Abstract
Introduction
There is scarce data regarding the incidence of venous thromboembolism (VTE) and arterial thromboembolism (ATE) in the molecular subtypes of non-small cell lung cancer (NSCLC). We aimed to investigate the association between Anaplastic Lymphoma Kinase (ALK)-positive NSCLC and thromboembolic events.
Methods
A retrospective population-based cohort study of the Clalit Health Services database, included patients with NSCLC diagnosed between 2012 and 2019. Patients exposed to ALK-tyrosine-kinase inhibitors (TKIs) were defined as ALK-positive. The outcome was VTE (at any site) or ATE (stroke or myocardial infarction) 6 months prior to the diagnosis of cancer, until 5 years post-diagnosis. The cumulative incidence of VTE and ATE and hazard-ratios (HR) with 95% CIs were calculated (at 6- 12- 24 and 60-months), using death as a competing risk. Cox proportional hazards multivariate regression was performed, with the Fine and Gray correction for competing risks.
Results
The study included 4762 patients, of which 155 (3.2%) were ALK-positive. The overall 5-year VTE incidence was 15.7% (95% CI, 14.7-16.6%). ALK-positive patients had a higher VTE risk compared to ALK-negative patients (HR 1.87 [95% CI, 1.31-2.68]) and a 12-month VTE incidence of 17.7% (13.9-22.7%) compared to 9.9% (9.1-10.9%) in ALK-negative patients. The overall 5-year ATE incidence was 7.6% [6.8-8.6%]. ALK positivity was not associated with ATE incidence (HR 1.24 [0.62-2.47]).
Conclusions
In this study, we observed a higher VTE risk, but not ATE risk, in patients with ALK-rearranged NSCLC relative to those without ALK rearrangement. Prospective studies are warranted to evaluate thromboprophylaxis in ALK-positive NSCLC.
Keywords: anaplastic lymphoma kinase (ALK), NSCLC, thrombosis, venous thromboembolism, arterial thromboembolism
There is scarce data regarding the incidence of venous thromboembolism and arterial thromboembolism in the molecular subtypes of non-small cell lung cancer (NSCLC). This study investigated the association between ALK-positive NSCLC and thromboembolic events.
Implications for Practice.
There is scarce data regarding the incidence of venous and arterial thromboembolic events (VTE and ATE, respectively) in the genetic subtypes of non-small cell lung cancer (NSCLC). This retrospective, population-based cohort study demonstrated that ALK-positive patients had 87% higher risk of developing VTE and that the ATE rates were comparable between ALK-positive and ALK-negative patients. In ALK-negative patients, both VTE and ATE were associated with increased 5-year all-cause mortality, but ALK-positive patients did not demonstrate a such association. These results suggest that the genetic profile of a tumor should be incorporated into clinical scores that aid in thromboprophylaxis decision-making.
Introduction
Solid malignancies are associated with an increased risk for venous thromboembolism (VTE)1-3 and arterial thromboembolism (ATE).4-6 While clinical scoring systems such as the Khorana score have been developed to identify patients with cancer at increased risk for VTE and can guide thromboprophylaxis, their performance in specific malignancies, especially lung cancer, is suboptimal.7-10 Given the implications and poor outcomes of patients with cancer-associated VTE and ATE,11-13 more accurate prediction tools are needed for patients with lung cancer, to select patients who may be candidates for thromboprophylaxis. Tumor-specific markers, such as somatic genomic alterations, may help improve VTE risk stratification, as exemplified in a recent study.14
In non-small cell lung cancer (NSCLC), advances in molecular testing in the last decade have introduced specific genomic subtypes with variable clinical behavior. Anaplastic Lymphoma Kinase (ALK) rearranged NSCLC is one such subtype identified in 5% of all patients with pulmonary adenocarcinoma, and usually affects younger, non-smoking patients.15 Recent retrospective studies, including from our group, have shown a 3- to 5-fold higher VTE rate in ALK-positive NSCLC.16-18 This is also the case in ROS1 rearranged NSCLC representing 1%-2% of the NSCLC population.19 Data on ATE incidence in specific molecular subtypes are scarce. Limitations of these studies include small sample size, selection bias of patients undergoing genomic sequencing, and mostly single-centre settings.
We aimed to address these knowledge gaps and investigate the association between ALK-positive NSCLC and VTE/ATE in a large nationwide cohort of patients with advanced lung cancer treated in tertiary and community-based centers.
Methods
Design and Patient Sample
A retrospective population-based cohort study was performed using the Clalit Health Services (CHS) database. CHS is both a healthcare payer and provider, covering over half of the Israeli population, with individual patient data recorded in a centralized electronic database. Accordingly, the CHS database has a sample that is fairly representative of the Israeli population.20 The study included patients diagnosed with advanced lung cancer between January 2012 and June 2019, defined as both of the following: International Classification of Diseases-9th revision (ICD-9) codes compatible with a diagnosis of pulmonary malignancy (162.X) and treatment with systemic anti-cancer therapy. No exclusion criteria were defined.
Patients were indexed on the day of lung cancer diagnosis. Records were followed from 6 months prior to index date, and until 5 years post index, or until death, whichever occurred first. The follow-up for outcomes began 6 months prior to the index date since outcomes occurring in this time-period can be considered to be cancer-associated.21
Variables and Measurements
Patient data documented at study index included the following: age, sex, smoking status (current/non-smoker), body mass index (BMI), platelet count, white blood cell count, hemoglobin level, Charlson’s comorbidity index,22ALK—inhibitor treatment. The Khorana score at the index was calculated according to data availability.7
Groups and Exposure
The study exposure was the presence of ALK rearrangement. ALK-positive NSCLC was defined as ≥2 ALK-inhibitor prescriptions (crizotinib or alectinib) at any time during follow-up since these medications require pre-authorization in Israel, and were the only ALK-inhibitors reimbursed and utilized in Israel as first-line therapy during the study period. NSCLC patients with 0-1 ALK-inhibitor prescriptions were classified as ALK-negative.
Outcomes
The outcomes of interest included a first diagnosis of VTE or ATE during the follow-up period. ATE was defined as primary ICD-9 diagnosis of myocardial infarction (MI) or stroke. VTE was defined using ICD-9 codes for deep vein thrombosis (DVT) at any site or pulmonary embolism (PE) (ICD-9 codes are detailed in Supplementary Table S1).
Statistical Analyses
Patient characteristics are presented as median (inter-quartile range [IQR]) for continuous variables and as frequency for categorical variables. Baseline characteristics were compared between patients with or without outcome events during follow-up, using t-test for continuous normal variables, Wilcoxon for skewed variables, and Fisher`s exact test for categorical variables. All analyses were performed separately for VTE and ATE.
The cumulative incidence of outcomes throughout follow-up, with corresponding 95% CIs, was calculated for the whole cohort and also separately for ALK-positive and ALK-negative subgroups. Death (not due to VTE or ATE) was considered as a competing risk using the Fine and Gray estimator.
A Cox proportional hazards model was used to calculate the hazard ratios (HR) and their corresponding 95% CI for outcome events (VTE or ATE) between ALK-positive and ALK-negative groups, with death as a competing risk (Fine and Gray method). Two separate multivariate cox proportional hazards regression models (one for VTE and the other for ATE) were used to calculate associations between the following baseline variables and VTE/ATE: ALK status (positive/negative), age (continuous), sex (male/female), smoker (past or current/none), Charlson comorbidity index (continuous), Khorana score (continuous). Death was considered a competing risk in this analysis.
Statistical analysis was performed using the SAS Vs 9.4 software (SAS Institute, North Carolina, USA).
Results
Patient Characteristics
A total of 4762 patients met the inclusion criteria for this study. Patient characteristics are detailed in Table 1. The majority of patients (63%) were male, 72% of patients had documented history of past/current smoking. Seventeen patients (0.35%) had only one ALK tyrosine kinase inhibitor (TKIs) prescription and were not defined as ALK-positive, while 155 (3.2%) had ≥2 ALK-inhibitor prescriptions and were defined as ALK-positive. The median follow-up time was 15.2 months (IQR 7-29.61). During the follow-up period, 45.16% of patients in the ALK-positive group, and 70.26% of patients in the ALK-negative group died. The VTE and ATE incidence in all patients is detailed in the Supplemental Results and Supplementary Fig. S1.
Table 1.
Characteristics of the participants at baseline, stratified for VTE/ATE status during follow-up*.
| Variable (at NSCLC diagnosis) | All, n (%) | VTE*, n (%) | No VTE*, n (%) | ATE*, n (%) | No ATE*, n (%) | |
|---|---|---|---|---|---|---|
| All patients, n (% of all) | 4762 (100) | 673 (14) | 4089 (86) | 311 | 4451 | |
| Age, median (range) | 67 (21-98) | 65 (21-94) | 67 (26-98) | 68 (39-93) | 67 (21-98) | |
| Sex | Male | 3017 (63) | 387 (57) | 2630 (65) | 204 (66) | 2813 (63) |
| Female | 1731 (36) | 282 (43) | 1449 (35) | 106 (34) | 1625 (37) | |
| ALK rearranged‡ | 155 (3.2) | 45 (6) | 110 (3) | 12 (4) | 143 (3) | |
| BMI | <35 | 4328 (91) | 617 (92) | 3711 (91) | 276 (89) | 4052 (91) |
| ≥35 | 257 (5) | 28 (4) | 229 (5) | 19 (6) | 238 (5) | |
| Missing | 177 (4) | 28 (4) | 149 (4) | 16 (5) | 161 (4) | |
| Smoker§ | 3447 (72) | 440 (65) | 3007 (74) | 217 (70) | 3230 (73) | |
| Charlson comorbidity index, mean (range) [n = 4542] | 2.50 (0-20) | 2.29 (0-12) | 2.53 (0-20) | 2.51 (0-20) | 2.50 (0-18) | |
| Khorana score, mean (range) [n = 3513] | 1.5 (1-5) | 1.4 (1-4) | 1.5 (1-5) | |||
*VTE/ATE at any point from 6 months prior study index to 5 years post-index. Index date defined as date of NSCLC diagnosis.
‡Defined as ≥2 ALK-inhibitor prescriptions (crizotinib or alectinib).
§Past or current smoking.
Abbreviations: ALK, anaplastic lymphoma kinase; ATE, arterial thromboembolism; BMI, body mass index; NSCLC, non-small cell lung cancer; VTE, venous thromboembolism
VTE According to ALK Status
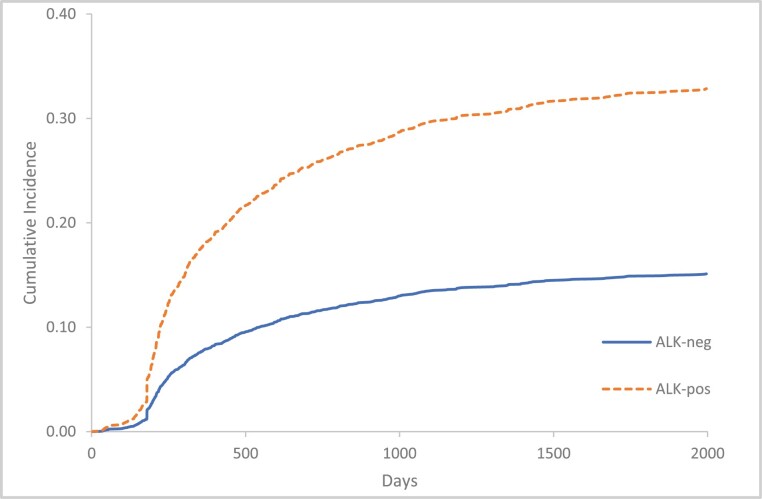
During the follow-up period, 45/155 (29%) patients with ALK-positive NSCLC experienced a VTE compared to 628/4607 (13%) in ALK-negative patients. The cumulative incidence of VTE among patients with ALK-positive NSCLC (95% CI) at index (representing the prior 6 months) and 6-, 12-, 24-, and 60-months post-index were 5% (3.7%-6.6%), 17.7% (13.9%-22.7%), 22.6% (17.7%-29.7%), 27.5% (21.2%-35.7%), and 32.8% (26.3%-41.4%), respectively (Fig. 1).
Figure 1.
Cumulative incidence of VTE, according to ALK rearrangement status. Outcome events counted from 6 months prior to study index to 5 years post-index. Index date defined as date of NSCLC diagnosis. Death considered as a competing risk. ALK, anaplastic lymphoma kinase; VTE, venous thromboembolism.
The cumulative incidence of VTE among patients with ALK-negative NSCLC (95% CI) at index and 6-, 12-, 24-, and 60 months post-index were 2.1% (1.7%-2.5%), 7.7% (7.1%-8.4%), 9.9% (9.1%-10.9%), 12.4% (11.5%-13.3%), and 15.1% (14.2%-16.1%) (Fig. 1).
The multivariate cox proportional hazards regression model (Table 2) demonstrated a higher risk for VTE in ALK-positive NSCLC as compared with ALK-negative NSCLC (HR 1.87 [95% CI, 1.31-2.68]). Past/current smoking was associated with a decreased risk of VTE (HR 0.69 [95% CI, 0.56-0.85]).
Table 2.
Association of confounding variables with VTE or ATE*†.
| Variable (at NSCLC diagnosis) |
VTE | ATE |
|---|---|---|
| HR (95% CI) † | HR (95% CI) † | |
| ALK positive§ | 1.87 (1.30-2.68) | 1.24 (0.62-2.47) |
| Age (increasing)¶ | 0.98 (0.97-0.99) | 1.01 (0.99-1.02) |
| Sex (male) | 0.86 (0.71-1.04) | 1.14 (0.86-1.53) |
| Smoker‡ | 0.69 (0.56-0.85) | 0.88 (0.65-1.20) |
| Charlson comorbidity index | 0.99 (0.95-1.03) | 0.99 (0.93-1.05) |
| Khorana score | 0.96 (0.83-1.10) | 0.94 (0.79-1.13) |
*At any point from 6 months prior study index to 5 years post-index. Index date defined as date of NSCLC diagnosis.
†Multivariate cox regression analysis with death as a competing risk.
§Defined as ≥2 ALK-inhibitor prescriptions (crizotinib or alectinib).
¶Hazard ratio for every 1-year increase.
‡Past or current smoking.
Abbreviations: ALK, anaplastic lymphoma kinase; ATE, arterial thromboembolism; CI, confidence interval; HR, hazard ratio; NSCLC, non-small cell lung cancer; VTE, venous thromboembolism.
ATE According to ALK status
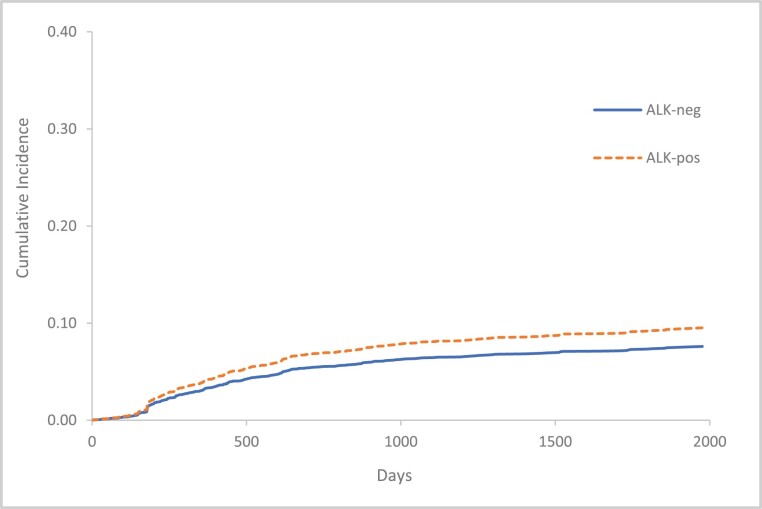
Patients with ALK-positive NSCLC had a numerically higher rate of ATE (12/155, 7.7%) compared to ALK-negative cases (299/4607, 6.5%); however, ALK-positive NSCLC was not significantly associated with a higher risk for ATE (HR 1.24 [0.62-2.47]) (Table 2).
The cumulative incidence of ATE among ALK-positive patients (95% CI) at index (representing the prior 6 months) and 6-, 12-, 24-, and 60-months post-index were 1.8% (0.9%-3.3%), 3.93% (2.2%-6.8%), 5.62% (3.1%-10.2%), 7.5% (4.7%-12.7%), 9.52% (5.3%-17.2%), respectively (Fig. 2).
Figure 2.
Cumulative incidence of ATE, according to ALK rearrangement status. Outcome events counted from 6 months prior study index to 5 years post-index. Index date defined as date of NSCLC diagnosis. Death considered as a competing risk. ALK, anaplastic lymphoma kinase; ATE, arterial thromboembolism.
The cumulative incidence of ATE among ALK-negative patients (95% CI) at index and 6-, 12-, 24-, and 60-months post-index were 1.4% (1.2%-1.7%), 3.1% (2.6%-3.7%), 4.5% (3.9%-5.1%), 6% (5.3%-6.7%), 7.6% (6.8%-8.5%) as shown in Fig. 2.
Discussion
This large population-based cohort study demonstrated a higher risk for VTE in ALK-positive NSCLC as compared with ALK-negative NSCLC. However, ALK-positive NSCLC was not significantly associated with a higher risk for ATE.
VTE in ALK-Positive NSCLC
In our study, ALK-positive patients had a 6-month VTE incidence of 17.7%, which is similar to prior studies which demonstrated 6-month incidence of 15.7%-22.8%.18,23 In contrast to prior studies, the current study includes unselected NSCLC patients treated nationwide at both community-based and tertiary medical centers, improving the generalizability of these findings. The high VTE incidence is comparable to VTE rates seen in patients with high VTE risk malignancies, such as pancreatic and gastric cancer,24,25 who are candidates for thromboprophylaxis with direct oral anticoagulants or low molecular weight heparin.26,27 In comparison, the VTE rate in unselected NSCLC patients ranges from 3% to 15% in historical studies.4,7 Of note, in our study, the Khorana score was not associated with VTE incidence similar to some prior studies of NCSLC patients.8-10 Increasing age and past/current smoking were both associated with decreased VTE incidence at 5 years, which may be reconciled by the association between these factors and absence of ALK-rearrangement, even after multivariable analysis.
The significant association between the presence of ALK alteration and VTE has been reported in several recent studies. In a systemic review and meta-analysis which included 8 trials that slightly vary in their methodology, Zhu et al.28 examined thromboembolic events among ALK/ROS1-positive patients and reported an odds ratio of 2.1 for VTE in patients with ALK-positive NSCLC, in line with our study findings. The high VTE incidence among ALK-positive patients with lung cancer may be hypothetically explained by the histopathological features of these cancer cells, which have a mucinous cribriform pattern.29,30 Mucin production has been associated with thromboembolism in a murine model,31 though evidence of this in patients with lung cancer is lacking.
Taken together, these findings confirm ALK-rearrangement as a strong risk factor for VTE in NSCLC and suggest the need for studies investigating thromboprophylaxis strategies in patients with ALK-positive NSCLC.
ATE in ALK-Positive NSCLC
Our study also examined ATE incidence, demonstrating that ALK-positive NSCLC was not significantly associated with a higher risk for ATE. Data regarding ATE incidence and risk factors among patients with lung cancer, especially in specific molecular subtypes, are scarce.
The association between ALK-rearrangement and ATE incidence was previously explored in a single-centre retrospective cohort that demonstrated a non-significant difference of 5% vs. 4.4% (P = .71) in the crude ATE rates, though a multivariate regression accounting for cardiovascular risk factors revealed a significantly higher ATE risk attributed to ALK genomic alterations (HR 3.15 [95% CI, 1.18-8.37]).23 In our study, the crude ATE rate among ALK-positive patients was 7.7%, comparable to those in Al-Samkari’s study. Multivariate analysis in the current study did not identify factors associated with ATE, but while age, sex, CCI, and smoking status were available, we did not have available data on specific cardiovascular risk factors such as hypertension and diabetes. These factors are more prevalent in ALK-negative lung cancer, as these patients are usually older than ALK-positive patients,15 possibly explaining why the multivariate analysis did not reveal an association between ALK rearrangement and ATE in our study.
Taken together, our study suggests that more research is warranted on ATE risk associated with ALK-positive NSCLC, while accounting for multiple ATE risk factors to further test the hypothesis that ALK-positive NSCLC might be associated with an increased ATE risk.
Strengths and Limitations
Strengths of the current study include a dataset from the largest healthcare provider in Israel, which includes all NSCLC patients treated between 2012 and 2019, across a variety of cancer centers nationwide, representing approximately 50% of Israeli population. Our study includes a long follow-up period starting 6 months prior to diagnosis and 5 years after.
Our study has several limitations, some of which are inherent to its retrospective design. First, the use of diagnostic and prescription codes (for VTEs/ATEs and anti-ALK tyrosine kinase inhibitors) to classify ALK status, rather than manual chart review, introduces possible misclassification bias. For instance, patients with only one anti-ALK TKI prescription were classified as ALK-negative, although a subset of such patients could potentially have ALK-positive NSCLC. This surrogate of ALK rearrangement may also lead to misclassification of ROS1 fusion (also associated with an increased VTE risk19) positive NSCLC as ALK-positive in a minority of patients (~15% of those classified as ALK-positive patients treated after January 2017, when this indication was approved32). Second, the registration quality of diagnosis and thromboembolic events may vary between different institutions and family physicians which may affect event rates and risk factor distribution. Third, the ALK-positive cohort was probably enriched with patients with adenocarcinoma, as this mutation is nearly exclusively found in patients with this histology.15,33 The ALK-negative cohort, on the other hand, probably has heterogeneous histologic distribution (where about half are adenocarcinoma and a third are squamous cell carcinoma). Some trials suggest adenocarcinoma in itself is a risk factor for VTE,34 while others did not demonstrate such an association.35,36 Therefore, this difference in histology may have led to overestimation of the VTE risk associated with ALK mutations in lung cancer.
Fourth, the VTE and ATE codes may have been misclassified. These codes have been used in prior studies of patients with and without cancer37-40 but have not been formally validated. Another possible limitation is that patients with ALK-positive NSCLC have a better prognosis and, supposedly, “more time” to develop thromboembolic events. Nevertheless, this potential confounder was addressed by adjusting for death as a competing risk in analyses with VTE/ATE as the dependent variable, and by treating VTE and ATE as time-dependent variables in analyses with death as the dependent variable. Last, residual confounding is possible since we did not evaluate all potential risk factors for ATE and VTE.
Conclusion
ALK-positive NSCLC was associated with VTE but not ATE. VTE incidence in patients with ALK-rearrangement may be sufficiently high to consider thromboprophylaxis, but this needs to be assessed in future studies.
Supplementary Material
Contributor Information
Oded Icht, Institute of Oncology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Avi Leader, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Institute of Hematology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel.
Erez Batat, The Community Medical Services Division, Clalit Health Services, Tel Aviv, Israel.
Lilach Yosef, Institute of Oncology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel.
Tzippy Shochat, Statistical consulting unit, Rabin Medical Center, Petah Tikva, Israel.
Daniel A Goldstein, Institute of Oncology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; The Community Medical Services Division, Clalit Health Services, Tel Aviv, Israel.
Elizabeth Dudnik, Lung Cancer Service, Assuta Medical Centers, Tel Aviv, Israel; Ben-Gurion University of the Negev, Beer-Sheva, Israel.
Galia Spectre, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Institute of Hematology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel.
Pia Raanani, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Institute of Hematology, Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel.
Ariel Hammerman, The Community Medical Services Division, Clalit Health Services, Tel Aviv, Israel.
Alona Zer, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Oncology Division, Rambam Health Care Campus, Haifa, Israel.
Funding
None declared.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: O.I., A.L., A.Z. Provision of study material or patients: E.D., A.Z. Collection and/or assembly of data: E.D., D.A.G., A.H. Data analysis and interpretation: T.S., D.A.G., A.H. Manuscript writing: O.I., A.L., G.S., P.R., A.Z. Final approval of manuscript: All authors.
Data Availability
The data underlying this article were provided by Clalit Health Services under licence, by permission. Data will be shared on request to the corresponding author with permission of Clalit Health Services.
Previous Presentation
This study was previously presented at the 2019 ASCO Annual Meeting.
References
- 1. Chew HK, Wun T, Harvey D, Zhou H, White RH.. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464. 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 2. Connolly GC, Menapace L, Safadjou S, Francis CW, Khorana AA.. Prevalence and clinical significance of incidental and clinically suspected venous thromboembolism in lung cancer patients. Clin Lung Cancer. 2013;14:713-718. 10.1016/j.cllc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH.. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632-634. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377-2381. 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926-938. 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulder FI, Horváth-Puhó E, van Es N, et al. Arterial thromboembolism in cancer patients: a Danish population-based Cohort Study. JACC CardioOncol. 2021;3(2):205-218. 10.1016/j.jaccao.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW.. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Austin K, George J, Robinson EJ, Scully M, Thomas MR.. Retrospective cohort study of venous thromboembolism rates in ambulatory cancer patients: association with Khorana Score and other risk factors. J Hematol. 2019;8:17-25. 10.14740/jh471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dapkeviciute A, Daskeviciute A, Zablockis R, et al. Association between the Khorana score and pulmonary embolism risk in patients with advanced stage lung cancer. Clin Respir J. 2020;14:3-8. 10.1111/crj.13092. [DOI] [PubMed] [Google Scholar]
- 10. van Es N, Ventresca M, Di Nisio M, et al. The Khorana score for prediction of venous thromboembolism in cancer patients: an individual patient data meta-analysis. J Thromb Haemost. 2020;18:1940-1951. [DOI] [PubMed] [Google Scholar]
- 11. Kourelis TV, Wysokinska EM, Wang Y, et al. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer. 2014;86:358-362. 10.1016/j.lungcan.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuzovic M, Herrmann J, Iliescu C, et al. Arterial thrombosis in patients with cancer. Curr Treat Options Cardiovasc Med. 2018;20:40. 10.1007/s11936-018-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. 2016;140(Suppl 1):S12-S17. 10.1016/S0049-3848(16)30092-5. [DOI] [PubMed] [Google Scholar]
- 14. Dunbar A, Bolton KL, Devlin SM, et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood. 2021;137(15):2103-2113. 10.1182/blood.2020007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247-4253. 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zer A, Moskovitz M, Hwang DM, et al. ALK-rearranged non-small-cell lung cancer is associated with a high rate of venous thromboembolism. Clin Lung Cancer. 2017;18:156-161. 10.1016/j.cllc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 17. Xiong W, Du H, Ding W, et al. The association between pulmonary embolism and the cancer-related genomic alterations in patients with NSCLC. Respir Res. 2020;21:185. 10.1186/s12931-020-01437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roopkumar J, Poudel SK, Gervaso L, et al. Risk of thromboembolism in patients with ALK- and EGFR-mutant lung cancer: a cohort study. J Thromb Haemost. 2020;19(3):822-829. [DOI] [PubMed] [Google Scholar]
- 19. Ng TL, Smith DE, Mushtaq R, et al. ROS1 gene rearrangements are associated with an elevated risk of peridiagnosis thromboembolic events. J Thorac Oncol. 2019;14:596-605. [DOI] [PubMed] [Google Scholar]
- 20. Cohen R, Rabin H.. National Insurance Institute of Israel. Membership in sick funds 2016 (In Hebrew). 2017; https://www.btl.gov.il/Publications/survey/Documents/seker289/seker_289.pdf
- 21. Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2018;133(8):781-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23. Al-Samkari H, Leiva O, Dagogo-Jack I, et al. Impact of ALK rearrangement on venous and arterial thrombotic risk in NSCLC. J Thorac Oncol. 2020;15(9):1497-1506. 10.1016/j.jtho.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 24. Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol. 2015;33(18):2028-2034. 10.1200/JCO.2014.55.1481. [DOI] [PubMed] [Google Scholar]
- 25. Agnelli G, George DJ, Kakkar AK, et al. ; SAVE-ONCO Investigators. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601-609. 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 26. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496-520. 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 27. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17(10):1772-1778. 10.1111/jth.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu VW, Zhao JJ, Gao Y, et al. Thromboembolism in ALK+ and ROS1+ NSCLC patients: a systematic review and meta-analysis. Lung Cancer. 2021;157:147-155. 10.1016/j.lungcan.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 29. Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol. 2010;63(12):1066-1070. 10.1136/jcp.2010.081166. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35(8):1226-1234. [DOI] [PubMed] [Google Scholar]
- 31. Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118(15):4015-4023. 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863-870. 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190-1203. 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 34. Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6(4):601-608. 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 35. Kuderer NM, Poniewierski MS, Culakova E, et al. Predictors of venous thromboembolism and early mortality in lung cancer: results from a global prospective study (CANTARISK). Oncologist. 2018;23(2):247-255. 10.1634/theoncologist.2017-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen Q, Dong X, Tang X, Zhou J.. Risk factors and prognosis value of venous thromboembolism in patients with advanced non-small cell lung cancer: a case-control study. J Thorac Dis. 2017;9(12):5068-5074. 10.21037/jtd.2017.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423. 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078-1090. 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leader A, Dagan N, Barda N, et al. Previously undiagnosed cancer in patients with arterial thrombotic events—a population‐based cohort study. J Thromb Haemost. 2021. [DOI] [PubMed] [Google Scholar]
- 40. Saliba W, Rennert HS, Gronich N, Gruber SB, Rennert G.. Association of atrial fibrillation and cancer: analysis from two large population-based case-control studies. PLoS One. 2018;13(1):e0190324. 10.1371/journal.pone.0190324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Clalit Health Services under licence, by permission. Data will be shared on request to the corresponding author with permission of Clalit Health Services.