Abstract
Background
Previous research has identified older age, African-American race, and female sex as meningioma risk factors, but there is limited information on their joint effects, or on how these demographic factors vary across strata of tumor grade.
Methods
The Central Brain Tumor Registry of the United States (CBTRUS) is a population-based registry combining data from the CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program which covers ~100% of the U.S. population and aggregates incidence data on all primary malignant and nonmalignant brain tumors. These data were used to explore the joint impacts of sex and race/ethnicity on average annual age-adjusted incidence rates of meningioma. We calculated meningioma incidence rate ratios (IRRs) by sex and race/ethnicity, across strata of age and tumor grade.
Results
Compared to individuals who are non-Hispanic White, individuals who are non-Hispanic Black had significantly higher risk of grade 1 (IRR = 1.23; 95% CI: 1.21–1.24) and grade 2–3 meningioma (IRR = 1.42; 95% CI: 1.37–1.47). The female-to-male IRR peaked in the fifth decade of life across all racial/ethnic groups and tumor grades, but was 3.59 (95% CI: 3.51–3.67) for WHO grade 1 meningioma and 1.74 (95% CI: 1.63–1.87) for WHO grade 2–3 meningioma.
Conclusions
This study reveals the joint effects of sex and race/ethnicity on meningioma incidence throughout the lifespan and across strata of tumor grade, highlighting incidence disparities among females and African-Americans that may inform future strategies for tumor interception.
Keywords: African Americans, epidemiology, incidence, meningioma, racial disparities
Key Points.
Compared to non-Hispanic Whites, non-Hispanic Blacks are at 42% greater risk of grade 2–3 meningioma.
In the fifth decade of life, female meningioma incidence is 3.4-fold higher than in males.
The female-to-male incidence rate ratio for meningioma differs by race/ethnicity.
Female sex is associated with a higher risk of grade 1 meningioma.
Female sex is associated with a higher risk of grade 2–3 meningioma.
Importance of the Study.
Sex-differences in meningioma incidence are investigated in a population-based study capturing more than 450 000 diagnoses, revealing significant variability in female-associated risk across decades of life, across racial/ethnic groups, and across strata of meningioma tumor grade.
Meningioma is the most common primary central nervous system (CNS) tumor in adults and accounts for >40% of all primary brain tumors diagnosed annually in the United States (U.S.). In comparison, glioma – including glioblastoma – accounts for <20%.1 The last decade has seen a rise in meningioma incidence that can partially be explained by increased diagnosis due to elevated utilization of brain imaging technology.2 Approximately 1% of the U.S. population will develop a meningioma in their lifetime,1,3 of which ~5.4% are classified as WHO grade 2 (eg, atypical) or WHO grade 3 (ie, anaplastic) and the remainder classified as WHO grade 1.4 Of note, the proportion of meningioma that is classified as grade 2–3 is substantially higher in tissue-based studies that include histopathologic review,5,6 as many grade 1 meningiomas are diagnosed radiographically and monitored by imaging, but never undergo surgical resection.
Higher-grade meningioma (WHO grades 2–3) is an aggressive tumor with 5-year survival rates as low as 37%.4 Due to its sensitive intracranial location, even WHO grade 1 meningioma can be a devastating diagnosis due to the morbidity associated with neurosurgical resection. A recent study investigating the long-term neurocognitive, psychological, and return-to-work outcomes of meningioma patients found that 68% exhibited global neurocognitive impairment at 18 months posttreatment, and 48% were unable to return to work.7 These data underscore the long-term impact of this disease and the substantial associated public health burden.
Meningioma epidemiology research has identified several strong demographic risk factors, including: older age, African-American race, and female sex.4,8 The Central Brain Tumor Registry of the United States (CBTRUS), a population-based registry covering ~100% of the US population and capturing >99% all newly diagnosed primary CNS tumors,1 reports an approximately 20% increased incidence of meningioma in African-American relative to non-Hispanic White individuals and an even higher risk of WHO grade 3 tumors in this population.1 Relative to biologic males, females have an approximately 2.3-fold greater risk of developing a meningioma during their lifetime,1 but sex differences in meningioma risk are likely to vary by age. Indeed, a recent registry-based analysis of a California birth cohort observed a 1.6-fold increase in meningioma risk at ages 20–39 among females, but similar risk to males prior to age 20.9 How sex differences in meningioma incidence vary by age in the U.S. population, or across strata of race/ethnicity and tumor grade, has not been thoroughly evaluated.
Meningioma epidemiology research seldom presents risk estimates by tumor grade, largely owing to a paucity of higher-grade cases for analysis. Among studies reporting grade-stratified analyses, such as the CBTRUS annual report, risk estimates are often split into strata of nonmalignant tumors (WHO grades 1–2) and malignant tumors (WHO grade 3). Emerging evidence from epigenomic and transcriptomic tumor profiling reveals substantial limitations in predicting meningioma clinical behavior using traditional histopathologic classification approaches.6,10 These molecularly-informed tumor classification techniques further indicate that grade 2 and 3 tumors often share a similar spectrum of somatic alterations that are generally absent in grade 1 meningioma.
To address these limitations and provide a more comprehensive understanding of meningioma epidemiology, we use CBTRUS data to evaluate population-level variation in incidence of WHO grade 1 meningioma and higher-grade meningioma (WHO grades 2–3). We examine the joint impacts of age, sex, and race/ethnicity on meningioma incidence and compare results across these clinically and molecularly meaningful strata of tumor grade. This work helps inform the underlying mechanisms of meningioma pathogenesis, highlights significant racial disparities, and nominates potential paths toward tumor interception in high-risk populations.
Methods
Data Source
Study data are from CBTRUS, which aggregates primary brain tumor incidence data from 52 central cancer registries (45 National Program of Cancer Registries [NPCRs], and 5 Surveillance Epidemiology and End Results [SEERs]).11 Collected data capture >99% of the U.S. population.12 While U.S. cancer registries must collect data on all cancers diagnosed in the U.S., all nonmalignant primary tumors of the CNS must also undergo registration in accordance with the Benign Brain Tumor Cancer Registries Amendment Act (Public Law 107-260). Meningioma incidence data included in the study cover the period from 2004 (when reporting of nonmalignant CNS tumors became mandatory) until 2019 (the most recent year that CBTRUS received data from NPCR and SEER) and cover all 50 U.S. states and Washington D.C. (excluding data from Nevada for diagnosis years 2018–2019). Data from Puerto Rico were not included.
Histopathology and behavior codes from International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) were used to identify meningioma diagnoses and assign WHO tumor grade as follows: grade 1 (9530-9534/0, 9537/0), grade 2 (9530-34/1, 9537-9539/1), and grade 3 (9530-35/3, 9537-39/3). Tumors with ICD-O-3 site codes C70.0 (cerebral meninges) or C70.9 (meninges not otherwise specified) are included in analyses; those with site code C70.1 (spinal meninges) were excluded. Because ~95% of all meningioma are located intracranially, tumors with site code C70.9 (meninges not otherwise specified) are overwhelmingly intracranial in location and retained in analyses, consistent with previous reports.4 The WHO grading criteria for meningioma saw revisions in 2004, 2016, and 2021. Revisions from 2021 will not impact our dataset, which includes only diagnoses prior to 2020. Revisions from 2004 may affect our dataset if the incorporation of these new criteria into clinical practice had slow uptake, as would the 2016 revisions (when brain invasion was added as a criterion that qualified a grade 2 designation). Age, sex, and race/ethnicity data were obtained from the CBTRUS database.
Statistical Analysis
Population data from the United States Census Bureau were obtained from the National Cancer Institute SEER program (http://seer.cancer.gov) and used to calculate incidence rates that are age-adjusted to the 2000 US standard population. Average annual age-adjusted incidence rates (AAAIR) with 95% confidence intervals (95% CIs) were generated using SEER*Stat, by tumor grade (grade 1, grades 2–3), combined race/ethnicity (non-Hispanic White, non-Hispanic Black, and Hispanic [all races]), sex, and 10-year age groups. Incidence rate ratios (IRRs) and 95% confidence intervals (95% CI) were generated using AAAIR, as previously described.13,14 IRRs were considered statistically significant when P < .05. Figures were created in R 4.1.3. To address issues relating to changes in WHO grading criteria, we performed sensitivity analyses comparing overall results (2004–2019) to results for diagnoses from 2007 to 2016.
Results
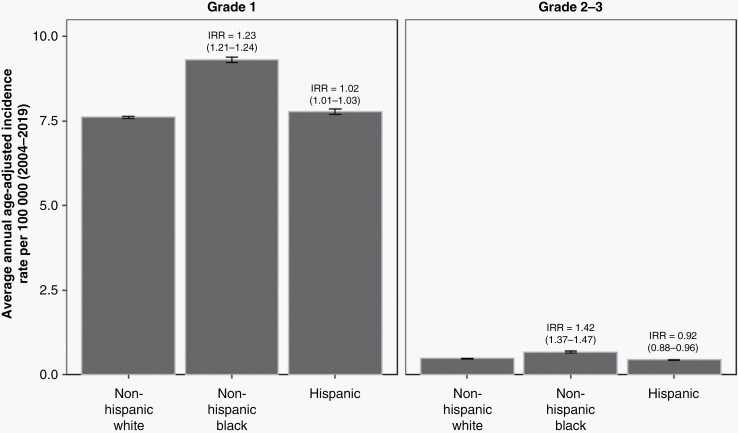
More than 450 000 new intracranial meningioma diagnoses were reported from 2004 to 2019 and aggregated within CBTRUS. The AAAIR for grade 1 tumors was highest among individuals who are non-Hispanic Black (9.31 cases per 100 000; 95% CI: 9.23–9.39), followed by individuals who are Hispanic (7.76 cases per 100 000; 95% CI: 7.68–7.84), and individuals who are non-Hispanic White (7.60 cases per 100 000; 95% CI: 7.57–7.62) (Supplementary Table 1). This corresponds to a 1.23-fold higher rate of WHO grade 1 meningioma among non-Hispanic Black individuals (95% CI: 1.21–1.24) and a 1.02-fold higher rate among individuals who are Hispanic, relative to individuals who are non-Hispanic White (Figure 1). The increased meningioma incidence among non-Hispanic Black individuals was even more pronounced in analysis of higher-grade meningioma. Specifically, individuals who are non-Hispanic Black had a 1.42-fold higher rate of grade 2–3 meningioma compared to individuals who are non-Hispanic White (95% CI: 1.37–1.47). Interestingly, individuals who are Hispanic had a reduced incidence of higher-grade meningioma than individuals who are non-Hispanic White (IRR = 0.92; 85% CI: 0.88–0.96) (Figure 1, Supplementary Table 1).
Figure 1.
Average annual age-adjusted incidence rate and 95% confidence interval (CI) for meningioma by race/ethnicity and stratified by grade. Incidence rate ratios (IRR) and their 95% CI appear above bars and are calculated relative to non-Hispanic White individuals as the reference. Rates are age-adjusted to the 2000 US standard population (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004-2019).
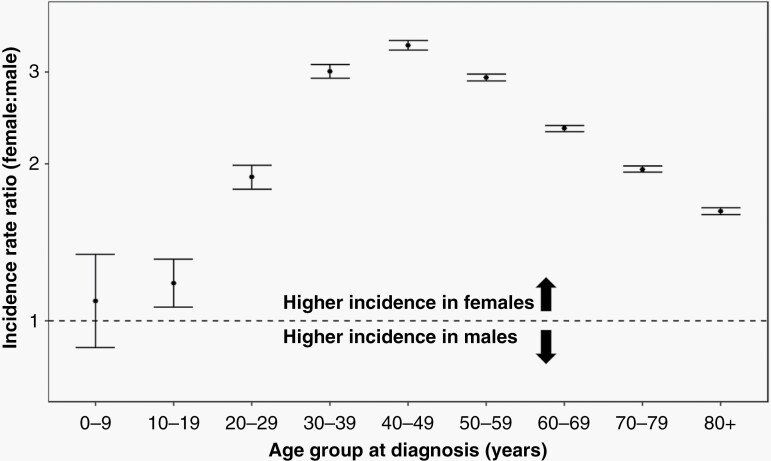
Among all intracranial meningioma diagnoses registered between 2004 and 2019, 73.3% were diagnosed in females. We evaluated the female-to-male meningioma IRR in all subjects, split across 10-year intervals of age at diagnosis (Table 1). From age 0 to 9 years, females were at nonsignificantly elevated risk of meningioma (IRR = 1.09; 95% CI: 0.89–1.34). Female-associated meningioma incidence increased in the second decade of life (age 10–19 years), with incidence rates significantly higher in females than in males (IRR = 1.18; 95% CI: 1.06–1.31). The female-to-male IRR continued to increase with age, reaching a zenith of 3.38 (95% CI: 3.31–3.45) among those age 40–49 years. The female-to-male IRR decreased monotonically across subsequent decades of life, reaching 1.62 (95% CI: 1.60–1.65) among those age 80+ years, but never returning to the prepubertal nadir observed in children (Figure 2).
Table 1.
Total Cases, Average Annual age-adjusted Incidence Ratea (AAAIR), Incidence Rate Ratioa (IRR), and 95% Confidence Interval (CI) for Meningioma by Sex and Stratified by 10-year Age Intervals (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004-2019)
| Age Group | Sex | Total Cases (2004–2019) | AAAIR (95% CI) | IRR (95% CI) | IRR P-value |
|---|---|---|---|---|---|
| 0–9 | Male | 187 | 0.06 (0.05–0.07) | Ref | .4168 |
| Female | 196 | 0.06 (0.05–0.07) | 1.09 (0.89–1.34) | ||
| 10–19 | Male | 662 | 0.19 (0.18–0.21) | Ref | .0020 |
| Female | 746 | 0.22 (0.21–0.24) | 1.18 (1.06–1.31) | ||
| 20–29 | Male | 2112 | 0.60 (0.57–0.62) | Ref | <.0001 |
| Female | 3864 | 1.13 (1.09–1.17) | 1.89 (1.79–1.99) | ||
| 30–39 | Male | 5262 | 1.62 (1.57–1.66) | Ref | <.0001 |
| Female | 15 748 | 4.87 (4.79–4.95) | 3.01 (2.92–3.11) | ||
| 40–49 | Male | 11 253 | 3.29 (3.22–3.35) | Ref | <.0001 |
| Female | 38 766 | 11.10 (10.99–11.22) | 3.38 (3.31–3.45) | ||
| 50–59 | Male | 20 459 | 6.21 (6.13–6.30) | Ref | <.0001 |
| Female | 62 663 | 18.20 (18.06–18.35) | 2.93 (2.88–2.98) | ||
| 60–69 | Male | 28 438 | 12.29 (12.14–12.43) | Ref | <.0001 |
| Female | 73 835 | 28.76 (28.55–28.97) | 2.34 (2.31–2.37) | ||
| 70–79 | Male | 28 931 | 22.25 (21.99–22.50) | Ref | <.0001 |
| Female | 69 817 | 43.47 (43.15–43.80) | 1.95 (1.93–1.98) | ||
| 80+ | Male | 23 328 | 34.44 (34.00–34.89) | Ref | <.0001 |
| Female | 65 156 | 55.94 (55.50–56.37) | 1.62 (1.60–1.65) |
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States.
aRates are per 100 000 and are age-adjusted to the 2000 US standard population.
Figure 2.
Female-to-male incidence rate ratios and 95% confidence intervals (CI) for meningioma, stratified by age group at diagnosis (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004-2019).
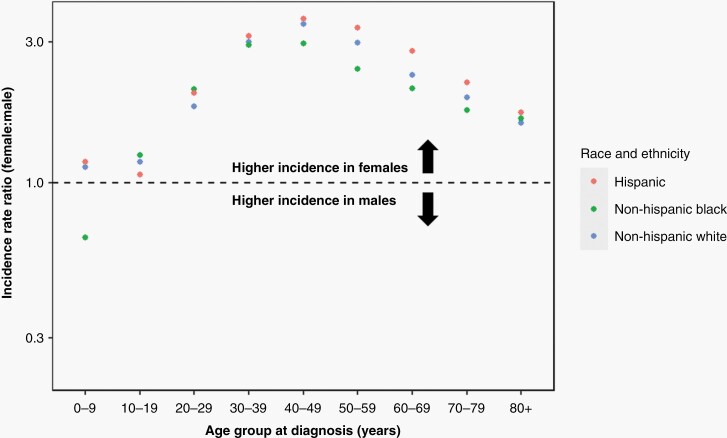
Because we observed significant meningioma incidence disparities in non-Hispanic Black individuals and a complex interplay between sex and age on meningioma risk, we next evaluated the female-to-male meningioma IRRs in 10-year age intervals across strata of race/ethnicity (Supplementary Table 2). The pattern previously observed across all racial/ethnic groups was largely recapitulated in the non-Hispanic White, non-Hispanic Black, and Hispanic subgroups (Figure 3). Meningioma incidence was higher among females than males across all age groups, with the exception of non-Hispanic Black children under 10 years of age. The lower female incidence observed in non-Hispanic Black children was nonsignificant (IRR = 0.65; 95% CI: 0.35–1.20) and based on small numbers within this subgroup (19 females and 30 males). The female-to-male IRR peaked in the 40 to 49-year age group among individuals who are non-Hispanic White (IRR = 3.45; 95% CI: 3.36–3.54), non-Hispanic Black (IRR = 2.96; 95% CI: 2.81–3.13), and Hispanic (IRR = 3.59; 95% CI: 3.39–3.81).
Figure 3.
Female-to-male incidence rate ratios for meningioma, by age group at diagnosis, and stratified by race/ethnicity (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004-2019).
Despite strong consistencies in age-stratified female-to-male IRRs across racial/ethnic groups, several key differences were observed. From 30 years of age onward, a range encompassing >98% of all diagnosed meningioma cases in our dataset, the female-to-male IRR was highest in individuals who are Hispanic. Furthermore, from age 50 to 79 years, a range encompassing 63% of all diagnosed meningioma in our dataset, 95% confidence intervals for the female-to-male IRR among individuals who are Hispanic did not overlap the 95% confidence intervals among individuals who are non-Hispanic White or non-Hispanic Black. Conversely, the female-to-male IRRs in individuals who are non-Hispanic Black tended to be lower than that in individuals who are Hispanic or non-Hispanic White, plateauing earlier and never exceeding a 3.0-fold increase in risk (Figure 3, Supplementary Table 2).
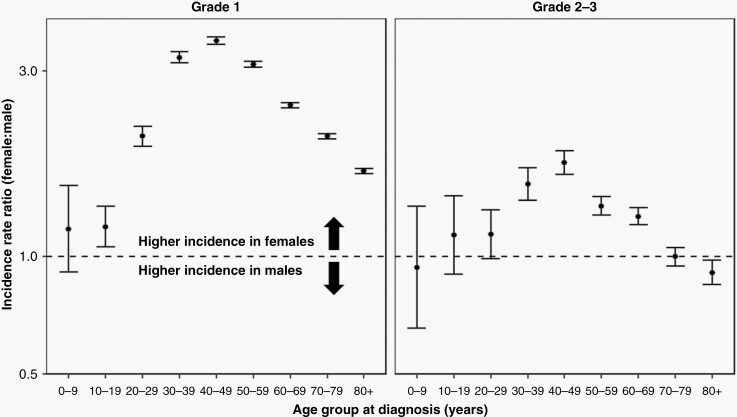
Finally, we evaluated the female-to-male meningioma IRRs in 10-year age intervals across strata of tumor grade, where we observed stark differences between WHO grade 1 and higher-grade meningioma. The female-to-male IRR peaked in the 40 to 49-year age group, irrespective of tumor grade, but peaked at 3.59 (95% CI: 3.51–3.67) for grade 1 meningioma and at 1.74 (95% CI: 1.63–1.87) for higher-grade meningioma (Figure 4, Supplementary Table 3). Compared to males, females did not experience statistically significantly increased incidence of higher-grade meningioma until the fourth decade of life. Within the 70 to 79-year age bracket, female incidence of higher-grade meningioma was no different than among males (IRR = 1.00; 95% CI: 0.95–1.06). Interestingly, female sex was also associated with an approximately 10% reduction in higher-grade meningioma incidence in the oldest (80+ years) group (IRR = 0.91; 95% CI: 0.85–0.98).
Figure 4.
Female-to-male incidence rate ratios and 95% confidence intervals (CI) for meningioma, by age group at diagnosis and stratified by WHO tumor grade (CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2004-2019).
Sensitivity analyses comparing results for diagnoses from 2004 to 2019 to results for diagnoses from 2007 to 2016 did not suggest any biases due to changes in WHO grading criteria. Similarly, restricting analyses to only those patients receiving a tissue-based diagnosis and tumor grade also had minimal effects on study results.
Discussion
This study leverages 16 years of population-based meningioma tumor registration data from CBTRUS, covering approximately 100% of the U.S. population. Analysis of more than 450 000 meningioma diagnoses helped clarify how sex and race/ethnicity jointly influence meningioma incidence, across the lifespan. We reveal significant racial disparities in risk of meningioma – particularly higher-grade meningioma – among non-Hispanic Black individuals residing in the U.S. Furthermore, our analyses demonstrated that the well-documented increased incidence of meningioma among females is present throughout adulthood, but this increase in incidence is most pronounced in the fourth, fifth, and sixth decades of life. Importantly, we also show that female sex is associated with elevated risk of meningioma across tumor grades, but that its contribution to risk of grade 1 meningioma is substantially stronger than its contribution to risk of higher-grade meningioma.
Our results regarding the elevated meningioma incidence in females and a peak female-to-male IRR observed in midlife are largely in-line with prior research.15 However, a particular strength of our analysis is its population-based collection and comprehensive coverage of U.S. racial and ethnic minority populations, which has allowed us to look at the joint effects of age, sex, and race/ethnicity on meningioma incidence. Importantly, due to the large number of cases available for analysis, we are able to make more strongly-supported inferences about demographic differences in the incidence of higher-grade meningioma, a clinically challenging disease that represents ~6% of intracranial cases and is often too uncommon to be adequately studied in smaller datasets.
Observed differences in the female-to-male IRR across strata of age at diagnosis support the hypothesized hormonal etiology of WHO grade 1 meningioma. Differences in age-stratified sex-ratios across racial/ethnic groups are intriguing and could implicate important differences in hormonal regulation, such as variation in age at menarche or age at menopause, in meningioma development, or tumor latency. Immunohistochemical analysis of meningioma tumor specimens has found up to 80% of tumors are progesterone receptor positive (PR+) and 40% are estrogen receptor positive (ER+).16–18 A recent genomic analysis of meningioma patient germline data showed that the lead meningioma risk allele from a prior genome-wide association study (GWAS) also conferred risk of ovarian and ER+ breast cancer, but that this same allele was associated with a reduced risk of ER− breast cancer.15 Transcriptional analyses further revealed that this heritable genetic variant was associated with upregulation of estrogen receptor 1 (ESR1) gene expression in both normal meninges and meningioma tumors,15 underscoring the potential importance of intracranial estrogenic signaling in meningioma development.
Despite conferring greater risk of WHO grade 1 meningioma than of higher-grade meningioma, female sex was demonstrated to be a strong and consistent risk factor for WHO grade 2–3 tumors and was associated with a 1.74-fold increased incidence among those age 40–49 years. Interestingly, the effect of female sex on higher-grade meningioma incidence waned over time, disappearing by the eighth decade of life and, ultimately, becoming protective among individuals age 80+ years. Whether this protective effect in octogenarians is biologically meaningful or is influenced by a more pronounced healthy survivor bias among elderly men remains to be seen, but could benefit from a more sophisticated analytic framework incorporating competing risks into the model.
The 20% higher incidence of WHO grade 1 meningioma and 40% higher incidence of WHO grade 2–3 meningioma in individuals who are non-Hispanic Black compared to non-Hispanic White merits further attention, particularly because similar disparities were not observed in U.S. Hispanics. Consequently, African-Americans have both an elevated burden of disease and would be anticipated to have poorer long-term recurrence-free and overall survival outcomes.4,12 Despite increased attention to racial/ethnic disparities in cancer risk and outcomes, disparities in meningioma incidence and survival among African-Americans has been largely overlooked. While African-Americans have been observed to have lower risks of both pediatric and adult glioma, partly due to differences in genetic ancestry and the distribution of underlying genetic risk factors,19–21 only two common heritable polymorphisms associated with meningioma risk have been discovered by GWAS.22
Whether environmental exposures could contribute to the elevated meningioma incidence observed in African-American individuals is unclear. In addition to ionizing radiation exposure, other modifiable/exogenous factors that have been associated with meningioma risk in high-quality studies include obesity,23 cigarette smoking,24 and a more recent potential association with exposure to airborne particulate pollution.25 The elevated meningioma incidence among African-American individuals and the lack of a clear genetic attribution suggest that environmental factors contributing to meningioma development may have differing distributions across U.S. racial groups and be more prevalent in locales where a greater proportion of African-Americans reside.
Our study has several important limitations. First, CBTRUS data lack central pathology review and detailed molecular information. Cases included in this analysis include those diagnosed by both radiography confirmation and molecular confirmation. Completeness of data collection for radiographic diagnosis of nonmalignant brain tumors varies by state, and this may contribute to variation seen between demographic groups that vary in distribution by region of the U.S. Additionally, a subset of cases had ICD-O-3 site codes C70.9 (meninges not otherwise specified), meaning that a small percent of individuals in our dataset likely had extracranial tumors. Recent multi-omic analysis of meningioma tumor DNA has revealed unique molecular clusters with distinct clinical outcomes that tend to outperform histopathologic grade in terms of predicting recurrence-free and overall survival.6,10 However, biomarkers have only been sparsely incorporated into the WHO classification of meningioma (eg, upgrading to grade 3 based on CDKN2A loss) and are not yet available for CBTRUS-based analyses.5 Finally, the latency period between exposures and meningioma development is likely to be quite long and may differ across strata of age, sex, and race/ethnicity in an unrecognized manner that could alter how the data presented here are interpreted.26
Our findings reveal important disparities in the incidence of intracranial meningioma across racial/ethnic groups and between sexes and show that associations with these demographic variables vary substantially across the life course and between tumor grades. Clinical nomograms have been developed to predict meningioma grade in the presurgical setting,27 and five-year tumor recurrence risk,28 although neither model includes age, sex, or race/ethnicity in the nomogram. Our results suggest that incorporating these demographic data may lead to meaningful gains in predictive power, helping to inform patient stratification, subclinical disease detection, and targeted interception efforts. Future research is needed to explore demographic risk factors within strata of molecular tumor classification, and also to explore the contributions of environmental exposures to meningioma incidence, with a particular focus on exposures having uneven geographic and socioeconomic distributions that could underlie structural inequalities in meningioma incidence.
Supplementary Material
Acknowledgments
The CBTRUS data were provided through an agreement with the Centers for Disease Control’s National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Supplement sponsorship. This supplement was sponsored by a generous donation from Mr. Paul Mielnik and his family to help raise awareness and advance the care of patients with meningiomas worldwide.
Conflict of interest statement. J.S.B.-S. is a full-time paid employee of the NIH/NCI.
Contributor Information
Kyle M Walsh, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke University Medical Center, Duke Cancer Institute, Durham, North Carolina, USA.
Mackenzie Price, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Corey Neff, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Jordan M Komisarow, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Courtney E Wimberly, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Jill S Barnholtz-Sloan, Center for Biomedical Informatics & Information Technology (CBIIT) and Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute (NCI), Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Quinn T Ostrom, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke University Medical Center, Duke Cancer Institute, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No:00002; the American Brain Tumor Association; The Sontag Foundation, Novocure; the Musella Foundation for Brain Tumor Research & Information, Inc.; National Brain Tumor Society; the Pediatric Brain Tumor Foundation; the Uncle Kory Foundation; the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Additional funding provided by the U.S. National Cancer Institute under P30CA014236 (K.M.W.) and P50CA190991 (K.M.W., Q.T.O.). JSBS is a full-time, paid employee of the NCI/NIH.
References
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022; 24(Supplement_5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin DD, Lin JL, Deng XY, et al. Trends in intracranial meningioma incidence in the United States, 2004-2015. Cancer Med. 2019; 8(14):6458–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huntoon K, Toland AMS, Dahiya SM.. A review of clinicopathological and molecular aspects. Front Oncol. 2020; 10:579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kshettry VR, Ostrom QT, Kruchko C, et al. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015; 17(8):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nassiri F, Liu J, Patil V, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021; 597(7874):119–125. [DOI] [PubMed] [Google Scholar]
- 7. Sekely A, Zakzanis KK, Mabbott D, et al. Long-term neurocognitive, psychological, and return to work outcomes in meningioma patients. Support Care Cancer. 2022; 30(5):3893–3902. [DOI] [PubMed] [Google Scholar]
- 8. Walsh KM. Epidemiology of meningiomas. Handb Clin Neurol. 2020; 169:3–15. [DOI] [PubMed] [Google Scholar]
- 9. Cote DJ, Wang R, Morimoto LM, et al. Birth characteristics and risk of meningioma in a population-based study in California. Neurooncol Adv. 2022; 4(1):vdac173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choudhury A, Magill ST, Eaton CD, et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet. 2022; 54(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Central Brain Tumor Registry of the United States SEER*Stat Database. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data, 2020 submission (2000–2018). 2021. [Google Scholar]
- 12. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021; 23(12 Suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SEER*Stat Software [Computer Program]. Version 8.4 . 2022. https://seer.cancer.gov/seerstat/download/.
- 14. Tiwari RC, Clegg LX, Zou Z.. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006; 15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 15. Walsh KM, Zhang C, Calvocoressi L, et al. Pleiotropic MLLT10 variation confers risk of meningioma and estrogen-mediated cancers. Neuro-Oncol Adv. 2022; 4(1):vdac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korhonen K, Salminen T, Raitanen J, et al. Female predominance in meningiomas can not be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol. 2006; 80(1):1–7. [DOI] [PubMed] [Google Scholar]
- 17. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M.. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006; 105(2):163–173. [DOI] [PubMed] [Google Scholar]
- 18. Hage M, Plesa O, Lemaire I, Raffin Sanson ML.. Estrogen and Progesterone Therapy and Meningiomas. Endocrinology. 2022; 163(2):bqab259. [DOI] [PubMed] [Google Scholar]
- 19. Walsh KM, Neff C, Bondy ML, et al. Influence of county-level geographic/ancestral origin on glioma incidence and outcomes in US Hispanics. Neuro Oncol. 2023; 25(2):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrom QT, Egan KM, Nabors LB, et al. Glioma risk associated with extent of estimated European genetic ancestry in African Americans and Hispanics. Int J Cancer. 2020; 146(3):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, Ostrom QT, Hansen HM, et al. European genetic ancestry associated with risk of childhood ependymoma. Neuro Oncol. 2020; 22(11):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Claus EB, Cornish AJ, Broderick P, et al. Genome-wide association analysis identifies a meningioma risk locus at 11p15.5. Neuro Oncol. 2018; 20(11):1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi H, Cornish AJ, Sud A, et al. Mendelian randomization provides support for obesity as a risk factor for meningioma. Sci Rep. 2019; 9(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Claus EB, Walsh KM, Calvocoressi L, et al. Cigarette smoking and risk of meningioma: the effect of gender. Cancer Epidemiol Biomarkers Prev. 2012; 21(6):943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer JD, Prasad RN, Cioffi G, et al. Exposure to radon and heavy particulate pollution and incidence of brain tumors. Neuro Oncol. 2023; 25(2):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D.. Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg. 2002; 97(5):1078–1082. [DOI] [PubMed] [Google Scholar]
- 27. Peng S, Cheng Z, Guo Z.. Diagnostic nomogram model for predicting preoperative pathological grade of meningioma. Transl Cancer Res. 2021; 10(9):4057–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nassiri F, Mamatjan Y, Suppiah S, et al. ; International Consortium on Meningiomas. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019; 21(7):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.