir, valacyclovir, or famciclovir. The duration of treatment is determined by immune status with immunocompetent individuals receiving 7–10 days, and immunocompromised individuals receiving 14–21 days. Prophylactic antiviral therapy may be indicated in immunocompromised individuals [30].
While double-contrast esophagography can assist in the diagnosis of HSV, CMV, and HIV esophagitis, upper endoscopy with biopsy is more readily available and is necessary for a tissue diagnosis [1••]. CMV esophagitis is treated with ganciclovir, as opposed to HIV esophagitis which is treated with steroids [1••]. Endoscopically, CMV lesions are more common in the distal esophagus and are deep, longitudinal, and linear. Histologically, under H&E staining, large eosinophilic or basophilic intranuclear inclusions are characteristic of CMV esophagitis [31]. CMV esophagitis is treated with ganciclovir or valganciclovir. Patients should receive IV ganciclovir daily for 3–6 weeks [32].
Inflammatory
Lichen Planus
Lichen planus is an inflammatory mucocutaneous disease of unknown etiology. The disease may be associated with infections, medications, or may be autoimmune [33]. Oral cutaneous lichen planus affects both sexes equally, however esophageal lichen planus disproportionately affects women [34, 35•]. Patients can display pruritic, violaceous papules and plaques, most commonly over extensor surfaces. The skin, nails, hair, genital, and oral mucosa may be involved. Patients with oral lichen planus can have a white lace-like pattern known as Wickham striae.
Esophageal involvement is seen in 26% to 50% of patients [36•]. Many patients with esophageal involvement are asymptomatic, however patients may develop dysphagia, odynophagia, heartburn, and regurgitation. Diagnosis should take into consideration characteristic skin and mucosal findings, endoscopy, histology, and immunofluorescence. Endoscopy may demonstrate findings typically seen in eosinophilic esophagitis such as concentric rings, mucosal edema and white plaques. Other endoscopic findings described include loss of vascularity, friability, and erythema. Esophageal strictures are a frequent finding in esophageal lichen planus [1, 33, 34]. Mucosal detachment during endoscopy or dilation may be a specific sign for esophageal lichen planus [36•]. Esophageal biopsy can demonstrate lichenoid inflammation, detachment of the epithelium, and necrotic keratinocytes (Civatte bodies). Immunofluorescence can show fibrin deposition along the basement membrane [34, 36•].
Severe esophageal lichen planus can be treated with systemic steroids followed by maintenance therapy with swallowed steroids [35•,37]. According to one case series, 44% of patients with severe esophageal lichen planus were treated with systemic steroids [35•]. Some patients received treatment with immunomodulators (azathioprine, tacrolimus, cyclosporine, rituximab). Patients with milder disease often do not require systemic or topical steroids but may be treated with swallowed steroids if needed [35•, 37]. Relapse rates after treatment may be as high as 85% [33]. Dysphagia may also be treated with endoscopic dilation and can lead to rapid improvement in dysphagia. Although dilations are often necessary, it should be noted that Koebner phenomenon (new lesions after trauma to uninvolved site) has been described after dilation. Squamous Cell Carcinoma (SCC) has been reported in the setting of long-standing esophageal lichen planus. According to one study, 6% of patients developed esophageal SCC, therefore periodic surveillance endoscopy with mucosal biopsies should be considered [33, 34, 38].
Crohn’s Disease
Crohn’s disease is an idiopathic inflammatory bowel disease with genetic and environmental influences. The incidence of disease is approximately 3.1 to 14.6 per 100,000 individuals per year [39••]. Patients can present with various dermatological manifestations including pyoderma gangrenosum, erythema nodosum, fistulas, Sweet syndrome and oral aphthous ulcers [40].
Esophageal Crohn’s disease is rare with a higher prevalence in children and potentially racial and ethnic minority populations [41]. Patient’s may present with dysphagia (54%), odynophagia (33%), reflux (25%), and chest pain (13%) [39••]. Esophageal Crohn’s disease most frequently involves the distal esophagus. Early stages of disease display erythema, edema, aphthous ulcers, and granularity. Later stages of disease may demonstrate ulcers, strictures, and fistulas.
Esophageal Crohn’s disease should be suspected in a patient with Crohn’s disease and atypical esophageal findings. Esophageal Crohn’s may be difficult to diagnose due to the potential for multiple diagnoses (GERD, Eosinophilic Esophagitis). Esophageal biopsies may reveal non-caseating granulomas, however this finding is frequently absent. In contrast to adults, screening endoscopy is recommended in all children newly diagnosed with Crohn’s disease [42]. Treatment of esophageal Crohn’s is similar to Crohn’s without esophageal involvement typically requiring the use of biologics and/or immunosuppressants. Patients often undergo a stepwise approach with PPIs, steroids, and 5-aminosalicilates. Many immunomodulators have low activity in the upper gastrointestinal tract, and therefore systemic steroids are often utilized for their superior efficacy [39••]. Topical swallowed steroids have also been shown to effectively treat esophageal Crohn’s disease [39••]. Infliximab is an effective therapy for patients with esophageal Crohn’s disease that have failed other treatment modalities. In one case series all five patients treated with infliximab had complete resolution of clinical symptoms. Furthermore, an additional two patients who were treated with infliximab after prednisone had complete resolution of esophageal symptoms [42•]. The study also revealed that a combination of balloon dilation, biologics, and immunomodulators is an effective treatment for structuring esophageal Crohn’s disease [42•]. One patient with a gastro-esophageal fistula was successfully treated with infliximab after they failed to respond to a combination of immunomodulators, systemic steroids, and PPIs [42•].
Endoscopic treatment is an alternative middle ground between medical and surgical therapy in the treatment of Crohn’s disease-related strictures. Endoscopic management can include balloon dilation, stricturotomy and stricturoplasty. Endoscopic balloon dilation is the first line therapy for non-complex strictures, strictures less than 4–5 cm, single strictures, or strictures without pre-stenotic dilation. If repeat endoscopic balloon dilation is unsuccessful at treating Crohn’s related strictures, endoscopic electroincision (stricturotomy and strictureplasty) may be utilized [43]. Rarely, patients may require esophagectomy for fistulas with the tracheobronchial tree, paraoesophageal abscesses, and mediastinitis [39••]
Genetic
Epidermolysis Bullosa
Epidermolysis bullosa is a rare genetic syndrome with blistering mucocutaneous lesions caused by a defect in an anchoring protein between the epidermis and the dermis. Esophageal involvement is normally associated with the autosomal recessive inheritance and the dystrophic epidermolysis bullosa subtype [44]. The cutaneous manifestations of disease are characterized by blistering lesions, erosions, scars, milia, and sometimes missing nails. The disorder is seen in children that present with dysphagia, odynophagia, or malnutrition. Esophageal involvement is most notable for the development of esophageal strictures, and proximal esophageal strictures are seen twice as frequently as distal strictures [4]. Management of esophageal stricture consists of dietary modification, careful balloon dilation, or placement of gastrostomy tube for nutritional purposes [44]. A dedicated anesthesia team may be used during endoscopy to assist in intravenous catheter placement and potential need for intubation during or after the procedure. Dietary management includes encouragement of soft foods, as well as avoidance of hot foods and hyperalimentation [45•]. Some patients develop microstomia due to oropharyngeal scarring, in which case an esophageal dilation may be attempted in a retrograde approach from the gastrostomy tube [45•, 46].
Cowden Syndrome
Cowden syndrome is an autosomal dominant disease secondary to a mutation in the PTEN gene. The prevalence of Cowden syndrome is approximately 1 in 200,000 to 1 in 250,000 [47]. The dominant features of Cowden syndrome are the development of multiorgan hamartomas and an increased risk of multiple malignancies including breast, thyroid, endometrium, renal and colon. Dermatologic manifestations of Cowden syndrome are near universal with 90 percent of affected individuals developing trichilemmommas (hamartomas of the infundibulum of hair follicles) and mucocutaneous papillomas [48]. Patients with Cowden syndrome can also develop polyposis throughout the gastrointestinal tract including hamartomatous polyps, hyperplastic changes, gangliocytomas, and adenomas [49]. Histologic examination of esophageal lesions displays glycogenic acanthosis. Glycogenic acanthosis is a benign finding that may be associated with gastroesophageal reflux and is an incidental finding discovered in approximately 3.5% of endoscopies [50]. One study reports that glycogenic acanthosis can be observed in upwards of 80% of patients with Cowden syndrome [51]. Endoscopists should consider the diagnosis of Cowden syndrome if glycogenic acanthosis is observed in conjunction with colonic polyposis.
The diagnosis of Cowden syndrome is made through clinical findings but has very specific pathognomonic criteria (trichilemmommas, acral keratosis, papillomatous papules, mucosal lesions), major criteria (breast thyroid endometrial carcinoma, macrocephaly, Lhermitt-Duclos disease), and minor criteria (other thyroid lesions, Intelligence Quotient < 75, GI hamartomas, fibrocystic disease of the breasts, lipomas, fibromas, genitourinary tumors) [48]. The diagnosis can be made if the patient has the pathognomonic skin findings, two major criteria, one major and 3 minor criteria, or four minor criteria [52]. PTEN genetic testing confirms the diagnosis. As noted above, Cowden syndrome is associated with many malignancies and therefore close follow up is required [47,53]. When Cowden syndrome is diagnosed, patients should receive a colonoscopy at age 35 or 10 years before the age of a first-degree relative at diagnosis of colorectal cancer. Surveillance endoscopy should be performed every 1–5 years depending on the number and characteristics of the polyps identified [51].
Focal Dermal Hypoplasia (Goltz Syndrome)
Focal dermal hypoplasia (FDH) is a rare X-linked dominant genetic disorder with approximately 300 cases reported worldwide. The disease has a 9:1 female to male ratio because it is often fatal in utero for males. FDH is caused by a mutation in the PORCN gene resulting in defective mesodermal and ectodermal development [54]. Patients have many dermatological manifestations including skin nodules, pigment changes, abnormal nails, dysmorphic facial features, and telangiectasias [55,56]. Patients also develop mucocutaneous papillomas over the mouth, nose, larynx, anus, and genitals [54]. The condition affects the skeleton frequently resulting in syndactyly, ectrodactyly, and malformations of the truncal skeleton [55]. Patients with FDH can present with dysphagia secondary to papillomas that develop within the esophagus. Many patients with FDH have GERD, and it is theorized that reflux contributes to the formation of esophageal papillomas [4, 54].
The diagnosis of FDH can be made with a combination of characteristic clinical findings including limb malformations, skin findings, and molecular genetics [56]. Patients should be monitored for GERD and dysphagia symptoms. One case details the use of argon plasma coagulation to debulk the papillomas and successfully relieve the patient’s dysphagia [54]. Malignant conversion of esophageal papillomas to SCC has been described, however has not been frequently observed in the literature [55].
Tylosis
Tylosis is a rare genetic disorder characterized by epidermolytic palmoplantar hyperkeratosis and an increased risk of developing SCC of the esophagus. The disorder has been associated with the RHBDF2 gene and is inherited in an autosomal dominant pattern. The prevalence of the disorder in the general population is thought to be less than 1 in 1,000,000 [57]. The disease is classified into two categories: early onset (before year 1) and late onset (age 5–15) [58]. Patients with early onset tylosis are unlikely to develop SCC of the esophagus, whereas those with late onset tylosis are at a very high risk of developing esophageal cancer. Patients develop esophageal cancer at an average age of 45 years, and the risk of developing SCC is approximately 40–92% [59••]. From a dermatologic perspective, patients can present with skin plaques of the hands and the feet, fissures on the extremities, and are at an increased risk of developing tinea pedis. Patients may be asymptomatic during the early stages of disease but can also present with the symptoms of esophageal cancer including dysphagia, odynophagia, and weight loss [57].
The diagnosis of tylosis can be made through a combination of family history, skin findings, endoscopic features, and evidence of a mutation in the RHBDF2 gene [57,57••]. Endoscopically, patients can display white polypoid lesions, diffuse hyperkeratosis, longitudinal grooves, and strictures [57, 57••, 60]. However, due to the rarity of the disease, there is no correlation between endoscopic findings and disease progression [60]. Esophageal neoplasms are most commonly discovered in the lower two-thirds of the esophagus. Esophageal histologic features are non-specific for the disease and can display keratohylaine granules, parakeratosis, and an inflammatory infiltrate. Patients diagnosed with tylosis should undergo surveillance endoscopy starting at age 30 every 1–3 years [59••]. If tylosis is diagnosed in an individual, family members should receive genetic counseling. Family members with tylosis should also be screened with biopsy of suspicious lesions as well as quadratic biopsies of the upper, middle, and lower esophagus. Patients should be counseled to avoid risk factors that lead to the development of SCC including alcohol and smoking cessation [57]. There is evidence that carotenoids may delay the progression or possibly revert lesions back to normal [60]. The dermatological manifestations can be treated with footwear, emollients, retinoids, and early treatment of fissures and infections.
Conclusion
Dermatological conditions that affect the esophagus are not particularly common, however it is important to connect the two in a patient with dysphagia of unknown etiology. The diagnosis of many of the conditions is made through characteristic clinical features, endoscopy, and histology. The autoimmune conditions are unique in that they utilize serology. The infectious conditions are diagnosed with endoscopy and biopsy and are treated with antimicrobials. The inflammatory conditions often rely heavily on clinical features, and the genetic diseases rely on clinical features and are confirmed with genetic testing. The treatment for many of the conditions is similar with steroids, immunosuppressants, and proton pump inhibitors. Many of the conditions result in esophageal strictures that may require endoscopic treatment with dilation. Importantly, many of the diseases are pre-malignant where proper cancer surveillance and vigilance is important.
Figure 1:
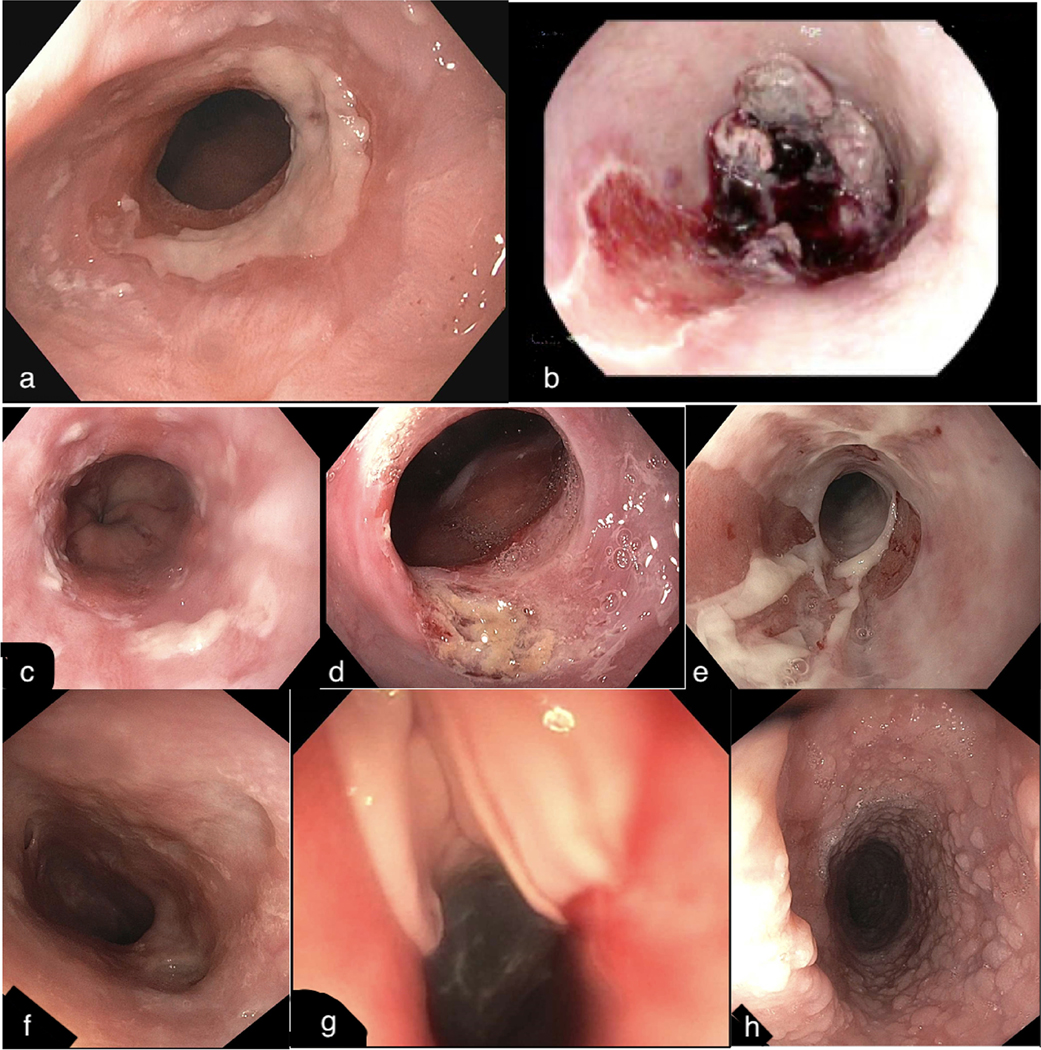
Endoscopic Features of Dermatological Diseases
a) stricture and esophagitis seen in a patient with systemic sclerosis b) large ulcer with associated blood clot and liquified tissue seen in a patient with bullous pemphigoid c) small numerous ulcers seen in HSV esophagitis d) large ulcer seen in CMV esophagitis e) granularity, sloughing, and ulceration seen in lichen planus f) stricturing and ulceration seen in esophageal Crohn’s Disease g) mucosal disruption and sloughing after dilation in epidermolysis bullosa h) glycogenic acanthosis seen in Cowden syndrome
Table 1:
Dermatological and Esophageal Characteristics of Autoimmune and Infectious Diseases
| Disease | Cutaneous Findings | Esophageal Symptoms | Endoscopic/ Manometry Findings | Histological Findings | Treatment |
|---|---|---|---|---|---|
| Scleroderma | Raynaud’s Telangiectasias Sclerodactyly Calcinosis Digital scars Contractures Digital ulcers | Heartburn Dysphagia Chest pain Regurgitation Abdominal pain Weight loss | Strictures Esophagitis Telangiectasias Absent contractility Ineffective motility |
Barrett’s esophagus Adenocarcinoma | Pro-kinetic agents PPIs Systemic steroids Immunosuppressants Buspirone Bethanechol Pyridostigmine |
| Dermatomyositis | Gottron papules Gottron’s sign Heliotrope rash Shawl sign Periungual telangiectasias | Dysphagia Heartburn Regurgitation Odynophagia | Ineffective esophageal motility Absent motility Jackhammer esophagus |
Barrett’s esophagus Adenocarcinoma | PPI Systemic steroids Immunosuppressants |
| Pemphigus | Intraepithelial skin and mucous membrane vesiculobullous lesions Nikolsky sign positive |
Odynophagia Dysphagia | Strictures Erosions Ulcers Blisters Furrows Vesiculobullous lesions |
Suprabasal epidermal acantholysis Intraepithelial bullae Deposition of IgG, IgA, and C3 complement in a net-like pattern |
Systemic steroids Immunosuppressants Esophageal dilation |
| Pemphigoid | Sub-epidermal bullae Nikolsky sign negative |
Dysphagia Odynophagia Weight loss | Exudates with fibrin Esophageal webs Stenosis Erosions Subepithelial hematomas Proximal esophageal strictures |
Sub-epithelial blister formation Linear deposits of IgG at the basement membrane (anti- BP-180, antiBP-230) |
Systemic steroids Immunosuppressants Esophageal dilation |
| HSV | Cold sores Genital herpes Herpetic whitlow Herpetic eczema | Dysphagia Odynophagia | Punched-out ulcers with a yellow-rim and vesicles | Intranuclear eosinophilic inclusions otherwise known as Cowdry A bodies | Acyclovir Valacyclovir Famciclovir |
| CMV | Petechiae Morbilliform rashes Cutaneous vasculitis | Dysphagia Odynophagia | Deep, longitudinal and linear ulcers | Large eosinophilic or basophilic intranuclear inclusions | Ganciclovir |
| HIV | Kaposi sarcoma Anal papillomas Candida Eosinophilic folliculitis | Dysphagia Odynophagia | Deep ulcers in the middle of the esophagus | Eosinophilic infiltrate Inflammatory infiltrate |
Steroids |
Table 2:
Dermatological and Esophageal Characteristics of Inflammatory and Genetic Diseases
| Disease | Cutaneous Findings | Esophageal Symptoms | Endoscopic Findings | Histological Findings | Treatment |
|---|---|---|---|---|---|
| Lichen Planus | Pruritic violaceous papules and plaques Wickham striae | Dysphagia Odynophagia Heartburn Regurgitation | Ringed esophagus Strictures Peeling mucosa Papular lesions Submucosal plaques White papules |
Lichenoid inflammation Necrotic keratinocytes (civatte bodies) Fibrin deposition along the basement membrane |
Systemic steroids Immunosuppressants Swallowed topical steroids Esophageal dilation |
| Crohn’s | Pyoderma gangrenosum Erythema nodosum Skin fistulas Sweet syndrome Oral aphthous ulcers |
Dysphagia Odynophagia Heart burn Chest pain | Erythema Edema Aphthous ulcers Strictures Fistulas Ulcers |
Non-caseating granulomas Intraepithelial lymphocytes |
PPIs H2 blockers Systemic steroids Aminosalicylates Swallowed topical steroids Immunosuppressants Esophageal dilation |
| Epidermolysis Bullosa | Blistering lesions Erosions scars Milia Nail changes |
Dysphagia Odynophagia Malnutrition | Proximal strictures | Route light microscopy not recommended given difficult to interpret. Immunofluorescence antigenic mapping and transmission electron microscopy can provide diagnostic information on diagnostic subtype [61]. | Dietary modification Esophageal dilation Gastrostomy tube |
| Cowden Syndrome | Trichilemmomas Acral keratosis Papillomatous papules Mucosal lesions Lipomas Fibromas |
Dysphagia | Hamartomatous Polyps Hyperplastic changes Gangliocytomas Adenomas | Glycogenic acanthosis | Cancer screening |
| Focal Dermal Hypoplasia | Skin nodules Pigment changes Abnormal nails Dysmorphic facial features Telangiectasias Mucocutaneous papilloma Skeletal defects | Dysphagia Heart burn | Papillomas | Esophageal papilloma Squamous cell carcinoma | Esophageal dilation PPI Aragon plasma coagulation debulking |
| Tylosis | Skin plaques of the hands and the feet Fissures Tenia pedis |
Dysphagia Odynophagia Weight loss | Polypoid lesions Hyperkeratosis Longitudinal grooves Strictures | Keratohylaine granules Parakeratosis Inflammatory infiltrate | Carotenoids Regular cancer screening after age 30 Avoid smoking and alcohol |
Footnotes
Disclosure
Amr M. Arar declares that he has no potential conflicts of interest
Kelli DeLay declares that she has no potential conflicts of interest
David A. Leiman declares that he has no potential conflicts of interest
Paul Menard-Katcher declares that he has no potential conflicts of interest
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1. Lee MH, Lubner MG, Peebles JK, Hinshaw MA, Menias CO, Levine MS, et al. Clinical, Imaging, and Pathologic Features of Conditions with Combined Esophageal and Cutaneous Manifestations. Radiographics. 2019;39(5):1411–34. ••Highly recmomended recent review article on esophgeal manifestations of dermatological illness from radiologist perspective.
- 2.Gualtierotti R, Marzano AV, Spadari F, Cugno M. Main Oral Manifestations in Immune-Mediated and Inflammatory Rheumatic Diseases. J Clin Med. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun-Moscovici Y, Brun R, Braun M. Systemic Sclerosis and the Gastrointestinal Tract-Clinical Approach. Rambam Maimonides Med J. 2016;7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise JL, Murray JA. Esophageal manifestations of dermatologic disease. Curr Gastroenterol Rep. 2002;4(3):205–12. [DOI] [PubMed] [Google Scholar]
- 5.Rohof WOA, Bredenoord AJ. Chicago Classification of Esophageal Motility Disorders: Lessons Learned. Curr Gastroenterol Rep. 2017;19(8):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowell MD, Umar SB, Griffing WL, DiBaise JK, Lacy BE, Vela MF. Esophageal Motor Abnormalities in Patients With Scleroderma: Heterogeneity, Risk Factors, and Effects on Quality of Life. Clin Gastroenterol Hepatol. 2017;15(2):207–13 e1. doi: 10.1016/j.cgh.2016.08.034. •Interesting article on the prevalence of manometry findings in scleroderma.
- 7.Christopher P Denton M. Overview of the treatment and prognosis of systemic sclerosis (scleroderma) in adults [Web page]. Wolters Kluwer; 2021. [updated Jan 2021; cited 2021 2/14/2021]. Available from: https://www.uptodate.com/contents/overview-of-the-treatment-and-prognosis-of-systemic-sclerosis-scleroderma-in-adults. [Google Scholar]
- 8.Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–39. [DOI] [PubMed] [Google Scholar]
- 9.Karamanolis GP, Panopoulos S, Denaxas K, Karlaftis A, Zorbala A, Kamberoglou D, et al. The 5-HT1A receptor agonist buspirone improves esophageal motor function and symptoms in systemic sclerosis: a 4-week, open-label trial. Arthritis Res Ther. 2016;18:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castell DO. The lower esophageal sphincter. Physiologic and clinical aspects. Ann Intern Med. 1975;83(3):390–401. [DOI] [PubMed] [Google Scholar]
- 11.McMahan ZH, Hummers LK. Gastrointestinal involvement in systemic sclerosis: diagnosis and management. Curr Opin Rheumatol. 2018;30(6):533–40. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanov I, Kazandjieva J, Darlenski R, Tsankov N. Dermatomyositis: Current concepts. Clin Dermatol. 2018;36(4):450–8. [DOI] [PubMed] [Google Scholar]
- 13.Casal-Dominguez M, Pinal-Fernandez I, Mego M, Accarino A, Jubany L, Azpiroz F, et al. High-resolution manometry in patients with idiopathic inflammatory myopathy: Elevated prevalence of esophageal involvement and differences according to autoantibody status and clinical subset. Muscle Nerve. 2017;56(3):386–92. [DOI] [PubMed] [Google Scholar]
- 14.Waldman R, DeWane ME, Lu J. Dermatomyositis: Diagnosis and treatment. J Am Acad Dermatol. 2020;82(2):283–96. [DOI] [PubMed] [Google Scholar]
- 15.Amos J, Baron A, Rubin AD. Autoimmune swallowing disorders. Curr Opin Otolaryngol Head Neck Surg. 2016;24(6):483–8. [DOI] [PubMed] [Google Scholar]
- 16.Yuan H, Pan M. Endoscopic characteristics of oesophagus involvement in mucous membrane pemphigoid. Br J Dermatol. 2017;177(4):902–3. [DOI] [PubMed] [Google Scholar]
- 17.de Macedo AG, Bertges ER, Bertges LC, Mendes RA, Bertges T, Bertges KR, et al. Pemphigus Vulgaris in the Mouth and Esophageal Mucosa. Case Rep Gastroenterol. 2018;12(2):260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecinato P, Laterza L, De Marco L, Casali A, Zanelli M, Sassatelli R. Esophageal involvement by pemphigus vulgaris resulting in dysphagia. Endoscopy. 2015;47 Suppl 1 UCTN:E271–2. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura R, Omori T, Suda K, Wada N, Kawakubo H, Takeuchi H, et al. Endoscopic findings of laryngopharyngeal and esophageal involvement in autoimmune bullous disease. Dig Endosc. 2017;29(7):765–72. [DOI] [PubMed] [Google Scholar]
- 20.Benoit S, Scheurlen M, Goebeler M, Stoevesandt J. Structured Diagnostic Approach and Risk Assessment in Mucous Membrane Pemphigoid with Oesophageal Involvement. Acta Derm Venereol. 2018;98(7):660–6. [DOI] [PubMed] [Google Scholar]
- 21.DeGrazia T, Eisenstadt R, Willingham FF, Feldman R. Mucous Membrane Pemphigoid Presenting With Esophageal Manifestations: A Case Series. Am J Gastroenterol. 2019;114(10):1695–7. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez Prudencio S, Domingo Senra D, Martin Rodriguez D, Botella Mateu B, Esteban Jimenez-Zarza C, de la Morena Lopez F, et al. Esophageal Cicatricial Pemphigoid as an Isolated Involvement Treated with Mycophenolate Mofetil. Case Rep Gastrointest Med. 2015;2015:620374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeda Y, Tanaka K, Yamada R. An Unusual Web Stricture in the Cervical Esophagus. Gastroenterology. 2020;158(1):54–5. [DOI] [PubMed] [Google Scholar]
- 24.Gopal P, Gibson JA, Lisovsky M, Nalbantoglu I. Unique causes of esophageal inflammation: a histopathologic perspective. Ann N Y Acad Sci. 2018;1434(1):219–26. [DOI] [PubMed] [Google Scholar]
- 25.Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune Subepidermal Bullous Diseases of the Skin and Mucosae: Clinical Features, Diagnosis, and Management. Clin Rev Allergy Immunol. 2018;54(1):26–51. [DOI] [PubMed] [Google Scholar]
- 26. Ozeki KA, Zikos TA, Clarke JO, Sonu I. Esophagogastroduodenoscopy and Esophageal Involvement in Patients with Pemphigus Vulgaris. Dysphagia. 2020;35(3):503–8. •Relatively large retrospective study of 111 pemphigus vulgaris patients examining the incidence of esophgeal involvement.
- 27.Galloro G, Mignogna M, de Werra C, Magno L, Diamantis G, Ruoppo E, et al. The role of upper endoscopy in identifying oesophageal involvement in patients with oral pemphigus vulgaris. Dig Liver Dis. 2005;37(3):195–9 [DOI] [PubMed] [Google Scholar]
- 28.Usman RM, Jehangir Q, Bilal M. Recurrent Esophageal Stricture Secondary to Pemphigus Vulgaris: A Rare Diagnostic and Therapeutic Challenge. ACG Case Rep J. 2019;6(2):e00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zehou O, Raynaud JJ, Le Roux-Villet C, Alexandre M, Airinei G, Pascal F, et al. Oesophageal involvement in 26 consecutive patients with mucous membrane pemphigoid. Br J Dermatol. 2017;177(4):1074–85. ••Retrospective study of MMP patients with esophgeal involvement. Within this study there is a good description of physical exam findings, endoscopic findings, and response to therapeutics.
- 30.Peter AL Bonis CNK. Herpes simplex virus infection of the esophagus UpToDate: Wolters Kulwer; 2020. [updated Jan 2021; cited 2021 2/14/21]. Available from: https://www.uptodate.com/contents/herpes-simplex-virus-infection-of-the-esophagus. [Google Scholar]
- 31.Bannoura S, Barada K, Sinno S, Boulos F, Chakhachiro Z. Esophageal Cytomegalovirus and Herpes Simplex virus co-infection in an immunocompromised patient: Case report and review of literature. IDCases. 2020;22:e00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Chakinala RC. Cytomegalovirus Esophagitis. StatPearls. Treasure Island (FL) 2020. [PubMed] [Google Scholar]
- 33.Sato Y, Takenaka R, Matsumi A, Takei K, Okanoue S, Yasutomi E, et al. A Japanese Case of Esophageal Lichen Planus that Was Successfully Treated with Systemic Corticosteroids. Intern Med. 2018;57(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Makey IA, Francis DL, Wolfsen HC, Bowers SP. Squamous Cell Carcinoma in Lichen Planus of the Esophagus. Ann Thorac Surg. 2020;109(2):e83–e5. [DOI] [PubMed] [Google Scholar]
- 35. Schauer F, Monasterio C, Technau-Hafsi K, Kern JS, Lazaro A, Deibert P, et al. Esophageal lichen planus: towards diagnosis of an underdiagnosed disease. Scand J Gastroenterol. 2019;54(10):1189–98. •Retrospective study of 52 patients with esophgeal lichen planus examining disease severity, diagnosis, and respone to therapeutics.
- 36. Kern JS, Technau-Hafsi K, Schwacha H, Kuhlmann J, Hirsch G, Brass V, et al. Esophageal involvement is frequent in lichen planus: study in 32 patients with suggestion of clinicopathologic diagnostic criteria and therapeutic implications. Eur J Gastroenterol Hepatol. 2016;28(12):1374–82. •Retrospective study of 32 patients with esophgeal lichen planus commenting on diagnosis and therapeutics.
- 37.Zamani F, Haghighi M, Roshani M, Sohrabi M. Esophageal Lichen Planus Stricture. Middle East J Dig Dis. 2019;11(1):52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravi K, Codipilly DC, Sunjaya D, Fang H, Arora AS, Katzka DA. Esophageal Lichen Planus Is Associated With a Significant Increase in Risk of Squamous Cell Carcinoma. Clin Gastroenterol Hepatol. 2019;17(9):1902–3 e1. doi: 10.1016/j.cgh.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 39. Schwartzberg DM, Brandstetter S, Grucela AL. Crohn’s Disease of the Esophagus, Duodenum, and Stomach. Clin Colon Rectal Surg. 2019;32(4):231–42. ••Great review article on the clincal manifestations, diagnosis, and treatment of upper gastrointestinal Crohn’s disease.
- 40.Greuter T, Navarini A, Vavricka SR. Skin Manifestations of Inflammatory Bowel Disease. Clin Rev Allergy Immunol. 2017;53(3):413–27. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen GC, Torres EA, Regueiro M, Bromfield G, Bitton A, Stempak J, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. 2006;101(5):1012–23. [DOI] [PubMed] [Google Scholar]
- 42. De Felice KM, Katzka DA, Raffals LE. Crohn’s Disease of the Esophagus: Clinical Features and Treatment Outcomes in the Biologic Era. Inflamm Bowel Dis. 2015;21(9):2106–13. •Study of 24 patients with esophgeal Crohn’s diesase examining clinical symptoms, endoscopic findings, and therapeutic response.
- 43.Pokala A, Shen B. Update of endoscopic management of Crohn’s disease strictures. Intest Res. 2020;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makker J, Bajantri B, Remy P. Rare case of dysphagia, skin blistering, missing nails in a young boy. World J Gastrointest Endosc. 2015;7(2):154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson BT, Feinstein JA, Kramer RE, Narkewicz MR, Bruckner AL, Brumbaugh DE. Approach and Safety of Esophageal Dilation for Treatment of Strictures in Children With Epidermolysis Bullosa. J Pediatr Gastroenterol Nutr. 2018;67(6):701–5. •Retrospective study examining the rate of adverse events after esophageal balloon dilation in patients with epidermolysis bullosa.
- 46.Vowinkel T, Laukoetter M, Mennigen R, Hahnenkamp K, Gottschalk A, Boschin M, et al. A two-step multidisciplinary approach to treat recurrent esophageal strictures in children with epidermolysis bullosa dystrophica. Endoscopy. 2015;47(6):541–4. [DOI] [PubMed] [Google Scholar]
- 47.Kang YH, Lee HK, Park G. Cowden Syndrome Detected by FDG PET/CT in an Endometrial Cancer Patient. Nucl Med Mol Imaging. 2016;50(3):255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37(11):828–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inukai K, Takashima N, Fujihata S, Miyai H, Yamamoto M, Kobayashi K, et al. Arteriovenous malformation in the sigmoid colon of a patient with Cowden disease treated with laparoscopy: a case report. BMC Surg. 2018;18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz N. The relationship between reflux symptoms and glycogenic acanthosis lesions of the oesophagus. Prz Gastroenterol. 2020;15(1):39–43.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modi RM, Arnold CA, Stanich PP. Diffuse Esophageal Glycogenic Acanthosis and Colon Polyposis in a Patient With Cowden Syndrome. Clin Gastroenterol Hepatol. 2017;15(8):e131–e2. [DOI] [PubMed] [Google Scholar]
- 52.Cowden syndrome National Institutes of Health: Health and Human Services; 2017. [updated 1/6/2017; cited 2021 2/14/2021]. Available from: https://rarediseases.info.nih.gov/diseases/6202/cowden-syndrome. [Google Scholar]
- 53.Parvataneni S, Chaudhari D, Swenson J, Young M. Cowden syndrome. Indian J Gastroenterol. 2015;34(6):468. [DOI] [PubMed] [Google Scholar]
- 54.Pasman EA, Heifert TA, Nylund CM. Esophageal squamous papillomas with focal dermal hypoplasia and eosinophilic esophagitis. World J Gastroenterol. 2017;23(12):2246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hafiz M, Sundaram S, Naqash AR, Speicher J, Sutton A, Walker P, et al. A Rare Case of Squamous Cell Carcinoma of the Esophagus in a Patient With Goltz Syndrome. ACG Case Rep J. 2019;6(3):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bostwick B, Van den Veyver IB, Sutton VR. Focal Dermal Hypoplasia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al. , editors. GeneReviews((R)). Seattle (WA)1993. [Google Scholar]
- 57.Ellis A, Risk JM, Maruthappu T, Kelsell DP. Tylosis with oesophageal cancer: Diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maillefer RH, Greydanus MP. To B or not to B: is tylosis B truly benign? Two North American genealogies. Am J Gastroenterol. 1999;94(3):829–34. [DOI] [PubMed] [Google Scholar]
- 59. Ramai D, Lai JK, Ofori E, Linn S, Reddy M. Evaluation and Management of Premalignant Conditions of the Esophagus: A Systematic Survey of International Guidelines. J Clin Gastroenterol. 2019;53(9):627–34. ••Interesting review article on premalignant conditions of the esophagus. This article provides clinicans with guidance on the clinical presentation, surveillance, and management of tylosis.
- 60.Surapaneni BK, Kancharla P, Vinayek R, Dutta SK, Goldfinger M. Uncommon Endoscopic Findings in a Tylosis Patient: A Case Report. Case Rep Oncol. 2019;12(2):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lykova SG, Maksimova YV, Nemchaninova OB, Guseva SN, Omigov VV, Aidagulova SV. [Inherited epidermolysis bullosa]. Arkh Patol. 2018;80(4):54–60. [DOI] [PubMed] [Google Scholar]


