Abstract
Background
There is a lack of consensus regarding the optimal method of assessing health-related quality of life (HR-QOL) among patients with metastatic renal cell carcinoma (mRCC). This study explored the perceived relevance of items that make up the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19), as judged by patients with mRCC.
Methods
This was a multinational cross-sectional survey. Eligible patients responded to a questionnaire composed of 18 items that assessed the perceived relevance of each item in the FKSI-19 questionnaire. Open-ended questions assessed additional issues deemed relevant by patients. Responses were grouped as relevant (scores 2-5) or nonrelevant (score 1). Descriptive statistics were collated, and open-ended questions were analyzed and categorized into descriptive categories. Spearman correlation statistics were used to test the association between relevance and clinical characteristics.
Results
A total of 151 patients were included (gender: 78.1 M, 21.9F; median age: 64; treatment: 38.4 immunotherapy, 29.8 targeted therapy, 13.9 immuno-TKI combination therapy) in the study. The most relevant questions evaluated fatigue (77.5), lack of energy (72.2), and worry that their condition will get worse (71.5). Most patients rated blood in urine (15.2), fevers (16.6), and lack of appetite (23.2) as least relevant. Qualitative analysis of open-ended questions revealed several themes, including emotional and physical symptoms, ability to live independently, effectiveness of treatment, family, spirituality, and financial toxicity.
Conclusion
There is a need to refine widely used HR-QOL measures that are employed among patients diagnosed with mRCC treated with contemporary therapies. Guidance was provided for the inclusion of more relevant items to patients’ cancer journey.
Keywords: renal cell carcinoma, health-related quality of life, Functional Assessment of Cancer Therapy Kidney Symptom Index-19, health care survey, patient-reported outcomes
This study explored the perceived relevance of items that make up the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19), as judged by patients with metastatic renal cell carcinoma.
Implications for Practice.
Current health-related quality-of-life measures appear to be outdated based on patients’ perceptions. Results showed that some items in the FKSI-19 are relevant, but most were rated as nonrelevant with respect to patients’ quality of life.
Introduction
The treatment of renal cell carcinoma (RCC) is associated with significant side-effects, with an inevitable impact on patients’ health-related quality of life (HR-QOL). The assessment of HR-QOL with validated measures has provided greater insight into the patient experience and prompted the FDA to outline several core domains that serve as the foundation for patient reported-outcome measurement. These include adverse events, physical function, and disease-related symptoms.5 Despite these broad recommendations, we lack consensus concerning which of the many validated measures should be used in patient care or clinicals trials. Further, randomized clinical trials in this space have used a variety of HR-QOL measures, thus limiting our understanding of the true effect of novel therapies among patients diagnosed with metastatic renal cell carcinoma (mRCC).6-9
Five phase III trials, which have led to the approval of first-line immune checkpoint blockade-based regimens for patients with mRCC, used varying measures to assess HR-QOL.6-10 Given the importance of HR-QOL data in the comprehensive assessment of treatment benefit and potential toxicity, it is important that evidence-based, consensus-driven and valid measures be used. In the trials noted above, HR-QOL was assessed using either generic tools (eg, 36-Item Short Form Survey, SF-36) or cancer-specific tools (eg, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, EORTC QLQ-C30 and Functional Assessment of Cancer Therapy Kidney Symptom Index-19, FKSI-19).11 The methodology followed to develop the FKSI-19, for example, includes information gathered from patients and expert clinicians to prioritize symptoms and concerns and to determine best way to word items.12,13 Despite their proven sensitivity, these questionnaires were tested and validated among patients receiving now outdated therapeutic regimens, and thus may not fully capture the impact of current standard of care treatments on patients’ HR-QOL. In light of these limitations, the current study examined the perceived relevance of questions contained within one of the most widely used cancer-specific measures in renal cell carcinoma (FKSI-19) to patients with mRCC on systemic treatment.14 In addition, this study sought to differentiate between treatment and disease-related HR-QOL issues, to determine the optimal number of questions and frequency of assessment, and to garner suggestions for the inclusion of additional questions to accurately measure HR-QOL in the context of mRCC.
Methods
This cross-sectional survey study was conducted at 4 institutions located in the US (1 NCI designated Comprehensive Cancer Center located in Duarte, California) and Brazil (3 private cancer centers located in Brasilia, Porto Alegre and Salvador) from November 2021 to April 2022. Patients diagnosed with mRCC were eligible and those who agreed to participate signed a consent form and responded to the survey questionnaire. Ethics approval was obtained from the Review Boards at City of Hope and the Santa Marta Hospital, respectively.
Survey Structure
The survey questionnaire was composed of 18 questions divided into 2 parts: (1) sociodemographic characteristics (eg, age, gender, race, education level), and (2) HR-QOL measurement feedback and treatment related questions. In the second part, patients were asked to evaluate the various elements of the FKSI-19,14 including rating their current HR-QOL, rating each item in terms of its relevance to their overall HR-QOL on a 5-point Likert scale (ranging from 1 “not at all relevant to HR-QOL” to 5 “extremely relevant to HR-QOL”), as well as responding to a 2 open-ended questions regarding topics not covered by the FKSI-19:
“What questions should we be asking to assess your QOL?”
“If it was possible to only ask 3 questions, what do you feel are the most important questions for us to assess your quality of life?”
Statistical Analysis
Our primary objective was to identify questions in the FKSI-19 that were the least relevant to patients with mRCC receiving systemic therapy. The responses for each question were categorized using thresholds as (a) relevant (scores 2-5), or (b) nonrelevant (score 1). The proportion of patients each group was calculated. Notably, if a question was scored as nonrelevant by ≥70 of respondents, this suggested that the item is not meaningful to contemporary patients with mRCC and should not be preserved in future surveys. This threshold was decided from steering committee consensus.
Descriptive statistics were used to summarize the patient demographic and clinical characteristics, to assess severity (rating) and relevance for each of 19 issues, and to summarize all other questions on the survey. Notably, no significant difference was found between samples (US and Brazil). Thus, we have analyzed the data conjoined. The Spearman correlation statistic was used to test the association between items rated as relevant and type of treatment. Furthermore, a qualitative content analysis was completed for the 2 open-ended questions. Each response was reviewed by 2 independent reviewers (C.B. and P.B.) and categorized into one of 6 descriptive themes, including emotional symptoms, physical symptoms, ability to live well, effectiveness of treatment, family/spirituality, and financial toxicity. Discrepancies were discussed and adjudicated by consensus (Cohen’s kappa coefficient = 0.91).
Results
Baseline Characteristics
A total of 151 patients were recruited (119 from US and 32 from Brazil), with a median age of 64 years (range between 38 and 87). The majority of patients were male (78.1), White (64.9), married (79.5), and had at least a college degree (68.9) (Table 1). Patients were primarily receiving immunotherapy (38.4; 22/38 patients were on multiple immunotherapy agents), targeted therapy (29.8) or a combination of these agents (13.9) at the time of survey response. Overall, based on patients’ experience, side effects from treatment tended to fluctuate (65.9), rather than consistently improve, or worsen. Few patients (8.0) noted side effects that had worsened over time, while 26.1 noted that they had improved. The 8 most common side effects reported by patients were fatigue (52.3), lack of energy (51.7), worry (46.3), weight loss (36.4), trouble sleeping (34.3), diarrhea (33.1), and pain (31.8).
Table 1.
Patients’ characteristics (N = 151).
| Characteristics | N ()/M (min-max) |
|---|---|
| Gender [N ()] | |
| Male | 118 (78.1) |
| Female | 33 (21.9) |
| Age [M (min-max)] | 64 (38-87) |
| Marital status [N ()] | |
| Single | 15 (9.9) |
| Married | 120 (79.5) |
| Divorced | 11 (7.3) |
| Widowed | 5 (3.3) |
| Education [N ()] | |
| No formal education | 10 (6.6) |
| Elementary school | 6 (3.9) |
| High school | 31 (20.5) |
| College degree | 85 (56.3) |
| Beyond college | 19 (12.6) |
| Race [N ()] | |
| White | 98 (64.9) |
| Hispanic | 29 (19.2) |
| Mulato | 5 (3.3) |
| Southeast Asian | 5 (3.3) |
| Chinese | 4 (2.6) |
| Black | 3 (2.0) |
| Japanese | 3 (2.0) |
| East Asian | 3 (2.0) |
| Native Hawaiian | 1 (0.7) |
| Employment status [N ()] | |
| Retired | 81 (53.6) |
| More than 32 hours | 41 (27.2) |
| Disability | 13 (8.6) |
| Less than 32 hours | 9 (6.0) |
| Employed (on medical leave) | 3 (2.0) |
| Other | 4 (2.6) |
| Treatment [N ()] | |
| Immunotherapy | 58 (38.4) |
| Targeted therapy | 45 (29.8) |
| Immuno-TKI combination | 21 (13.9) |
| No treatment | 27 (17.9) |
Patient-Assessed Relevance of FKSI-19 Items
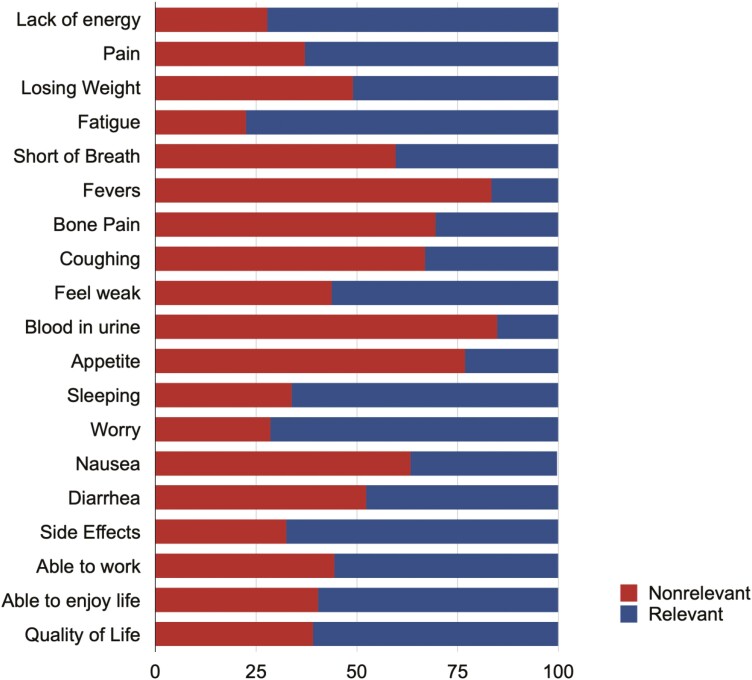
When assessing the relevance of FKSI-19 items to patients’ overall HR-QOL, few items were meaningful (items scored by >70 of respondent as 2-5) to patients, as illustrated in Fig. 1, including fatigue (77.5), lack of energy (72.2), and worry that their condition will get worse (71.5). A total of 8/19 questions were deemed non-relevant at lower levels of scrutiny (defined as >50 of patients considered this relevant), including blood in urine (15.2), fevers (16.6), appetite (23.2), bone pain (30.5), coughing (33.1), nausea (36.4), short of breath (40.4), diarrhea (47.7). Notably, no question was reported as very relevant (score 4 to 5) for > 50 of patients. Significant associations between items rated as relevant (score 2-5) to patients’ HR-QOL and the type of treatment patients were receiving at the time of the survey were identified. Patients receiving immuno-TKI combination considered the fatigue (P = .006), losing weight (P = .014), and nausea (P = .003) the most relevant to their HR-QOL, while those receiving targeted therapy considered diarrhea (P = .001). No association was found between items rated as relevant to patients’ HR-QOL and immunotherapy.
Figure 1.
Items of the FKSI-19 ranked as relevant (scores 2-5) or nonrelevant (score 1) for patients’ HR-QOL.
Furthermore, when asking patients about the length of the survey, we identified that 43.1 of patients considered that the optimal number of questions to assess HR-QOL was 15 to 25 questions, while one-third (29.1) of patients suggested 30 or more questions would be optimal. In addition, the maximum tolerable number of questions for 57.6 of patients would be between 15 and 25 items. Furthermore, 59.6 of patients suggested reassessment was warranted every 3 months, while 32.5 of patients suggested every month.
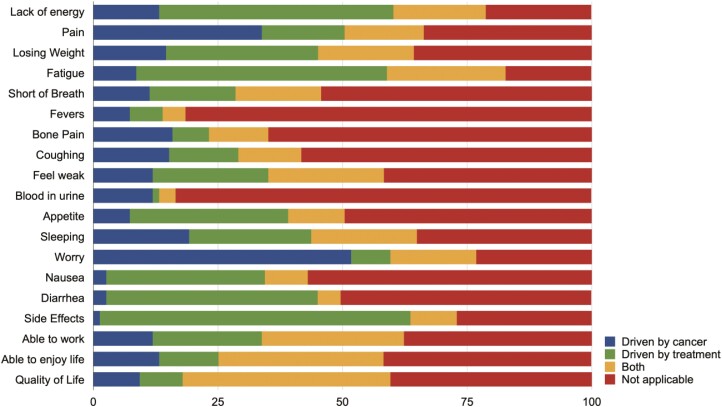
Patient Attribution of FKSI-19 Items to Treatment Vs Disease
When assessing which FKSI-19 items were associated with disease or treatment, the most common items in the FKSI-19 that were primarily associated with disease were fear of progression (51.7) and pain (33.8). In contrast, items primarily associated with treatment were fatigue (50.3), lack of energy (47.0), diarrhea (42.4), appetite (31.8), and nausea (31.8). Patients’ quality of life (41.7), ability to enjoy life (33.1), and ability to work (28.5) were associated with both cancer and treatment (Fig 2). However, most patients were not able to ascribe a great proportion of questions to either cancer or treatment.
Figure 2.
Symptoms suggested by patients to be driven by cancer, treatment, both or not applicable.
Assessment of Open-Ended Feedback on FKSI-19
The majority of patients (60.3) provided feedback on items that should be included in the assessment HR-QOL (Table 2). Emotional symptoms (37.4), the ability to live well (15.6), and additional physical symptoms (13.9) were the most frequently endorsed categories of items suggested for inclusion. In addition, 7.9 of patients suggested that some form of an open-ended question should be included in validated measures. When asked about the 3 most important questions to assess HR-QOL, 31.8 of respondents thought that the FKSI-19 included their most important concerns. Among the remaining patients, emotional (34.4), physical symptoms (19.5), and the ability to live well (11.3) were the most frequently endorsed as absent from the assessment measure.
Table 2.
Additional topics suggested by patients to be covered by a Health-Related Quality of Live measure.
| Theme | Quotation | Frequency |
|---|---|---|
| Emotional symptoms | “Are you happy?” “Do you have depression, do you feel hopeless, are you able to enjoy life?” “Fear of cancer progression, treatment option.” “How many times a day thoughts of cancer arise? How intrusive those thoughts might be? How are you managing/coping with that?” |
37.4 |
| Ability to live well | “Ability to live a normal life. Comparison of before and after diagnosis/treatment.” “How cancer/treatment has affected ability to do hobbies, meet with family/friends.” “Can you do what you need to do? Can you do what you want to do?” “Ability to work and to live a normal life.” |
15.6 |
| Physical symptoms | “About eating—not just weight loss—about whether or not you are eating.” “Addressing intimacy.” “Itching and dermatological effects.” “Issues from surgery” “Hand-foot syndrome issues” |
13.9 |
| Effectiveness of treatment | “How effective treatment is helping with symptoms? Ask about how patient feels about dose reduction/increases.” “How confident am I to receive this treatment?” |
9.6 |
| Family/spirituality | “Family relationship and effect on family members.” “How does it affect your family life?” “Touch on issues related to spirituality as related to end of life issues.” |
9.4 |
| Financial distress | “Financial considerations and the impact on quality of life.” “Insurance company responsive and treating patients fairly.” |
6.2 |
Discussion
The current study suggests that some items (8/19) used to measuring of HR-QOL in mRCC may not be relevant in the context of current treatment paradigms. Fatigue, the lack of energy and worry that patients’ condition will get worse were rated as meaningful for >70 of respondent in the context of their HR-QOL. In addition, patients have suggested items that are not fully addressed by the FKSI-19. Collectively, our findings highlight items that could potentially be excluded from current measures, including blood in urine, fevers, and lack of appetite, with those deemed most relevant providing more accurate insight into their HR-QOL.
This work also highlights the challenges of developing HR-QOL measures for patients with the same disease but undergoing different treatments with varying side effect profiles. We identified different priorities amongst patients receiving targeted therapy versus immuno-TKI combination. However, no differences were noted among patients receiving immunotherapy. Given the need for patients to frequently manage disease and treatment related symptoms, it is important to identify which adverse events are most bothersome to patients and should be routinely assessed as part of their care. This may require the inclusion of open-ended questions in future measures, including asking patients how they are coping with treatment and which adverse events are most bothersome to them. Additional themes suggested by patients were primarily related to their ability to cope emotionally with their disease, treatment, and prognosis. These last components appear to be underrepresented in the FKSI-19.
We asked patients whether they felt items on the FKSI-19 scale were more relevant to either cancer therapy or cancer itself. As one might expect, symptoms considered most associated to treatment (eg, fatigue, diarrhea, lack of energy, appetite) tended to correspond to adverse events reported in corresponding clinical trials.1-4,10 “Worry that their condition will get worse”, pain, and sleep were primarily associated with their cancer. Finally, the ability to work and to enjoy life were the most common factors suggested by patients to be associated with both cancer and its treatment.
Our open-ended questions highlighted themes that should be explored further, including a desire amongst patients to address “fear of cancer recurrence”. Advances in treatment have brought new hope for patients with mRCC; however, gaining an accurate expectation of cure or prognosis is still very relevant,1,15 which underlies the desire of patients to be able to respond to open-ended questions regarding their own clinical situation (eg, “Is my treatment plan changing or has there been a change in prognosis”). It is notable that past research has found that patients with an inaccurate expectation of cure tend to report higher levels of anxiety.15
There are several limitations of this work. First, we focused our survey on one validated questionnaire, the FKSI-19. Future studies should include other measures that are used in clinical trials, including the EORTC-QLQ C30 and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 5 Dimensions (EQ-5D). Second, we did not include patients receiving adjuvant therapy, which may have affected the perceived relevance of some of the items from the FKSI-19. Third, no comparisons between localized and metastatic disease or number of previous therapies were made. This will be the focus of future funded projects. Further, we did not collected data regarding histology, sites of metastases and line of treatment at the time of study enrollment. Patients’ perceptions may differ based on these characteristics. The relatively small sample size of our cohort, lack of diversity among respondents, and the small number of sites (total of 4 sites; 1 site located in the US and 3 sites in Brazil) may limit the generalizability of our findings. Notably, the main goal of this study was not to validate a new questionnaire but to determine patients’ perceptions regarding a current measure used in phase III trials. Thus, no psychometric tests were conducted. Finally, the current study did not address how perceptions of items might change over time, and thus there is a need to examine the perceived relevance of items at different treatment time points.
In summary, the current study suggests that there is a need to refine current, widely used, HR-QOL measures to better assess patient experience and the impact of targeted and immunotherapy-based agents. Patients deemed relatively few items to be relevant to their HR-QOL and provided several suggestions for refinement. These findings will be validated and extended in an upcoming study of patients with RCC who are receiving treatment in both the metastatic and adjuvant setting.
Contributor Information
Cristiane Decat Bergerot, Centro de Câncer de Brasília, Instituto Unity de Ensino e Pesquisa, Brasília, DF, Brazil.
Jasnoor Malhotra, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Paulo Bergerot, Centro de Câncer de Brasília, Instituto Unity de Ensino e Pesquisa, Brasília, DF, Brazil.
Errol J Philip, School of Medicine, University of California San Francisco, San Francisco, CA, USA.
Daniela V Castro, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
JoAnn Hsu, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Augusto Cesar de Andrade Mota, Medical Oncology, Clinica AMO, Salvador, BA, Brazil.
Andressa Cardoso de Azeredo, Medical Oncology, Instituto de Oncologia Kaplan, Porto Alegre, RS, Brazil.
João Nunes de Matos Neto, Centro de Câncer de Brasília, Instituto Unity de Ensino e Pesquisa, Brasília, DF, Brazil.
Thomas Hutson, Urologic Oncology Program, Texas Oncology at Baylor Sammons Cancer Center, Dallas, TX, USA.
Viktor Grünwald, Clinic for Medical Oncology, Clinic for Urology, University Hospital Essen, Essen, Alemanha, Germany.
Axel Bex, UCL Division of Surgical and Interventional Science, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Sarah P Psutka, Urology Clinic, University of Washington, Seattle, WA, USA.
Brian Rini, Department of Medicine, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
Elizabeth R Plimack, Department of Hematology/Oncology and Chief, Fox Chase Cancer Center, Philadelphia, PA, USA.
Viraj Master, Department of Urology, Emory University Hospital, Atlanta, GA, USA.
Laurence Albiges, Department of Cancer Medicine, Gustave Roussy Institute, Paris, France.
Toni K Choueiri, Lank Center for Genitourinary (GU) Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Sumanta Pal, Department of Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Thomas Powles, Barts Cancer Centre, Barts Cancer Centre at St. Bartholomew’s Hospital, London, UK.
Funding
This study was funded by the Kidney Cancer Association’s 2021 Psychosocial Focus Award (PI: C.D. Bergerot).
Conflict of Interest
Augusto Cesar de Andrade Mota reported consulting/advisory relationships with AstraZeneca, Janssen, Pfizer, and Astellas; research funding from Janssen, AstraZeneca, MSD, Merck, and Nektar Therapeutics; honoraria from Janssen and Pfizer; and Scientific Advisory Board member for Janssen, Pfizer, Bayer, and Astellas. Thomas Hutson reported research support, honorarium, advisory board, and speaker fees for Pfizer, Exelexis, Eisai, Aveo, Merck, and BMS. Viktor Grünwald reported stock and other ownership interests in AstraZeneca, Bristol-Myers Squibb, and MSD; honoraria from Asklepios Kliniken, AstraZeneca, Bayer, Bristol-Myers Squibb, Clinic of Oldenburg, Diakonie Clinic, Dortmund Hospital, Eisai, EUSA Pharma, Ipsen, Janssen-Cilag, Lilly, Merck Serono, MSD Oncology, Novartis, Pfizer, PharmaMar, and Roche; consulting or advisory role for Bristol-Myers Squibb, Cor2Ed, Ipsen, Janssen-Cilag, Lilly, MSD Oncology, Novartis, Onkowissen, and Pfizer; research funding from Novartis (institutional); and travel, accommodations, expenses from AstraZeneca, Bayer, Bristol-Myers Squibb, Ipsen, and Pfizer. Axel Bex reported speakers fees from ESAI, steering committee membership of adjuvant trials of Roche and BMS, and a restricted educational grant from Pfizer for a neoadjuvant trial made to institution. Sarah P. Psutka reported advisory board member for Merck (2021), honoraria from AstraZeneca and Medtronic, research funding from Prime Education Inc. and the Bladder Cancer Advocacy Network; and speaker fees from the American Urological Association Guidelines Panel, American Urological Association. Brian Rini reported research funding to institution from Pfizer, Hoffman-LaRoche, Incyte, AstraZeneca, Seattle Genetics, Arrowhead Pharmaceuticals, Immunomedics, BMS, Mirati Therapeutics, Merck, Surface Oncology, Aravive, Exelixis, Jannsen, and Pionyr; consulting relationships with BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Merck, Corvus, Surface Oncology, Aravive, Alkermes, Arrowhead, Shionogi, Eisai, Nikang Therapeutics, EUSA, Athenex, Allogene Therapeutics, and Debiopharm; and stock in PTC Therapeutics. Elizabeth R. Plimack reported scientific advisory board member for Pharma, Astellas, AstraZeneca, BMS, EMD Serrono, Exelexis, IMV, Merck, Natera, Pfizer, and Seattle Genetics; and grants for clinical research from Astellas, BMS, Genentech, and Merck. Viraj Master serves on the advisory boards for Merck, Pfizer, and Exelixis. Laurence Albiges has received consulting/advisory fees from BMS, Pfizer, Novartis, Sanofi, Amgen, Bristol-Myers Squibb, Bayer, and Cerulean, and research funding from Pfizer and Novartis. Toni K. Choueiri reports institutional and personal, paid and unpaid, support for research, advisory boards, consultancy, and honoraria from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, Merck, Nikang, Nuscan, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, CME events (Peerview, OncLive, MJH and others), outside the submitted work; institutional patents filed on molecular mutations and immunotherapy response/toxicity, and ctDNA; equity in Tempest, Pionyr, Osel, and Precede Bio; service on committees for NCCN, GU Steering Committee, ASCO/ESMO, ACCRU, KidneyCan; medical writing and editorial assistance support may have been funded by communications companies in part; Mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components; the institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter. Sumanta Pal reported research funding from Eisai, Genentech, Roche, Exelixis, Pfizer, CRISPR Therapeutics, and Allogene Therapeutics, and travel reimbursement from Roche and CRISPR Therapeutics. Thomas Powles reported consultancy/honorarium from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono (EMD in US), Astellas, Johnson & Johnson, Eisai, Roche, and Mashup Ltd.; grant/funding to institution from AstraZeneca, Roche, BMS, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono (EMD in US), Astellas, Johnson & Johnson, and Eisai; and travel/accommodation expenses from Roche, Pfizer, MSD, AstraZeneca, and Ipsen. The other authors indicated no financial relationships.
Author Contributions
Conception/design: C.D.B., S.K.P. Provision of study material or patients: C.D.B., P.B., A.C.d.A.M., A.C.d.A., J.N.d.M.N., T.H., V.G., A.B., S.P.P., B.R., E.R.P., V.M., L.A., T.K.C., S.P., T.P. Collection and/or assembly of data: C.D.B., J.M., P.B., D.C., J.A.H., S.P., T.P. Data analysis and interpretation: all authors. Drafting manuscript review of the literature: C.D.B., E.J.P., P.G.B., S.K.P., T.P. Critical revision of the manuscript: all authors. Final approval of manuscript: All authors.
Data Availability
The data that support the findings of the paper are available from the corresponding author upon reasonable request.
References
- 1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 3. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 5. Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin. Cancer Res. 2016;22(7):1553-1558. [DOI] [PubMed] [Google Scholar]
- 6. Cella D, Grunwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate214): a randomized, phase 3 trial. Lancet Oncol. 2019;20(2):297-310. 10.1016/S1470-2045(18)30778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bedke J, Rini B, Plimack E, et al. Health-related quality of life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). 35th Annual EAU Congress 2022;82(4):427-439. https://resource-centre.uroweb.org/resource-centre/EAU20V/212868/Abstract. [DOI] [PubMed] [Google Scholar]
- 8. Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomized, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. 10.1016/S1470-2045(21)00693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Motzer RJ, Porta C, Alekseev B, et al. Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of Lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2021;39(15_suppl):4502-4502. 10.1200/jco.2021.39.15_suppl.4502. [DOI] [Google Scholar]
- 10. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergerot CD, Bergerot PG, Philip EJ, Pal SK.. Patient-reported outcome measures in metastatic urinary cancers. Eur Urol Focus. 2020;6(1):26-30. 10.1016/j.euf.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 12. Cella D, Yount S, Du H, et al. Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). J Support Oncol. 2006;4(4):191-199. [PubMed] [Google Scholar]
- 13. Rosenblad AK, Sundqvist P, Westman B, Ljungberg B.. A psychometric evaluation of the Functional assessment of cancer therapy-kidney symptom index (FKSI-19) among renal cell carcinoma patients suggesting an alternative two-factor structure. Qual Life Res. 2021;30(9):2663-2670. 10.1007/s11136-021-02839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanesvaran R, Le Saux O, Motzer R, et al. Elderly patients with metastatic renal cell carcinoma: position paper from the International Society of Geriatric Oncology. Lancet Oncol. 2018;19(6):e317-e326. 10.1016/S1470-2045(18)30125-6. [DOI] [PubMed] [Google Scholar]
- 15. Bergerot CD, Bergerot PG, Philip EJ, et al. Perception of cure among patients with metastatic genitourinary cancer initiating immunotherapy. J ImmunoTher Cancer. 2019;7(1):71. 10.1186/s40425-019-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the paper are available from the corresponding author upon reasonable request.