Abstract
Background
Plexiform neurofibromas (PN) are a manifestation of neurofibromatosis type 1 (NF1) that may cause morbidity and impact health-related quality of life (HRQoL). Selumetinib (ARRY-142886, AZD6244) is an orally available, selective, mitogen-activated protein kinase kinase 1/2 inhibitor approved for children with NF1 and symptomatic, inoperable PN in regions including the USA (aged ≥2 years), EU (≥3 years), and Japan (≥3 years). This open-label, single-arm, phase I study evaluated selumetinib in Japanese children with NF1 and symptomatic, inoperable PN.
Methods
Eligible patients (aged 3–18 years) received oral selumetinib (25 mg/m2 twice daily) continuously in 28-day cycles in a fasted state. Primary objectives were safety and tolerability. Secondary objectives included pharmacokinetics, efficacy, PN-related morbidities, and HRQoL.
Results
Twelve patients (median age 13.3 years) were enrolled, received ≥1 selumetinib dose (data cutoff: cycle 13 day 1) with median follow-up of 11.5 months. All patients had baseline PN-related morbidities, most commonly disfigurement (91.7%) and pain (58.3%). Most frequently reported any-grade adverse events were dermatologic and gastrointestinal. Objective response rate was 33.3%; median duration of response was not reached. Most patients (83.3%) had target PN volume reduction versus baseline. No patients reported worsening of PN-related morbidities. Selumetinib was rapidly absorbed with moderate-to-high inter-patient variability in maximum plasma concentration and area under the concentration–time curve from time 0–6 hours.
Conclusions
Consistent with results of the phase II SPRINT trial, 25 mg/m2 selumetinib twice daily was well tolerated with a manageable safety profile in Japanese children with NF1 and symptomatic, inoperable PN.
Keywords: Neurofibromatosis 1, plexiform neurofibroma, selumetinib, MEK inhibitor
Key Points.
Selumetinib 25 mg/m2 twice daily was well tolerated in Japanese children with symptomatic, inoperable PN.
Results of this Japanese phase I study were consistent with the phase II SPRINT trial.
No adjustment to selumetinib starting dose was required in Japanese children.
Importance of the Study.
Selumetinib is the first and only medicine approved by the US Food and Drug Administration (patients aged ≥2 years) and the European Medicines Agency (patients aged ≥3 years) for pediatric patients with NF1 and symptomatic, inoperable PN based on the phase II SPRINT trial. No Japanese pediatric patients were included in the SPRINT trial, and until the recent approval of selumetinib in Japan there was an unmet medical need for this patient population. This phase I study showed that the safety profile of selumetinib in Japanese patients was consistent with that reported in the SPRINT study. The pharmacokinetic profile in Japanese pediatric patients was also consistent with that seen in the SPRINT study, and so no adjustment to starting dose of selumetinib was needed for Japanese pediatric patients with NF1 and symptomatic, inoperable PN.
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder, arising from an NF1 mutation resulting in RAS dysfunction, and can affect every organ system in the body.1 Although NF1 is considered a rare disease, it is one of the most common genetic disorders worldwide, with an estimated prevalence of 1 in 3000 people in Japan and a global prevalence ranging from 1 in 3000 to 1 in 6000 people.2–5 NF1 is a heterogeneous disease with diverse clinical manifestations that may cause life-changing morbidities, lead to malignant neoplasms and cardiovascular disease, and impact patient health-related quality of life (HRQoL).6–8 It is reported that patients with NF1 experience a 10–15-year reduction in life expectancy compared with the general population.2
Characteristic manifestations of NF1 include pigmented lesions, multiple skin neurofibromas, brain tumors, skeletal manifestations, and plexiform neurofibromas (PN).2,9,10 PN are benign nerve sheath tumors that develop in up to 50% of patients with NF1,11 many of whom have clinical features that are not apparent on physical examination.12 PN tend to develop early in childhood, growing most rapidly during this period (often increasing by over 20% in volume each year) and can continue to manifest through late adolescence and early adulthood; therefore, timely intervention is critical.9,10,13 PN are associated with considerable morbidities including pain, neurological dysfunction, disfigurement, and breathing difficulties. PN also have the potential to transform into malignant peripheral nerve sheath tumors (MPNSTs) in approximately 10%–15% of cases.14–16 Surgery is a treatment option for NF1-PN17; however, complete resection of PN is frequently impossible because of their size and location, and partially resected tumors often continue to increase in size.16,17
Selumetinib (ARRY-142886, AZD6244), an orally administered, selective, mitogen-activated protein kinase kinase (MEK) 1/2 inhibitor, is the first and only medical treatment approved for pediatric patients with NF1 and symptomatic, inoperable PN, in regions including the USA (aged ≥ 2 years), the EU (aged ≥ 3 years), and Japan (aged ≥ 3 years).18–21 US and EU approval of selumetinib was based on results from the pivotal SPRINT trial.18–20 In the phase II part of the SPRINT trial, selumetinib elicited durable PN shrinkage and an overall clinical benefit with a manageable safety profile at a dosage of 25 mg/m2 twice daily, which was amenable to the long-term treatment of children aged 2–18 years with NF1 and symptomatic, inoperable PN.18 In SPRINT, MPNSTs (among other adverse events [AEs]) led to the permanent discontinuation of selumetinib treatment in 2 of the 50 patients (4%).18,19
In Japan, selumetinib was recently approved as the first and only medicine for pediatric patients with NF1 and symptomatic, inoperable PN; fulfilling a previously unmet medical need.21 In SPRINT, selumetinib was not evaluated in Japanese pediatric patients with NF1-PN.18 The objectives of this phase I study were to evaluate the safety and tolerability (primary objective) and pharmacokinetics (PK), efficacy, HRQoL, and changes in PN-related morbidities (secondary objectives) of selumetinib in Japanese pediatric patients with NF1 and inoperable, symptomatic PN.
Materials and Methods
Study Design and Patients
This was an open-label, single-arm, phase I study designed to evaluate the safety, tolerability, PK, and efficacy of selumetinib in Japanese pediatric patients with NF1 and inoperable, symptomatic PN (Supplementary Figure S1).
Eligible patients were aged 3–18 years, were required to be able to swallow capsules, had a clinical diagnosis of NF122 with inoperable, symptomatic PN, had at least one measurable PN (≥3 cm or ≥ 2 cm with significant symptoms), and either a Karnofsky (if aged >16 years) or Lansky (if aged ≤ 16 years) performance status of ≥ 70. Full inclusion and exclusion criteria are provided in Supplementary Methods. Target PN were defined as the most clinically relevant PN amenable to volumetric MRI analysis and classified as either typical or nodular. Additional PN were captured as non-target PN. Inoperable PN was defined as PN that cannot be completely resected by surgery without the risk of substantial morbidity due to the encasement of, or proximity to, vital structures, or invasiveness or high vascularity of the PN. Patients were excluded if they had received prior treatment with a MEK, RAS, or RAF inhibitor or systemic treatment for NF1-PN in the previous 4 weeks, or if they displayed evidence of MPNSTs.
Patients were screened within 28 days before the first selumetinib administration. At the time of screening, PN type was classified by site as typical PN (nodular component accounting for < 30% of the PN), nodular PN (nodular component accounting for ≥ 30% of the PN), or solitary nodular PN (the whole body of the PN is an encapsulated mass and could not be classified as a target or non-target PN).
All patients received oral selumetinib 25 mg/m2 twice daily (as 10 mg or 25 mg capsules) from cycle 1 day 1 continuously in 28-day cycles in a fasted state until disease progression (as determined by the investigator) or unacceptable selumetinib-related toxicity. Selumetinib dose was individualized based on body surface area (BSA), with a minimum allowable BSA of 0.55 m2 and a maximum dosage of 50 mg twice daily for patients with a BSA of ≥1.9 m2.
Objectives and Assessments
The primary objective was the safety and tolerability of selumetinib. Secondary objectives included the PK of selumetinib and its metabolite N-desmethyl selumetinib, the efficacy of selumetinib as determined by volumetric MRI analysis, and the impact of selumetinib treatment on PN-related morbidities and HRQoL.
Safety.
AEs were collected from the time of signing the informed consent form and throughout treatment and follow-up periods. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.1 and graded according to the Common Terminology Criteria for Adverse Events version 5.0. Data from all treatment cycles were combined in the presentation of AEs, unless stated otherwise. All AEs, in terms of MedDRA system organ class, preferred term, and Common Terminology Criteria for Adverse Events grade were listed and summarized descriptively by count and percentage.
Pharmacokinetics.
PK parameters evaluated after a single dose and at steady state for selumetinib and N-desmethyl selumetinib included area under the concentration–time curve from time 0 to 6 hours (AUC0–6), AUC from time 0 to last measurable concentration (AUC0–t), maximum plasma concentration (Cmax), and time to maximum plasma concentration (tmax).
Efficacy.
PN response was assessed by independent central review (ICR) according to Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria.23 REiNS criteria represent standardized consensus recommendations to evaluate imaging response in clinical trials for benign neurofibromatosis (NF) tumors and include guidelines for image acquisition, target lesion selection, image interpretation by volumetric analysis, and trial design. REiNS criteria use volumetric MRI analysis to evaluate response in NF tumors and reproducibly detect small changes; a 20% volume change indicates a decrease or increase in tumor size.23
As per REiNS criteria, complete response was defined as a complete disappearance of the target PN and partial response (PR) was determined as a ≥20% reduction in target PN volume compared with baseline, (PR was considered unconfirmed when first detected and a response required confirmation within 3–6 months).23 Progressive disease (PD) was ascertained by a ≥20% increase in target PN volume compared with baseline and stable disease was identified by an insufficient volume change to qualify as a PR or PD.23 Histologic tumor confirmation was not needed in the presence of consistent clinical and radiographic findings but was required to be considered if malignant degeneration of a PN were clinically suspected. Response was evaluated in both target and non-target PN. Duration of response (DoR) was defined as the time from the first documentation of response (which was subsequently confirmed) until the date of documented PD per REiNS criteria, death by any cause, or the last evaluable REiNS assessment for patients without PD or death at the time of the analysis. No efficacy data were collected after cycle 13 day 1.
PN-related morbidities and quality of life.
Clinical Global Impression of Change (CGIC) assessments were used to evaluate PN-related morbidities. CGIC was measured on a 7-point scale for all patients. The investigator performed a comprehensive evaluation of the changes in PN-related morbidities (symptoms or complications) from baseline, for example, vision loss, facial motor dysfunction, auditory loss, difficulty swallowing, abnormal speech, airway obstruction, respiratory compromise, bladder dysfunction, bowel dysfunction, motor weakness, decreased range of motion, sensory deficit, PN-related disfigurement, pain, and relevant findings from imaging studies and physical examinations. General HRQoL was measured using the generic Pediatric Quality of Life Inventory (PedsQL; self and parent reported).24 PedsQL scores range from 0 to 100, with higher scores indicating better HRQoL.
Data and Statistical Analysis
Safety and efficacy data are reported up to cycle 13 day 1 (approximately 12 months of selumetinib treatment). Some safety data were collected beyond cycle 13 day 1 for patients who were enrolled early in the study. PK sampling was performed after single dosing (cycle 1 day 1) and following multiple dosing to steady state (cycle 2 day 1).
No formal statistical hypothesis testing or sample size calculation was performed. All data are described descriptively. Confidence intervals (CIs) were calculated using appropriate methods, where applicable. Median DoR and 95% CI were calculated using the Kaplan–Meier method. Objective response rate (ORR) was presented with corresponding 2-sided exact 95% CI based on the Clopper–Pearson method.
PK data were listed and summarized. The derived PK parameters (including AUC0–6, AUC0–12, AUC0–t, and Cmax, tmax) after single dosing and at steady state were summarized by analyte and evaluation day. Individual and geometric mean plasma concentrations for selumetinib and N-desmethyl selumetinib were presented graphically.
Ethics
The clinical study protocol, informed consent form, investigator brochure, and other relevant documents were reviewed and approved by each Institutional Review Board or Independent Ethics Committee before study initiation. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and were consistent with the International Council for Harmonisation, Good Clinical Practice, applicable regulatory requirements, and AstraZeneca’s policy on bioethics.
Results
Patients
The study was conducted at four centers in Japan. The first patient was enrolled on August 31, 2020 and data are available up to December 8, 2021 (~12 months after the last enrolled patient received the first selumetinib dose). In total, 12 patients were enrolled and received at least one dose of selumetinib. As of cycle 13 day 1, all patients remained on selumetinib treatment, with a median duration of follow-up of 11.5 months; the median actual treatment duration (excluding dose interruptions) was 11.5 months (range 9.0–11.5).
All patients were Japanese (nine female and three male) with a median age at baseline of 13.3 years (range 7.5–18.2; Table 1). The median duration from NF1 diagnosis to the start of selumetinib treatment was 9.3 years (range 4.2–17.9). All patients experienced ≥1 PN-related symptom at the time of enrollment, the most common of which were disfigurement, pain, and motor weakness (Table 1). Reported PN-related symptoms were heterogeneous in nature. The median target PN volume at baseline was 207.7 mL (range 17.6–3089.9) and target PN were primarily located in the neck or trunk (n = 4; 33.3%) and trunk (n = 3; 25%); other target PN locations were the head, extremity (n = 2 each; 16.7%), and head and neck (n = 1; 8.3%; Table 1). Seven patients had undergone at least one previous NF1- or PN-related surgical procedure. Three of the 12 patients also had non-target PN at baseline. These non-target PN were located in the trunk (n = 1; PN volume 18.5 mL), neck/trunk (n = 1; PN volume 3.7 mL), and the head (n = 1; PN volume 3.5 mL).
Table 1.
Demographic and Clinical Characteristics of Patients at Baseline
| Characteristic | Patients N = 12 |
|---|---|
| Sex, n (%) | |
| Female | 9 (75.0) |
| Male | 3 (25.0) |
| Age, years, median (range) | 13.3 (7.5–18.2) |
| Height, cm, median (range) | 142.4 (117.4–156.8) |
| Weight, kg, median (range) | 34.0 (20.7–58.9) |
| Body surface area, m2, median (range) | 1.2 (0.8–1.6) |
| Time from NF1 diagnosis,a years, median (range) | 9.3 (4.2–17.9) |
| NF1 diagnostic criteria present, n (%) | |
| ≥6 café-au-lait macules | 12 (100) |
| Freckling in axillary or inguinal regions | 9 (75.0) |
| ≥2 Lisch nodules | 6 (50.0) |
| First-degree relative with NF1 | 5 (41.7) |
| Distinctive osseous lesion | 4 (33.3) |
| Optic glioma | 1 (8.3) |
| Target PN location, n (%) | 12 (100) |
| Neck or trunk | 4 (33.3) |
| Trunk | 3 (25.0) |
| Head | 2 (16.7) |
| Extremity | 2 (16.7) |
| Head and neck | 1 (8.3) |
| Any target PN-related symptoms, n (%) | 12 (100) |
| Disfigurement | 11 (91.7) |
| Pain | 7 (58.3) |
| Motor weakness | 4 (33.3) |
| Abnormal speech | 2 (16.7) |
| Airway obstruction | 2 (16.7) |
| Compromised respiration | 2 (16.7) |
| Decreased range of motion | 2 (16.7) |
| Sensory deficit | 2 (16.7) |
| Auditory loss | 1 (8.3) |
| Facial motor dysfunction | 1 (8.3) |
| ≥1 prior PN-related or NF1-related surgery, n (%) | 7 (58.3) |
| Target PN volume, mL, median (range) | 207.7 (17.6–3089.9) |
aTo start of selumetinib treatment.
Abbreviations: NF1, neurofibromatosis type 1; PN, plexiform neurofibroma.
Safety
No AEs leading to death or treatment discontinuation were reported. All patients experienced at least one AE and 11 patients experienced at least one AE that was determined by the investigator as possibly related to selumetinib (Table 2). At cycle 13 day 1, the most common AEs of any grade were dermatologic and gastrointestinal (eczema [n = 7], dermatitis acneiform [n = 6], paronychia and diarrhea [n = 5 each], and dry skin, stomatitis, and vomiting [n = 4 each]). Two grade 3 AEs of paronychia were reported in one patient, one of which was assessed as selumetinib related; both instances resolved without any modifications to selumetinib treatment.
Table 2.
Summary of AEs at Cycle 13 Day 1
| Patients Experiencing AEs, n (%) | All N = 12 |
Treatment Related N = 12 |
|---|---|---|
| Any AE (any grade) | 12 (100) | 11 (91.7) |
| Any AE (grade ≥3) | 1 (8.3) | 1 (8.3)a |
| AEs leading to dose modifications | 2 (16.7)b | 2 (16.7)b |
| AEs occurring in ≥25% of patients | ||
| Eczema | 7 (58.3) | 7 (58.3) |
| Dermatitis acneiform | 6 (50.0) | 5 (41.7) |
| Paronychia | 5 (41.7) | 3 (25.0) |
| Diarrhea | 5 (41.7) | 5 (41.7) |
| Stomatitis | 4 (33.3) | 4 (33.3) |
| Vomiting | 4 (33.3) | 3 (25.0) |
| Dry skin | 4 (33.3) | 2 (16.7) |
| Abdominal pain | 3 (25.0) | — |
| Cheilitis | 3 (25.0) | 3 (25.0) |
| Nausea | 3 (25.0) | 2 (16.7) |
| Alopecia | 3 (25.0) | 3 (25.0) |
| Pyrexia | 3 (25.0) | — |
aOne patient reported 2 grade 3 AEs of paronychia; one instance was considered to be treatment related.
bPatients with asymptomatic grade 2 ejection fraction decrease leading to dose interruption and subsequent dose reduction.
AE, adverse event.
Dose interruptions were reported in six patients (50.0%) as of cycle 13 day 1. Two patients (16.7%) had their dose interrupted and then decreased because of AEs (grade 2 ejection fraction decrease); both patients were able to continue selumetinib treatment at a lower dose after ejection fraction normalization (one patient) or improvement (one patient). When all data, including those collected beyond cycle 13 day 1, were considered, one additional patient had an AE leading to dose interruption. The additional patient experienced grade 3 alanine aminotransferase (ALT) and aspartate aminotransferase (AST) increase (deemed as possibly related to selumetinib). ALT and AST levels for this patient had not normalized at the data cutoff date.
Efficacy
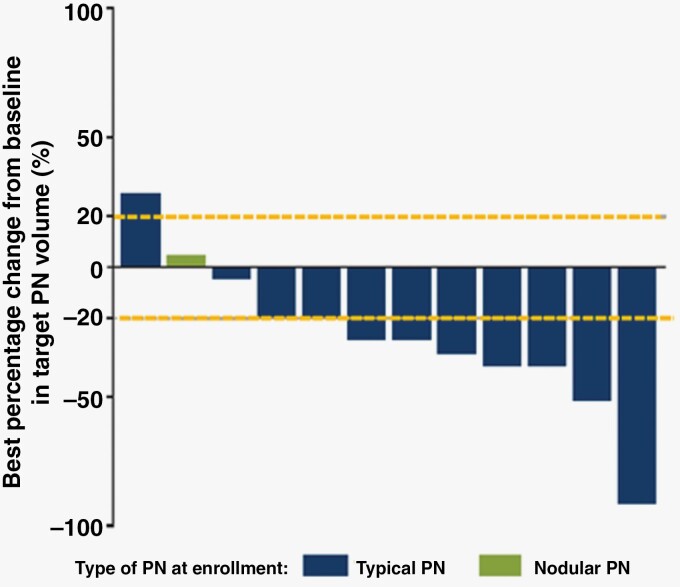
All 12 patients had measurable PN volume at baseline and were evaluable for change in PN volume. As of cycle 13 day 1, the ORR assessed by ICR as per REiNS criteria was 33.3% (Table 3). Four patients (33.3%) had a confirmed PR as their best response, two (16.7%) had an unconfirmed PR, four (33.3%) had stable disease, and two (16.7%) had PD. The two patients with unconfirmed PR had a PR that was first observed during the evaluation prior to data cutoff; therefore, confirmation was not possible. One patient was assessed as having PD as of cycle 13 day 1 due to progression in a non-target PN at cycle 5 day 1 and cycle 9 day 1, despite a >20% reduction in target PN volume from baseline at all time points. This patient experienced a considerable reduction of −43.4% in target PN volume from baseline (905.2 mL) to cycle 13 day 1 (511.9 mL). Their non-target lesion increased in volume from 18.5 mL at baseline to 25.1 mL at cycle 5 day 1 and to 26.6 mL at cycle 9 day 1, followed by a reduction to 16.8 mL at cycle 13 day 1. Of the four patients who had a confirmed PR, one patient subsequently had PD. The median duration of response was not reached.
Table 3.
Response Assessed by ICR as Per REiNS Criteria as of Cycle 13 Day 1a
| Response Assessment, n (%) | Patients N = 12 |
|---|---|
| Objective response rate | 4 (33.3) |
| Best objective response | 4 (33.3) |
| Complete response | 0 |
| Confirmed partial response | 4 (33.3) |
| Unconfirmed partial responseb | 2 (16.7) |
| Stable disease | 4 (33.3) |
| Progressive disease as per REiNS criteria | 2 (16.7)c |
No efficacy data were collected after cycle 13 day 1.
aMRI assessments were available at cycle 13 day 1 for all patients.
bPR achieved but either no assessment for confirmation was performed, or a confirmation assessment was performed but response was not confirmed. For the 2 unconfirmed PRs here, PR was first observed at the evaluation just prior to the data cutoff.
cOne patient was assessed as PD overall due to PD in a non-target PN at cycle 5 day 1 and cycle 9 day 1, despite PR (>20% reduction in volume from baseline) reported in target PN.
ICR, independent central review; PD, progressive disease; PN, plexiform neurofibroma; PR, partial response; REiNS, Response Evaluation in Neurofibromatosis and Schwannomatosis.
As of cycle 13 day 1, most patients (10 of 12; 83.3%) experienced a reduction in target PN volume compared with baseline; of these, seven patients (58%) had a >20% reduction in target PN volume up to cycle 13 day 1 (Figure 1). The mean best percentage change from baseline in target PN volume was −26.55% (range −91.2% to + 28.3%) (Supplementary Figure S2). A reduction in mean target PN volume was observed across all time points during the study. Of the two patients who did not experience a reduction in target PN volume from baseline to cycle 13 day 1, one patient had PD, and the other had stable disease.
Figure 1.
Best percentage change in target PN volume from baseline as of cycle 13 day 1. No efficacy data were collected after cycle 13 day 1. Two patients were classified as PD: Best reduction in target PN volume was 37.9% for one of these patients (best response was PD due to PD in a non-target PN) and was a volume increase of 28.3% for the other. One patient was assessed as having PD due to progression in a non-target PN at cycle 5 day 1 and cycle 9 day 1, despite a >20% reduction in target PN volume from baseline at all time points. Reference lines represent ± 20% change in target PN volume, which represent PD and PR, respectively. PD, progressive disease; PN, plexiform neurofibroma; PR, partial response.
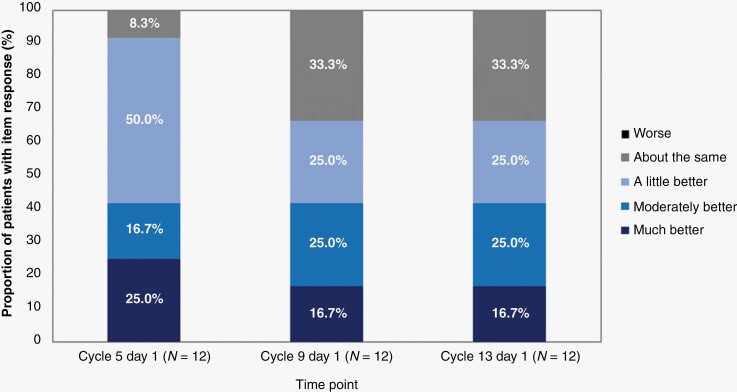
Clinical Global Impression of Change
CGIC evaluation for PN-related morbidities was performed for all 12 patients at three-time points. At cycle 13 day 1, eight patients (67%) were deemed by the investigator to demonstrate an improvement from baseline in PN-related morbidities, and no patients were reported as having worsening CGIC responses at any time point during the study (Figure 2). Overall improvement from baseline was reported as early as cycle 5 day 1 in 11 of 12 patients (91.7%). As assessed by CGIC, pain palliation (explicitly reported for five patients [41.7%]) was not associated with increased use of analgesics. Only one patient received two analgesics (milnacipran and tramadol) concomitantly with selumetinib; this patient discontinued milnacipran and underwent dose reduction of tramadol.
Figure 2.
CGIC assessments over time up to cycle 13 day 1. CGIC, Clinical Global Impression of Change.
Health-Related Quality of Life
Compliance for self- and parent-reported PedsQL questionnaires was 100%. As all patients were aged ≥ 5 years, self-reported PedsQL scores were applicable for all patients. Self-reported PedsQL score at baseline was 85.5 out of 100, indicating a relatively good HRQoL at baseline for these patients. At cycle 13 day 1, patients and their parents reported a consistent improvement in PedsQL scores versus baseline at all time points during selumetinib treatment, whereby a higher score indicates a better HRQoL (Supplementary Figure S3, Table S1). Mean (standard deviation [SD]) change from baseline in self-reported PedsQL total score was 6.4 (6.1) and mean (SD) change from baseline in parent-reported PedsQL total score was 1.3 (11.7). For self-reported PedsQL total score at cycle 13 day 1 compared with baseline, a numerical increase was reported in nine patients (75.0%), no change in two patients (16.7%), and a numerical decrease in one patient (8.3%). For parent-reported PedsQL total score at cycle 13 day 1 compared with baseline, a numerical increase was reported in 6 patients (50.0%), no change in one patient (8.3%), and a numerical decrease in five patients (41.7%).
Pharmacokinetics
All patients were evaluable for the PK assessment of selumetinib (Table 4). Selumetinib was rapidly absorbed after oral administration. Median tmax after single (cycle 1 day 1) and multiple oral doses leading to steady state (cycle 2 day 1) were 1.5 hours. Exposure to selumetinib at steady state was greater than after single-dose administration. Accumulation was minimal, geometric mean Cmax was 1.1-fold at steady state versus single dosing (869.4 ng/mL; geometric coefficient of variation [gCV] 53.5% vs. 783.1 ng/mL; gCV 52.7%) and geometric mean AUC0–6 was 1.3-fold at steady state versus single dosing (2396 ng·h/mL; gCV 40.3% versus 1926 ng·h/mL; gCV 41.6%). Moderate-to-high inter-patient variability was observed for Cmax and AUC0–6 at single dosing and steady state (range 40.3–53.5%). Lastly, single-dose geometric mean and gCV AUC0–12 were 2523 ng·h/mL and 24.2%, respectively. Conversion from selumetinib parent to active metabolite N-desmethyl selumetinib was low. Geometric mean metabolite:parent ratios at single dosing and steady state was 7.1% and 4.7% for Cmax and 7.6% and 5.2% for AUC0–t, respectively.
Table 4.
Key Plasma PK Parameters of Selumetinib and Selumetinib Metabolite (N-desmethyl Selumetinib)
| Parameter (Units) |
Statistic | Single Dose (Cycle 1 day 1; N = 12) |
Steady State (Cycle 2 day 1; n = 11) |
||
|---|---|---|---|---|---|
| Selumetinib | N-desmethyl Selumetinib | Selumetinib | N-desmethyl Selumetinib | ||
| Cmax (ng/mL) | Geometric mean | 783.1 | 55.5 | 869.4 | 40.9 |
| gCV% | 52.7 | 49.1 | 53.5 | 53.1 | |
| tmax (h) | Median | 1.5 | NA | 1.5 | NA |
| Min–Max | 0.5–3.1 | NA | 0.4–2.9 | NA | |
| AUC0–ta (ng·h/mL) | Geometric mean | 2224 | 169.7 | 2395 | 123.6 |
| gCV% | 37.6 | 35.4 | 40.3 | 37.3 | |
| AUC0–6 (ng·h/mL) | Geometric mean | 1926 | 140.8 | 2396 | 123.6 |
| gCV% | 41.6 | 38.9 | 40.3 | 37.3 | |
| AUC0–12 (ng·h/mL) | Geometric mean | 2523b | 188.7c | NA | NA |
| gCV% | 24.2 | 24.3 | NA | NA | |
| Ctrough (ng/mL) | Geometric mean | NA | NA | 92.7 | 6.7 |
| gCV% | NA | NA | 63.2 | 57.1 | |
| Rac Cmax | Geometric mean | NA | NA | 1.10 | 0.7 |
| gCV% | NA | NA | 34.3 | 42.2 | |
| Rac AUCd | Geometric mean | NA | NA | 1.27 | 0.9 |
| gCV% | NA | NA | 17.3 | 43.1 | |
| Metabolite:parent ratio Cmax | Geometric mean | NA | 0.07 | NA | 0.05 |
| gCV% | NA | 18.3 | NA | 62.8 | |
| Metabolite:parent ratio AUCe | Geometric mean | NA | 0.08 | NA | 0.05 |
| gCV% | NA | 15.9 | NA | 55.5 | |
aThe last sample was 10–12 hours post dose for cycle 1 day 1 and 6 hours post dose for cycle 2 day 1.
b n = 8.
c n = 7.
dRac AUC was calculated using AUC0–6 for cycle 1 day 1 and cycle 2 day 1.
eMetabolite: Parent ratio for AUC was calculated using AUC0–t for selumetinib and N-desmethyl selumetinib.
AUC, area under the concentration–time curve; AUC0–6, AUC from time 0 to 6 hours; AUC0–12, AUC from time 0 to 12 hours; AUC0–t, AUC from time 0 to last measurable concentration; Cmax, maximum plasma concentration; Ctrough, lowest concentration of selumetinib prior to next dose; gCV, geometric coefficient of variation; NA, not applicable; PK, pharmacokinetics; RAC, accumulation ratio; tmax, time to maximum plasma concentration.
Discussion
The results from this phase I trial show that selumetinib at a dosage of 25 mg/m2 twice daily is well tolerated with a manageable safety profile in Japanese pediatric patients with NF1 and symptomatic, inoperable PN. These findings are consistent with the results of the phase II SPRINT trial, which evaluated the same dosing regimen in children with NF1-PN in the USA, leading to approval by the US Food and Drug Administration and the European Medicines Agency.19,20
In this study, disease burden was considerable and protracted; all patients were experiencing PN-related morbidities at baseline, more than half of the patients had undergone a prior PN- or NF1-related surgical procedure, and patients were living with NF1 (and any related complications) for a median of 9.3 years prior to receiving selumetinib treatment. The characteristics of patients in this trial were generally similar to those of patients in the SPRINT trial, which enrolled pediatric patients with NF1 and symptomatic, inoperable PN. The median age of patients in the present study was slightly higher than in SPRINT (13.3 vs. 10.2 years, respectively), and the median target PN volume was lower than in the SPRINT trial (208 mL vs. 487 mL, respectively). In both studies, the most common PN-related morbidities were disfigurement, pain, and motor dysfunction.18
Selumetinib was well tolerated, with most AEs being grade 1 or 2; up to cycle 13 day 1, only one patient experienced grade 3 AEs (paronychia), and these resolved without any dose modifications. The results were generally consistent with the known safety profile of selumetinib; the most common AEs in SPRINT were grade 1 or 2 dermatologic or gastrointestinal symptoms,18 aligning with the observations in the present trial. In the present study, dose interruptions due to AEs were reported for three patients (25.0%; grade 2 ejection fraction decrease for two patients and grade 3 ALT and AST increase for one patient). Symptomatic changes in ejection fraction decrease were not observed in children enrolled in SPRINT, grade 2 asymptomatic ejection fraction decrease was identified in two patients (14%) in phase II, and one patient experienced grade 3 asymptomatic left ventricular ejection fraction decrease in phase I part.25 This is a known AE associated with MEK inhibition in adult patients with cancer.26 However, it should be noted that the number of patients in this present study is small (N = 12) and results should be interpreted with caution.
The ORR for patients treated with selumetinib was 33.3% (a confirmed PR was reported in four patients). Although PD was reported as best response in 2 patients, one of these patients had a considerable reduction in target PN volume from baseline to cycle 13 day 1 (−43.4% from 905.2 mL to 511.9 mL), but experienced PD in non-target PN at cycle 5 day 1 and cycle 9 day 1, hence, classified as PD overall. Overall, 10 patients experienced a reduction in target PN volume during the study, seven of whom experienced reductions of >20% from baseline. These results align with the data reported from the SPRINT trial, in which after a median follow-up of 26 months, 34 of the 50 patients had a confirmed PR (ORR 68%) and the median reduction in PN volume at best response was 27.9%.18 It must be noted that the median time to an initial response in SPRINT was 8 cycles and the median time to best response was 16 cycles,18 which has not yet been reached by patients in the present study. In addition, it should be noted that, unlike in the present study which evaluated response after 12 cycles from first dose, the ORR in SPRINT was reported after patients were followed for at least 12 months from first response.
To evaluate efficacy, this study used the REiNS criteria, which have been specifically developed to capture response in benign tumors associated with NF. It is important to note that standard response criteria for solid tumors have limited applicability in the assessment of response for benign tumors such as PN that cause substantial morbidity. The response evaluation criteria in solid tumors are designed to minimize inter-rater variability in differentiating malignant tumors that are shrinking, stable, or growing.23 For a condition such as NF1-PN, disease stabilization or minor shrinkage may result in symptomatic improvement; thus, volumetric measurements that reproducibly detect small changes in tumor size are more clinically relevant. In addition, the lack of standardized response criteria for NF tumors has meant that prior trials have used a range of primary and secondary objectives, further limiting comparison of results between trials.23
Overall, no patients reported worsening of PN-related morbidities in this study, with most patients reporting improvement. Although a CGIC pain-specific score was not calculated for this small patient population, selumetinib may be associated with pain palliation for five patients independent from the use of analgesics; the only patient receiving analgesics concomitantly underwent a dose reduction and discontinuation of analgesics during the study. At cycle 13 day 1, patients and their parents reported a consistent improvement in PedsQL scores with selumetinib treatment. The mean change from baseline in self-reported PedsQL total score was 6.4, which was similar to the self-reported PedsQL score in SPRINT (6.7).18 For most patient- and parent-reported outcomes and functional measures, no pre-defined, validated thresholds for clinically meaningful change exist for pediatric patients with NF1; therefore, we described the changes and trends over time. Across other studies of healthy young children and those with chronic medical conditions, a minimal clinically important difference in PedsQL score has been defined for total scores ranging from 4 to 6.24,27,28 In the SPRINT study, the authors defined changes greater than a half SD as the minimal clinically important difference (a change greater than 8.7 for self-reported and 8.1 for parent-reported PedsQL).18 Using this defined threshold, the authors determined that approximately half of the children in SPRINT reported clinically meaningful increases in PedsQL score after 1 year of treatment. Improvements in ≥ 1 patient-reported outcome were also observed in 68% of patients after one year of selumetinib treatment in SPRINT.18
In this study, selumetinib PK were characterized by rapid absorption with moderate-to-high inter-patient variability in Cmax and AUC0‒6 (gCV 40.3%‒53.5%) after single and multiple dosing (twice daily). A previous study in healthy adults reported that dose-normalized selumetinib exposure may be 35%–39% higher in Asian people compared with White or Black people after a single dose.29 Of the Asian race sub-categories investigated in that study, Japanese adults demonstrated the greatest increase (51%) in dose-normalized selumetinib exposure versus White adults.29 However, in the present study, geometric means of selumetinib exposure in Japanese pediatric patients after a single selumetinib dose (1926 ng·h/mL) and at steady state (2396 ng·h/mL) appear to be comparable, considering inter-patient variability, to that observed in SPRINT (single dose, 2009 ng·h/mL; steady state 1958 ng·h/mL).19,25,30 An exposure–response analysis of data (20–30 mg/m2) from pediatric patients with NF1-PN showed that there were no clear relationships between selumetinib exposure and safety events or common AEs, including rash acneiform, diarrhea, vomiting, and nausea.31
Limitations of this study include the small patient number, which limits the detectability of potentially uncommon AEs, the short follow-up duration, and the phase I study design to allow adequate evaluation of preliminary objective response. To avoid potential bias, ICR analysis was used to evaluate volumetric MRIs according to REiNS criteria.
These findings are consistent with those of the SPRINT trial: Selumetinib at a dosage of 25 mg/m2 twice daily was well tolerated in Japanese pediatric patients with NF1 and symptomatic, inoperable PN, and preliminary efficacy was demonstrated. No adjustments to the starting dose of 25 mg/m2 were required in Japanese pediatric patients. Therefore, selumetinib addresses a previously unmet need for Japanese pediatric patients with NF1 and symptomatic, inoperable PN who typically experience a high disease burden.
Supplementary Material
Acknowledgments
The authors would like to thank all the study volunteers.
Contributor Information
Souichi Suenobu, Department of Pediatrics, Oita University Hospital, Yufu, Japan.
Keita Terashima, Division of Neuro-Oncology, Children's Cancer Center, National Center for Child Health and Development, Tokyo, Japan.
Masaharu Akiyama, Department of Pediatrics, The Jikei University Hospital, Tokyo, Japan.
Tomoyo Oguri, Research and Development, AstraZeneca K.K., Osaka, Japan.
Asako Watanabe, Research and Development, AstraZeneca K.K., Osaka, Japan.
Masatoshi Sugeno, Research and Development, AstraZeneca K.K., Osaka, Japan.
Mitsuo Higashimori, Research and Development, AstraZeneca K.K., Osaka, Japan.
Karen So, Alexion, AstraZeneca Rare Disease Clinical Development, NF and Bone Metabolism Therapeutic Area, Cambridge, UK.
Yoshihiro Nishida, Department of Orthopedic Surgery, Nagoya University Hospital, Nagoya, Japan.
Funding
This research was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp and Dohme Corp, a subsidiary of Merck and Co., Inc., Rahway, NJ, USA (MSD). Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Emily Clark, PhD of OPEN Health Communications (London, UK) and was funded by Alexion, AstraZeneca Rare Disease and Merck Sharp and Dohme Corp, a subsidiary of Merck and Co., Inc., Rahway, NJ, USA (MSD), in accordance with Good Publications Practice (GPP 2022) guidelines.
Conflict of interest statement
SS’s institution received funding from AstraZeneca and received grants from Osaka University and ARTham Therapeutics. SS has also received grants for an endowed course from OITA City (Division of General Pediatrics and Emergency Medicine). SS is also a committee member of The Japanese Society of Hematology/Oncology and the Japan Children’s Cancer Group. KT has received grants or contracts from AstraZeneca, Novartis, and Ohara. KT has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Chugai Pharmaceutical and Bayer. KT has also received payment for participating on an advisory board or Data Safety Monitoring Board from AstraZeneca and Bayer. KT holds a leadership or fiduciary role in the Japan Children’s Cancer Group and the Japan Society for Neuro-Oncology. MA reports no conflicts of interest. TO, AW, MS, MH, and KS report employment at AstraZeneca and own stock at AstraZeneca. YN has received consulting fees from AstraZeneca and has received payment or honoraria for lectures from AstraZeneca.
Authorship statement
Conceptualization: SS, KT, MS, MH, and KS; Resources: SS, MA, KS, and YN; Data curation: KT, KS; Formal analysis: TO, MS, MH, and KS; Supervision: SS, KT, KS, and YN; Validation: SS, KT, MA, and YN; Visualization: TO, AW, MS, and MH; Investigation: SS, KT, and MA; Methodology: MS, MH, and KS; Writing (original draft): SS, KS; Project administration: MS; Writing (review and editing): SS, KT, MA, TO, AW, MS, MH, KS, and YN.
Data availability
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
References
- 1. Gutmann DH, Ferner RE, Listernick RH, et al. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [DOI] [PubMed] [Google Scholar]
- 2. Bergqvist C, Servy A, Valeyrie-Allanore L, et al. ; NF France Network. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am J Med Genet A. 2010;152a(2):327–332. [DOI] [PubMed] [Google Scholar]
- 4. Lammert M, Friedman JM, Kluwe L, Mautner VF.. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005;141(1):71–74. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida Y, Kuramochi A, Ota H, et al. . 神経線維腫症1型(レックリングハウゼン病)診療ガイドライン [Neurofibromatosis type 1 (Recklinghausen disease) clinical practice guidelines]. Jpn J Dermatol. 2018;128(1):17–34. [Google Scholar]
- 6. Hirbe AC, Gutmann DH.. Neurofibromatosis type 1: A multidisciplinary approach to care. Lancet Neurol. 2014;13(8):834–843. [DOI] [PubMed] [Google Scholar]
- 7. Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. [DOI] [PubMed] [Google Scholar]
- 8. Hamoy-Jimenez G, Kim R, Suppiah S, et al. Quality of life in patients with neurofibromatosis type 1 and 2 in Canada. Neurooncol Adv. 2020;2(suppl 1):i141–i149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baldo F, Magnolato A, Barbi E, Bruno I.. Selumetinib side effects in children treated for plexiform neurofibromas: First case reports of peripheral edema and hair color change. BMC Pediatr. 2021;21(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blakeley JO, Plotkin SR.. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonsgard JH, Kwak SM, Short MP, Dachman AH.. CT imaging in adults with neurofibromatosis-1: Frequent asymptomatic plexiform lesions. Neurology. 1998;50(6):1755–1760. [DOI] [PubMed] [Google Scholar]
- 13. Williams VC, Lucas J, Babcock MA, et al. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123(1):124–133. [DOI] [PubMed] [Google Scholar]
- 14. Kar M, Deo SV, Shukla NK, et al. Malignant peripheral nerve sheath tumors (MPNST)–clinicopathological study and treatment outcome of twenty-four cases. World J Surg Oncol. 2006;4:55. https://wjso.biomedcentral.com/articles/10.1186/1477-7819-4-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landry JP, Schertz KL, Chiang YJ, et al. Comparison of cancer prevalence in patients with neurofibromatosis type 1 at an academic cancer center vs in the general population from 1985 to 2020. JAMA Netw Open. 2021;4(3):e210945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: A retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armstrong AE, Brossier NM, Hirbe AC.. Neurofibromatosis type 1-related tumours in paediatrics: An evolving treatment landscape. Lancet Child Adolesc Health. 2020;4(7):488–490. [DOI] [PubMed] [Google Scholar]
- 18. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. AstraZeneca Pharmaceuticals LP. Selumetinib (Koselugo) Full Prescribing Information. 2020. https://medicalinformation.astrazeneca-us.com/home/prescribing-information/koselugo-pi.html. Accessed October 18, 2022.
- 20. European Medicines Agency. Koselugo. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/koselugo. Accessed October 18, 2022.
- 21. Selumetinib (Koselugo) Japanese Prescribing Information. 2022.
- 22. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45(5):575–578. [PubMed] [Google Scholar]
- 23. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varni JW, Seid M, Kurtin PS.. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 25. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1–related plexiform neurofibromas. New Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdel-Rahman O, ElHalawani H, Ahmed H.. Risk of selected cardiovascular toxicities in patients with cancer treated with MEK inhibitors: A comparative systematic review and meta-analysis. J Glob Oncol. 2015;1(2):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hilliard ME, Lawrence JM, Modi AC, et al. ; SEARCH for Diabetes in Youth Study Group. Identification of minimal clinically important difference scores of the PedsQL in children, adolescents, and young adults with type 1 and type 2 diabetes. Diabetes Care. 2013;36(7):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varni JW, Limbers C, Burwinkle TM.. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. J Pediatr Psychol. 2007;32(9):1151–1163. [DOI] [PubMed] [Google Scholar]
- 29. Dymond AW, Elks C, Martin P, et al. Pharmacokinetics and pharmacogenetics of the MEK1/2 inhibitor, selumetinib, in Asian and Western healthy subjects: A pooled analysis. Eur J Clin Pharmacol. 2017;73(6):717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen-Rabbie S, Zhou L, Vishwanathan K, et al. Physiologically based pharmacokinetic modeling for selumetinib to evaluate drug–drug interactions and pediatric dose regimens. J Clin Pharmacol. 2021;61(11):1493–1504. https://pubmed.ncbi.nlm.nih.gov/34196005/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schalkwijk S, Zhou L, Cohen-Rabbie S, et al. Population pharmacokinetics and exposure–response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas. Cancer Chemother Pharmacol. 2021;88(2):189–202. https://pubmed.ncbi.nlm.nih.gov/33903938/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).