Abstract
Background
ZFTA-RELA (formerly known as c11orf-RELA) fused supratentorial ependymoma (ZFTAfus ST-EPN) has been recognized as a novel entity in the 2016 WHO classification of CNS tumors and further defined in the recent 2021 edition. ZFTAfus ST-EPN was reported to portend poorer prognosis when compared to its counterpart, YAP1 ST-EPN in some previously published series. The aim of this study was to determine the treatment outcome of molecularly confirmed and conventionally treated ZFTAfus ST-EPN patients treated in multiple institutions.
Methods
We conducted a retrospective analysis of all pediatric patients with molecularly confirmed ZFTAfus ST-EPN patients treated in multiple institutions in 5 different countries (Australia, Canada, Germany, Switzerland, and Czechia). Survival outcomes were analyzed and correlated with clinical characteristics and treatment approaches.
Results
A total of 108 patients were collated from multiple institutions in 5 different countries across three continents. We found across the entire cohort that the 5- and 10-year PFS were 65% and 63%, respectively. The 5- and 10-year OS of this cohort of patients were 87% and 73%. The rates of gross total resection (GTR) were high with 84 out of 108 (77.8%) patients achieving GTR. The vast majority of patients also received post-operative radiotherapy, 98 out of 108 (90.7%). Chemotherapy did not appear to provide any survival benefit in our patient cohort.
Conclusion
This is the largest study to date of contemporaneously treated molecularly confirmed ZFTAfus ST-EPN patients which identified markedly improved survival outcomes compared to previously published series. This study also re-emphasizes the importance of maximal surgical resection in achieving optimal outcomes in pediatric patients with supratentorial ependymoma.
Keywords: central nervous system tumors, molecular , pediatric, supratentorial ependymoma, ZFTA-RELA
Key Points.
This retrospective analysis of a large cohort of ZFTAfus ST-EPN identified markedly improved survival outcomes compared to some previously published series.
Maximal safe surgical resection remains to be an important outcome predictor in the treatment of pediatric ZFTAfus ST-EPN.
Chemotherapy did not show survival benefit in our cohort of patients.
Importance of the Study.
This study reported more favorable outcome in a large cohort of contemporaneously treated patients with ZFTAfus ST-EPN compared to previously published series. It provides updates to our knowledge of this tumor’s behavior. Albeit a retrospective study, it re-emphasizes the importance of maximal surgical resection and confirms the relative lack of benefit of chemotherapy in this tumor. This study will aid clinicians in counseling and decision making for this class of tumor and make suggestions that clinical trialists can consider for the next generation of ependymoma studies.
With the recent advancement of (epi)genomic profiling technology in pediatric oncology, molecular classifications have supplanted conventional histopathological or clinical classification in many tumor types.1 Pediatric ependymoma is one of the central nervous system (CNS) tumors whereby development of in-depth understanding of its driver of tumorigenesis from a genomic standpoint has provided significant insight into its prognosis in the recent decade.
Ependymomas are neuroepithelial tumors that can arise in all compartments of the CNS at all ages but are most common in childhood, especially in young children. The majority (>90%) of pediatric ependymomas occur intracranially either in the supratentorial (ST) compartment or posterior fossa (PF). In their seminal paper, Pajtler et al. identified 9 molecular subgroups in a large cohort of 500 ependymal tumors.2 Within the supratentorial compartment, ependymomas can be driven by distinct gene fusions initially described as involving the NF-kB subunit RELA, c11orf- or the HIPPO signaling regulator YAP1.3 Since these initial descriptions, it was found that the open reading frame component of the c11orf95-RELA fusion is the recurrent component of most variants of supratentorial disease. ZFTA-RELA fused supratentorial ependymoma (ZFTAfus ST-EPN) characterized by an oncogenic fusion between zinc finger translocation associated (ZFTA, formerly known as C11orf95) and in most cases v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA).3–6 Other alternative genes fused to ZFTA have been described in additional cases.5,7ZFTAfus ST-EPN, account for more than 70% of supratentorial ependymomas and primarily occur in children and young adults. A retrospective report found that ZFTAfus ST-EPN was associated with a poor 10-year overall survival (OS) of only 49% and progression free survival (PFS) of 19%.2 Previous reports on pre-clinical mouse models have shown that C11orf95-RELA fusion is potent oncogenes that most may transform neural stem cells by driving an aberrant NF-kB transcription program. Pathological nuclear accumulation of p65-RELA subsequently occurs which represents the hallmark of ST-RELA-EPN tumors.8,9 The 3 complementary reports by Kupp et al. in 2021 provided important and novel insight into the molecular and cancer phenotype characteristics of ZFTAfus tumors.4–6 The management of ependymoma is evolving. The current mainstays of treatment for pediatric ependymal tumors include maximal safe surgical resection followed by conformal radiotherapy.10–12 The role of chemotherapy remains contentious. To date, no chemotherapeutic regimen has proven to have any survival benefit for these patients and is currently under investigation by both the COG and International Society of Pediatric Oncology (SIOP) (NCT02265770).
Given the increased appreciation of this relatively new tumor class, we sought to better understand and define the clinical behavior of contemporaneously treated and molecularly confirmed ZFTAfus tumors to help guide future therapeutic interventions. We retrospectively collected and collated the molecular and clinical features of 108 patients treated between 1995 and 2020 at institutions across 3 continents (Europe, North America, and Australia). Herein, we present our study showing markedly improved outcomes than those reported in the seminal ependymoma paper for children with ZFTAfus - ST EPN.2 This study will aid clinicians in counseling and decision-making for this class of tumor and make suggestions that clinical trialists can consider for the next generation of ependymoma studies.
Materials and Methods
We conducted a retrospective analysis of all pediatric patients with molecularly confirmed and reported supratentorial ependymomas treated in multiple institutions in 5 countries (Australia, Canada, Germany, Switzerland, and Czechia). All patients with molecularly classified ZFTAfus ST-EPN diagnosed between 1995 and 2020 were included for analysis. Non-ZFTA-classified ependymal fusions were excluded from this analysis, including YAP fusions. In our local molecular characterization program, no patients with YAP fusions were found in over 5 years (unpublished) and in the international molecular profiling series, only one case was found.5 As such, our capacity to collect substantive and informative data on these entities would be meaningless. Demographic information, extent of surgical resection, histological grading according to World Health Organization (WHO) classification of CNS tumors, use of radiotherapy and/or chemotherapy, disease recurrence, treatment at recurrence, and clinical outcome data were collected. Patients were identified through local review of pathology databases, electronic, or paper medical records unique to each institution. The data was collected at each collaborating site through patient chart review at the respective institutions. Data from each institution was then collated for final analysis. This study was approved by local and collaborating institutions’ research ethics boards.
PFS and OS were analyzed by the Kaplan–Meier method and P-values were reported using the log-rank test. Associations between covariates and risk groups were tested by the Fishers exact test. Univariable and multivariable Cox proportional hazard regression was used to estimate hazard ratios including 95% confidence intervals. All statistical analyses were performed in the R statistical environment (v4.2.1), using R packages survival (v3.4-0), and ggplot2 (v3.3.6).
Results
Using DNA methylation profiling, both retrospectively and prospectively, we identified a total of 108 pediatric patients with ZFTAfus supratentorial ependymoma Patients were diagnosed and treated between 1995 and 2020 at the author’s respective institutions. The patient’s clinical characteristics are summarized in Table 1.
Table 1.
Clinical Characteristics of ZFTA-RELA Fused ST-EPN Patients
| Characteristic | Patients (N = 108) |
|---|---|
| Sex | |
| Male – no. (%) | 65 (60.2%) |
| Female – no. (%) | 43 (39.8%) |
| Age | |
| Median, y | 6 y 7 months |
| Range, y | 5 months–18 y 7 months |
| WHO grading | |
| Grade II | 14 (13%) |
| Grade III | 73 (67.6%) |
| unknown | 21 (19.4%) |
| Extent of surgical resection | |
| Gross total resection (GTR) | 84 (77.8%) |
| Subtotal resection (STR) | 24 (22.2%) |
| Radiotherapy | |
| Yes | 98 (90.7%) |
| No | 10 (9.3%) |
| Chemotherapy | |
| Yes | 63 (58.3%) |
| E-HIT Stratum A | 29 (26.9%) |
| E-HIT Stratum B | 16 (14.8%) |
| E-HIT Stratum C | 4 (3.7%) |
| ACNS 0831 | 5 (4.6%) |
| ICE | 3 (2.8%) |
| Personalized treatment | 2 (1.9%) |
| HIT 91 | 1 (0.9%) |
| CCLG 2007 | 1 (0.9%) |
| SIOP ependymoma II | 1 (0.9%) |
| No | 45 (41.7%) |
| Relapse/recurrence disease | |
| Local | 21 (19.4%) |
| Distant | 3 (2.8%) |
| Combined local/distant | 1 (0.9%) |
| Unknown | 14 (13%) |
There was a male predominance with 65 (60.2%) male patients and 43 (39.8%) female patients. Median age at diagnosis was 6 years 7 months (range: 5 months–18 years 7 months). The majority of the tumors were classed as histopathologic WHO grade III (14 grade II (13%); 73 grade III (67.6%) and 21 unknown (19.4%)). 84 (77.8%) patients underwent gross total resection (GTR) and 24 (22.2%) patients underwent subtotal resection (STR). The vast majority of patients, 90.7% (98/108), received radiotherapy post-operatively with 10 (9.3%) patients not. A total of 63 (58.3%) patients received multiagent chemotherapy, whilst 45 (41.7%) patients did not receive any chemotherapy. The chemotherapy regimens varied according to the institutions that the patients were treated in. Forty nine (45.7%) patients were treated on E-HIT series in 3 different stratums (Stratum A n = 29; Stratum B n = 16; Stratum C n = 4). Five (4.6%) patients were treated as per ACNS 0831. Three (2.8%) patients received ICE (Ifosfamide, Carboplatin, and Etoposide) chemotherapy regimen. Two (1.9%) patients were treated on personalized treatment as per their respective treating physicians. There were individual patients who were treated on HIT91, CCLG 2007, and SIOP ependymoma II respectively.
With a median follow-up time of 5.69 years (range: 0.23 years–20.46 years), a total of 25 patients (23%) relapsed. Fourteen patients did not have sufficient follow-up data to ascertain their relapse status. The majority of relapses 21/25 (84%) were local, with 3 (12%) patients with distant relapses and 1 (4%) patient with combined local and distant relapses.
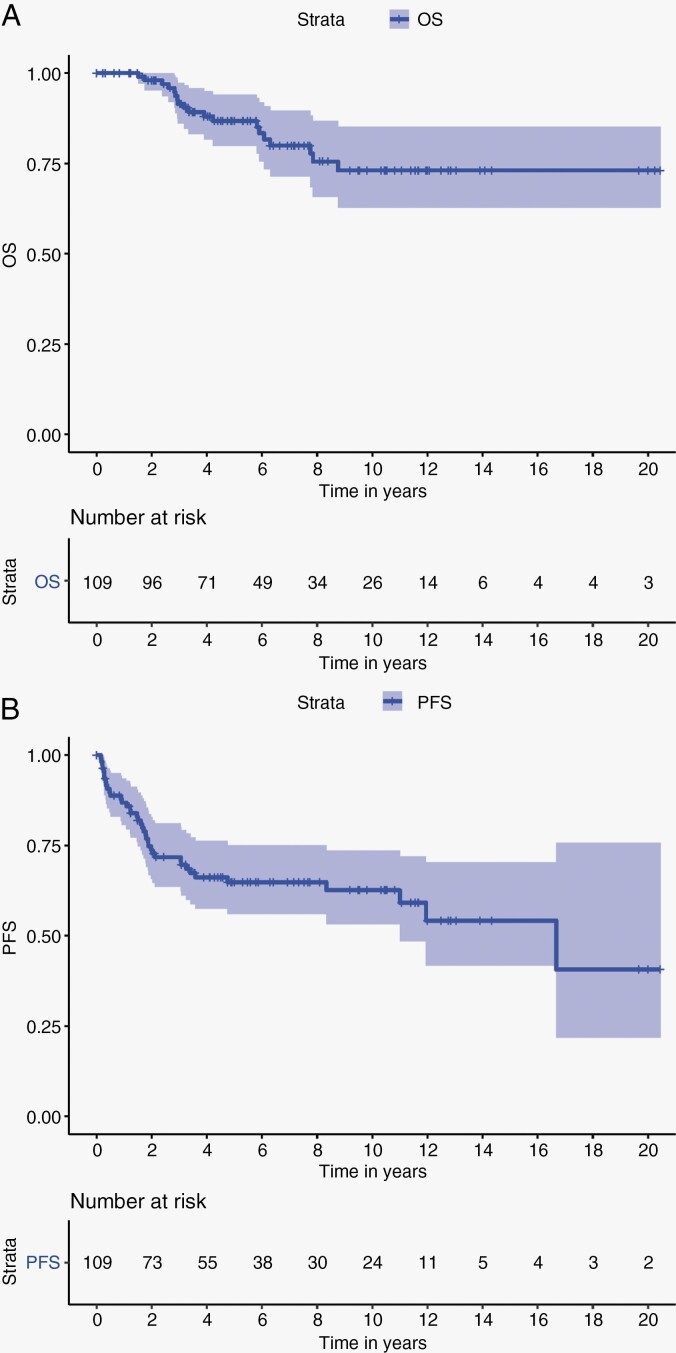
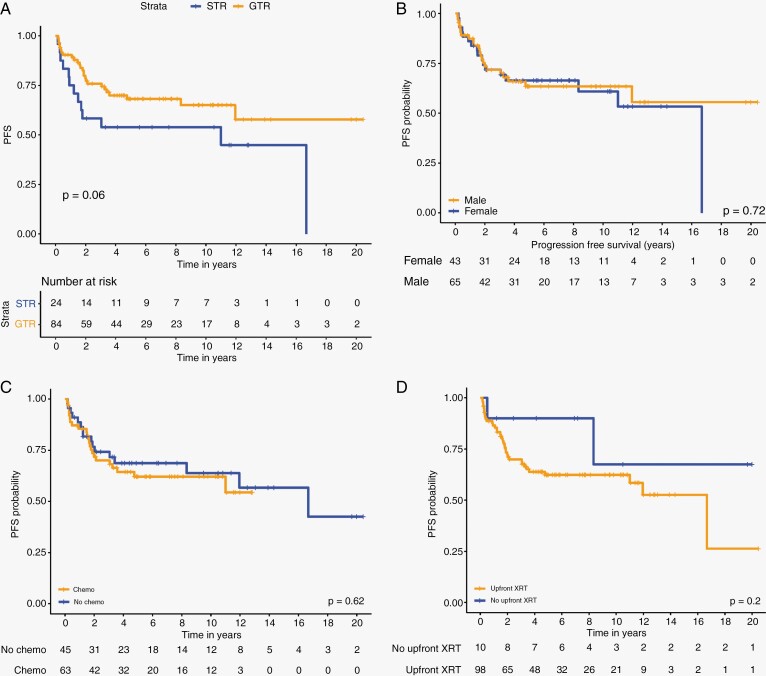
The OS at 1, 3, 5, and 10 years for the whole cohort are 100%, 91%, 87%, and 73% respectively (Figure 1a). The PFS at 1, 3, 5, and 10 years of the whole cohort are 87%, 72%, 65%, and 63% respectively (Figure 1b). Univariable analysis revealed that patients who underwent a GTR had a 5-year PFS of 68.1% (95% CI; 0.582–0.797) compared with 53% (95% CI; 0.371–0.782) for patients who underwent STR (P = .06) (Figure 2A). Age at diagnosis, gender (Figure 2B), upfront chemotherapy (Fig 2C), receipt of upfront radiotherapy (Figure 2D), and WHO status were not predictors of inferior outcome (Table 2). Multivariable analysis revealed a strong trend to poor survival with a STR compared to those with a GTR (HR 1.88; 95% CI 0.9633–3.66, P = .06) (Table 3).
Figure 1.
(A) Overall survival of whole cohort. (B) Progression free survival of whole cohort.
Figure 2.
(A) PFS analysis for patients undergoing GTR vs. STR. (B) PFS analysis by gender. (C) PFS analysis for patients by use of chemotherapy. (D) PFS analysis by use of radiation therapy.
Table 2.
Univariable Analysis of Survival in ST-RELA
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Progression free survival (n=108) | |||
| Age | 1.03 | 0.96–1.09 | .44 |
| Incomplete resection | 1.88 | 0.96–3.66 | .06 |
| Upfront radiotherapy | 2.50 | 0.59–10.61 | .21 |
| Male gender | 0.89 | 0.47–1.68 | .72 |
| Chemotherapy | 1.18 | 0.62–2.27 | .62 |
| WHO Grade III | 1.65 | 0.56–4.82 | .36 |
Table 3.
Multivariable Analysis of Survival in ST-RELA
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Progression f ree s urvival (n = 108) | |||
| Age | 1.02 | 0.96–1.10 | .47 |
| Incomplete resection | 1.22 | 0.55–2.71 | .62 |
| Upfront radiotherapy | 2.30 | 0.44–12.12 | .32 |
| Male gender | 0.75 | 0.37–1.52 | .43 |
| Chemotherapy | 0.96 | 0.44–2.07 | .92 |
| WHO Grade III | 1.10 | 0.31–3.91 | .88 |
Multivariable analysis performed on the entire cohort of patients did not find any difference in survival according to age, sex, or receipt of post-operative radiotherapy. A separate multivariable analysis was also performed on patients who underwent GTR to investigate the impact of chemotherapy on survival outcomes. For this group of patients, no statistically significant difference was seen between patients who received chemotherapy vs. those who did not (HR: 1.15; 95% CI (0.52–2.56), P = .73) (Table 3).
Discussion
There has been an explosion and rapid increase in knowledge over the past decade in the understanding of the molecular features of CNS tumors and their genetic drivers. Due to the high rates of interobserver variability and its lack of predictability in prognosticating patients’ outcomes in some studies, traditional histopathological grading has been slowly supplemented and in some tumor types supplanted by molecular subgrouping in risk stratifying patients for their management.2ZFTAfus ST-EPN was identified as a novel entity in the 2016 WHO classification of CNS tumors.13 Since then, there has been an increasing understanding of its clinical behavior and treatment outcome. In Pajtler’s published large series on the molecular classification of 500 ependymal tumors, 88 out of 500 patients had ZFTAfus tumors.2 Collectively, these patients had a much inferior outcome when compared to the other ependymoma subgroups with a 10-year PFS of 19% and 10-year OS of 49%. This retrospective series was collected from multiple institutions over decades with little clinical data. As a seminal publication on ependymoma, it has since been believed that ZFTAfus ST-EPN portends poor prognosis in contrast to historical studies with markedly better outcomes.11,12,14,15 Hukin and Palma reported on 8 and 6 patients with supratentorial ependymoma treated with surgery only.14,15 They showed that 12/14 were free of disease without intervention at the time of their publications.14,15 Merchant et al. reported on the St Jude experience using conformal radiation therapy +/- chemotherapy in upfront ependymoma treatment.11 In this prospective study, 31 supratentorial patients were enrolled with 5-year EFS of 82.9% (CI: 66.6–99.2), 5-year OS of 89.5% (CI: 76.8–100.0), and a hazard ratio of 0.52 (CI: 0.20–1.32). More recently, the Children’s Oncology Group (COG) reported on the ACNS0121 study including ZFTAfus ST-EPN. This study included enrollment of a cohort of ST-EPN with GTR and classical histology that had expectant observation only postoperatively.12 Eleven patients were enrolled and at the time of their publication, the 5-year EFS was 61.4% (95% CI, 33.2% to 89.6%). Local control was achieved in 6 patients (54.55%); local failure occurred in 4 patients (36.36%), and local and distant failure occurred in 1 patient (9.09%). Importantly the 5-year OS was 100%. Following these studies, Upadayayay and colleagues reported on infants and young children treated at St Jude in the SJYC07 infant study.16 They showed a PFS of 83.1% (+/- 17%) with only 1 of 8 patients dying from disease. In line with these findings, Jünger et al. reported a 5-year OS of 92.6% and a 5-year PFS of 74.1% in a HIT ependymoma cohort of 54 patients with ZFTA-RELA fusion-positive ependymoma.9 Given these findings, it is possible that many patients with ZFTAfus ST-EPN are being over-treated. Similar to the COG ACNS0121 study, prospective de-escalation of therapy for this tumor class might be considered.
We have also observed that patients who achieved GTR for their primary tumor have higher PFS as compared to their counterparts who only achieved STR, 5-year PFS 68.1% vs. 53% (P = .06), although this did not reach statistical significance. This observation is consistent with previously published cohorts again emphasizing the importance of achieving maximal surgical resection safely for patients with ST-EPN.
The implementation of systematic postoperative radiotherapy in clinical trials during the past 20 years has increased the proportion of patients attaining durable disease control with excellent results.17 90.7% of the patients in our cohort received radiotherapy again accentuating its contribution to the favorable overall outcome as compared to Pajtler’s paper whereby 74% of ZFTAfus EPN received postoperative radiotherapy. In combination, a higher rate of GTR followed by higher rate of radiotherapy provided superior survival outcomes in this current cohort of patients. This is an excellent reflection of the result of advances in surgery and radiotherapy through new technologies, increased participation in clinical trials, more centers with pediatric neuro-oncology expertise, improved care, and better collaboration among investigators.17 Interestingly, in our cohort, patients not receiving radiation therapy had similar PFS to those that received radiation therapy (Figure 2B). Though the numbers are small and the retrospective nature of our cohort introduces bias, this may suggest a subgroup of patients in whom surgery only approaches might be considered prospectively. Consistent with this finding, Merchant et al showed excellent outcomes for a small subset of prospectively enrolled ST-EPN on ACNS0121.12 Given the known neurocognitive issues young children in particular acquire with time post radiation therapy, this will be an important question to answer prospectively in the next series of ependymoma studies.
As for the utility of chemotherapy in the management of pediatric ependymoma, we did not find any differences in survival outcomes for those patients who received multi-agent chemotherapy. With 58.3% of the cohort had received chemotherapy with no improvement in survival outcomes (hazard ratio of 0.98), it is very possible we are overtreating children with this entity. This observation is consistent with previously published studies in ependymoma. Traditionally, investigators have explored the utility of chemotherapy in very young patients to avoid or delay irradiation. Through several international studies, its efficacy remains indeterminate.18–21 The neuro-oncology community anxiously awaits the publication of large collaborative group studies on ependymoma from the COG and SIOP-Europe conducted in the past decade.
With molecular biomarkers gaining importance in providing ancillary and diagnostic information, WHO CNS tumor classification 2021 has incorporated numerous molecular changes with clinicopathologic utility that are important for the most accurate classification.22 Ependymomas should be classified by anatomic site and by molecular group or an associated genetic alteration so that classification of the disease reflects its underlying biology. As such ST-EPN can be classified according to their key diagnostic gene i.e., ZFTA, RELA, YAP1, MAML2. Resonating cIMPACT-NOW update 7’s recommendation, an integrated and tiered approach to reporting the diagnosis is advocated for capturing information on molecular characteristics alongside histopathological features.23 Molecular subclassification is expected to significantly support treatment decisions and simplify risk stratification processes in the immediate future and should impact clinical trial design and operation in both children and adults.24 Despite the increasing importance of molecular characterization on ependymal tumors, DNA methylation studies or gene panel sequencing are not always readily available for diagnostic neuropathologists worldwide. Thankfully, ZFTAfus tumors can be diagnosed with specific FISH break apart probes making diagnosis much easier and robust. The majority of ZFTAfus tumors carry fusions with RELA and these tumors show strong nuclear accumulation of p65-RelA protein detectable by immunohistochemistry. Both methods, immunohistochemistry as well as FISH can be performed in most diagnostic units worldwide.25 It is important to emphasize, while FISH is excellent for ZFTAfus tumors, they do not reliably diagnose other known fusions.
Though our study adds to the knowledge about this tumor’s behavior, it does have limitations. Retrospective studies by nature lack knowledge of the treatment intent and decision-making around the timing of surgery, chemo- and radiotherapy. As well, the treatment approaches were heterogeneous across contributing centers making conclusions about the use of chemotherapy difficult. Since commencement of this study, other risk factors for this group and other ependymomas have emerged including CDK2NA9 and 1q/6q status26 that were not examined and will need confirmation. Prospective, worldwide clinical trials are critical as we further define treatment risk and disease stratification to standardize and harmonize therapy for children with ZFTAfus tumors.
Moving forward, we have entered an era whereby molecular genetic information is inseparable from histological and clinical information in treating patients with various tumors. We have reported the largest cohort to date of contemporaneously treated patients ZFTAfus ST-EPN and demonstrated more favorable survival outcomes compared to previously published series. High rates of GTR in particular likely have contributed to the patients’ outcomes. Given these findings, our team strongly advocates for second look surgery in cases of less than GTR where safe. Chemotherapy did not appear to provide any survival benefit in this cohort of patients. The role of radiotherapy remains unclear as the small number of patients in our series that did not receive radiation therapy did exceptionally well. International prospective clinical trial incorporating molecular risk stratification is required to further evaluate these findings, perhaps with the inclusion of new and novel agents as we learn more about the biology of this new entity.
Acknowledgments
The authors thank all participating centres and the patients and families that have contributed to this work.
Contributor Information
Chia Huan Ng, Children’s Cancer Centre, Royal Children’s Hospital, Murdoch Children’s Research Institute, University of Melbourne, Melbourne, Australia.
Denise Obrecht, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Olivia Wells, Children’s Cancer Centre, Royal Children’s Hospital, Murdoch Children’s Research Institute, University of Melbourne, Melbourne, Australia.
Michal Zapotocky, Department of Paediatric Haematology and Oncology, Charles University, 2nd Faculty of Medicine and Faculty Hospital Motol, Prague.
David Sumerauer, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Department of Paediatric Haematology and Oncology, Charles University, 2nd Faculty of Medicine and Faculty Hospital Motol, Prague.
Hallie Coltin, Developmental and Stem Cell Biology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, Toronto, Canada; Division of Haematology/Oncology, Hospital for Sick Children, Toronto, ON, Canada; Division of Pediatric Hematology-Oncology, Charles-Bruneau Cancer Centre, CHU Sainte-Justine, University of Montreal, Montreal, Quebec, Canada.
Dong-Anh Khuong-Quang, Children’s Cancer Centre, Royal Children’s Hospital, Murdoch Children’s Research Institute, University of Melbourne, Melbourne, Australia.
David D Eisenstat, Children’s Cancer Centre, Royal Children’s Hospital, Murdoch Children’s Research Institute, University of Melbourne, Melbourne, Australia; Hudson Institute of Medical Research, Melbourne, Australia; Department of Molecular and Translational Science, Monash University, Melbourne, Australia.
Kathryn M Kinross, Hudson Institute of Medical Research, Melbourne, Australia; Australia and New Zealand Children’s Haematology/Oncology Group, Melbourne, Australia.
Christine L White, Hudson Institute of Medical Research, Melbourne, Australia; Department of Molecular and Translational Science, Monash University, Melbourne, Australia; Victorian Clinical Genetics Services, Melbourne, Australia.
Elizabeth M Algar, Hudson Institute of Medical Research, Melbourne, Australia; Department of Molecular and Translational Science, Monash University, Melbourne, Australia.
Amanda Luck, Michael Rice Cancer Centre, Women’s and Children’s Hospital; South Australian Health and Medical Research Institute, Adelaide, Australia.
Hendrik Witt, German Cancer Research Centre, DKFZ, Heidelberg, Germany.
Ulrich Schüller, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Martin Mynarek, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Torsten Pietsch, Department of Neuropathology and DGNN Brain Tumor Reference Center, University Bonn Medical Centre, Germany.
Nicolas U Gerber, Children’s Hospital of Zurich, Switzerland.
Martin Benesch, Medical University of Graz, Austria.
Monika Warmuth-Metz, University Hospital Leipzig, Leipzig, Germany.
Rolf Kortmann, University Hospital Leipzig, Leipzig, Germany.
Brigitte Bison, University of Wuerzburg, Würzburg.
Michael D Taylor, Developmental and Stem Cell Biology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, Toronto, Canada.
Stefan Rutkowski, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Stefan M Pfister, Hopp Children’s Cancer Center Heidelberg (KiTZ) and Division of Pediatric Neurooncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Department of Pediatric Oncology, Hematology, and Immunology, University Hospital Heidelberg, Heidelberg, Germany.
David TW Jones, Hopp Children’s Cancer Center Heidelberg (KiTZ), Pediatric Glioma Research Group, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Nicholas G Gottardo, Hudson Institute of Medical Research, Melbourne, Australia; Perth Children’s Hospital, Telethon Kid’s Institute, Western Australia, Perth, Australia.
Katja von Hoff, Charité Universitätsmedizin Berlin, Germany.
Kristian W Pajtler, Hopp Children’s Cancer Center Heidelberg (KiTZ) and Division of Pediatric Neurooncology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Vijay Ramaswamy, Developmental and Stem Cell Biology, Labatt Brain Tumour Research Centre, The Hospital for Sick Children, Toronto, Canada; Division of Haematology/Oncology, Hospital for Sick Children, Toronto, ON, Canada; Departments of Medical Biophysics and Pediatrics, University of Toronto, Toronto, ON, Canada.
Jordan R Hansford, Children’s Cancer Centre, Royal Children’s Hospital, Murdoch Children’s Research Institute, University of Melbourne, Melbourne, Australia; Hudson Institute of Medical Research, Melbourne, Australia; Michael Rice Cancer Centre, Women’s and Children’s Hospital; South Australia Health and Medical Research Institute; South Australia ImmunoGENomics Cancer Institute, University of Adelaide, Adelaide SA, Australia.
Funding
J.R.H. is supported by grants from the McClurg Foundation, Hospital Research Foundation, Robert Connor Dawes Foundation, and My Room Children’s Cancer Charity; Analysis of Australian and New Zealand patients was supported by project funding from the Australian Government through Cancer Australia, the Robert Connor Dawes Foundation, Carrie’s Beanies for Brain Cancer and the Victorian Government’s Operational Infrastructure Support Program. VR is funded through a Canadian Cancer Society Emerging Scholar Award.
Conflict of interest statement: None declared.
Authorship Statement
Experimental design: C.H.N., V.R., J.R.H.; data collection: all authors; data analysis and interpretation of data: C.H.N., V.R., J.R.H.; manuscript writing: C.H.N., V.R., J.R.H.
References
- 1. Hübner JM, Kool M, Pfister SM, Pajtler KW.. Epidemiology, molecular classification and WHO grading of ependymoma. J Neurosurg Sci. 2018;62(1):46–50. [DOI] [PubMed] [Google Scholar]
- 2. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arabzade A, Zhao Y, Varadharajan S, et al. ZFTA-RELA dictates oncogenic transcriptional programs to drive aggressive supratentorial ependymoma. Cancer Discov. 2021;11(9):2200–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng T, Ghasemi DR, Okonechnikov K, et al. Cross-species genomics reveals oncogenic dependencies in ZFTA/C11orf95 fusion-positive supratentorial ependymomas. Cancer Discov. 2021;11(9):2230–2247. [DOI] [PubMed] [Google Scholar]
- 6. Kupp R, Ruff L, Terranova S, et al. ZFTA translocations constitute ependymoma chromatin remodeling and transcription factors. Cancer Discov. 2021;11(9):2216–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zschernack V, Junger ST, Mynarek M, et al. Supratentorial ependymoma in childhood: more than just RELA or YAP. Acta Neuropathol. 2021;141(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28(19):3182–3190. [DOI] [PubMed] [Google Scholar]
- 9. Jünger ST, Andreiuolo F, Mynarek M, et al. CDKN2A deletion in supratentorial ependymoma with RELA alteration indicates a dismal prognosis: a retrospective analysis of the HIT ependymoma trial cohort. Acta Neuropathol. 2020;140(3):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merchant TE, Bendel AE, Sabin ND, et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol. 2019;37(12):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 14. Hukin J, Epstein F, Lefton D, Allen J.. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29(1):40–45. [DOI] [PubMed] [Google Scholar]
- 15. Palma L, Celli P, Mariottini A, Zalaffi A, Schettini G.. The importance of surgery in supratentorial ependymomas. Long-term survival in a series of 23 cases. Childs Nerv Syst. 2000;16(3):170–175. [DOI] [PubMed] [Google Scholar]
- 16. Upadhyaya SA, Robinson GW, Onar-Thomas A, et al. Molecular grouping and outcomes of young children with newly diagnosed ependymoma treated on the multi-institutional SJYC07 trial. Neuro Oncol. 2019;21(10):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merchant TE. Current clinical challenges in childhood ependymoma: a focused review. J Clin Oncol. 2017;35(21):2364–2369. [DOI] [PubMed] [Google Scholar]
- 18. Grundy RG, Wilne SA, Weston CL, et al. ; Children's Cancer and Leukaemia Group (formerly UKCCSG) Brain Tumour Committee. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8(8):696–705. [DOI] [PubMed] [Google Scholar]
- 19. Massimino M, Gandola L, Barra S, et al. Infant ependymoma in a 10-year AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) experience with omitted or deferred radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80(3):807–814. [DOI] [PubMed] [Google Scholar]
- 20. Grill J, Le Deley MC, Gambarelli D, et al. ; French Society of Pediatric Oncology. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19(5):1288–1296. [DOI] [PubMed] [Google Scholar]
- 21. Venkatramani R, Ji L, Lasky J, et al. Outcome of infants and young children with newly diagnosed ependymoma treated on the “Head Start” III prospective clinical trial. J Neurooncol. 2013;113(2):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellison DW, Aldape KD, Capper D, et al. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30(5):863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khatua S, Ramaswamy V, Bouffet E.. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur J Cancer. 2017;70:34–41. [DOI] [PubMed] [Google Scholar]
- 25. Andreiuolo F, Mazeraud A, Chrétien F, Pietsch T.. A global view on the availability of methods and information in the neuropathological diagnostics of CNS tumors: results of an International Survey among neuropathological units. Brain Pathol. 2016;26(4):551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baroni LV, Sundaresan L, Heled A, et al. Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro Oncol. 2021;23(8):1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]