Abstract
Background
Patients with advanced biliary tract cancers (BTCs) have poor prognoses and limited therapeutic options. Renin-angiotensin antagonists (ACE-I/ARBs), statins, and aspirin may have potential anti-tumorigenic effects and decrease mortality per retrospective analyses in some solid tumors.
Objective
To evaluate the efficacy of ACE-Is/ARBs, statins, and/or aspirin concurrent to first-line systemic therapy in patients with advanced or metastatic BTC.
Methods
Adult patients at University of Michigan with pathologic confirmation of BTC between January 2010 and December 2020 were included in this retrospective analysis.
Results
Of 1140 patients who met eligibility, a total of 509 patients received one or more concomitant medication(s) of interest in conjunction with systemic therapy for advanced cancer. In the total cohort, the overall survival for locally advanced patients (N = 305) was 16.3 months (95% CI: 12.1-18.6), and metastatic patients (N = 512) 8.6 months (95% CI: 7.6-9.5); P < .0001. Within this concomitant medication cohort, patients with locally advanced stage (n = 132) experienced significantly longer progression-free survival (9.8 vs 4.5; P < 0.0001), and overall survival (17.4 vs 10.6; P < 0.0001) than those with metastatic (n = 297) cancer, respectively. Patients who received ACE-Is/ARBs, statins, and/or aspirin (n = 245) versus not (n = 264) concurrent with systemic anti-cancer therapy did not experience improved progression-free (5.5 vs 5.5 months; hazard ratio (HR) 1.1; P = 0.51), or overall survival (12.3 vs 12.6 months; HR 1.1; P = 0.18), respectively.
Conclusion
In contrast to prior studies, no progression free or overall survival benefit in patients with advanced BTC from concurrent use of ACE-I/ARBs, statin, and/or aspirin with systemic therapy was observed when assessed by BTC subtype or specific systemic therapy regimen.
Keywords: biliary tract neoplasms, cholangiocarcinoma, aspirin, angiotensin receptor antagonist, angiotensin-converting enzyme inhibitor, statin
Patients with advanced biliary tract cancers have poor prognoses and limited therapeutic options. This study evaluated the efficacy of renin-angiotensin antagonists (ACE-Is/ARBs), statins, and/or aspirin when given concurrently with first-line systemic therapy in patients with advanced or metastatic biliary tract cancer.
Implications for Practice.
In contrast to prior studies, this study did not identify any survival benefit in patients who were prescribed blood pressure medications (ACE-I/ARBs class), aspirin, and/or statins in conjunction with systemic anti-cancer therapy for advanced or metastatic biliary tract cancer.
Introduction
Biliary tract cancers (BTCs) are uncommon and often fatal malignancies that develop from the biliary tract or gallbladder epithelium and are subtyped into intrahepatic cholangiocarcinoma (IHCCA), hilar cholangiocarcinoma (HCCA), distal cholangiocarcinoma (DCCA), and gallbladder cancer (GBCA). While these sub-categories were initially identified based on the anatomic location of the primary tumor, the molecular profiles of each subtype are now known to vary significantly, further highlighting the heterogeneity and significance of BTC subtypes.1-6
Surgery is the only potentially curable treatment modality and is preferred in patients with localized disease. Patients with advanced, unresectable BTC have poor prognoses and limited treatment options. Systemic therapy is considered standard of care for previously untreated BTC and often includes gemcitabine and cisplatin chemotherapy as established by the phase III ABC-02 trial.7 A phase III randomized trial, S1815 that explored the potential benefit of the addition of nab-paclitaxel to gemcitabine and cisplatin8 reported no additional benefit in efficacy. Recently, the phase III TOPAZ-1 trial reported a statistically significant improvement in median overall survival (OS) with the addition of durvalumab to gemcitabine and cisplatin.9,10
Renin-angiotensin antagonists, statins, and aspirin are frequently used medications in patients for the management of cardiovascular disease (CVD) and dyslipidemia. There is accumulating evidence to indicate these medications might have potential anti-tumorigenic effects. Specifically, the renin-angiotensin system (RAS) has been implicated in tumorigenesis, metastasis, and resistance to immunotherapy in multiple malignancies.11-14 A key regulator of RAS, angiotensin-converting enzyme (ACE), has been shown to be upregulated in HCCA and DCCA.15 Additional studies have found that angiotensin II, a main effector molecule of RAS, promotes CCA tumor progression and that angiotensin II receptor blockers (ARBs) attenuate CCA growth.16-18 RAS is increasingly being considered a potential target for cancer treatment and indeed, retrospective studies in multiple malignancies have observed survival benefit from concurrent use of RAS inhibitors such as ACE inhibitors (ACE-Is) or ARBs.19-26 The use of concurrent ACE-Is or ARBs with chemotherapy was associated with improved median OS for patients with gastric and lung cancer receiving platinum-based therapy and reduced risk of both progression and death for pancreatic cancer patients receiving gemcitabine.22-25
Statins have also been shown to independently suppress the RAS pathway by reducing angiotensin II, its receptors and intracellular signaling, as well as RAS-dependent oxidative stress and inflammation.27-31 In addition, statins have displayed anti-tumorigenic effects by destabilizing and degrading mutant p53 protein in multiple tumor types.32-34 A pilot clinical trial assessing the efficacy of statins in reducing the level of mutant p53 in tumor tissue is ongoing.35 In BTC, statins have been shown to sensitize GBCA cells to cisplatin, enhance anti-tumor effects of gemcitabine, induce CCA apoptosis, and inhibit CCA cellular proliferation.36-40 A retrospective analysis of 394 EHCCA patients observed that 28 DCCA patients who took statins experienced longer survival.41
Aspirin has also been shown to suppress RAS by lowering plasma renin activity.42,43 Aspirin use may elicit other anti-cancer effects via downregulation of the COX-1, COX-2 enzymes and BCL2 gene expression, and upregulation of tumor-suppressor protein p53 and DNA mismatch repair proteins.44-51 The CAPP2 randomized, placebo-controlled trial found aspirin significantly protects mismatch repair-deficient Lynch syndrome patients against developing colorectal cancer.52 Aspirin has been shown to improve survival for breast, bladder, and colorectal cancer patients and, in BTC, to inhibit CCA cellular proliferation and promote apoptosis.45,53-55 Retrospective, epidemiologic studies of 16,057 and 2,934 BTC patients found aspirin decreased the risk of BTC mortality and increased survival.56,57
Prior reports in BTC, however, have had several methodological limitations. First, studies investigated the impact of independent ACE-I/ARB, aspirin or statins use on incidence or mortality, even though these medications are commonly co-prescribed due to the inter-relationship of cardiovascular disease and dyslipidemia. Second, a majority of the studies do not account for the impact of disease stage, anatomic subtype, surgical resection and use of concomitant anti-cancer systemic therapy. As such, the lack of these covariates may lead to over-estimation of the anti-cancer effect of these medications in BTC.
In this context, the aim of our investigation was to evaluate the association of ACE-I/ARBs, aspirin and/or statins in combination with systemic anti-cancer therapy in patients with advanced or metastatic BTC.
Methods
Study Design and Cohort Identification
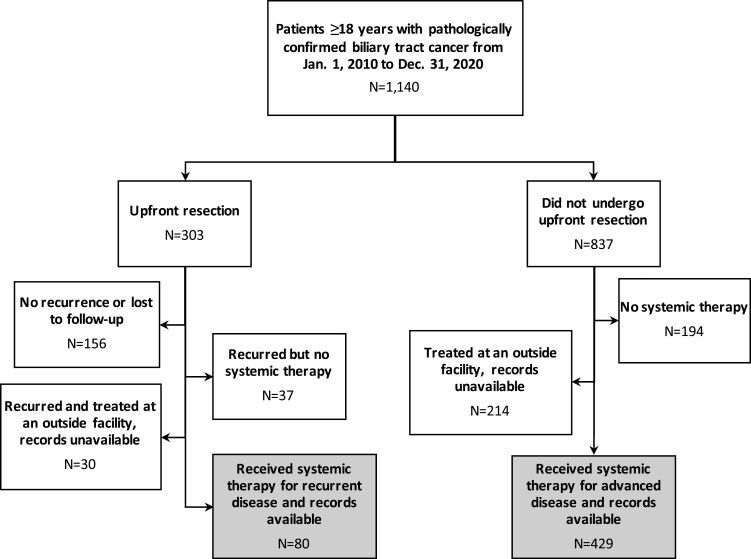
This single-center, retrospective cohort study was approved by the institutional review board (HUM00149617) at the University of Michigan. Informed consent was not required due to the retrospective nature and minimal risk of the study. All patients aged 18 years and older with a pathologic diagnosis of BTC at the University of Michigan Health System between January 2010 and December 2020 were included (Figure 1).
Figure 1.
Flow diagram of cohort selection.
Data Collection
Patient demographics, comorbidities, disease characteristics, and treatment were collected using DataDirect, Electronic Medical Record Search Engine (EMERSE), and manual review of electronic medical records (EMRs).58 Public databases were reviewed for survival data. Disease stage at diagnosis, at the start of first-line systemic therapy, and progression data were collected from EMR notes as established by the treating physician, multi-disciplinary tumor board evaluation, and/or radiologist. Patients were considered resectable at diagnosis if they were judged able to undergo resection with curative intent prior to systemic therapy. If patients were recommended upfront systemic therapy at diagnosis, they were either staged as locally advanced or metastatic based on clinician judgment. Patients were considered to have recurrent disease if cancer recurred after a curative resection. Simple cholecystectomy alone for incidental GBCA was not considered curative resection.
Comorbidity status was collected for diabetes, hypertension, dyslipidemia, chronic kidney disease, and CVD. The latter included arrhythmias, atherosclerosis, congenital heart disease, coronary artery disease, deep vein thrombosis, pulmonary embolism, heart failure, cardiomyopathy, pericardial disease, stroke, and vascular disease.
ACE-I, ARB, statin, and/or aspirin use concurrent with first-line systemic therapy given for advanced cancer was based on administration dates recorded in the EMR. First-line systemic therapy regimens for advanced disease were grouped as containing gemcitabine, 5-fluorouracil, platinum, immunotherapy, or other drugs (including taxanes and targeted therapies, among others).
Statistical Analysis
Progression-free survival (PFS) was calculated as the time from the start of systemic therapy for advanced disease to the date of clinical/radiologic progression or death, or censored at their last date of contact in the EMR. Overall survival (OS) was defined as the time from the date of pathologic diagnosis to death, unless otherwise specified, or censored at last contact if still alive or lost to follow-up.
Survival probabilities, PFS and OS, were estimated using the Kaplan-Meier method and compared using the log-rank test. Categorical variables were described using the χ2-test or Fisher’s exact test. Continuous variables were assessed with the Mann-Whitney U-test or the Kruskal-Wallis test. Univariate and multivariate analyses were completed using Cox proportional hazards regression model. Univariate analyses were completed for the whole BTC group and within IHCCA, HCCA, DCCA, and GBCA subtypes. Prognostic factors from univariate analyses of BTC or IHCCA were included in multivariate models for BTC, IHCCA, HCCA, and GBCA subtypes if represented by at least 10 patients and deemed clinically relevant and/or statistically significant (P < .05). Comorbidities potentially prompting the use of ACE-I, ARB, statin, or aspirin were included in multivariate models as clinically appropriate. Univariate mixed cholangiocarcinoma-hepatocellular carcinoma (CCA-HCC) and multivariate DCCA and CCA-HCC models were not assessed due to limited cohort sizes and subtype-specific concurrent medication groups considered unevaluable if fewer than 20 patients received the concurrent medication.
To assess the effects of concomitant medication(s) both independently of and in combination with each systemic anti-cancer therapy group, all potential combinations of concurrent medications (ACE-I, ARBs, statins, aspirin, ≥1 medication) and systemic therapy were modeled for PFS and OS assessment. The multivariate models assessing concurrent ACE-I or ARBs accounted for CVD, CKD, hypertension, and diabetes. Models evaluating statin use included CVD and dyslipidemia, and aspirin-containing models were adjusted for CVD, hypertension, diabetes, and dyslipidemia. Models assessing the use of one or more ACE-I, ARB, statin, and/or aspirin accounted for all comorbidities. Statistical analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Population
A total of 1140 patients had a confirmed pathologic diagnosis of BTC between January 2010 and December 2020 at the University of Michigan. The median (range) age at diagnosis was 65 years (20-92) and included 536 women (47.0%). Most patients were Caucasian (N, %) (947, 83.1%), non-Hispanic or Latino (952, 83.5%), and had IHCCA subtype (571, 50.1%). Metastatic disease at diagnosis was more common than other stages (512, 44.9%) and most patients did not undergo resection (798, 70.0%). A majority of patients had hypertension (719, 63.1%), while 381 patients had CVD (33.4%), 290 had diabetes (25.4%), 314 had dyslipidemia (27.5%), and 135 had CKD (11.8%). Age, gender, race, stage at diagnosis, resection status, diabetes, and hypertension differed significantly between BTC subtypes (P < 0.05); Table 1.
Table 1.
Patient characteristics of BTC cohort and subtypes.
| Characteristic | Total (N = 1140) | IHCCA (N = 571) | HCCA (N = 209) | DCCA (N = 88) | GBCA (N = 249) | CCA-HCC (N = 23 | P-value† |
|---|---|---|---|---|---|---|---|
| Median age, years, range | 65 (20-92) | 63 (20-90) | 66 (27-92) | 67 (33-91) | 67 (27-92) | 64 (21-78) | .0003 |
| Gender (F/M) | 536/604 | 256/315 | 94/115 | 28/60 | 151/98 | 7/16 | <.0001 |
| Race | .01 | ||||||
| African American | 75 | 33 | 13 | 0 | 28 | 1 | |
| Asian | 23 | 7 | 6 | 1 | 9 | 0 | |
| Caucasian | 947 | 488 | 173 | 78 | 187 | 21 | |
| Other/unknown | 95 | 43 | 17 | 9 | 25 | 1 | |
| Ethnicity | .12 | ||||||
| Hispanic | 25 | 12 | 4 | 1 | 7 | 1 | |
| Non-Hispanic | 952 | 486 | 172 | 65 | 208 | 21 | |
| Unknown | 163 | 73 | 33 | 22 | 34 | 1 | |
| Stage at diagnosis | <.0001 | ||||||
| Resectable | 324 | 135 | 42 | 60 | 79 | 7 | |
| Locally advanced | 304 | 142 | 88 | 15 | 52 | 8 | |
| Metastatic | 512 | 294 | 79 | 13 | 118 | 8 | |
| Resected | <.0001 | ||||||
| Yes | 342 | 149 | 52 | 58 | 78 | 5 | |
| No | 798 | 422 | 157 | 30 | 171 | 18 | |
| Comorbidities* | |||||||
| CVD | 381 | 185 | 65 | 38 | 83 | 10 | .23 |
| Diabetes | 290 | 140 | 47 | 22 | 68 | 13 | .01 |
| Dyslipidemia | 314 | 144 | 60 | 33 | 70 | 7 | .19 |
| CKD | 135 | 68 | 22 | 7 | 32 | 6 | .18 |
| Hypertension | 719 | 360 | 129 | 64 | 146 | 20 | .02 |
*Groups are not mutually exclusive and should not necessarily sum to the row or column total.
† P-value compares biliary tract cancer subtypes, thereby describing variation between BTC subtypes.
Abbreviations: CCA-HCC, mixed cholangiocarcinoma and hepatocellular carcinoma; CKD, chronic kidney disease; CVD, cardiovascular disease; DCCA, distal cholangiocarcinoma; HCCA, hilar cholangiocarcinoma; IHCCA, intrahepatic cholangiocarcinoma; GBCA, gallbladder carcinoma.
Patients who received systemic therapy for advanced cancer at diagnosis or recurrence and had available therapy records (509), were included in the concurrent medication cohort analysis; Table 2 and Supplementary Table S1. This cohort had a median (range) age at diagnosis of 62 years (20-86) and included 240 women (47.2%). Overall, the concurrent medication cohort (Supplementary Table S1) was similar to the entire BTC population (Table 1) with respect to gender, diabetes, dyslipidemia, CDK, and hypertension; however, the concurrent medication cohort varied slightly but significantly from the entire BTC population in race (85.7% vs. 81.0% Caucasian, P = .04), ethnicity (90.6% vs. 77.8% Non-Hispanic, P < .0001), included a higher percentage of patients with IHCCA (58.6% vs.43.3%, P < .0001), a lower percentage of resected patients (22.6% vs. 36.0%, P < .0001), and encompassed slightly younger patients at diagnosis (62 vs. 65 mean age at diagnosis, P < .0001). CVD was less prevalent in the concurrent medication (36.7% vs. 30.7%, P = .03). Again, most patients were Caucasian (N, %) (436; 85.7%), non-Hispanic or Latino (461; 90.6%), and had IHCCA (298; 58.5%). Most had metastatic disease at diagnosis (297; 58.3%) and did not undergo resection (394, 77.4%). Many had hypertension (308, 60.5%), while 187 had CVD (36.7%), 120 had diabetes (23.6%), 131 had dyslipidemia (25.7%), and 65 had CKD (12.8%). Gender, stage at diagnosis, and resection status were significantly different between BTC subtypes.
Table 2.
Patient characteristics of concurrent medication cohort by concurrent ACE-I/ARB, statin, and/or aspirin.
| Characteristic | Total (N = 509) | One or more concurrent medication of interest (N = 245) |
None (N = 264) | P-value† | |||
|---|---|---|---|---|---|---|---|
| ACE* | ARB* | Statin* | Aspirin* | ||||
| (N = 106) | (N = 49) | (N = 111) | (N = 109) | ||||
| Median age, years (range) | 62 (20-86) | 64 (35-79) | 65 (46-86) | 66 (37-86) | 66 (46-86) | 59 (20-84) | <.0001 |
| Gender (F/M) | 240/269 | 43/63 | 26/23 | 44/67 | 41/68 | 136/128 | .04 |
| Race | .28 | ||||||
| African American | 35 | 8 | 5 | 7 | 6 | 20 | |
| Asian | 7 | 0 | 0 | 0 | 1 | 6 | |
| Caucasian | 436 | 92 | 41 | 95 | 96 | 223 | |
| Other/unknown | 31 | 6 | 3 | 9 | 6 | 15 | |
| Ethnicity | .06 | ||||||
| Hispanic | 11 | 1 | 3 | 2 | 1 | 5 | |
| Non-Hispanic | 461 | 101 | 45 | 104 | 101 | 233 | |
| Unknown | 37 | 4 | 1 | 5 | 7 | 26 | |
| Type of cancer | .85 | ||||||
| IHCCA | 298 | 62 | 33 | 55 | 65 | 150 | |
| HCCA | 79 | 16 | 3 | 21 | 18 | 45 | |
| DCCA | 27 | 5 | 3 | 6 | 7 | 13 | |
| GBCA | 97 | 20 | 10 | 27 | 17 | 52 | |
| Mixed CCA-HCC | 8 | 3 | 0 | 2 | 2 | 4 | |
| Stage at diagnosis | .59 | ||||||
| Resectable | 80 | 15 | 9 | 16 | 21 | 39 | |
| Locally advanced | 141 | 24 | 10 | 35 | 31 | 78 | |
| Metastatic | 288 | 67 | 30 | 60 | 57 | 147 | |
| Resected | .26 | ||||||
| Yes | 115 | 19 | 9 | 20 | 25 | 199 | |
| No | 394 | 87 | 40 | 91 | 84 | 65 | |
| Stage at first-line systemic therapy start | .45 | ||||||
| Locally advanced | 132 | 22 | 9 | 31 | 29 | 74 | |
| Metastatic | 297 | 67 | 31 | 63 | 60 | 152 | |
| Recurrent | 80 | 17 | 9 | 17 | 20 | 38 | |
| Comorbidities* | |||||||
| CVD | 187 | 44 | 26 | 68 | 55 | 72 | <.0001 |
| Diabetes | 120 | 44 | 22 | 51 | 41 | 26 | <.0001 |
| Dyslipidemia | 131 | 44 | 22 | 65 | 50 | 34 | <.0001 |
| CKD | 65 | 21 | 11 | 27 | 21 | 19 | <.0001 |
| Hypertension | 308 | 101 | 45 | 90 | 86 | 107 | <.0001 |
| First-line systemic therapy included* | |||||||
| Gemcitabine | 417 | 93 | 44 | 96 | 94 | 209 | .09 |
| Fluoropyrimidine | 103 | 19 | 10 | 26 | 21 | 57 | .43 |
| Platinum | 393 | 77 | 41 | 85 | 82 | 211 | .13 |
| Immunotherapy | 34 | 11 | 2 | 5 | 5 | 17 | .82 |
| Other | 52 | 14 | 4 | 10 | 11 | 20 | .04 |
*Groups are not mutually exclusive and should not necessarily sum to the row or column total.
†P-value compares patients who took one or more medications versus none.
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCA-HCC, mixed cholangiocarcinoma and hepatocellular carcinoma; CKD, chronic kidney disease; CVD, cardiovascular disease; DCCA, distal cholangiocarcinoma; HCCA, hilar cholangiocarcinoma; IHCCA, intrahepatic cholangiocarcinoma; GBCA, gallbladder carcinoma.
The majority of the concurrent medication cohort received first-line systemic regimens containing gemcitabine (417, 81.9%) and were not using an ACE-I, ARB, statin, or aspirin concurrently with systemic therapy (264, 51.9%); Table 2 and Supplementary Table S1. ACE-Is were used concurrently with systemic therapy in 106 (20.8%), ARBs in 49 (9.6%), statins in 111 (21.8%), and aspirin in 109 (21.4%) patients. Women, younger patients, and those without comorbidities were significantly less likely to receive ACE-I, ARB, statin, and/or aspirin with systemic therapy; Table 2.
Survival Outcomes
Entire BTC cohort (N = 1140)
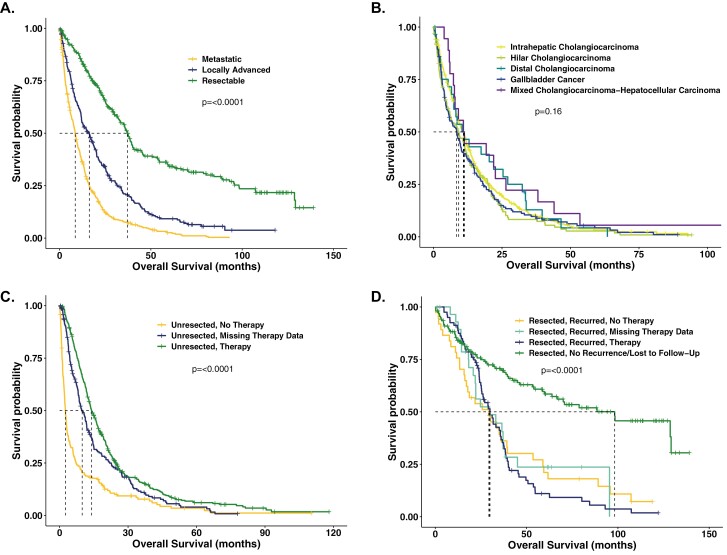
Median OS for all treated and untreated BTC patients was 14.4 months (95% confidence interval (CI): 13.2-16.0). The median OS of patients deemed resectable at diagnosis (N = 323) was 37.1 months (95% CI: 33.1-39.9), locally advanced patients (N = 305) 16.3 months (95% CI: 12.1-18.6), and metastatic patients (N = 512) 8.6 months (95% CI: 7.6-9.5); Figure 2A. The median OS in unresected BTC patients (N = 798) by anatomic subtype was: IHCCA (N = 422) 10.9 months (95% CI: 9.2-11.9), HCCA (N = 157) 9.2 months (95% CI: 6.0-11.4), DCCA (N = 30) 11.3 months (95% CI: 6.6-21.9), GBCA (N = 171) 8.4 months (5.7-9.9), CCA-HCC (N = 18) 11.1 months (95% CI: 7.3-22.6); Figure 2B.
Figure 2.
Biliary tract cancer survival analysis by stage at diagnosis, anatomic subtype, and therapy status. A. All biliary tract cancer (BTC) patients (N = 1140) by stage at diagnosis: resectable patients experienced a median OS of 37.1 months (95% CI: 33.1-39.9); locally advanced patients (N = 305) 16.3 months (95% CI: 12.1-18.6), and metastatic patients (N = 512) 8.6 months (95% CI: 7.6-9.5); P < 0.0001. B. Unresected BTC patients (N = 798) per anatomic subtype: IHCCA (N = 422) 10.9 months (95% CI: 9.2-11.9), HCCA (N = 157) 9.2 months (95% CI: 6.0-11.4), DCCA (N = 30) 11.3 months (95% CI: 6.6-21.9), GBCA (N = 171) 8.4 months (5.7-9.9), CCA-HCC (N = 18) 11.1 months (95% CI: 7.3-22.6); P = 0.16. 2C. Upfront therapy patients (N = 837): patients unresected and received no therapy (N = 194) 2.6 months (95% CI: 2.1-3.0); unresected and missing therapy data (N = 214) 9.9 months (95% CI: 8.4-11.9); upfront systemic therapy (N = 429) 14.0 months (95% CI: 12.9-15.9); P < 0.0001. 2D. Upfront resection patients (N = 303): resected patients who recurred and received no systemic therapy (N = 37) 29.3 months (95% CI: 16.2-39.2); recurred and are missing therapy data (N = 30) 29.8 months (95% CI: 20.8-38.3); recurred and received systemic therapy (N = 80) 29.9 months (95% CI: 25.2-35.6); did not recur or were lost to follow-up (N = 156) 98.2 months (95% CI: 59.8-129.1); P < 0.0001. Adv, advanced; CCA-HCC, mixed cholangiocarcinoma and hepatocellular carcinoma; DCCA, distal cholangiocarcinoma; HCCA, hilar cholangiocarcinoma; IHCCA, intrahepatic cholangiocarcinoma; GBCA, gallbladder carcinoma.
Upfront systemic therapy (N = 837)
Unresected patients who received no therapy (N = 194) experienced a median OS of 2.6 months (95% CI: 2.1-3.0). Patients treated with upfront systemic therapy for a new diagnosis of advanced disease (N = 429) and with available treatment records had a median OS of 14.0 months (95% CI: 12.9-15.9); Figure 2C.
Upfront resection (N = 303)
Patients treated with upfront resection who had no documented recurrence or were lost to follow-up (N = 156) had a median OS of 98.2 months (95% CI: 59.8-129.1). Patients with documented recurrence but no subsequent palliative systemic therapy (N = 37) showed a median OS of 29.3 months (95% CI: 16.2-39.2); those with documented recurrence but missing records of subsequent systemic therapy (N = 30) experienced a median OS of 29.8 months (95% CI: 20.8-38.3). Patients with documented recurrence with available records of systemic therapy (N = 80) experienced a median OS of 29.9 months (95% CI: 25.2-35.6; Figure 2D).
Concurrent medication cohort (N = 509)
A total of 509 patients who received systemic therapy for new or recurrent advanced cancer and had available treatment records were included in the concurrent medication cohort analysis. OS was calculated as the time from the start of systemic therapy to death in the concurrent medication category. The variables age, gender, and disease stage at the start of therapy met inclusion standards and were included in multivariate models.
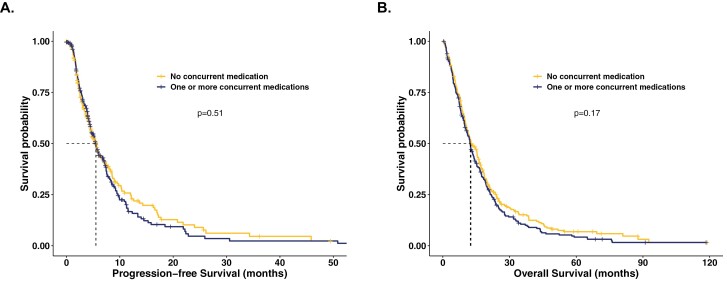
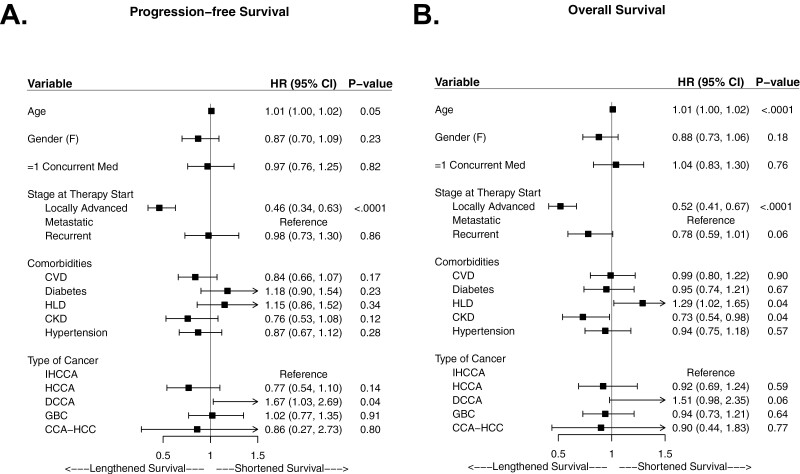
No difference in PFS (5.5 vs 5.5 months; HR 1.1; 95% CI: 0.9-1.3; P = 0.51) or OS (12.3 vs 12.6 months; HR 1.1; 95% CI; 0.9-1.4; P = 0.18) was observed for patients who received one or more concurrent RAS-affecting medications versus those who did not in univariate analyses; Table 3 and Figure 3 with additional details in Supplementary Table S2. Similarly, no significant differences in PFS or OS were observed in multivariate analyses; Figure 4.
Table 3.
Univariate and multivariate analysis: impact of concomitant medication(s) of interest and systemic anti-tumor therapy on survival in patients with advanced BTC.
| Characteristic | Concurrent medication | None (N = 264) | |||||
|---|---|---|---|---|---|---|---|
| ACE-I* (N = 106) |
ARB* (N = 49) |
ACE-I or ARB* (N = 152) |
Statin* (N = 111) |
Aspirin* (N = 109) |
One or more* (N = 245) |
||
| Univariate | |||||||
| Progression-free survival | |||||||
| Median (months) | 5.4 | 5.6 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| HR, (95% CI) | 0.9 (0.7-1.1) | 1.1 (0.8-1.6) | 0.9 (0.7-1.2) | 1.1 (0.9-1.4) | 1.2 (0.9-1.5) | 1.1 (0.9-1.3) | 0.9 (0.8-1.2) |
| P-value† | .26 | .47 | .55 | .52 | .25 | .51 | |
| Overall survival | |||||||
| Median (months) | 11 | 12.3 | 12.1 | 12.1 | 12.3 | 12.3 | 12.6 |
| HR, (95% CI) | 0.9 (0.8-1.2) | 1.3 (0.9-1.8) | 1.1 (0.9-1.3) | 1.3 (1.0-1.6) | 1.1 (0.9-1.4) | 1.1 (0.9-1.4) | 0.9 (0.7-1.1) |
| P-value† | .89 | .12 | .48 | .04 | .25 | .18 | |
| Multivariate | |||||||
| Progression-free survival | |||||||
| HR, (95% CI) | 0.8 (0.6-1.1) | 1.9 (0.7-1.6) | 0.8 (0.7-1.1) | 1.1 (0.8-1.4) | 1.1 (0.8-1.4) | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) |
| P-value† | .16 | .66 | .23 | .60 | .49 | .82 | |
| Overall survival | |||||||
| HR, (95% CI) | 0.9 (0.7-1.2) | 1.2 (0.8-1.6) | 1.0 (0.8-1.3) | 1.1 (0.9-1.5) | 1.1 (0.8-1.4) | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) |
| P-value† | .65 | .36 | .97 | .31 | .61 | .76 | |
*Groups are not mutually exclusive and should not necessarily sum to the row or column total.
†P-value compares patients who did or did not receive a concurrent medication of interest.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BTC, biliary tract cancer; CI, confidence interval; HR, hazard ratio.
Figure 3.
Kaplan-Meier progression-free and overall survival curves in patients with advanced BTC in the concurrent medication cohort. A. PFS. Median PFS was 5.5 months (95% CI: 4.5-6.7) and 5.5 months (95% CI: 4.6-6.5) for those who did and did not, respectively, receive an ACE-inhibitor, ARB, statin, and/or aspirin concurrently with 1st line systemic therapy (HR 1.1; 95% CI: 0.9-1.3; P = 0.51). B. OS. Median OS 12.3 months (95% CI: 10.5-13.8) and 12.6 months (95% CI: 11.2-15.4) for those who did and did not, respectively, receive an ACE-inhibitor, ARB, statin, and/or aspirin concurrently with systemic therapy (HR 1.1; 95% CI; 0.9-1.4; P = 0.18). P-values displayed on plots are from log rank comparison of the Kaplan-Meier curves. OS defined as the time from therapy start to death. The following HRs, 95% CIs, and P-values are from univariate analysis; N = 509.
Figure 4.
Forest plots depicting variables of interest impacting progression-free and overall survival in BTC in multivariate analysis. Receipt of one or more concurrent medication of interest was not statistically significant in multivariate analysis for either A. PFS (HR 0.87; 95% CI: 0.70-1.25; P = 0.82), or B. OS (HR 1.04; 0.83-1.30; P = 0.76).
Patients who received concurrent statins experienced shorter OS (HR 1.3; 95% CI: 1.0-1.6; P = 0.04) in univariate analysis; however, after adjusting for age, gender, stage at therapy start, and relevant comorbidities, statins were no longer significantly associated with OS (HR 1.1, 95% CI: 0.9-1.5; P = 0.31); Table 3. Aspirin use was not significantly associated with PFS (5.5 vs 5.5 months; HR 1.2; 95% CI: 0.9-1.5; P = 0.25) or OS (12.3 vs 12.6 months; HR 1.1; 95% CI: 0.9-1.4; P = 0.25) in our cohort.
Discussion
This retrospective study is the first to assess the efficacy of concurrent ACE-Is/ARBs, statin, and/or aspirin in combination with first-line systemic therapy for advanced BTC patients to our knowledge. Per our literature review, this is also the largest retrospective advanced BTC cohort in the literature with both OS and PFS data reported.41,56,57,59-75 No survival benefit was observed from these concomitant medications independent of or in combination with any specific regimen for patients with advanced BTC or within any subtype. While our univariate analysis indicated that statin users experienced shorter OS, it was not significant after adjustment for covariates such as age, gender, disease stage, and relevant comorbidities in multivariate analysis.
The major strengths of this study include (a) evaluation of separate and combined use of ACE-I/ARBs, aspirin and statins; (b) a relatively large sample size of consecutive patients with pathologically confirmed advanced BTC with a balance of medication users and non-users; (c) adjustment for multiple covariates including disease stage, resection status and anatomic subtype; (d) accounting for the impact of concurrent systemic anti-cancer therapy and reporting PFS in addition to OS; and (e) reliable data collection on medication use through manual medication reconciliation at each visit, and not limited to prescription tracking, use of aspirin or missed prescriptions via an alternate provider.
{Cho, 2017 #618}A retrospective study of 394 EHCCA patients had reported improvement in the survival of 28 patients with DCCA with the use of statin but the study did not account for other relevant survival variables such as disease stage, systemic therapy, or resection status.41 Two additional retrospective BTC studies observed a lower risk of BTC-specific death and extended survival in aspirin users, but neither assessed PFS or accounted for important survival variables describing disease stage and chemotherapy.56,57 The research letter describing a large retrospective study of 2,934 patients with BTC in the UK reported aspirin use was significantly associated with improved OS.57 However, the study did not account for cancer stage, treatment and resection status, and as previously reported, included a high survival censorship in the aspirin-using group in addition to using a database enriched with younger patients.57,76,77 A follow-up study assessed only BTC-specific mortality and did not consider specifics of disease stage or chemotherapy overlap.56 Another large retrospective study of 795 BTC patients from Canada reported no significant benefit of statin or aspirin on recurrence free or overall survival, although the effect of chemotherapy was not studied and study spanned 26 years during which standard of care therapy has significantly changed.78 A smaller study from Japan of 287 patients reported lack of survival benefit from concurrent ACE-Is and ARBs but neither aspirin nor statins were assessed; BTC subtype, systemic therapy regimens, race, and ethnicity were also not included in analysis.65 In comparison to these assessments, our current study accounts for all these factors and provides a clinically focused assessment in a large, real-world patient population. This study has low survival censorship and variation (1.5-7.9%) between medication users and non-users.
This study also highlights real-world outcomes of patients with BTC when accounting for stage, resection status and receipt of anti-cancer systemic therapy. We highlight the improved outcome of patients with locally advanced stage in median PFS (N = 132) and OS (N = 304) values of 9.8 months (HR (95% CI): 0.50 (0.38-0.66); P < .0001) and 16.3 months (HR (95% CI): 0.57 (0.46-0.71); P < .0001), respectively, in comparison to patients with metastatic cancer. Our data also indicate that first-line therapy containing gemcitabine is independently associated with significantly improved PFS compared to non-gemcitabine containing regimens (HR (95% CI): 0.73 (0.55-0.96), P = 0.02). In our advanced BTC cohort, female gender was associated with a trend toward improved survival. Interestingly, females with IHCCA experienced significantly longer median PFS and OS (6.0 vs 4.1 months, P = 0.04; 13.4 vs 10.5 months, P = 0.02, respectively) but gender-based differences were not observed across other subtypes. Multiple studies support that females with IHCCA experience longer survival but not in general BTC or other subtypes.62,65,75,79-85 A retrospective study in 104 BTC patients in Brazil did observe that women with BTC experienced longer OS but over half of the cohort had IHCCA.67
Several limitations to this study should be noted. While we used a large real-world BTC dataset with both PFS and OS data, it remains a retrospective single-center study. Additionally, we did not evaluate dose- and duration-response relationships, thus potentially diluting survival benefit of higher dose or longer term use. Data were abstracted from EMR which may not reflect accurate data if not updated, although per Cancer Center policy medication reconciliation is completed at each visit. While this cohort provides data on concurrent medications and systemic anti-cancer therapy for BTC patients in a western population, a majority of these patients were non-Hispanic and white and thus this work does not fully assess effects in African American, Asian, or Hispanic populations. Similar analyses in these populations should be explored as CCA mortality and OS have been shown to vary by race and ethnicity.85-88 We did not include tumor and/or pharmacogenomic alteration data in our analyses, but this should be a future consideration, exemplified by patients with wild-type PIK3CA, Lynch syndrome and KRAS-mutated colorectal cancers experiencing survival benefit from aspirin.54,89,90
Conclusion
The present study suggests no survival benefit in patients with advanced biliary cancer from concomitant use of ACE-I/ARBs, aspirin and/or statin with chemotherapy, generally or when analyzed by disease subtype and in combination with specified systemic therapies.
Supplementary Material
Contributor Information
Valerie Gunchick, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Rachel L McDevitt, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Elizabeth Choi, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
Katherine Winslow, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
Mark M Zalupski, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Vaibhav Sahai, Division of Hematology and Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Funding
The study was supported by the Beverly Mitchell Fund (PI: V. Sahai).
Conflict of Interest
Mark M. Zalupski reported institutional grant funding from AstraZeneca, MedImmune, and Seattle Genetics. Vaibhav Sahai reported institutional grant funding from Agios, Bristol-Myers Squibb, Celgene, Clovis, Exelixis, Fibrogen, Incyte, Ipsen, Medimmune, Merck, and Cornerstone, and consultant fees from AstraZeneca, GlaxoSmithKline, Histosonics, Incyte, Lynx Group, QED, and Cornerstone. The other authors indicated no financial relationships.
Author Contributions
Conception/design: V.G., R.L.M., V.S. Provision of study material or patients: M.M.Z., V.S. Financial support: V.S. Collection and/or assembly of data: V.G., E.C., K.W., V.S. Data analysis and interpretation: V.G., V.S. Manuscript writing: V.G., V.S. Final approval of manuscript: All authors.
Data Availability
The data underlying this article cannot be shared publicly due to institutional IRB limitations. The data will be shared on reasonable request to the corresponding author.
References
- 1. Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX.. Biliary tract cancer. Lancet. 2021;397(10272):428-444. [DOI] [PubMed] [Google Scholar]
- 2. Hezel AF, Deshpande V, Zhu AX.. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol. 2010;28(21):3531-3540. 10.1200/JCO.2009.27.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ross JS, Wang K, Javle MM, et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinically relevant genomic alterations. J Clin Oncol. 2015;33(15_suppl):4009-4009. 10.1200/jco.2015.33.15_suppl.4009. [DOI] [Google Scholar]
- 4. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43(10):1552-1558. 10.1016/j.humpath.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 6. Personeni N, Lleo A, Pressiani T, et al. Biliary tract cancers: molecular heterogeneity and new treatment options. Cancers (Basel). 2020;12(11):3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8. Shroff RT, Guthrie KA, Scott AJ, et al. : SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41:LBA490-LBA490. [Google Scholar]
- 9. Oh D-Y, He AR, Qin S, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. 2022;40(4_suppl):378-378. 10.1200/jco.2022.40.4_suppl.378. [DOI] [Google Scholar]
- 10. Oh D-Y, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence. 0(0):EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 11. Takiguchi T, Takahashi-Yanaga F, Ishikane S, et al. Angiotensin II promotes primary tumor growth and metastasis formation of murine TNBC 4T1 cells through the fibroblasts around cancer cells. Eur J Pharmacol. 2021;909:174415. 10.1016/j.ejphar.2021.174415. [DOI] [PubMed] [Google Scholar]
- 12. Xie G, Cheng T, Lin J, et al. Local angiotensin II contributes to tumor resistance to checkpoint immunotherapy. J ImmunoTher Cancer. 2018;6(1):88. 10.1186/s40425-018-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu ZW, Yan SX, Wu HX, et al. The influence of TNF-α and Ang II on the proliferation, migration and invasion of HepG2 cells by regulating the expression of GRK2. Cancer Chemother Pharmacol. 2017;79(4):747-758. 10.1007/s00280-017-3267-z. [DOI] [PubMed] [Google Scholar]
- 14. Ishikane S, Hosoda H, Nojiri T, et al. Angiotensin II promotes pulmonary metastasis of melanoma through the activation of adhesion molecules in vascular endothelial cells. Biochem Pharmacol. 2018;154:136-147. 10.1016/j.bcp.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 15. Beyazit Y, Purnak T, Suvak B, et al. Increased ACE in extrahepatic cholangiocarcinoma as a clue for activated RAS in biliary neoplasms. Clin Res Hepatol Gastroenterol. 2011;35(10):644-649. 10.1016/j.clinre.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 16. Okamoto K, Tajima H, Ohta T, et al. Angiotensin II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through an interaction with hepatic stellate cells. Int J Oncol. 2010;37(5):1251-1259. 10.3892/ijo_00000776. [DOI] [PubMed] [Google Scholar]
- 17. Saikawa S, Kaji K, Nishimura N, et al. Angiotensin receptor blockade attenuates cholangiocarcinoma cell growth by inhibiting the oncogenic activity of Yes-associated protein. Cancer Lett. 2018;434:120-129. 10.1016/j.canlet.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 18. Samukawa E, Fujihara S, Oura K, et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int J Oncol. 2017;51(6):1674-1684. 10.3892/ijo.2017.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantini L, Pecci F, Hurkmans D, et al. Statin treatment improves response to anti-PD1 agents in patients with malignant pleural mesothelioma and non-small cell lung cancer. J Clin Oncol. 2020;38(15):3074-3074. 10.1200/jco.2020.38.15_suppl.3074.32634335 [DOI] [Google Scholar]
- 20. Keith SW, Maio V, Arafat HA, et al. Angiotensin blockade therapy and survival in pancreatic cancer: a population study. BMC Cancer. 2022;22(1):150. 10.1186/s12885-022-09200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun H, Li T, Zhuang R, Cai W, Zheng Y.. Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients?: Evidence from a meta-analysis including 55 studies. Medicine (Baltim). 2017;96(13):e6394e6394. 10.1097/md.0000000000006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim ST, Park KH, Oh SC, et al. How does inhibition of the renin-angiotensin system affect the prognosis of advanced gastric cancer patients receiving platinum-based chemotherapy?. Oncology (Huntingt). 2012;83(6):354-360. 10.1159/000337979. [DOI] [PubMed] [Google Scholar]
- 23. Nakai Y, Isayama H, Sasaki T, et al. The inhibition of renin-angiotensin system in advanced pancreatic cancer: an exploratory analysis in 349 patients. J Cancer Res Clin Oncol. 2015;141(5):933-939. 10.1007/s00432-014-1873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilop S, von Hobe S, Crysandt M, et al. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135(10):1429-1435. 10.1007/s00432-009-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakai Y, Isayama H, Ijichi H, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103(11):1644-1648. 10.1038/sj.bjc.6605955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlos-Escalante JA, de Jesús-Sánchez M, Rivas-Castro A, et al. The use of antihypertensive drugs as coadjuvant therapy in cancer. Front Oncol. 2021;11:660943-660943. 10.3389/fonc.2021.660943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delbosc S, Cristol J-P, Descomps B, Mimran A, Jover B.. Simvastatin prevents angiotensin II–induced cardiac alteration and oxidative stress. Hypertension. 2002;40(2):142-147. 10.1161/01.hyp.0000024348.87637.6f. [DOI] [PubMed] [Google Scholar]
- 28. Strazzullo P, Kerry SM, Barbato A, et al. Do statins reduce blood pressure?. Hypertension. 2007;49(4):792-798. 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 29. Straznicky NE, Howes LG, Lam W, Louis WJ.. Effects of pravastatin on cardiovascular reactivity to norepinephrine and angiotensin II in patients with hypercholesterolemia and systemic hypertension. Am J Cardiol. 1995;75(8):582-586. 10.1016/s0002-9149(99)80621-3. [DOI] [PubMed] [Google Scholar]
- 30. Han SH, Koh KK.. Cross-talk between statins and the renin-angiotensin system. Asia-Pacific Cardiology. 2011;3(1):33-36. [Google Scholar]
- 31. Drapala A, Sikora M, Ufnal M.. Statins, the renin-angiotensin-aldosterone system and hypertension - a tale of another beneficial effect of statins. J Renin Angiotensin Aldosterone Syst. 2014;15(3):250-258. [DOI] [PubMed] [Google Scholar]
- 32. Chou C-W, Lin C-H, Hsiao T-H, et al. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci Rep. 2019;9(1):20403. 10.1038/s41598-019-56532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tutuska K, Parrilla-Monge L, Di Cesare E, Nemajerova A, Moll UM.. Statin as anti-cancer therapy in autochthonous T-lymphomas expressing stabilized gain-of-function mutant p53 proteins. Cell Death Disease. 2020;11(4):274. 10.1038/s41419-020-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Grady S, Crown J, Duffy MJ.. Statins inhibit proliferation and induce apoptosis in triple-negative breast cancer cells. Med Oncol. 2022;39(10):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Telfah M, Iwakuma T, Bur A, et al. A window of opportunity trial of atorvastatin in p53-mutant and p53 wild type malignancies. J Clin Oncol. 2019;37(15_suppl):TPS3165-TPS3165. 10.1200/jco.2019.37.15_suppl.tps3165. [DOI] [Google Scholar]
- 36. Vallejo A, Erice O, Entrialgo-Cadierno R, et al. FOSL1 promotes cholangiocarcinoma via transcriptional effectors that could be therapeutically targeted. J Hepatol. 2021;75(2):363-376. 10.1016/j.jhep.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 37. Kitagawa K, Moriya K, Kaji K, et al. Atorvastatin augments gemcitabine-mediated anti-cancer effects by inhibiting yes-associated protein in human cholangiocarcinoma cells. Int J Mol Sci . 2020;21(20):7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Liu Y, Duan J, et al. Cholesterol depletion sensitizes gallbladder cancer to cisplatin by impairing DNA damage response. Cell Cycle. 2019;18(23):3337-3350. 10.1080/15384101.2019.1676581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamigaki M, Sasaki T, Serikawa M, et al. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int J Oncol. 2011;39(3):561-568. 10.3892/ijo.2011.1087. [DOI] [PubMed] [Google Scholar]
- 40. Buranrat B, Senggunprai L, Prawan A, Kukongviriyapan V.. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016;153:41-49. 10.1016/j.lfs.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 41. Lavu S, Therneau TM, Harmsen WS, et al. Effect of statins on the risk of extrahepatic cholangiocarcinoma. Hepatology. 2020;72(4):1298-1309. 10.1002/hep.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magagna A, Abdel-Haq B, Favilla S, Taddei S, Salvetti A.. Hemodynamic and humoral effects of low-dose aspirin in treated and untreated essential hypertensive patients. Blood Press. 1994;3(4):236-241. 10.3109/08037059409102263. [DOI] [PubMed] [Google Scholar]
- 43. Snoep JD, Hovens MMC, Pasha SM, et al. Time-dependent effects of low-dose aspirin on plasma renin activity, aldosterone, cortisol, and catecholamines. Hypertension. 2009;54(5):1136-1142. 10.1161/HYPERTENSIONAHA.109.134825. [DOI] [PubMed] [Google Scholar]
- 44. Lichtenberger LM, Vijayan KV.. Are platelets the primary target of aspirin’s remarkable anticancer activity?. Cancer Res. 2019;79(15):3820-3823. 10.1158/0008-5472.CAN-19-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boueroy P, Aukkanimart R, Boonmars T, et al. Inhibitory effect of aspirin on cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2017;18(11):3091-3096. 10.22034/APJCP.2017.18.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langley RE, Burdett S, Tierney JF, et al. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy?. Br J Cancer. 2011;105(8):1107-1113. 10.1038/bjc.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim KM, Song JJ, An JY, Kwon YT, Lee YJ.. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J Biol Chem. 2005;280(49):41047-41056. 10.1074/jbc.m503713200. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Z, Chen F, Shang L.. Advances in antitumor effects of NSAIDs. Cancer Manag Res. 2018;10:4631-4640. 10.2147/CMAR.S175212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meune C, Mourad JJ, Bergmann JF, Spaulding C.. Interaction between cyclooxygenase and the renin-angiotensin-aldosterone system: rationale and clinical relevance. J Renin Angiotensin Aldosterone Syst. 2003;4(3):149-154. [DOI] [PubMed] [Google Scholar]
- 50. Ho CC, Yang XW, Lee TL, et al. Activation of p53 signalling in acetylsalicylic acid-induced apoptosis in OC2 human oral cancer cells. Eur J Clin Invest. 2003;33(10):875-882. 10.1046/j.1365-2362.2003.01240.x. [DOI] [PubMed] [Google Scholar]
- 51. Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR.. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9(1):383-390. [PubMed] [Google Scholar]
- 52. Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. The Lancet. 2020;395(10240):1855-1863. 10.1016/s0140-6736(20)30366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loomans-Kropp HA, Pinsky P, Umar A.. Evaluation of aspirin use with cancer incidence and survival among older adults in the prostate, lung, colorectal, and ovarian cancer screening trial. JAMA Network Open. 2021;4(1):e2032072-e2032072. 10.1001/jamanetworkopen.2020.32072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gebauer L, Nist A, Mernberger M, et al. Superior overall survival in patients with colorectal cancer, regular aspirin use, and combined wild-type PIK3CA and KRAS-mutated tumors. Cancers (Basel). 2021;13(19):4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi T, Gong J, Fujita K, et al. Aspirin inhibits cholangiocarcinoma cell proliferation via cell cycle arrest in vitro and in vivo. Int J Oncol. 2021;58(2):199-210. 10.3892/ijo.2020.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liao SF, Koshiol J, Huang YH, et al. Postdiagnosis aspirin use associated with decreased biliary tract cancer-specific mortality in a large nationwide cohort. Hepatology. 2021;74(4):1994-2006. 10.1002/hep.31879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackson SS, Pfeiffer RM, Liu Z, et al. Association between aspirin use and biliary tract cancer survival. JAMA Oncol. 2019;5(12):1802-1804. 10.1001/jamaoncol.2019.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hanauer DA. EMERSE: the electronic medical record search engine. AMIA Annu Symp Proc. 2006;2006:941-941. [PMC free article] [PubMed] [Google Scholar]
- 59. Beaulieu C, Lui A, Yusuf D, et al. A population-based retrospective study of biliary tract cancers in Alberta, Canada. Current Oncology. 2021;28(1):417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Frega G, Garajová I, Palloni A, et al. Brain metastases from biliary tract cancer: a monocentric retrospective analysis of 450 patients. Oncology (Huntingt). 2018;94(1):7-11. 10.1159/000479929. [DOI] [PubMed] [Google Scholar]
- 61. Hyung J, Kim B, Yoo C, et al. Clinical benefit of maintenance therapy for advanced biliary tract cancer patients showing no progression after first-line gemcitabine plus cisplatin. Cancer Res Treat. 2019;51(3):901-909. 10.4143/crt.2018.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho K-M, Park H, Oh D-Y, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. 2017;8(2):2329-2341. 10.18632/oncotarget.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suzuki Y, Kan M, Kimura G, et al. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J Gastroenterol. 2019;54(3):281-290. 10.1007/s00535-018-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer. 2014;110(9):2165-2169. 10.1038/bjc.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nakai Y, Isayama H, Sasaki T, et al. No survival benefit from the inhibition of renin-angiotensin system in biliary tract cancer. Anticancer Res. 2016;36(9):4965-4970. 10.21873/anticanres.11065. [DOI] [PubMed] [Google Scholar]
- 66. Kim BJ, Hyung J, Yoo C, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017;80(1):209-215. 10.1007/s00280-017-3353-2. [DOI] [PubMed] [Google Scholar]
- 67. Felismino TC, Oliveira FMA, Fogassa CAZ, et al. Evaluation of prognostic factors in patients undergoing first-line chemotherapy for advanced biliary tract cancer: a retrospective analysis from a South American cancer centre. Ecancermedicalscience. 2022;16:1345. 10.3332/ecancer.2022.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Möhring C, Feder J, Mohr RU, et al. First line and second line chemotherapy in advanced cholangiocarcinoma and impact of dose reduction of chemotherapy: a retrospective analysis. Front Oncol. 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bisello S, Buwenge M, Palloni A, et al. Radiotherapy or chemoradiation in unresectable biliary cancer: a retrospective study. Anticancer Res. 2019;39(6):3095-3100. 10.21873/anticanres.13445. [DOI] [PubMed] [Google Scholar]
- 70. Lowery MA, Goff LW, Keenan BP, et al. Second-line chemotherapy in advanced biliary cancers: A retrospective, multicenter analysis of outcomes. Cancer. 2019;125(24):4426-4434. 10.1002/cncr.32463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Z, Alsaggaf R, McGlynn KA, et al. Statin use and reduced risk of biliary tract cancers in the UK Clinical Practice Research Datalink. Gut. 2019;68(8):1458-1464. 10.1136/gutjnl-2018-317504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yadav S, Xie H, Bin-Riaz I, et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: a propensity score matched analysis. Eur J Surg Oncol. 2019;45(8):1432-1438. [DOI] [PubMed] [Google Scholar]
- 73. Choi J, Ghoz HM, Peeraphatdit T, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64(3):785-796. 10.1002/hep.28529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109(12):1881-1890. 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kommalapati A, Tella SH, Goyal G, et al. Association between treatment facility volume, therapy types and overall survival in patients with intrahepatic cholangiocarcinoma. HPB. 2019;21(3):379-386. [DOI] [PubMed] [Google Scholar]
- 76. Bergquist JR, Shariq OA, Visser BC.. Questionable survival benefit of aspirin use in patients with biliary tract cancer. JAMA Oncol. 2020;6(5):783-784. 10.1001/jamaoncol.2020.0122. [DOI] [PubMed] [Google Scholar]
- 77. Arhi CS, Bottle A, Burns EM, et al. Comparison of cancer diagnosis recording between the Clinical Practice Research Datalink, Cancer Registry and Hospital Episodes Statistics. Cancer Epidemiol. 2018;57:148-157. 10.1016/j.canep.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 78. McNamara MG, Aneja P, Le LW, et al. Effects of statin, aspirin, or metformin use on recurrence free and overall survival in patients with biliary tract cancer (BTC). J Clin Oncol. 2014;32(3_suppl):303-303. 10.1200/jco.2014.32.3_suppl.303. [DOI] [PubMed] [Google Scholar]
- 79. Clark CJ, Wood-Wentz CM, Reid-Lombardo KM, et al. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: a population-based study using the National Cancer Institute SEER database. HPB. 2011;13(9):612-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shiosaki JR, Sempokuya T, Hernandez BY, Wong LL.. Cholangiocarcinoma in Pacific islanders compared to Asians. Hawaii J Health Soc Welf. 2021;80(4):80-87. 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goto T, Saito H, Sasajima J, et al. High response rate and prolonged survival of unresectable biliary tract cancer treated with a new combination therapy consisting of intraarterial chemotherapy plus radiotherapy. Front Oncol. 2020;10:597813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Balachandran P, Agarwal S, Krishnani N, et al. Predictors of long-term survival in patients with gallbladder cancer. J Gastrointest Surg. 2006;10(6):848-854. 10.1016/j.gassur.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 83. Zhu X, Zhang X, Hu X, et al. Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: a retrospective study based on SEER data. Medicine (Baltimore). 2020;99(40):e22292-e22292. 10.1097/MD.0000000000022292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park HS, Park JS, Chun YJ, et al. Prognostic factors and scoring model for survival in metastatic biliary tract cancer. Cancer Res Treat. 2017;49(4):1127-1139. 10.4143/crt.2016.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ellington TD, Momin B, Wilson RJ, et al. Incidence and mortality of cancers of the biliary tract, gallbladder, and liver by sex, age, race/ethnicity, and stage at diagnosis: United States, 2013 to 2017. Cancer Epidemiol Biomarkers Prevent. 2021;30(9):1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Antwi SO, Mousa OY, Patel T.. Racial, ethnic, and age disparities in incidence and survival of intrahepatic cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol. 2018;17(2):604274-604614. 10.5604/01.3001.0012.0929. [DOI] [PubMed] [Google Scholar]
- 87. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA.. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117-117. 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jaruvongvanich V, Yang JD, Peeraphatdit T, Roberts LR.. The incidence rates and survival of gallbladder cancer in the USA. Eur J Cancer Prev. 2019;28(1):1-9. 10.1097/cej.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 89. Mädge JC, Stallmach A, Kleebusch L, Schlattmann P.. Meta-analysis of aspirin-guided therapy of colorectal cancer. J Cancer Res Clin Oncol. 2022;148(6):1407-1417. 10.1007/s00432-022-03942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hua X, Phipps AI, Burnett-Hartman AN, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35(24):2806-2813. 10.1200/jco.2017.72.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to institutional IRB limitations. The data will be shared on reasonable request to the corresponding author.