Abstract
Spinal meningiomas (SM) are lesions with a mostly favorable oncological and surgical prognosis and a low incidence of tumor recurrence. SM account for approximately 1.2–12.7% of all meningiomas and 25% of all spinal cord tumors. Typically, SM are located in the intradural extramedullary space. SM grow slowly and spread laterally into the subarachnoid space, stretching and sometimes incorporating the surrounding arachnoid but rarely the pia. Standard treatment is surgery with the primary aims of achieving complete tumor resection as well as improving and recovering neurologic function. Radiotherapy may be considered in case of tumor recurrence, for challenging surgical cases, and for patients with higher-grade lesions (World Health Organization grade 2 or 3); however, radiotherapy is mostly used as an adjuvant therapy for SM. New molecular and genetic profiling increases the understanding of SM and may uncover additional treatment options.
Keywords: molecular and genetic targets, recurrence rate, surgical therapy
Spinal meningiomas (SM) account for approximately 1.2–12.7% of all meningiomas and 25% of all spinal cord tumors.1–5 In 1938, Cushing and Eisenhardt performed the first surgical resection of a spinal meningioma and described it as “one of the most satisfying of all surgical procedures.” From a surgical and oncological perspective, SM are lesions with a mostly favorable prognosis. Primary surgical resection is the therapy of choice with a favorable outcome and with a lower incidence of tumor recurrence than intracranial meningiomas. Here, we review the current knowledge of managing spinal meningiomas.
Epidemiology
Spinal intradural tumors have an incidence of 64 per 100,000 person-years and account for 3% of primary tumors of the central nervous system (CNS).6 Spinal intradural extramedullary tumors account for two-thirds of all spinal neoplasms. Spinal meningiomas (SM) are the second most common intradural spinal lesion after spinal schwannomas.7,8 SM are intradural extramedullary lesions that originate from meningothelial arachnoid membranes within the spinal dura mater.8 SM almost always adhere to the inner layer of the dura and are thus mainly located in the intradural compartment. SM generally respect the pial layer of the spinal cord, which can be used as an anatomical dissection plane during resection.9
SM can grow along anywhere along the neuraxis/spinal column but are predominantly observed in the thoracic (mid-spine) region (67–84%), the cervical spine (14–27%), and—in rare cases—in the lumbar spine (2–14%).4,10–15 SM may develop at any age, but the incidence increases with age and decreases after reaching a peak. Based on an analysis of the SEER database from 2004 to 2018, the peak age of benign SM is 80–84 years, 75–79 years for borderline SM, and 70–74 years for malignant SM.16
The age-adjusted incidence rate at the peak age of malignant SM is less than 0.25/100 000, whereas the reported age-adjusted incidence rate of supratentorial meningioma between 70 and 74 years is higher than 0.4/100 000.16 The prevalence of intracranial and spinal meningiomas is higher in women with a female:male ratio of 3:1 to 4:1 for both sites.4,17 Although a clinical series of SM in the 1980s had shown an even stronger female predominance in SM, this finding was never affirmed in other, more modern clinical series.17 Nevertheless, there is ample evidence that meningiomas can express receptors for sex hormones such as androgen, estrogen, and progesterone. Furthermore, endogenous and exogenous hormone exposure may be linked to a higher risk of developing meningioma.18 However, there is no clear evidence that this aspect may be different for SM because the expression pattern of receptors of sex hormones is similar to that of intracranial meningiomas.19
Neurofibromatosis 2 (NF2) is associated with the development of meningioma.20 14% of all spinal tumors found in NF2 patients are meningiomas.21 Detection of a spinal meningioma raises suspicion for NF2, particularly in younger patients, and when found in NF2 patients, tend to have a more aggressive course than in non-NF2 patients.22
Classification
The majority of SM are histopathologically benign and represent World Health Organization (WHO) grade 1 tumors. Only a small percentage are atypical (WHO grade 2) (5–25%) or anaplastic (WHO grade 3) meningiomas (1–5%).8,23 The histological subtypes of SM are similar to those seen in cranial meningiomas.24 The most common histological subtypes of SM are WHO grade 1 meningothelial, psammomatous, and transitional meningiomas. In general, these lesions are usually solitary, well-delineated, not invading the spinal cord and do not normally metastasize to any other parts of the central nervous system (CNS) or the body.1,25,26 SM have lower recurrence rates after surgical resection (between 1.3% and 6.4%) than cranial meningiomas.23 In the past decade, the molecular characterization of meningiomas has increased substantially, improving the original histological classification by integrating molecular data.27–29 Recently, a DNA methylation-based classification of intracranial meningiomas has been proposed to capture clinically more homogenous groups and to improve the power of predicting tumor recurrence and prognosis.30 For WHO grade 3 meningiomas, TERT promoter mutations or homozygous deletions of CDKN2A and/or CDKN2B have been described as additional defining molecular alterations.31 While this classification has increased the prediction of the clinical course of this tumor entity, the identification of molecular targets has not yet led to any meaningful novel therapeutic approaches. Most sporadic intracranial meningiomas exhibit frequent somatic mutations in NF2, TRAF7, KLF4, AKT1, SMO, and PIK3CA. Homozygous deletion of the NF2 gene is found in up to 80% of nonfamilial meningiomas and in 100% of patients with NF2 and SM.20 SM and intracranial meningiomas have historically been considered as the same entity, just developing at different anatomical sites. However, recent molecular data may change this paradigm because homozygous chromosome 22 deletions are more commonly detected in spinal meningiomas.32–34 In a microarray-based study of spinal and intracranial meningiomas, Sayagues and colleagues reported that in 86% of the spinal tumors versus 56% of the intracranial tumors, the detected ancestral tumor cell clone showed either absence of any chromosomal abnormality or monosomy 22/22q alone.35 Other chromosomal aberrations reported in SM include loss of 1p, 9p, and 10q or gain of 5p and 17q but these were mostly detected in atypical or anaplastic meningiomas supporting the notion that SM may be genetically more stable than intracranial meningiomas36,37 In addition, other genes such as of the DUSP family, the NR4 family, CMKOR, and FOSL2 have been identified to play a role in SM.32 SM were underrepresented in the cohorts of previous molecular studies on meningioma, but a recent genome-wide DNA methylation analysis identified SM to be different from cranial meningiomas. The analysis showed that SM consist of two major genetically and epigenetically distinct tumor groups.38 Targeted next-generation sequencing of frequently mutated genes in meningiomas identified two mutually exclusive molecular subtypes of SM WHO 1 characterized by AKT1E17K or NF2 mutations.39 While NF2-mutant tumors were strongly associated with female sex and most frequently occur at the dorsal thoracic spine, AKT1-mutant tumors occur in the cervical spine ventral to the spinal cord and are predominantly meningothelial. Smith et al. recently identified germline SMARCE1 mutations to be associated with multiple spinal meningiomas and with a clear-cell histological subtype.40,41
Further studies are needed to establish the clinical impact of a further refined classification of SM and to enable the development of targeted therapies for molecular subgroups of SM—if molecular key players can be identified.
Clinical Presentation and Diagnosis
The typical clinical presentation of SM consists of pain followed by gait, sensory, and bowel or bladder dysfunction.2 However, the clinical presentation of SM is often rather unspecific and may be marked by chronic or acute spinal cord compression causing neurologic dysfunction and progressive myelopathy, depending on the tumor site. About 50% of affected patients experience unspecific back pain, whereas radiating pain, motor deficits, and sensory loss often only progress very slowly.
In advanced stages of spinal cord compression, dissociated long tract signs or in rare cases Brown-Séquard syndrome may occur. Many SM are asymptomatic for a long time because of their slow growth rate and become symptomatic when the spinal cord or nerve roots are compressed to a critical level. Due to the “unspecific nature and indolent course of symptoms and signs may delay timely diagnosis.1,9,42,43
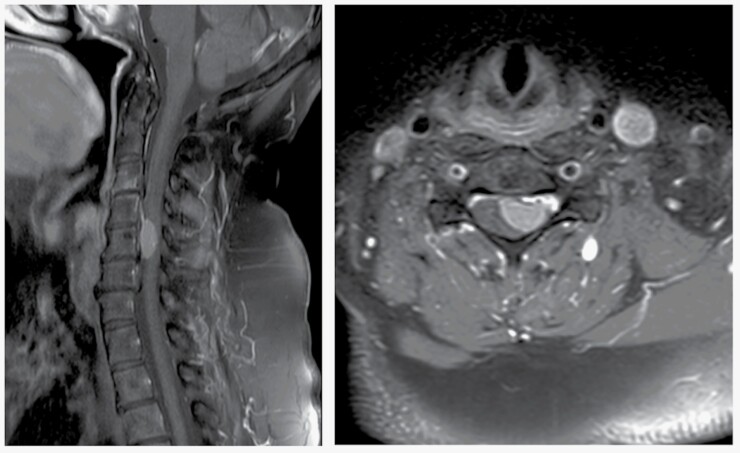
Spinal magnetic resonance imaging (MRI) represents the gold standard and has greatly improved preoperative diagnosis and treatment.26 SM display a similar spectrum of MRI characteristics as cranial meningiomas.44–46 MRI allows the assessment of the extent of involvement and compression of neural and neurovascular structures and the presence of myelopathy (Figure 1). The diagnostic work-up can be extended by computed tomography (CT) to assess any bony involvement, which is rather rare in SM as compared to cranial meningiomas. Primary intraosseous meningioma of the spine is an exceptionally rare tumor entity with only one case reported in the literature so far.47 More importantly, CT provides information about the presence and extent of tumor calcification, which is a relevant factor to consider in surgical planning as calcified tumors can be more difficult to resect. Tumor texture can influence surgical morbidity and outcome as recently shown for intracranial meningiomas.48 Based on imaging characteristics and growth patterns, SM can usually be differentiated from other intradural extramedullary spinal cord lesions such as neurofibroma and schwannoma. Signal intensity ratio of the spinal tumor and fat on T2 weighted images is useful for differentiating schwannomas from meningiomas to obtain an accurate diagnosis.49 Furthermore, bony changes such as enlarged neural foramina or pedicle erosion are suspicious for spinal nerve sheath tumors.
Figure 1.
Preoperative MR image showing a classic case of homogeneously enhancing cervical spinal meningioma in (A) sagittal and (B) axial T1 contrast-enhanced sequences and significant compression of the spinal cord.
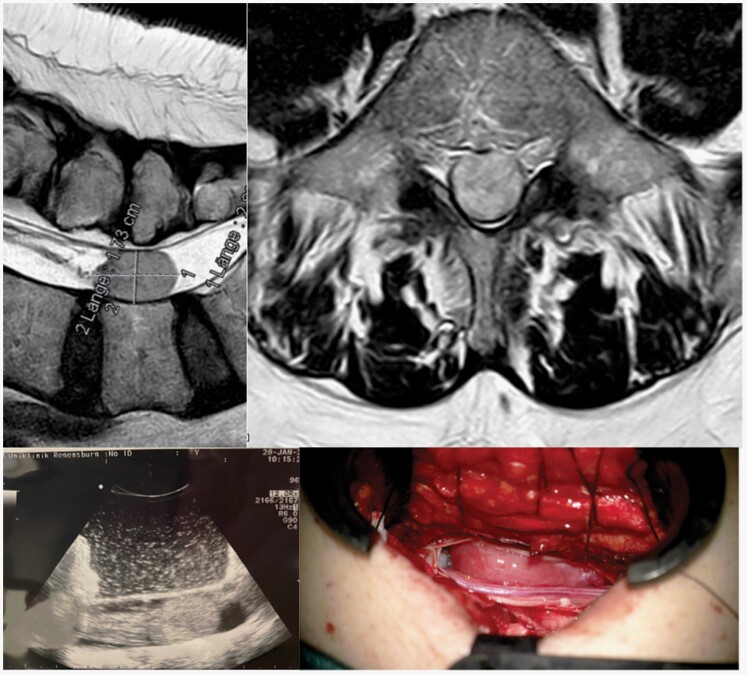
However, solitary fibrous tumors, as previously termed hemangiopericytoma, have a similar appearance on MRI, and differential diagnosis can only be established by a neuropathologist after surgical resection (Figure 2A,B). Intraoperatively, these very rare tumors may display higher vascularization and/or higher adherence or infiltration of surrounding neural structures that must be assessed during surgical resection to avoid surgical morbidity. More importantly, establishing the diagnosis of solitary fibrous tumors or hemangiopericytoma is highly relevant for patient management because adjuvant radiotherapy must be considered particularly in patients in whom gross total resection (GTR) cannot be achieved because these tumors have a significantly higher recurrence rate than SM.50
Figure 2.
(A) Preoperative T2-MR image of an intradural space-occupying lesion at level L5, showing (B) homogenous contrast enhancement on T1-MR imaging. This lesion was a lumbal spinal solitary fibrous tumor, also termed hemangiopericytoma, WHO II, which reflects one of the possible differential diagnoses when a spinal meningioma is suspected in preoperative MR imaging. (C) The intraoperative ultrasound image confirmed the correct level and extent of surgical access before dural opening. (D) In contrast to most spinal meningiomas, this tumor showed a more reddish coloration during surgery, which is indicative of higher vascularization.
Indication for Treatment
SM are much rarer than intracranial meningiomas, but, in general, the same principles for therapeutic decisions apply.51 There is no evidence to suggest the growth dynamics of spinal meningiomas differ from the majority of intracranial meningiomas.52 Therefore, a potentially indolent clinical course has to be considered when SM are incidentally detected during neuroimaging. Nonsymptomatic and small SM may be managed with a wait-and-see strategy using annual MR imaging as recommended for meningiomas in general.51 However, given that the spinal canal is a narrow, confined anatomical compartment containing the vulnerable spinal cord, any decision on an observational strategy has to consider that further growth may lead to compression of functionally relevant neural structures, and of course that the spinal canal is proportionally much smaller than the cranial vault, therefore, a smaller amount of growth of a smaller meningioma in the spine, may cause more neurological morbidity than a meningioma of the same size in the cranium. Enlarging, untreated SM may result in serious neurologic deficits. Therefore, the primary treatment for most growing and symptomatic SM is surgical resection of the space-occupying tumor; such surgical interventions have the potential to be curative, are generally quite safe, and usually result in rapid functional recovery12–14 The decision on surgery or observation should balance the benefit of tumor removal versus the risk of surgical morbidity and outcome on an individual basis including comorbidities and patient expectations.
The occurrence of multiple SM is mostly associated with NF2.20 In such patients, treatment preferably by surgery should be reserved for symptomatic or significantly growing SM and take into account the stage of disease and any previous treatment. Therapeutic strategies should be discussed in a multidisciplinary setting, particularly in case of patients with NF2.53
Surgical Therapy
Advances in the development of imaging and surgical techniques (e.g. MR imaging, intraoperative neuromonitoring (IOM), intraoperative ultrasound, microsurgical techniques, and surgical ultrasonic aspirator) have resulted in earlier diagnosis and in the ability to achieve GTR while preserving or even improving neurological dysfunction. Surgical resection is the first-line therapy for the treatment of symptomatic SM, with a GTR often leading to cure for most patients.
Furthermore, surgery allows the immediate relief of neurologic symptoms instead of primary radiotherapy or gamma knife which takes much longer to control tumor growth and shrink tumor, and the establishment of a histopathological diagnosis. The surgical approach to resection of SM requires the dissection of the musculoligamentous structures of the spine. Various vertebral components must be removed to create a corridor to the intraspinal compartment. The cardinal principles of intraspinal tumor resection are to minimize the intraoperative risks of vertebral column deformity, and neurologic injury to the spinal cord.54 The optimal and safest surgical approach to SM resection normally depends on the site and extension of the tumor. In most dorsal and dorsolateral tumors, one- or two-level hemilaminectomy or laminectomy is adequate, allowing the surgeon to work in an adequate surgical corridor to achieve complete resection, even of anterior and anterolateral lesions.2,55 Raco et al. described the posterior approach as the gold standard in the majority of cases.2 However, some tumors located anterior or anterolateral to the spinal cord require a more lateral exposure, especially in case intradural lesions with extradural extension infiltrating the vertebral body or in case of massively calcified lesions and recurrent tumors with spinal cord invasion. As mentioned above, SM are typically classified as intradural extramedullary lesions, although 5–14% of SM may have an extradural component.10,11 In these cases, costotransversectomy or partial vertebrectomy may be required to improve exposure and allow safer tumor removal.46
In case of ventral extension or origin of the SM, removal of the articular process laterally may be useful to provide a corridor to the ventral spinal cord. In case of anterior or anterolateral cervical SM, an anterior cervical approach with corpectomy and grafting with fusion may provide an ideal corridor for tumor resection. With this approach, primary dural repair can be difficult and necessitate CSF drainage (CSF) using a intra- or postoperative lumbar drain.8 For lesions located in the lateral or anterior corridor between T3 and L2, a lateral extracavitary approach can be used to allow circumferential neural decompression with direct visualization and extrapleural or extravisceral dissection.56 This approach may require posterior instrumentation with screws in the setting of extensive pedicle removal and disruption of the ipsilateral facet joint. In general, the necessity of spinal fusion in the setting of SM surgery depends on the dimensions of the lesion, its site along the spinal axis, its position relative to the spinal cord, and the presence of extradural or vertebral column extension that defines the extent of the surgical approach.54 Predictors of spinal instability after SM resection are well established and include multilevel laminectomy, disruption of the facet joints, and corpectomy.56–58 In women, SM most often occur in the posterior, posterolateral, or lateral thoracic region (80%) of the spine, followed by the anterior cervical region (15%), and least often in the lumbosacral region (5%).26 In men, 50% of SM occur in the thoracic region and 40% in the cervical region.59 SM more often develop in the upper cervical region and foramen magnum than other tumors.26 With regard to the predisposed anterior or anterolateral development in the upper cervical region, SM may encase or surround but rarely infiltrate the vertebral artery.60
Another important aspect when planning surgical resection of thoracic SM is the fact that radiculomedullary arteries (RMA) can be found at any thoracic vertebral level (ventral and posterior). RMA play an important role in spinal cord vascularization, and their preservation during surgery can be critical to functional outcome. RMA injury may constitute a significant pitfall during surgical procedures and may result in complete paraplegia. The best known RMA is the anterior radicular artery (Adamkiewicz artery).61
The gold standard for the surgical treatment of SM is complete tumor resection because it is a predictor of good prognosis with potential cure. The individual outcome depends on the size and site of the tumor, its preoperative neurologic state, and the age and medical comorbidities of the patient.62 The Simpson grading classification is commonly used to define the extent of SM resection, although the classification was originally established to describe the extent of tumor and dural resection of cranial meningiomas.37 According to the Simpson grading score, which is based on Simpson’s description of tumor recurrence of intracranial meningioma after incomplete surgical removal in 1957, Simpson grade I resection (representing complete tumor removal including the dural and arachnoid matrixes) is the most promising surgical approach to avoid tumor regrowth. However, Simpson grade I is rarely feasible, especially in patients with anterior dural attachment because of the risk of damaging the spinal cord or the difficulty of dural repair after radical excision.62 Therefore, Simpson grade II (complete removal of exophytic tumor with coagulation of suspicious dural attachment) has been proposed as an acceptable option with a recurrence rate of 1–8%.63
Many authors have suggested Simpson grade II removal as the achievable standard.9 Consequently, in most published series, the dural attachment of SM is cauterized rather than resected because of the variable site of the dural attachment and the difficulty of repairing a dural defect in the anterior and lateral corridor. Another technique for resecting the dural attachment is to separate the dura into its inner and outer leaflets and resect the inner layer containing the tumor attachment.8 However, it is not clear if this technique leads to a lower recurrence rate than a Simpson grade 2 resection.
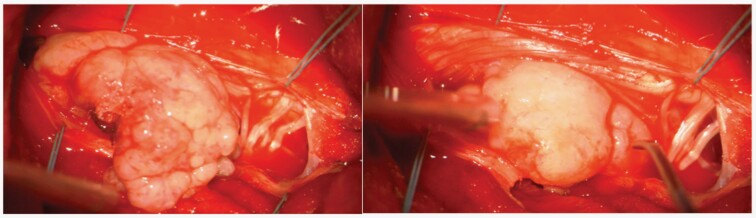
Surgery for SM involves surgery involves a wide spectrum of technical procedures that bear the risk of damaging the spinal cord, nerve roots, or main blood vessels. One of the most important surgical risks is postoperative neurologic worsening, including, but not limited to, motor weakness, sensory loss, and bowel and bladder dysfunction. Even when relevant steps are taken to mitigate neurological damage, some risk remains. The introduction of the surgical microscope in neurosurgery has made microsurgical resection of SM the standard of care. Neuronavigation for spinal cord tumors has not become as commonplace as it has for intracranial lesions, because the main use would be to localize spinal level, and this can be done with C-arm or O-arm preoperatively. However, the standardized use of intraoperative ultrasound can be a helpful adjunct in identifying and localizing meningiomas by providing information about the size, shape, and degree of displacement of the spinal cord, thereby helping to intraoperatively optimize the surgical approach, especially to confirm the meningioma location after laminectomy before opening the dura and whether additional bony removal is needed,8 (Figure 2C,D). A key element of successful surgical resection is minimizing manipulation and displacement of the spinal cord by using an exposure wide enough to safely and efficiently access the tumor and associated dural attachment8 (Figure 3).
Figure 3.
Intraoperative view of a large intradural cervical meningioma.
IOM provides the opportunity to assess the functional integrity of susceptible neural elements during surgery. The domain of IOM is to provide information about the spinal cord or nerve root manipulation and resection as well as the preservation of preservation of motor and/or sensory function. IOM enables the continuous evaluation of the sensory and motor functions of the spinal cord by means of somatosensory-evoked potentials (SSEP), motor evoked-potentials (MEP), neurogenic motor-evoked potentials (NMEP), D-waves (D-waves directly from the epidural space are generated by the direct activation of the axons of fast-conducting fibers of the cortico-spinal tract). This continuous evaluation reduces the incidence of subsequent neurological complications after spinal surgery.9,64 Spinal surgery involves some additional and commonly used monitoring modalities, such as spontaneous and stimulated electromyography, direct spinal cord stimulation, and reflex monitoring.65 Surgeons should choose the appropriate monitoring technique and take into account the individual findings and effects of anesthesia for each patient.
Surgical Outcome
As previously mentioned, surgical resection is first-line therapy for SM and generally considered a safe and efficient procedure in experienced centers. The rate of complete resection of SM mentioned in the literature range between 82% and 98%.9,23,66 It is important to emphasize that complete resection in SM includes Simpson grade I and II resection. Compared to intracranial meningiomas, resection of the dural attachment for spinal meningiomas is less radical and is not routinely performed for spinal meningiomas, with rates ranging from 14% and 58%.23,42
Generally, the recurrence rates of SM after neurosurgical resection are low, ranging between 1.3% and 6.4%; recurrence occurs within 1–17 years.10,11,67 SM generally have a more indolent clinical course, which is most likely due to the lack of genetic abnormalities often found in recurrent intracranial meningiomas.8,68 In addition, the slow growth rate and propensity of SM to present in older age also contributes to the lower recurrence rate.8 Subtotal or partial resection has been implicated as a cofactor in tumor progression but subtotal removal does not necessarily lead to progression.69,70 Surgery on recurrent or reoperation on progressive SM is more challenging because of the presence of arachnoid scarring, making extensive resection difficult and increasing the risk of surgically morbidity. As with intracranial meningioma, atypical or anaplastic SM subtypes have been associated with increased recurrence.1
In the majority of patients who have undergone Simpson grade II resection, long-term follow-up can be achieved without developing recurrent SM at the same site. A series conducted by Cohen-Gadol et al. showed much higher recurrence rates in patients younger than 50 years because of a higher frequency of cervical SM, extradural tumor extension, and en plaque growth, all of which are barriers to complete resection and implicate more difficult primary surgery.71
Only a few of the published contemporary reports have consistently analyzed distinct functional neurological performance and recovery after surgery in a large sample size with sufficiently long follow-up to detect true recurrence.72,73
The majority of patients have improved neurological function after tumor removal. A large nationwide population-based study including 2844 patients with SM reported a rate of 86.8% of patients with good functional outcome and without any symptoms specific after three years of surgery. Multivariable logistic regression analysis defined older age at the time of surgery, a high level of comorbidities, and aggressive tumor pathology as risk factors for decreased functional outcome.72 This finding is in line with that of previous smaller case series showing a higher risk of mortality in patients of very advanced age with severe comorbidities.66 Prognostic factors in patients with motor deficits due to SM seem to include the site of the SM in relation to the spinal cord.73 Moreover, perioperative morbidity is mostly related to the extent of manipulation of the surrounding spinal cord structures. Temporary deterioration of neurological function usually does not last longer than 6 months otherwise, deficits are generally fixed or permanent.74 Even with the use of IOM, surgical resection is challenging and bears a risk of transient or permanent neurological deterioration.
However, IOM adds to the modern surgical armamentarium of spinal surgery and improves outcome after SM resection.75 Many authors have reported rates of up to 19.5% for new neurological deficits or worsening of pre-existing neurological impairment, or both.9,76 Sandalcioglu et al. found in their series of SM that higher age and complete calcification of the tumor (based on intraoperative findings) were risk factors for permanent neurological deterioration. Surprisingly and in contrast to other authors, Sandalcioglu et al. found no association between the site of dural insertion, the extent of the tumor, or the degree of resection and postoperative neurological deterioration.23
So far, few studies have examined quality of life and return to work (RTW) after surgical treatment of SM. Pettersson-Segerlind et al. showed that each of the working patients in their study (n = 41/48.8%) had returned to work, most of them within three months. 96% of their patients would accept surgery for the same diagnosis again.77 In cranial meningiomas, quality of life and return to work have been extensively studied, for example by Sekely et al. who found that neurocognitive and psychological factors contribute to the RTW status in patients with meningioma.78
Radiotherapy and Chemotherapy
Primary radiotherapy or radiosurgery for SM represent alternative treatment options to microsurgical resection and should be considered in patients with a high risk of surgery because of the tumor site or comorbidities.8 Historically, radiosurgery of SM lacked the precision to safely deliver a large enough dose in the vicinity of the spinal cord. However, modern techniques have solved this problem. Accuracy of patient setup and planning and irradiation techniques have been improved dramatically. Nowadays patients are treated utilizing online image-guided tracking systems using stereoscopic radiographs of the involved spine segment and compared with planning system generated digitally reconstructed radiographs (e.g. CyberKnife system equipped with Xsight Spine tracking software of Accuray Inc. Sunnyvale CA) or using gantry-mounted cone beam CT scanner (e.g. Elekta Synergy S 6-MV linear accelerator with CBCT image guidance). The Cone beam CT is performed in treating position before each treatment and compared software based with the planning CT-scan. The automatic calculated correction is implemented by a robotic couch (e.g. HexaPOD evo RT Couchtop) using six degrees of freedom for precise positioning with an accuracy less than 1 mm in all directions.79 The development of intensity-modulated radiotherapy (IMRT) is a new method for delivering highly conformal radiation to tumors and used everywhere. IMRT allows conformal dose distribution in the tumor while simultaneous protection of risk structures such as the spinal cord even if the structures are adjacent.80 Response rates similar to those of cranial meningiomas have been reported, but long-term data are still lacking.81,82 Primary radiosurgery is an evolving field and may be a safe and effective alternative to surgery in selected patients.83
Adjuvant radiotherapy should be considered following subtotal resection, for recurrent SM with higher risk of reoperation, or higher grade SM such as atypical or anaplastic tumors.8 A possible complication of radiotherapy is the development of delayed myelopathy. This complication needs to be considered because of the mostly benign course of most SM, as these patients have a long life expectancy.84 However, the incidence of radiation-induced complications is remains low, ranging below 5%.85
The role of adjuvant chemotherapy in SM is very limited, similar to that in cranial meningiomas. Many studies using different medical-based approaches were assessed for their effectiveness to inhibit or stabilize meningioma growth but failed to demonstrate any clinical benefits for the patients. Therefore, adjuvant chemotherapy is not part of the standard therapy for meningiomas, independent of the tumor site. In cases of recurrent SM, however, chemotherapeutic options need to be considered on an individual basis within an interdisciplinary setting. Graillon et al. summarized that despite a low level of evidence, some systemic therapies can be considered for patients with recurrent meningioma who are unsuitable for further surgery or radiotherapy.86 In recurrent high-grade meningioma, everolimus-octreotide combination, bevacizumab, sunitinib, and peptide receptor radionuclide therapy exhibit a signal of activity that may justify their clinical use. Despite a lack of clear signal of activity till date, immunotherapy may offer new perspectives in the treatment of these refractory tumors. Taking into account the rapidly evolving molecular classification of SM and the increasing pharmacological options in other oncological entities, a personalized therapeutic option may be established if an individual case-specific molecular analysis identifies a druggable molecular target.
Conclusion and Future Directions
SM are mostly benign, slow-growing lesions and represent the most common spinal tumors in adults. Primary treatment consists of surgical resection to remove the tumor completely. Multimodal IOM is significantly predictive of postoperative deficits after the resection of spinal cord tumors.75 Most patients will benefit from improved neurological function after tumor resection. Rates of recurrence of SM after surgical resection are low, ranging between 1.3% and 6.4%.
Radiation therapy is an important adjuvant concept after subtotal resection and for grade 2 or grade 3 lesions. Modern concepts in molecular and genetic profiling have the potential to improve prognostication and to enable adjuvant treatment of patients with recurrent SM or higher-grade lesions. However, whereas the genetic and molecular landscape of intracranial meningiomas is well characterized the differences to SM are not fully unraveled yet. Further studies are needed to clarify if recent and further advancements in the understanding of SM will be able to translate into a meaningful clinical impact.
Conflict of interest statement. None declared.
Supplement sponsorship. This supplement was sponsored by a generous donation from Mr. Paul Mielnik and his family to help raise awareness and advance the care of patients with meningiomas worldwide.
Contributor Information
Christoph Hohenberger, Department of Neurosurgery, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
Peter Hau, Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany; Wilhelm Sander Neuro-Oncology Unit, University Medical Center Regensburg, Regensburg, Germany.
Karl-Michael Schebesch, Department of Neurosurgery, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
Oliver Kölbl, Department of Radiotherapy, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
Markus J Riemenschneider, Department of Neuropathology, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
Fabian Pohl, Department of Radiotherapy, University Medical Center Regensburg, Regensburg, Germany.
Martin Proeschold, Department of Neurosurgery, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
Nils Ole Schmidt, Department of Neurosurgery, University Medical Center Regensburg, Regensburg, Germany; Brain Tumor Center, University Medical Center Regensburg, Regensburg, Germany.
References
- 1. Jamilson Araújo Pereira B, Nogueira de Almeida A, Silva Paiva W, Henrique Pires de Aguiar P, Jacobsen Teixeira M, Kazue Nagahashi Marie S.. Neuro-oncological features of spinal meningiomas: Systematic review. Neurochirurgie. 2020;66:41–44.http://www.ncbi.nlm.nih.gov/pubmed/31672597.Accessed February 5, 2022. [DOI] [PubMed] [Google Scholar]
- 2. Raco A, Pesce A, Toccaceli G, et al. Factors leading to a poor functional outcome in spinal meningioma surgery: remarks on 173 cases. Neurosurgery. 2017;80:602–609. http://www.ncbi.nlm.nih.gov/pubmed/28362922. Accessed 2019 April 5. [DOI] [PubMed] [Google Scholar]
- 3. Helseth A, Mørk SJ.. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842–845.http://www.ncbi.nlm.nih.gov/pubmed/2585075. Accessed Febraury 12, 2022. [DOI] [PubMed] [Google Scholar]
- 4. Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH.. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14:1e2–1e7..http://www.ncbi.nlm.nih.gov/pubmed/15669787.Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura M, Tsuji O, Fujiyoshi K, et al. Long-Term Surgical Outcomes of Spinal Meningiomas. Spine. 2012;37:E617–E623.http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00007632-201205010-00020.Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 6. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol 2014;16:iv1–i63.https://academic.oup.com/neuro-oncology/article-lookup/doi/10.1093/neuonc/nou223.Accessed Febraury 12, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engelhard HH, Villano JL, Porter KR, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine 2010;13:67–77.http://www.ncbi.nlm.nih.gov/pubmed/20594020.Accessed November 6, 2019. [DOI] [PubMed] [Google Scholar]
- 8. Ravindra VM, Schmidt MH.. Management of Spinal Meningiomas. Neurosurg Clin N Am. 2016;27:195–205. [DOI] [PubMed] [Google Scholar]
- 9. Hohenberger C, Gugg C, Schmidt NO, Zeman F, Schebesch K-M.. Functional outcome after surgical treatment of spinal meningioma. J Clin Neurosci. 2020;77:62–66. http://www.ncbi.nlm.nih.gov/pubmed/32409209.Accessed October 26, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Solero CL, Fornari M, Giombini S, et al. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25:153–160.http://www.ncbi.nlm.nih.gov/pubmed/2671779.Accessed April 6, 2019. [PubMed] [Google Scholar]
- 11. Levy WJ, Bay J, Dohn D.. Spinal cord meningioma. J Neurosurg. 1982;57:804–812http://www.ncbi.nlm.nih.gov/pubmed/7143063.Accessed Febraury 15, 2022. [DOI] [PubMed] [Google Scholar]
- 12. Gezen F, Kahraman S, Canakci Z, Bedük A.. Review of 36 cases of spinal cord meningioma. Spine. 2000;25:727–731.http://www.ncbi.nlm.nih.gov/pubmed/10752106.Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 13. King AT, Sharr MM, Gullan RW, Bartlett JR.. Spinal meningiomas: a 20-year review. Br J Neurosurg. 1998;12:521–526. http://www.ncbi.nlm.nih.gov/pubmed/10070460. Accessed April 6, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Roux FX, Nataf F, Pinaudeau M, et al. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458–634.http://www.ncbi.nlm.nih.gov/pubmed/8874546.Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 15. Klekamp J, Samii M.. Surgical results for spinal meningiomas. Surg Neurol. 1999;52:552–562.http://www.ncbi.nlm.nih.gov/pubmed/10660020.Accessed Febraury 15, 2022. [DOI] [PubMed] [Google Scholar]
- 16. Cao J, Yan W, Li G, et al. Incidence and survival of benign, borderline, and malignant meningioma patients in the United States from 2004 to 2018. Int J Cancer. 2022;151:1874–1888.http://www.ncbi.nlm.nih.gov/pubmed/35779059.Accessed Dec 15, 2022. [DOI] [PubMed] [Google Scholar]
- 17. Westwick HJ, Shamji MF.. Effects of sex on the incidence and prognosis of spinal meningiomas: a Surveillance, Epidemiology, and End Results study. J Neurosurg Spine 2015;23:368–373.http://www.ncbi.nlm.nih.gov/pubmed/26023898. Accessed Jun 20, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Supartoto A, Sasongko MB, Respatika D, et al. Relationships between neurofibromatosis-2, progesterone receptor expression, the use of exogenous progesterone, and risk of orbitocranial meningioma in females. Front Oncol. 2018;8:651..http://www.ncbi.nlm.nih.gov/pubmed/30687635. Accessed Dec 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Portet S, Banor T, Bousquet J, et al. New insights into expression of hormonal receptors by meningiomas. World Neurosurg 2020;140:e87–e96.http://www.ncbi.nlm.nih.gov/pubmed/32371078. Accessed Dec 15, 2022. [DOI] [PubMed] [Google Scholar]
- 20. Goutagny S, Kalamarides M.. Meningiomas and neurofibromatosis. J Neurooncol. 2010;99:341–347.http://www.ncbi.nlm.nih.gov/pubmed/20714782. Accessed Dec 15, 2022. [DOI] [PubMed] [Google Scholar]
- 21. Parry DM, Eldridge R, Kaiser-Kupfer MI, et al. Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994;52:450–461. http://www.ncbi.nlm.nih.gov/pubmed/7747758.Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 22. Wang X-Q, Zeng X-W, Zhang B-Y, et al. Spinal meningioma in childhood: Clinical features and treatment. Childs Nerv Syst. 2012;28:129–136.http://www.ncbi.nlm.nih.gov/pubmed/21947034. Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 23. Sandalcioglu IE, Hunold A, Müller O, et al. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–1041.http://www.ncbi.nlm.nih.gov/pubmed/18481118. Accessed April 5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riemenschneider MJ, Perry A, Reifenberger G.. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006:1045–1054.http://www.ncbi.nlm.nih.gov/pubmed/17110285. Accessed December 15, 2022. [DOI] [PubMed]
- 25. McCormick PC, Post KD, Stein BM.. Intradural extramedullary tumors in adults. Neurosurg Clin N Am. 1990:591–608. http://www.ncbi.nlm.nih.gov/pubmed/2136160Accessed November 6, 2019. [PubMed]
- 26. Saraceni C, Harrop JS.. Spinal meningioma: chronicles of contemporary neurosurgical diagnosis and management. Clin Neurol Neurosurg. 2009;111:221–226.http://www.ncbi.nlm.nih.gov/pubmed/19101080. Accessed Febraury 14, 2022. [DOI] [PubMed] [Google Scholar]
- 27. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080.http://www.ncbi.nlm.nih.gov/pubmed/23348505. Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289.http://www.ncbi.nlm.nih.gov/pubmed/23334667. Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maas SLN, Stichel D, Hielscher T, et al. Integrated olecular-morphologic meningioma classification: a multicenter retrospective analysis, retrospectively and prospectively validated. J Clin Oncol. 2021;39:3839–3852.http://www.ncbi.nlm.nih.gov/pubmed/34618539. Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18:682–694.http://www.ncbi.nlm.nih.gov/pubmed/28314689. Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 31. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol 2021;23:1231–1251.http://www.ncbi.nlm.nih.gov/pubmed/34185076.Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moussalem C, Massaad E, Minassian GB, et al. Meningioma genomics: a therapeutic challenge for clinicians. J Integr Neurosci. 2021:463–469.http://www.ncbi.nlm.nih.gov/pubmed/34258948. Accessed Febraury 12, 2022. [DOI] [PubMed]
- 33. Zadnik PL, Gokaslan ZL, Burger PC, Bettegowda C.. Spinal cord tumours: advances in genetics and their implications for treatment. Nat Rev Neurol. 2013;9:257–266.http://www.ncbi.nlm.nih.gov/pubmed/23528542.Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sayagués JM, Tabernero MD, Maíllo A, et al. Microarray-based analysis of spinal versus intracranial meningiomas: different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol. 2006;65:445–454.http://www.ncbi.nlm.nih.gov/pubmed/16772868.Accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 35. Sayagués JM, Tabernero MD, Maíllo A, et al. Microarray-based analysis of spinal versus intracranial meningiomas: different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol. 2006;65:445–454.http://www.ncbi.nlm.nih.gov/pubmed/16772868. Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 36. Pemov A, Dewan R, Hansen NF, et al. Comparative clinical and genomic analysis of neurofibromatosis type 2-associated cranial and spinal meningiomas. Sci Rep 2020;10:12563..http://www.ncbi.nlm.nih.gov/pubmed/32724039Accessed Jan 31, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arslantas A, Artan S, Oner U, et al. Detection of chromosomal imbalances in spinal meningiomas by comparative genomic hybridization. Neurol Med Chir. 2003;43:1219–1218.http://www.ncbi.nlm.nih.gov/pubmed/12568317Accessed Febraury 14, 2022. [DOI] [PubMed] [Google Scholar]
- 38. Ricklefs FL, Fita KD, Mohme M, et al. Genetic and epigenetic profiling identifies two distinct classes of spinal meningiomas. Acta Neuropathol. 2022;144:1057–1059.http://www.ncbi.nlm.nih.gov/pubmed/36163595. Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hua L, Alkhatib M, Podlesek D, et al. Two predominant molecular subtypes of spinal meningioma: thoracic NF2-mutant tumors strongly associated with female sex, and cervical AKT1-mutant tumors originating ventral to the spinal cord. Acta Neuropathol. 2022;144:1053–1055.http://www.ncbi.nlm.nih.gov/pubmed/35943573Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith MJ, O’Sullivan J, Bhaskar SS, et al. Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45:295–298.http://www.ncbi.nlm.nih.gov/pubmed/23377182. Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 41. Smith MJ, Wallace AJ, Bennett C, et al. Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. J Pathol. 2014;234:436–440.http://www.ncbi.nlm.nih.gov/pubmed/25143307. Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 42. Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005;75:157–161.http://www.ncbi.nlm.nih.gov/pubmed/16132511. Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 43. Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD.. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007;23:E14..http://www.ncbi.nlm.nih.gov/pubmed/17961038.Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 44. Huang RY, Bi WL, Griffith B, et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol 2019;21:i44–i61.http://www.ncbi.nlm.nih.gov/pubmed/30649491.Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park BJ, Dougherty MC, Noeller J, et al. Spinal meningioma in adults: imaging characteristics, surgical outcomes, and risk factors for recurrence. World Neurosurg 2022;164:e852–e860.http://www.ncbi.nlm.nih.gov/pubmed/35605940. Accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 46. Maiti TK, Bir SC, Patra DP, et al. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6..http://www.ncbi.nlm.nih.gov/pubmed/27476848. Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 47. Ho U-C, Chang K, Lin Y-H, Huang Y-C, Tsuang F-Y.. Primary intraosseous meningioma of the vertebra: illustrative case. J neurosurgery Case lessons 2021;2:CASE21362..http://www.ncbi.nlm.nih.gov/pubmed/35855279.Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sauvigny T, Ricklefs FL, Hoffmann L, et al. Features of tumor texture influence surgery and outcome in intracranial meningioma. Neuro-oncology Adv 2020;2:vdaa113..http://www.ncbi.nlm.nih.gov/pubmed/33134922. Accessed Jan 27, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takashima H, Takebayashi T, Yoshimoto M, et al. Differentiating spinal intradural-extramedullary schwannoma from meningioma using MRI T2 weighted images. Br J Radiol. 2018;91:20180262..http://www.ncbi.nlm.nih.gov/pubmed/30052467.Accessed Jan 27, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Apra C, El Arbi A, Montero A-S, Parker F, Knafo S.. Spinal solitary fibrous tumors: an original multicenter series and systematic review of presentation, management, and prognosis. Cancers. 2022;14.http://www.ncbi.nlm.nih.gov/pubmed/35740510.Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldbrunner R, Stavrinou P, Jenkinson MD, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol 2021;23:1821–1834.http://www.ncbi.nlm.nih.gov/pubmed/34181733.Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang RY, Bi WL, Griffith B, et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol 2019;21:i44–i61.http://www.ncbi.nlm.nih.gov/pubmed/30649491. Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nguyen T, Chung LK, Sheppard JP, et al. Surgery versus stereotactic radiosurgery for the treatment of multiple meningiomas in neurofibromatosis type 2: illustrative case and systematic review. Neurosurg Rev. 2019;42:85–96.http://www.ncbi.nlm.nih.gov/pubmed/28900754.Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 54. Misra SN, Morgan HW.. Avoidance of structural pitfalls in spinal meningioma resection. Neurosurg Focus. 2003;14:1e1–1e6..http://www.ncbi.nlm.nih.gov/pubmed/15669784. Accessed February 16, 2022. [DOI] [PubMed] [Google Scholar]
- 55. Eicker SO, Mende KC, Dührsen L, Schmidt NO.. Minimally invasive approach for small ventrally located intradural lesions of the craniovertebral junction. Neurosurg Focus. 2015;38:E10..http://www.ncbi.nlm.nih.gov/pubmed/25828486. Accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 56. Papagelopoulos PJ, Peterson HA, Ebersold MJ, et al. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine. 1997:442–451.http://www.ncbi.nlm.nih.gov/pubmed/9055374. Accessed February 16, 2022. [DOI] [PubMed]
- 57. Inoue A, Ikata T, Katoh S.. Spinal deformity following surgery for spinal cord tumors and tumorous lesions: analysis based on an assessment of the spinal functional curve. Spinal Cord. 1996;34:536–542.http://www.ncbi.nlm.nih.gov/pubmed/8883188Accessed February 16, 2022. [DOI] [PubMed] [Google Scholar]
- 58. Herman JM, Sonntag VK.. Cervical corpectomy and plate fixation for postlaminectomy kyphosis. J Neurosurg. 1994;80:963–970.http://www.ncbi.nlm.nih.gov/pubmed/8189276. Accessed February 16, 2022. [DOI] [PubMed] [Google Scholar]
- 59. Van Goethem JWM, van den Hauwe L, Ozsarlak O, De Schepper AMA, Parizel PM.. Spinal tumors. Eur J Radiol. 2004;50:159–176.http://www.ncbi.nlm.nih.gov/pubmed/15081130. Accessed February 15, 2022. [DOI] [PubMed] [Google Scholar]
- 60. Parsa AT, Lee J, Parney IF, et al. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004;69:291–318.http://www.ncbi.nlm.nih.gov/pubmed/15527097.Accessed February 15, 2022. [DOI] [PubMed] [Google Scholar]
- 61. Gailloud P. The artery of von Haller: a constant anterior radiculomedullary artery at the upper thoracic level. Neurosurgery. 2013;73:1034–1043.http://www.ncbi.nlm.nih.gov/pubmed/24077585.Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 62. Tsuda K, Akutsu H, Yamamoto T, et al. Is Simpson grade I removal necessary in all cases of spinal meningioma? Assessment of postoperative recurrence during long-term follow-up. Neurol Med Chir. 2014;54:907–913.http://www.ncbi.nlm.nih.gov/pubmed/24759095.Accessed Jun 20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim CH, Chung CK, Lee S-H, Tae-Ahn Jahng, Hyun S-J, Kim K-J, et al. Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur Spine J 2016;25:4025–4032. https://link.springer.com/content/pdf/10.1007%2Fs00586-015-4306-2.pdf. Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 64. Jesse CM, Alvarez Abut P, Wermelinger J, et al. Functional Outcome in Spinal Meningioma Surgery and Use of Intraoperative Neurophysiological Monitoring. Cancers. 2022;14. http://www.ncbi.nlm.nih.gov/pubmed/36010979.Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stecker MM. A review of intraoperative monitoring for spinal surgery. Surg Neurol Int 2012;3:S174–S187.http://www.ncbi.nlm.nih.gov/pubmed/22905324. Accessed February 17, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kwee LE, Harhangi BS, Ponne GA, et al. Spinal meningiomas: Treatment outcome and long-term follow-up. Clin Neurol Neurosurg. 2020;198:106238..http://www.ncbi.nlm.nih.gov/pubmed/33096449.Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 67. Pereira BJA, de Almeida AN, Paiva WS, et al. Natural history of intraventricular meningiomas: systematic review. Neurosurg Rev. 2020;43:513–523.http://www.ncbi.nlm.nih.gov/pubmed/30112665. Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 68. Ketter R, Henn W, Niedermayer I, et al. Predictive value of progression-associated chromosomal aberrations for the prognosis of meningiomas: a retrospective study of 198 cases. J Neurosurg. 2001;95:601–607. http://www.ncbi.nlm.nih.gov/pubmed/11596954. Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 69. Kim CH, Chung CK, Lee S-H, et al. Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur Spine J. 2016;25:4025–4032.http://www.ncbi.nlm.nih.gov/pubmed/26542390.Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 70. Barber SM, Konakondla S, Nakhla J, et al. Oncologic benefits of dural resection in spinal meningiomas: a meta-analysis of Simpson grades and recurrence rates. J Neurosurg Spine 2019:11. http://www.ncbi.nlm.nih.gov/pubmed/31703204. Accessed December 15, 2022. [DOI] [PubMed]
- 71. Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE.. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98:258–263.http://www.ncbi.nlm.nih.gov/pubmed/12691381Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 72. Champeaux-Depond C, Penet N, Weller J, Huec J-C Le, Jecko V.. Functional Outcome After Spinal Meningioma Surgery. A Nationwide Population-Based Study. Neurospine 2022;19:96–107.http://www.ncbi.nlm.nih.gov/pubmed/35378584Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kobayashi K, Ando K, Matsumoto T, et al. Clinical features and prognostic factors in spinal meningioma surgery from a multicenter study. Sci Rep. 2021;11:11630.http://www.ncbi.nlm.nih.gov/pubmed/34079036.Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roux FX, Nataf F, Pinaudeau M, et al. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458–463. discussion 674–685http://www.ncbi.nlm.nih.gov/pubmed/8874546. Accessed June 20, 2018. [DOI] [PubMed] [Google Scholar]
- 75. Lakomkin N, Mistry AM, Zuckerman SL, et al. Utility of Intraoperative Monitoring in the Resection of Spinal Cord Tumors. Spine (Phila Pa 1976) 2018. [DOI] [PubMed] [Google Scholar]
- 76. Gilard V, Goia A, Ferracci F-X, et al. Spinal meningioma and factors predictive of post-operative deterioration. J Neurooncol. 2018;140:49–54http://www.ncbi.nlm.nih.gov/pubmed/29926318. Accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- 77. Pettersson-Segerlind J, von Vogelsang A-C, Fletcher-Sandersjöö A, et al. Health-Related Quality of Life and Return to Work after Surgery for Spinal Meningioma: A Population-Based Cohort Study. Cancers (Basel) 2021;13http://www.ncbi.nlm.nih.gov/pubmed/34944991. Accessed December 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sekely A, Zakzanis KK, Mabbott D, et al. Long-term neurocognitive, psychological, and return to work outcomes in meningioma patients. Support Care Cancer. 2022;30:3893–3902http://www.ncbi.nlm.nih.gov/pubmed/35041087.Accessed December 15, 2022. [DOI] [PubMed] [Google Scholar]
- 79. Gerszten PC, Quader M, Novotny J, Flickinger JC.. Radiosurgery for benign tumors of the spine: clinical experience and current trends. Technol Cancer Res Treat. 2012;11:133–139http://journals.sagepub.com/doi/10.7785/tcrt.2012.500242. Accessed February 1, 2023. [DOI] [PubMed] [Google Scholar]
- 80. Yamada Y, Lovelock DM, Bilsky MH.. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226–235. http://www.ncbi.nlm.nih.gov/pubmed/17762734. Accessed February 1, 2023. [DOI] [PubMed] [Google Scholar]
- 81. Kufeld M, Wowra B, Muacevic A, Zausinger S, Tonn J-C.. Radiosurgery of spinal meningiomas and schwannomas. Technol Cancer Res Treat. 2012;11:27–34.http://www.ncbi.nlm.nih.gov/pubmed/22181328. Accessed October 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dodd RL, Ryu M-R, Kamnerdsupaphon P, et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674–678http://www.ncbi.nlm.nih.gov/pubmed/16575331. Accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 83. Meola A, Soltys S, Schmitt A, Gerszten PC, Chang SD.. Stereotactic Radiosurgery for Benign Spinal Tumors. Neurosurg Clin N Am. 2020;31:231–235.http://www.ncbi.nlm.nih.gov/pubmed/32147014. Accessed February 17, 2022. [DOI] [PubMed] [Google Scholar]
- 84. Dodd RL, Ryu M-R, Kamnerdsupaphon P, et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674–685.http://www.ncbi.nlm.nih.gov/pubmed/16575331. Accessed October 19, 2022. [DOI] [PubMed] [Google Scholar]
- 85. Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846.http://www.ncbi.nlm.nih.gov/pubmed/11564244Accessed October 10, 2022. [DOI] [PubMed] [Google Scholar]
- 86. Graillon T, Tabouret E, Chinot O.. Chemotherapy and targeted therapies for meningiomas: what is the evidence? Curr Opin Neurol. 2021;34:857–867.http://www.ncbi.nlm.nih.gov/pubmed/34629433.Accessed January 26, 2023. [DOI] [PubMed] [Google Scholar]