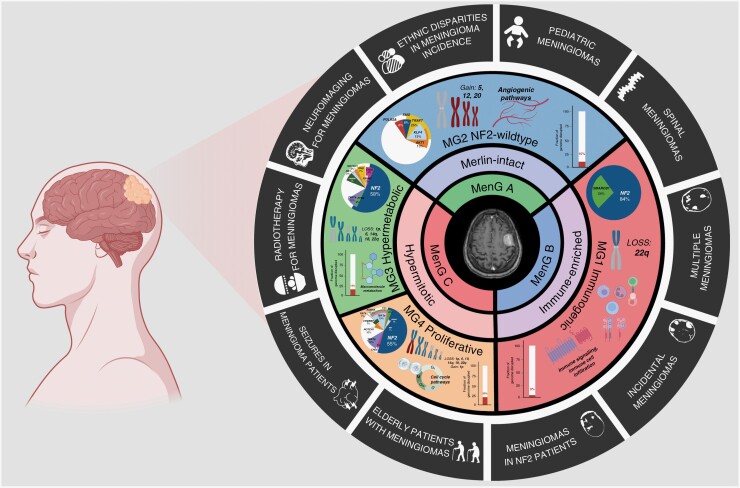
Meningiomas are the most common primary central nervous system (CNS) tumor in adults, making up 39.7% of all brain tumors and 55.4% of all benign brain tumors diagnosed in the United States.1 While these tumors are frequently treated by neurosurgeons, neuro-oncologists, and radiation oncologists, they remain understudied compared to malignant CNS tumors such as glioblastoma or medulloblastoma. This may be in part due to their perceived benign nature. However, there is increasing recognition of a significant subset of meningiomas that are biologically aggressive and resistant to the conventional treatments of surgery and radiotherapy. The development of other novel therapies through clinical trials have historically been hampered by a lack of known, targetable alterations in these tumors, and a classification system that has relied entirely on histopathology instead of objective molecular biomarkers.2,3 Recently, however, there has been a surge in meningioma studies aimed at filling these knowledge gaps. These studies have delved into the molecular biology of these tumors, identifying several key alterations associated with poor prognosis, including but not limited to homozygous loss of CDKN2A/B and TERT promoter mutation, both of which have been incorporated into the most recent iteration of the WHO classification as criteria for a grade 3 meningioma.4–9 Additionally, a significant amount of recent research has been dedicated to molecular classification, an area where meningiomas have lagged behind other CNS tumors, including glioma, medulloblastoma, and ependymoma, all of which have embraced an almost entirely molecular-based taxonomy.9–13 To this end, several international consortia, including the International Consortium for Meningiomas (ICOM), have come together with a shared goal of collaboratively sharing samples, data, and expertise across institutions and disciplines. This approach has yielded remarkable progress in the field of meningioma study, leading to landmark discoveries in the areas of classification and prognostication.3,14,15 Since Sahm et al. uncovered 2 main DNA methylation groups and 6 subgroups, several multiomic studies have aimed to improve and broaden classification by integrating data from multiple, different genomic platforms.16–20 We and others have found that when the most biologically aggressively meningiomas are included, 4 stable molecular groups consistently emerge from multiomic classification (Figure 1).21–23
Figure 1.
Schematic of recently published meningioma molecular groups and their representative molecular findings and alterations. The outside circle encompasses the clinical meningioma topics covered in this Special Issue. This figure was partly generated using BioRender.
Despite these significant strides in meningioma research, there are still important clinical questions that remain, particularly for patient populations that may have been excluded or underrepresented in genomics studies. These groups include patients with neurofibromatosis-2 (NF2) or meningiomatosis, multiple meningiomas, spinal meningiomas, incidental meningiomas, and pediatric patients with meningiomas. Moreover, in certain clinical scenarios, there remains clinical equipoise and even uncertainty surrounding the optimal treatment approaches. For instance, the role of radiotherapy or radiosurgery as a primary or adjunctive treatment modality for certain meningiomas, seizure management in meningioma patients, decision-making for elderly patients with meningiomas, and the use of PET imaging in meningiomas for diagnostic and therapeutic purposes, are all areas that require further exploration. The purpose of this special issue of Neuro-Oncology Advances (NOA) is to address these unresolved questions and uncertainties through articles written by a multidisciplinary group of experts in their respective fields. As an open-access publication, we hope this NOA special issue will offer worldwide accessibility to all interested readers, encourage new collaborations between different researchers and institutions, provide guidance to clinicians treating meningioma patients in their practice, and inspire future research projects aimed at answering some of these critically important questions. Below, we summarize each of the articles included in this special issue, sorted by their general category, and outline their importance (Figure 1).
Impacts of Sex, Race, and Age on Meningioma Diagnosis and Treatment
“The joint impacts of sex and race/ethnicity on incidence of grade 1 versus grades 2-3 meningioma across the lifespan”—Walsh et al.
“Meningioma in the elderly”—Amoo et al.
These two articles shed light on the current epidemiological landscape of clinically treated meningioma patients. Although it is widely accepted that meningiomas are more prevalent in females, the influence of race and ethnicity on the incidence rates of meningiomas, especially among various age groups, remains incompletely explored. Walsh et al. recognized this knowledge gap and utilized the comprehensive Central Brain Tumor Registry of the United States (CBTRUS) registry to examine the critical interplay between sex and race/ethnicity in meningioma patients. Their findings highlight significant incidence disparities that may inform future strategies for meningioma diagnosis and treatment. Similarly, as meningiomas are more frequently detected in elderly patients, it is imperative to develop strategies to tackle the challenges that come along with an aging demographic and the commensurate increase in tumor incidence rates seen clinically. Challenges in an elderly population include higher frailty rates, increased burden of comorbidities, and differences in risk stratification for observation versus treatment, be it surgery or radiotherapy. Addressing this critical issue, Amoo et al. offer a comprehensive review of the unique aspects of treating elderly patients with meningiomas, with a focus on preserving function and optimizing neurocognitive outcomes after treatment.
The Conundrum of Incidental and Multiple Meningiomas
“The management of incidental meningioma – an unresolved clinical conundrum”—Islim et al.
“Multiple Meningiomas: epidemiology, management, and outcomes”—Fahlström et al.
With the advent and increased availability of neuroimaging techniques, diagnoses of incidental meningiomas have substantially increased. This presents a formidable challenge for clinicians as most of these cases will remain asymptomatic and stable with time but the diagnosis itself and the possibility of progression can be a significant source of anxiety for patients. To address these points, Islim et al. comprehensively review the available clinical tools that may help predict the growth dynamics of incidental meningiomas and risk stratify these tumors into those that are more- or less-likely to progress in follow-up. While the majority of meningiomas are diagnosed as solitary tumors, certain patient populations can present with multiple meningiomas sporadically, as part of a syndrome such as NF2 or meningiomatosis, or due to childhood radiation exposure. Falström et al. provide a comprehensive review of the literature including etiology, pathophysiology, presentation, demographics, genomics, association with risk factors, and evidence-based management strategies for patients who present with multiple meningiomas.
Seizures in Meningioma Patients
“The clinical and genomic features of seizures in meningiomas”—Dincer et al.
“Clinical management of seizures In patients with meningiomas: efficacy of surgical resection for seizure control and post-operative anti-epileptic drug use”—Peart et al.
Given the commonality of seizures as a presentation in meningioma patients (affecting 10%–50% of patients) and their potential to be highly debilitating from a quality-of-life standpoint, we present two companion papers that address this clinical challenge. In the first paper, Dincer et al. predominantly explore the mechanisms of epileptogenesis in meningiomas including genomic susceptibility to seizure development as well as patient- and tumor-related factors that could increase seizure risk pre- and post-operatively. In the second paper, Peart et al. focus more on the role of resective surgery for seizure control and medical management of seizures in meningioma patients based on best available evidence and guidelines. These studies together underscore the importance of seizure management in meningioma patients that is necessary to help ensure optimal perioperative and postoperative outcomes.
Radiotherapy and Radio-Isotope Imaging for Meningiomas
“Radiotherapy and radiosurgery for meningiomas”—Chen et al.
“Advances in PET imaging for meningioma patients”—Galldiks et al.
Apart from surgical resection, other standard of care treatments for meningiomas include fractionated radiotherapy and stereotactic radiosurgery. While these are important tools, radiation may not be suitable for all meningiomas and there remains uncertainty about which patients would benefit most from these modalities. To help provide clarity on this matter, Chen et al. have conducted an extensive review of recent studies and trials that examine the efficacy of radiotherapy and radiosurgery in meningiomas. Their analysis offers valuable insights into the optimal use of radiation and provides evidence-based recommendations in line with contemporary guidelines. Furthermore, their review provides a glimpse into the future, highlighting ongoing randomized clinical trials and biological studies that will further elucidate the role of radiation in treating meningiomas moving forward. While structural magnetic resonance imaging and computed tomography have traditionally guided surgical and radiation treatments, the use of positron emission tomography (PET) has gained significant momentum as a helpful adjunct in meningioma management. Galldiks et al. have reviewed the various PET imaging techniques available for meningiomas and their efficacy in facilitating diagnosis, grading, treatment planning, and monitoring of treatment response and recurrence. Some of these techniques hold great promise and may even become integral components of the standard of care for these tumors in the future.
Rare Meningiomas in Unique Patient Populations
“The clinical, genetic, and immune landscape of meningioma in patients with NF2-schwannomatosis”—Gregory and Islim et al.
“Pediatric meningiomas: a literature review and diagnostic update”—Tauziede-Espariat et al.
“Spinal meningiomas”—Hohenberger et al.
In the concluding section of our Special Issue, we aim to highlight the importance of patient populations that have been historically underrepresented in meningioma studies. In the first article of this group, Islim et al. provide a comprehensive review of the epidemiology, management, and outcomes of meningiomas in patients with NF2. Although bilateral vestibular schwannomas are the hallmark of NF2, meningiomas are the second most frequent tumor type and found in over half of all NF2 patients.24 However, there is a dearth of knowledge about the natural history of NF2-associated meningiomas and managing them clinically can prove challenging as their presence often signifies higher severity disease and is associated with significantly increased mortality.25 The subsequent article authored by Tauziede-Espariat et al. provides a comprehensive overview of the literature on meningiomas in the pediatric population. As previously mentioned, meningiomas are primarily observed in older adults, and the occurrence of these tumors in children is rare, comprising less than 3% of primary intracranial tumors within this demographic. However, pediatric meningiomas are molecularly distinct from their adult counterparts and tend to be higher grade and more aggressive.26,27 This inherent heterogeneity makes managing and developing appropriate follow-up plans for pediatric meningioma patients a unique challenge. Our last article in the issue focuses on spinal meningiomas, which comprise only 1–10% of all CNS meningiomas.28 Although long presumed to be analogous to their intracranial counterparts, recent work has uncovered distinct molecular features that differentiate spinal from intracranial meningiomas. Importantly, the surgical techniques and management for spinal meningiomas are vastly different from those for intracranial tumors.29 Hohenberger et al. provide an overview of the current state of evidence on the epidemiology, presentation, molecular alterations, surgical management, and adjuvant therapies for spinal meningiomas to close out our Special Issue.
Conclusion
Although traditionally thought of as a benign tumor, meningiomas, particularly clinically aggressive variants, can be associated with significant morbidity and mortality. Despite recent advances in the molecular profiling of meningiomas, there still exists noteworthy challenges in the clinical management of these tumors that can create uncertainty for both healthcare providers and patients. The aim of this Special Issue is to provide a comprehensive review of the current evidence on the clinical management of select meningiomas and meningioma patient populations, written and curated by experts in their respective fields. In doing so, we hope this issue will help guide clinical treatment, address knowledge gaps, and identify topical areas of current and future research that will improve the care of meningioma patients worldwide.
Acknowledgments
This special edition and all the articles included were made possible by the generous contribution of Mr. Paul Mielnik and the Mielnik Family, who have a strong, steadfast commitment to advancing clinical meningioma research. They have been personally affected by the diagnosis of a meningioma and its subsequent treatment and it is their hope that their contribution will help others like themselves in the future. We and all the authors of this Special Issue express our gratitude for their incredible contribution and share their optimism that continued research and innovation will dramatically improve the care of meningioma patients in the future.
Supplement sponsorship. This supplement was sponsored by a generous donation from Mr. Paul Mielnik and his family to help raise awareness and advance the care of patients with meningiomas worldwide.
Conflict of interest statement. None declared.
Contributor Information
Justin Z Wang, MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada.
Farshad Nassiri, MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada.
Kenneth Aldape, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Gelareh Zadeh, MacFeeters Hamilton Neuro-Oncology Program, Princess Margaret Cancer Centre, University Health Network and University of Toronto, Toronto, Ontario, Canada; Division of Neurosurgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada; Princess Margaret Cancer Centre, University Health Network, Toronto, Ontario, Canada.
References
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Suppiah S, Nassiri F, Bi WL, et al. Molecular and translational advances in meningiomas. Neuro-oncology. 2019;21(Supplement_1):i4–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biczok A, Kraus T, Suchorska B, et al. TERT promoter mutation is associated with worse prognosis in WHO grade II and III meningiomas. J Neurooncol. 2018;139(3):671–678. [DOI] [PubMed] [Google Scholar]
- 5. Goutagny S, Nault JC, Mallet M, et al. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spiegl-Kreinecker S, Lötsch D, Neumayer K, et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018;20(12):1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boström J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159(2):661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat Rev Dis Primers. 2019;5(1):11. [DOI] [PubMed] [Google Scholar]
- 13. Bayliss J, Mukherjee P, Lu C, et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med. 2016;8(366):366ra161–366ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nassiri F, Mamatjan Y, Suppiah S, et al. ; International Consortium on Meningiomas. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olar A, Wani KM, Wilson CD, et al. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017;133(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 17. Bayley JC, Hadley CC, Harmanci AO, et al. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci Adv. 2022;8(5):eabm6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Driver J, Hoffman SE, Tavakol S, et al. A molecularly integrated grade for meningioma. Neuro Oncol. 2022;24(5):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hielscher T, Sill M, Sievers P, et al. Clinical implementation of integrated molecular-morphologic risk prediction for meningioma. Brain Pathol. 2022:e13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maas SLN, Stichel D, Hielscher T, et al. ; German Consortium on Aggressive Meningiomas (KAM). Integrated molecular-morphologic meningioma classification: a multicenter retrospective analysis, retrospectively and prospectively validated. J Clin Oncol. 2021;39(34):3839–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nassiri F, Liu J, Patil V, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choudhury A, Chen WC, Lucas CG, et al. Hypermitotic meningiomas harbor DNA methylation subgroups with distinct biological and clinical features. Neuro Oncol. 2023;25(3):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhury A, Magill ST, Eaton CD, et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet. 2022;54(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goutagny S, Bah AB, Henin D, et al. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: clinical, radiological, and molecular features. Neuro Oncol. 2012;14(8):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans LT, Van Hoff J, Hickey WF, et al. SMARCE1 mutations in pediatric clear cell meningioma: case report. Journal of Neurosurgery: Pediatrics. 2015;16(3):296–300. [DOI] [PubMed] [Google Scholar]
- 27. Kirches E, Sahm F, Korshunov A, et al. Molecular profiling of pediatric meningiomas shows tumor characteristics distinct from adult meningiomas. Acta Neuropathol. 2021;142(5):873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin HK, Park JH, Cho YH, et al. Risk factors for high-grade meningioma in brain and spine: systematic review and meta-analysis. World Neurosurg. 2021;151:e718–e730. [DOI] [PubMed] [Google Scholar]
- 29. Hua L, Alkhatib M, Podlesek D, et al. Two predominant molecular subtypes of spinal meningioma: thoracic NF2-mutant tumors strongly associated with female sex, and cervical AKT1-mutant tumors originating ventral to the spinal cord. Acta Neuropathol. 2022;144(5):1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]