Abstract
Populations face a suite of anthropogenic stressors acting simultaneously, which can combine additively or interact to have complex effects on population persistence. Yet we still know relatively little about the mechanisms underlying population-level responses to multifactorial combinations of stressors because multiple stressor impacts across organisms’ life cycles have not been systematically considered in population models. Specifically, different anthropogenic stressors can have variable effects across an organism’s life cycle, resulting in non-intuitive results for long-term population persistence. For example, synergistic or antagonistic interactions might exacerbate or alleviate the effects of stressors on population dynamics, and different life-history stages or vital rates might contribute unequally to long-term population growth rates. Demographic modelling provides a framework to incorporate individual vital rate responses to multiple stressors into estimates of population growth, which will allow us to make more informed predictions about population-level responses to novel combinations of anthropogenic change. Without integrating stressors’ interactive effects across the entire life cycle on population persistence, we may over- or underestimate threats to biodiversity and risk missing conservation management actions that could reduce species’ vulnerability to stress.
Keywords: Antagonistic interactions, demographic modelling, multifactorial, multiple stressor, perturbation analysis, population growth rate, synergistic interactions
As humans alter multiple facets of the environment simultaneously, addressing multiple, potentially interacting stressors will be critical for predicting species' responses. Yet the effects of multiple stressors across organisms' life cycle has not been systematically considered in demographic studies. Here, Zettlemoyer proposes a framework to explore the role of multiple stressors on population dynamics in order to make realistic predictions about population viability under different conservation scenarios. Specifically, demographic modelling provides a method to integrate stressors' potential additive and non-additive effects across the entire life cycle, provide mechanistic explanations of species loss and spread under combinations of novel conditions, and improve conservation and management plans.
Introduction
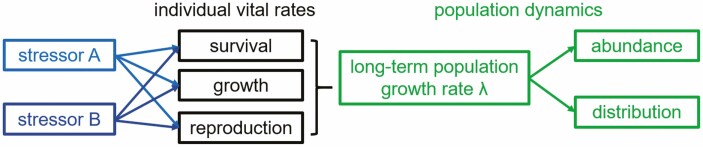
Anthropogenic environmental changes, or stressors (Crain et al. 2008; see Glossary for bolded terms), such as habitat loss, climate change and invasive species, represent some of the most severe threats to biodiversity worldwide (Urban 2015). These and other stressors can have complex interactive effects (i.e. additive or non-additive) on population persistence (Piggott et al. 2015). As humans alter multiple facets of the environment simultaneously, addressing multiple, interacting stressors will be critical for predicting species’ responses (e.g. abundance and distributions) to future environmental conditions (Fig. 1). Studies increasingly document the effects of multiple stressors on individuals, species, communities and ecosystems, yet research on interactive effects of multiple stressors remains relatively rare in population ecology (Capdevila et al. 2022; but see Nomoto and Alexander 2021; Zettlemoyer 2022). However, threats rarely impact a population individually (Hernández-Yáñez et al. 2016; Wake 2019; Orr et al. 2020; Rillig et al. 2021) and threat effects may be inconsistent across an organism’s life cycle (Paniw et al. 2019; Speißer et al. 2022). Therefore, single-threat, single-life-stage approaches can ignore or oversimplify how combinations of stressors ultimately impact species and communities (Capdevila et al. 2022; Litchman and Thomas 2022). Ultimately, conserving species will depend on our ability to determine both the underlying population-level processes modulating species’ responses to multiple stressors (Capdevila et al. 2020) and which threats have the largest impact on population health (Bernardo et al. 2019). Determining threats to long-term population health will require parameterizing population models (e.g. matrix population models, integral projection models [IPM]) that quantify the cumulative impact of multiple anthropogenic stressors on population growth rates (Earl 2019).
Figure 1.
Conceptual approach for predicting population dynamics in response to multiple stressors. Effects on population growth rate (λ) depend on the responses of individual vital rates, which can have complex responses to different stressors, and the sensitivity of λ to each of those vital rates.
Most work on population-level responses to anthropogenic stressors has focused on one or more targeted vital rates (e.g. survival, growth or reproduction) in size-, age- or stage-structured populations. Reviews report that only 136 studies integrate multiple vital rate responses into plant demographic models (Ehrlén et al. 2016) and only 106 studies link climate conditions to multiple vital rates in terrestrial mammals (Paniw et al. 2021). However, interacting stressors can have complex effects on different vital rates (Speißer et al. 2022), individual vital rates can respond uniquely to stressors (Villellas et al. 2015), and vital rates can contribute unequally to overall population growth rates (Ehrlén et al. 2016). Therefore, studies that do not integrate across multiple vital rates could under- or over-inflate the importance of an individual vital rate, making predicting the cumulative effects of global change on long-term fitness or population growth difficult. Studies that do not integrate across multiple vital rates could also bias conservation plans, which need to target activities at specific life stages that will benefit population viability (Côté et al. 2016; Orr et al. 2020). Developing management plans for at-risk species, therefore, needs to consider the non-additive effects of stressors across the entire life cycle. Unfortunately, the long-term demographic data needed for such stage- or age-based population analyses rarely exist (Morris and Doak 2002; Earl et al. 2018; Bernardo et al. 2018; Johnston et al. 2019) despite the fact that population modelling is an essential tool for understanding the effects of multiple stressors on several vital rates and translating those effects into population growth rates (Ehrlén et al. 2016).
Here, I argue that demographic studies that consider multiple stressors can partition additive vs. non-additive effects of interacting stressors across the life cycle and allow for more mechanistic explanations of population decline and species loss. Focusing on plant species, I will discuss how (i) interactions among anthropogenic stressors and (ii) counteracting effects of stressors on different vital rates might influence population dynamics. Finally, I outline potential experimental designs that integrate multiple stressor effects across the life cycle to predict extinction risk under future environmental conditions.
Additive and Non-additive Interactions Among Multiple Stressors
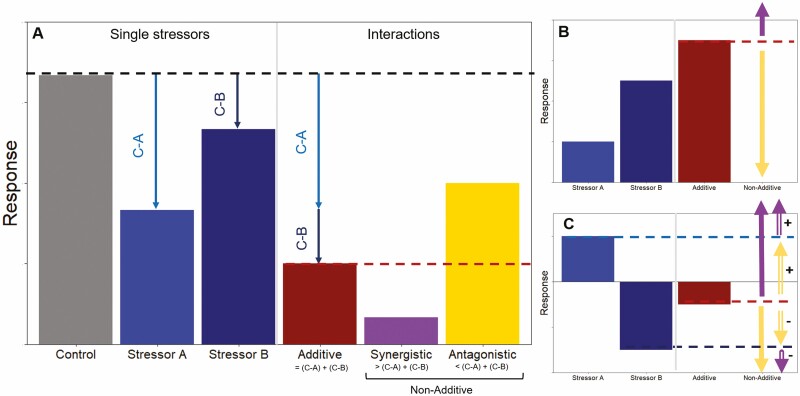
Understanding how multiple stressors combine to affect biodiversity is an ongoing challenge (Schäfer and Piggott 2018; Speißer et al. 2022; Turschwell et al. 2022). To date, research has focused on synthesizing studies on single stressors to estimate the possible effects of ecological interactions, but we often detect ‘ecological surprises’ (sensuPaine et al. 1998) when an interaction has a greater effect than expected (Burgess et al. 2021). The expectation is typically additive effects, wherein the combined effects of two stressors are equal to the sum of their individual effects (Fig. 2; Crain et al. 2008) or when two stressors act entirely independently of each other (Haller-Bull and Bode 2019). For instance, invasion by Alliaria petiolata (garlic mustard) interacts additively with deer herbivory to reduce population growth rates of Trillium erectum (Bialic-Murphy et al. 2019). In these cases, it may be possible to infer the effects of interacting stressors by combining results from single-factor studies. However, stressors can also show non-additive interactions wherein the effect of one stressor can change with the intensity of the second stressor (Folt et al. 1999; Piggott et al. 2015). These non-additive interactions can be synergistic when their combined effect has a greater impact than the algebraic sum of their individual impacts (Fig. 2; Darling and Côté 2008; Côté et al. 2016). In the invasive thistle Cirsium vulgare (common thistle), only the combination of high levels of interspecific competition and insect herbivory results in population decline (Tenhumberg et al. 2015). In contrast, non-additive interactions are antagonistic when the combined stressor impact is less than their sum (Fig. 2). For instance, the combined negative effects of rising temperatures and more humid climates are less negative than expected based on their individual contributions to survival for two (sub)alpine forbs, Veronica alpina (alpine speedwell) and Viola palustris (alpine marsh violet) (Tӧpper et al. 2018). Altogether, accounting for potential non-additive effects of individual drivers is critical for making quantitative predictions about population-level responses to combined anthropogenic changes (Fig. 3).
Figure 2.
Interactions among stressors can be additive, synergistic or antagonistic. (A) The black dashed line indicates a measurement under a control treatment (grey). The light and dark blue bars and arrows represent responses to single stressors A and B, respectively (left of grey line). An additive response (red bar) occurs when the difference between the control and each stressor (C-A and C-B) is equal to their algebraic sum (indicated with the red dashed line). A synergistic interaction (purple) occurs when the combined effect of two stressors is larger than the sum of their individual effects, while an antagonistic interaction (gold) occurs when their combined effects are less than their sum. Synergistic and antagonistic interactions represent non-additive responses. The additive sum provides a null hypothesis that provides a threshold (red dashed line) for distinguishing the two types of non-additive interactions. (B) When the responses to two single stressors A and B are in the same direction, values below their sum represent antagonistic interactions (gold arrows) while values above their sum represent synergistic interactions (purple arrows). (C) However, these categories become complicated when two stressors induce opposing responses, with some studies considering any value above or below the null synergistic versus antagonistic, respectively, while others advocate for positive or negative classifications of these interactions (double-barred arrow in C, with ± indicating positive vs. negative synergy and antagonism) depending on thresholds based on the single stressor responses (blue dashed lines) (cf. Fig. 2 in Piggott et al. 2015).
Figure 3.
Multiple stressors can have additive, synergistic or antagonistic interaction effects on long-term population growth rates (λ). In the case of additive interactions, the combined effects of stressor A and stressor B (medium grey arrow; width of the arrows represents combined effect size) on λ equal the sum of their individual effects (medium grey lines). If the interaction between A and B is synergistic, their combined effect on λ will be greater than their sum, exacerbating their single effects (black arrow), while if the interaction between A and B is antagonistic, their combined effects will be less than their sum (light grey arrow), negating the potential negative effects of a single stressor.
Assessing the additive vs. non-additive effects of multiple interacting threats on population dynamics will therefore help prioritize conservation efforts under future combinations of novel conditions. For example, climate warming threatens the viability of a rare forb, Eurybia furcata (forked aster), but only when site-level woody encroachment and deer herbivory are high (Bernardo et al. 2018). This suggests that management at a local scale (e.g. woody invasive species removal) could reduce this species’ extinction risk under warming. Similarly, invasive species removal is an important management strategy for the rare orchid Cypripedium candidum (white lady’s slipper) under moderate climate change scenarios. However, as drought stress increases, protecting groundwater recharge zones in sites near this hydrologically sensitive species becomes increasingly important (Phillips-Mao et al. 2016). Both of these studies inform conservation decision-making by assessing multiple interacting stressors and identifying the most important stressor. Applying this process to prioritize management under climate change is critical because while stressors like invasion can exacerbate the negative effects of climate change, their removal provides a ‘low-risk’ short-term management strategy. These low-risk strategies can be important initial actions to reduce the impact of climate change on vulnerable populations (Galatowitch et al. 2009), especially given the often-inadequate resources for conservation of rare plant populations (Heywood 2017; Westwood et al. 2021).
Counteracting Effects of Stressors on Different Vital Rates
Understanding responses to stressors across the life cycle can inform which vital rates are most susceptible to a given stressor, but relatively few studies integrate potentially disparate effects of several stressors on multiple aspects of performance into comprehensive assessments of population growth (Ehrlén et al. 2016). However, long-term demographic data can be used to identify the specific vital rates altered by a given stressor(s) and whether/how those altered vital rates ultimately influence population growth. Specifically, population models such as matrix projection models or IPMs incorporate the effects of stressors on individual vital rates, which are then used to estimate cumulative population growth rates (λ) (Easterling et al. 2000; Caswell 2001; Ellner and Rees 2006; Dalgleish et al. 2011; Merow et al. 2014; Doak et al. 2021). Thus, population models can be used to predict λ as a function of multiple interacting (a)biotic environmental conditions (Nicolé et al. 2011; Adler et al. 2012; Merow et al. 2017). There are two important considerations here. First, it is important to note that the values of an individual vital rate (e.g. survival ranges from 0 to 1) will not linearly correspond with λ values (which can range from 0 to infinity). In order to examine differences in λ between multiple stressors and determine the most relevant vital rate, log-transforming λ will help interpret the impact of vital rates on λ (Orzack and Tuljapurkar 1989). Second, if a study examines multiple stressors, a series of models can be fit against the stressors’ various combinations to then select the most parsimonious model using information criterion approaches (e.g. AICc; Burnham and Anderson 2004; Dalgleish et al. 2011) or model averaging (Dahlgren 2010).
Furthermore, perturbation analyses (e.g. integrated elasticities and Life Table Response Experiments [LTRE]) can quantify the sensitivity of λ to changes in each vital rate and/or environmental variable (van Tienderen 2000). Elasticity analyses decompose direct vs. indirect effects of single vital rates on λ; LTREs additionally identify which vital rate(s) drive differences in λ. For example, invasion and deer herbivory affect multiple vital rates in T. erectum (red trillium), including growth and seedling recruitment, but an LTRE revealed that decreases in fertility largely drive declines in plant fitness (Bialic-Murphy et al. 2019). Perturbation analyses can reveal not only which vital rate matters most but also whether a particular stressor influences λ at all. In a suite of tallgrass prairie species, an LTRE demonstrated that nitrogen fertilization drives population declines via decreasing survival, but deer herbivory has relatively small effects on individual vital rates and ultimately made no contribution to differences in λ (Box 1; Zettlemoyer 2022). Perturbation analyses are critical for predicting responses to multiple stressors because they can capture the demographic mechanisms that drive biological change (Dawson et al. 2011; Johnston et al. 2019; Schuwirth et al. 2019; Otto et al. 2020).
In the two studies above, variation in λ was driven by changes in a single vital rate. However, variation in λ can also be driven by trade-offs among vital rates (Stearns 1989; Reed et al. 2013; Compagnoni et al. 2016; Fung et al. 2022). Specifically, the combined effects of two vital rates that demonstrate opposing responses to a stressor might result in no net observed effect on λ (i.e. demographic compensation; Villellas et al. 2015). For example, negative covariance between stasis and retrogression alleviates yearly variation in λ in Primula farinosa subsp. modesta (bird’s-eye primrose; Jeong et al. 2022). In the alpine plant Silene acaulis (moss campion), growth and survival show compensatory responses to warming that buffers overall population growth (DeMarche et al. 2018). In this case, the negative effects of lower survival under warming are counteracted by the positive response of growth to warming, resulting in no net effect on λ. This approach can be expanded to consider contrasting vital rate responses to simultaneous stressors. In Phoenix loureiroi (voyavoy palm), compensatory responses of ramet growth and clonal reproduction buffer populations from the effects of grazing and harvest (Mandle et al. 2015). In addition to interactions among individual stressors, density-dependent processes can mitigate or enhance stressor impacts at the population level (Moe et al. 2005; Dahlgren et al. 2014). For example, mortality due to a stressor can reduce competition, such that density-related mortality can compensate for stressor-related mortality (Moe et al. 2013). In the weed Echinochloa crus-galli (barnyard grass), density-dependent mortality due to post-dispersal seed consumption decreases seedling density, resulting in increased fecundity and therefore compensating for low seedling numbers (Pannwitt et al. 2021). In contrast, in Pityopsis aspera var. aspera (pineland silkgrass), increasing density decreases λ, but this effect is alleviated by fire (Gornish 2013). Altogether, understanding how individual vital rate responses scale up (or do not) to consequences at the population level will be critical for conservation in landscapes experiencing multiple simultaneous stressors that can negate or exacerbate their effects at different stages of the life cycle.
A Need for Added Realism in Modelling Population-Level Responses to Multiple Anthropogenic Stressors
Single-stressor population ecology studies may yield less realistic, if simpler, answers to complex environmental problems (Töpper et al. 2018; Litchman and Thomas 2022). Despite their potential to disentangle interactions across life cycles, few demographic studies explicitly link multiple environmental drivers to multiple vital rates across the life cycle (Paniw et al. 2021), a concerning limitation given that demographic studies are often aimed at informing management (Crone et al. 2012; Earl 2019). I now offer four research priorities aimed at adding realism to studies on multifactorial anthropogenic stressors, including factorial experiments, stressor removal, stressor gradients, and long-term demographic data.
First, multifactorial studies provide the opportunity to investigate the effects of two or more variables simultaneously, a critical future avenue for understanding species’ responses to more realistic present and future conditions (Pazzaglia et al. 2022). This approach includes observational studies that take advantage of natural variation in environmental conditions to assess the influence of multiple stressors and experiments wherein stressors are applied while controlling for other influences. The latter represents our best tool for demonstrating causal relationships between stressor and response (Rillig et al. 2021), but multifactorial experiments in the field remain rare (Wake 2019; Reich et al. 2020). Factorial experiments can be difficult to implement due to the inevitable trade-off between number of replicates and number of treatments. Limited replication can result in low statistical power to detect interactions, defeating the purpose of a factorial design. Meanwhile, investigating a large number of factors results in the ‘combinatorial explosion problem’ (Katzir et al. 2019) wherein manipulating a larger number of factors, while more biologically realistic, becomes increasingly difficult due to the rapid increase in the possible number of combinations (although dimension reduction techniques such as Principal Components Analysis can facilitate analysis; Donham et al. 2023). Factor number can also influence results; for instance, manipulating an increasing number of global change factors resulted in directional changes in soil properties, but the magnitude of response was variable (Rillig et al. 2019). Ultimately, the possibility of non-additive effects (see above) suggests that we cannot rely on a thorough understanding of the effects of single factors to inform us about possible interactions (Norby and Luo 2004). Indeed, increasing the number of anthropogenic factors acting on a community affects plant composition and productivity in ways that differ from expected single-factor effects, suggesting that accounting for multifactorial interactions is crucial for developing a holistic understanding of the consequences of environmental change (Speißer et al. 2022).
Multifactorial experiments should also consider two critical differences between current research on multiple stressors and management. First, experimental research often adds stressors to a system, whereas management focuses on removing stressors (Côté et al. 2016). Therefore, designing experiments that quantify demographic responses to reductions in key stressors may be more informative for conservation practices. For instance, one approach might be to analyse the effects of invasive species removal efforts in addition to the per-capita effects of invasive species on native abundance or performance (Sofaer et al. 2018; Green and Grosholz 2021). The relationship between invasive species abundance and effect (cf. Fig. 1 in Sofaer et al. 2018) could affect the optimal allocation of management resources (i.e. the amount of money, time and effort going into eradication programs), especially if invasive species’ impacts on native populations are low until high invasive species densities are achieved. In this scenario, quantifying the effect of low invasive species abundances via removal experiments might avoid over-investment in invasive species control (Yokomizo et al. 2009). Second, experimental research often considers global-scale stressors that cannot be alleviated at local scales, so including interactions with manageable local-scale stressors will be useful for on-the-ground conservation efforts. For example, Maxwell et al. (2022) considered mechanical thinning to reduce local fire intensity when quantifying the effects of increasing wildfires under future climate scenarios on forest mortality in the western United States, and removal of woody invasives could reduce extinction risk under rising temperatures in E. furcata (Bernardo et al. 2018). These studies demonstrate that when such local and regional threats interact, local management can reduce species’ vulnerability to broader-scale stressors.
Third, identifying the processes underpinning stressor interactions will benefit from experiments with treatments across stress gradients rather than experiments that manipulate the presence/absence of stressors (Jackson et al. 2021; Pirotta et al. 2022). This can be done in two ways. First, we can examine how performance varies across natural environmental gradients in climate, resource availability or species interactions. For example, Hegland et al. (2010) utilized a natural gradient of red deer grazing intensity and a resource gradient to examine the effects of herbivory and resource availability on population dynamics of the boreal shrub Vaccinium myrtillus, finding that negative effects of grazing on λ were strongest in high-resource environments. While this approach maximizes spatial variation in potential drivers and vital rates, other factors along an environmental gradient may vary in addition to the driver of interest (Ehrlén et al. 2016). Alternatively, we can add multiple levels of a stressor to experimental manipulations to better visualize response curves. Reaction norms for most vital rates, if sampled across a range of temperatures, are curved such that vital rates peak at an optimal temperature value, a pattern that is obscured by fitting linear reaction norms between two points (Fig. 4; Arnold et al. 2019). These multi-level designs, as well as studies across stress gradients, also allow for regression analyses, which are more robust for estimating multifactorial effects than analysis of variance designs (Collins et al. 2022). For example, Merow et al. (2017) use regression-based IPM to compare the effects of climate (temperature and precipitation) and microsite conditions (light, nitrogen and pH levels) on the population dynamics of phylogenetically paired native versus invasive species, using a modest number of populations and individuals (n = 21 sites and 147 individuals/species). Thus, IPMs, by incorporating environmental covariates in sub-regressions and allowing the use of size-based regression than discrete size classes, can overcome potential pitfalls of underpowered designs or smaller datasets (Ross and Weegman 2022). Moreover, the use of stress gradients can allow tests of correlations between population metrics and a continuous environmental parameter (e.g. a gradient of nitrogen addition rather than nitrogen addition vs. control [Zettlemoyer 2022] or climatic gradients and rankings of habitat suitability [Merow et al. 2017]), allowing us to link demography with environmental correlates.
Figure 4.
(A) Using only two levels of a stressor (points) may oversimplify the shape of a reaction norm in response to stress (dashed curve), potentially missing biologically meaningful responses to stress, whereas (B) adding even one additional level of a stressor may capture more of the underlying shape. (C) However, five points are considered a minimum to fit nonlinear response curves using a regression design (Collins et al. 2022).
Finally, short-term studies often report a lack of interactions (Komatsu et al. 2019), which more often emerge from longer term, more realistic studies (Mueller et al. 2016; Komatsu et al. 2019; Reich et al. 2020; Cusser et al. 2021). Additionally, although short-term responses to multiple stressors rarely qualitatively reflect long-term responses (Leuzinger et al. 2011), most multistressor studies are conducted over short timeframes (Turschwell et al. 2022). More specifically, population ecology studies often focus on a single targeted vital rate (e.g. reproduction) or correlative measures of population size (e.g. species abundance) rather than measuring multiple vital rates across many years (Iler et al. 2021), which limits our ability to account for trade-offs in responses to stressors that could limit the effects of individual vital rates on population growth. Additionally, studies that focus on a single vital rate might miss important vital rate responses that will ultimately affect population growth. In the rare endemic plant Pulsatilla vulgaris subsp. gotlandica (common pasqueflower), temperature and precipitation affect fecundity but not growth or survival (Lindell et al. 2022), in contrast to most previous studies linking demography to climate (Morris et al. 2020). Measuring only survival or growth would therefore mislead conclusions about weather effects on population dynamics. Understanding how multiple environmental stressors affect long-term population dynamics is a significant challenge in global change ecology, and collecting long-term field data on vital rates across the life cycle will be essential for understanding the interacting effects of global change on demography (Clutton-Brock and Sheldon 2010; Johnston et al. 2019; Paniw et al. 2021).
Conclusion
Interactions among stressors will continue to generate uncertainty and surprises in ecological research (Côté et al. 2016; Burgess et al. 2021), but synergisms and antagonisms are commonly documented in both experiments and natural systems (Murdoch et al. 2020). Determining how multiple stressors interact will be key to attempts to mitigate their effects. However, studies also need to systematically consider how multiple stressor effects on demographic parameters across the entire life cycle will scale up to affect population- and community-level patterns (Moe et al. 2013; Haller-Bull and Bode 2019; Simmons et al. 2021). Multifactorial demographic studies that integrate responses across organisms’ life cycles will allow us to better predict long-term responses to co-occurring anthropogenic changes, particularly if the goal of such studies is to inform conservation and management efforts under future environmental conditions.
Glossary
Stressors: Aiotic and biotic environmental factors that exceed their natural range of variation due to human activity.
Population models: Type of mechanistic model that links individual state variables to changes in population growth, density and structure over discrete time steps; types of population models include (i) matrix population models, which use stage- or age-structured state variables and (ii) IPM, which use continuous (often size-dependent) state variables to predict population growth rates as well as (iii) population viability analyses that estimate population size (e.g. count data) from one monitoring event to the next.
Vital rates: Rates corresponding to an organism’s individual life stages or progression through development (e.g. birth/germination, growth, survival, reproduction, mortality).
Additive effects: Type of interaction when the combined impact of two or more stressors on an ecological response is equal to than their algebraic sum; in this case, studies of single stressors can be added to estimate their combined effect.
Non-additive interactions: Type of interaction when the combined impact of two or more stressors on an ecological response is not equal to their algebraic sum, such that the intensity of response to one stressor changes with the presence of the other; can be synergistic or antagonistic.
Synergistic: Type of non-additive interaction when the combined impact of two or more stressors on an ecological response is greater than their algebraic sum; one stressor can exacerbate the effects of another.
Antagonistic: Type of non-additive interaction when the combined impact of two or more stressors on an ecological response is less than their algebraic sum; one stressor can alleviate the effects of another.
Perturbation analyses: Demographic analyses exploring how population growth rates respond to changes in vital rates; types of perturbation analysis include (i) prospective analyses (e.g. sensitivity and elasticity) that test how much λ depends on vital rates independent of variability and (ii) retrospective analyses (e.g. LTRE) that decompose variance in λ as a function of variance in vital rates.
Demographic compensation: Opposing vital rate responses to stressors wherein the positive effects of one vital rate can negate the negative effects of another.
Density-dependent processes: Population-level processes (e.g. vital rates) that are sensitive to fluctuations in population density (i.e. the number of individuals per unit area).
Kernel: A probability density function that maps the change in the size distribution of individuals at time t to the size distribution after one timestep (t + 1).
Acknowledgements
I would like to thank the DeMarche lab at the University of Georgia and Dr S. Gascoigne and Dr M. Paniw for their valuable feedback on this manuscript.
Populations and Communities. Chief Editor:
Sources of Funding
There is no funding associated with this manuscript.
Contributions by the Author
M.A.Z. conceived and wrote this viewpoint article.
Conflict of Interest
None declared.
Data Availability
No new data were generated or analysed in support of this research.
Literature Cited
- Adler PB, Dalgleish HJ, Ellner SP.. 2012. Forecasting plant community impacts of climate variability and change: when do competitive interactions matter? Journal of Ecology 100:478–487. [Google Scholar]
- Arnold PA, Kruuk LEB, Nicotra AB.. 2019. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytologist 222:1235–1241. [DOI] [PubMed] [Google Scholar]
- Bernardo HL, Goad R, Vitt P, Knight TM.. 2019. Nonadditive effects among threats on rare plant species. Conservation Biology 34:1029–1034. [DOI] [PubMed] [Google Scholar]
- Bernardo HL, Vitt P, Goad R, Masi S, Knight TM.. 2018. Count population viability analysis finds that interacting local and regional threats affect the viability of a rare plant. Ecological Indicators 93:822–829. [Google Scholar]
- Bialic-Murphy L, Brouwer NL, Kalisz S.. 2019. Direct effects of a non-native invader erode native plant fitness in the forest understory. Journal of Ecology 108:189–198. [Google Scholar]
- Burgess BJ, Purves D, Mace G, Murrell DJ.. 2021. Classifying ecosystem stressor interactions: theory highlights the data limitations of the additive null model and the difficulty in revealing ecological surprises. Global Change Biology 27:3052–3065. [DOI] [PubMed] [Google Scholar]
- Burnham, K. P., and Anderson D. R.. 2004. Multimodal inference: understanding AIC and BIC in model selection. Sociological Methods and Research 33:261–304. [Google Scholar]
- Capdevila P, Stott I, Beger M, Salguero-Gómez R.. 2020. Towards a comparative framework of demographic resilience. Trends in Ecology and Evolution 35:776–786. [DOI] [PubMed] [Google Scholar]
- Capdevila P, Stott I, Cant J, Beger M, Rowlands G, Grace M, Salguero-Gómez R.. 2022. Life history mediates the trade-offs among different components of demographic resilience. Ecology Letters 25:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell, H. 2001. Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- Clutton-Brock T, Sheldon BC.. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends in Ecology and Evolution 25:562–573. [DOI] [PubMed] [Google Scholar]
- Collins S, Whittaker H, Thomas MK.. 2022. The need for unrealistic experiments in global change biology. Current Opinion in Microbiology 68:102151. [DOI] [PubMed] [Google Scholar]
- Compagnoni A, Bibian AJ, Ochoki BM, Rogers HS, Schultz EL, Sneck ME, Elderd BD, Iler AM, Inouye DW, Jacquemyn H, et al. 2016. The effect of demographic correlations on the stochastic population dynamics of perennial plants. Ecological Monographs 86:480–494. [Google Scholar]
- Côté IM, Darling ES, Brown CJ.. 2016. Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B 283:20152592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain CM, Kroeker K, Hapern BS.. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters 11:1304–1315. [DOI] [PubMed] [Google Scholar]
- Crone EE, Menges ES, Ellis MM, Bell T, Bierzychudek P, Ehrlén J, Kaye TN, Knight TM, Lesica P, Morris WF, Oostermeijer G, et al. 2012. How do plant ecologists use matrix population models? Ecology Letters 14:1–8. [DOI] [PubMed] [Google Scholar]
- Cusser S, Helms IV J, Bahlai CA, Haddad NM.. 2021. How long do population level field experiments need to be? Utilising data from the 40-year-old LTER network. Ecology Letters 24:1103–1111. [DOI] [PubMed] [Google Scholar]
- Dahlgren JP. 2010. Alternative regression methods are not considered in Murtaugh (2009) or by ecologists in general. Ecology Letters 13:E7–E9. [DOI] [PubMed] [Google Scholar]
- Dahlgren JP, Östergård H, Ehrlén J.. 2014. Local environment and density-dependent feedbacks determine population growth in a forest herb. Oecologia 176:1023–1032. [DOI] [PubMed] [Google Scholar]
- Dalgleish HJ, Koons DN, Hooten MB, Moffet CA, Adler PB.. 2011. Climate influences the demography of three dominant sagebrush steppe plats. Ecology 92: 75–85. [DOI] [PubMed] [Google Scholar]
- Darling ES, Côté IM.. 2008. Quantifying the evidence for ecological synergies. Ecology Letters 11:1278–1286. [DOI] [PubMed] [Google Scholar]
- Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM.. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332:53–58. [DOI] [PubMed] [Google Scholar]
- DeMarche ML, Doak DF, Morris WF.. 2018. Both life-history plasticity and local adaptation will shape range-wide responses to climate warming in the tundra plant Silene acaulis. Global Change Biology 24:1614–1625. [DOI] [PubMed] [Google Scholar]
- Doak DF, Waddle E, Langendorf RE, Louthan AM, Chardon NI, Dibner RR, Keinath DA, Lombardi E, Steenbock C, Shriver RK, et al. 2021. A critical comparison of integral projection and matrix population models for demographic analysis. Ecological Monographs 91:e01447. [Google Scholar]
- Donham EM, Flores I, Hooper A, O’Brien E, Vylet K, Takeshita Y, Freiwald J, Kroeker KJ.. 2023. Population-specific vulnerability to ocean change in a multistressor environment. Science Advances 9:eade2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl JE. 2019. Evaluating the assumptions of population projection models used for conservation. Biological Conservation 237:145–154. [Google Scholar]
- Earl JE, Nicol S, Wiederholt R, Diffendorfer JE, Semmens D, Flockhart DTT, Mattsson BJ, McCracken G, Norris DR, Thogmartin WE, et al. 2018. Quantitative tools for implementing the new definition of significant portion of the range in the U.S. Endangered Species Act. Conservation Biology 32:35–49. [DOI] [PubMed] [Google Scholar]
- Easterling MR, Ellner SP, Dixon PM.. 2000. Size-specific sensitivity: applying a new structured population model. Ecology 81:694–708. [Google Scholar]
- Ehrlén J, Morris WF, von Euler T, Dahlgren JP.. 2016. Advancing environmentally explicit structured population models of plants. Journal of Ecology 104:292–305. [Google Scholar]
- Ellner SP, Rees M.. 2006. Integral projection models for species with complex demography. American Naturalist 167:410–428. [DOI] [PubMed] [Google Scholar]
- Folt CL, Chen CY, Moore MV, Burnaford J.. 1999. Synergism and antagonism among multiple stressors. Limnology and Oceanography 44:864–877. [Google Scholar]
- Fung YL, Newman K, King R, de Valpine P.. 2022. Building integral projection models with nonindependent vital rates. Ecology and Evolution 12:e8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatowitsch S, Frelich L, Phillips-Mao L.. 2009. Regional climate change adaptation strategies for biodiversity conservation in a midcontinental region of North America. Biological Conservation 142:2012–2022. [Google Scholar]
- Gornish E. 2013. Effects of density and fire on the vital rates and population growth of a perennial goldenaster. AoB Plants 5:plt041. [Google Scholar]
- Green SJ, Grosholz ED.. 2021. Functional eradication as a framework for invasive species control. Frontiers in Ecology and the Environment 19:98–107. [Google Scholar]
- Haller-Bull V, Bode M.. 2019. Superadditive and subadditive dynamics are not inherent to the types of interacting threat. PLoS One 14:e0211444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegland SJ, Jongejans E, Rydgren K.. 2010. Investigating the interaction between ungulate grazing and resource effects on Vaccinium myrtillus populations with integral projection models. Oecologia 163:695–706. [DOI] [PubMed] [Google Scholar]
- Hernández-Yáñez H, Kos JT, Bast MD, Griggs JL, Hage PA, Killian A, Loza MI, Whitmore MB, Smith AB.. 2016. A systematic assessment of threats affecting the rare plants of the United States. Biological Conservation 203:260–267. [Google Scholar]
- Heywood VH. 2017. Plant conservation in the Anthropocene - challenges and future prospects. Plant Diversity 39:314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iler AM, CaraDonna PJ, Forrest JRK, Post E.. 2021. Demographic consequences of phenological shifts in response to climate change. Annual Review of Ecology, Evolution, and Systematics 52:221–245. [Google Scholar]
- Jackson MC, Pawar S, Woodward G.. 2021, The temporal dynamics of multiple stressor effects: from individuals to ecosystems. Trends in Ecology and Evolution 36:402–410. [DOI] [PubMed] [Google Scholar]
- Jeong H, Cho Y-C, Kim E.. 2022. Site-specific temporal variation of population dynamics in subalpine endemic plant species. Scientific Reports 12:19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston ASA, Boyd RJ, Watson JW, Paul A, Evans LC, Gardner EL, Boult VL.. 2019. Predicting population responses to environmental change from individual-level mechanisms: towards a standardized mechanistic approach. Proceedings of the Royal Society B 286:20191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir I, Cokol M, Aldridge BB, Alon U.. 2019. Prediction of ultra-high-order antibiotic combinations based on pairwise interactions. PLoS Computational Biology 15:e1006774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu KJ, Avolio ML, Lemoine NP, Isbell F, Grman E, Houseman GR, Koerner SE, Johnson DS, Wilcox KR, Alatalo JM, et al. 2019. Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proceedings of the National Academy of Sciences 116:17867–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C.. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends in Ecology and Evolution 26:236–241. [DOI] [PubMed] [Google Scholar]
- Lindell T, Ehrlén J, Dahlgren JP.. 2022. Weather-driven demography and population dynamics of an endemic perennial plant during a 34-year period. Journal of Ecology 110:582–592. [Google Scholar]
- Litchman E, Thomas MK.. 2022. Are we underestimating the ecological and evolutionary effects of warming? Interactions with other environmental drivers may increase species vulnerability to high temperatures. Oikos 2023:e09155. [Google Scholar]
- Mandle L, Ticktin T, Zuidema PA.. 2015. Resilience of palm populations to disturbance is determined by interactive effects of fire, herbivory and harvest. Journal of Ecology 103:1032–1043. [Google Scholar]
- Maxwell C, Scheller R, Long J, Manley P.. 2022. Forest management under uncertainty: the influence of management versus climate change and wildfire in the Lake Tahoe Basin, USA. Ecology and Society 27:15. [Google Scholar]
- Merow C, Dahlgren JP, Metcalf CJE, Childs DZ, Evans MEK, Jongejans E, Record S, Rees M, Salguero-Gómez R, McMahon SM.. 2014. Advancing population ecology with integral projection models: a practical guide. Methods in Ecology and Evolution 5:99–110. [Google Scholar]
- Merow C, Treanor Bois S, Allen JM, Xie Y, Silander JA.. 2017. Climate change both facilitates and inhibits invasive plant ranges in New England. Proceedings of the National Academy of Sciences 114:e3276–e3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SJ, Kristoggersen AB, Smith RH, Stenseth NC.. 2005. From patterns to processes and back: analysing density-dependent responses to an abiotic stressor by statistical and mechanistic modelling. Proceedings of the Royal Society B 272:2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SJ, De Schamphelaere K, Clements WH, Sorensen MT, Van den Brink PJ, Liess M.. 2013. Combined and interactive effects of global climate change and toxicants on populations and communities. Environmental Toxicology and Chemistry 32:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WF, Doak DF.. 2002. Quantitative conservation biology: theory and practice of population viability analysis. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- Morris WF, Ehrlén J, Dahlgren JP, Loomis AK, Louthan AL.. 2020. Biotic and anthropogenic forces rival climatic/abiotic factors in determining global plant population growth and fitness. Proceedings of the National Academy of Sciences 117:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KE, Blumenthal DM, Pendall E, Carrillo Y, Dijkstra FA, Williams DG, Follett RF, Morgan JA.. 2016. Impacts of warming and elevated CO2 on a semi-arid grassland are non-additive, shift with precipitation, and reverse over time. Ecology Letters 19:956–966. [DOI] [PubMed] [Google Scholar]
- Murdoch A, Mantyka-Pringle C, Sharma S.. 2020. The interactive effects of climate change and land use on boreal stream fish communities. Science of the Total Environment 700:134518. [DOI] [PubMed] [Google Scholar]
- Nicolé F, Dahlgren JP, Vivat A, Till-Bottraud I, Ehrlén J.. 2011. Interdependent effects of habitat quality and climate on population growth of an endangered plant. Journal of Ecology 99:1211–1218. [Google Scholar]
- Nomoto HA, Alexander JM.. 2021. Drivers of local extinction risk in alpine plants under warming climate. Ecology Letters 24:1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, Luo Y.. 2004. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytologist 162:281–293. [Google Scholar]
- Orr JA, Vinebrooke RD, Jackson MC, Kroeker KJ, Kordas RL, Mantyka-Pringle C, Van den Brink PJ, De Laender F, Stocks R, Holmstrup M, et al. 2020. Towards a unified study of multiple stressors: divisions and common goals across research disciplines. Proceedings of the Royal Society B 287:20200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzack SH, Tuljapurkar S.. 1989. Population dynamics in variable environments. VII. The demography and evolution of iteroparity. American Naturalist 133:901–923. [Google Scholar]
- Otto SA, Niiranen S, Blenckner T, Tomczak MT, Müller-Karulis B, Rubene G, Möllmann C.. 2020. Life cycle dynamics of a key marine species under multiple stressors. Frontiers in Marine Science 7:296. [Google Scholar]
- Paine RT, Tegner MJ, Johnson EA.. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1:535–545. [Google Scholar]
- Paniw M, James TD, Archer CR, Römer G, Levin S, Compagnoni A, Che-Castaldo J, Bennett JM, Mooney A, Childs DZ, et al. 2021. The myriad of complex demographic responses of terrestrial mammals to climate change and gaps of knowledge: a global analysis. Journal of Animal Ecology 90:1398–1407. [DOI] [PubMed] [Google Scholar]
- Paniw M, Maag N, Cozzi G, Clutton-Brock T, Ozgul A.. 2019. Life history responses to meerkats to seasonal changes in extreme environments. Science 363:631–635. [DOI] [PubMed] [Google Scholar]
- Pannwitt H, Waterman PR, De Mol F, Gerowitt B.. 2021. Demographic processes allow Echinocloa crus-galli to compensate seed losses by seed predation. Agronomy 11:65. [Google Scholar]
- Pazzaglia J, Reusch TBH, Terlizzi A, Marín-Guirao L, Procaccini G.. 2022. Phenotypic plasticity under rapid global changes: the intrinsic force for future seagrasses survival. Evolutionary Applications 14:1181–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Mao L, Galatowitsch SM, Snyder SA, Haight RG.. 2016. Model-based scenario planning to develop climate change adaptation strategies for rare plant populations in grassland reserves. Biological Conservation 193:103–114. [Google Scholar]
- Piggott JJ, Townsend CR, Matthaei CD.. 2015. Reconceptualizing synergism and antagonism among multiple stressors. Ecology and Evolution 5:1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Thomas L, Costa DP, Hall AJ, Harris CM, Harwood J, Kraus SD, Miller PJO, Moore MJ, Photopoulou T, et al. 2022. Understanding the combined effects of multiple stressors: a new perspective on a longstanding challenge. Science of the Total Environment 821:153322. [DOI] [PubMed] [Google Scholar]
- Reed TE, Grotan V, Jenouvrier S, Saether B-E, Visser ME.. 2013. Population growth in a wild bird is buffered against phenological mismatch. Science 340:488–491. [DOI] [PubMed] [Google Scholar]
- Reich PB, Hobbie SE, Lee TD, Rich R, Pastore MA, Worm K.. 2020. Synergistic effects of four climate change drivers on terrestrial carbon cycling. Nature Geoscience 13:787–793. [Google Scholar]
- Rillig MC, Lehmann A, Orr JA, Waldman WR.. 2021. Mechanisms underpinning nonadditivity of global change factor effects in the plant-soils system. New Phytologist 232:1535–1539. [DOI] [PubMed] [Google Scholar]
- Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang G.. 2019. The role of multiple global change factors in driving soil functions and microbial diversity. Science 366:886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BE, Weegman MD.. 2022. Relative effects of sample size, detection probability, and study duration on estimation in integrated population models. Ecological Applications 32:e2686. [DOI] [PubMed] [Google Scholar]
- Schäfer RB, Piggott JJ.. 2018. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Global Change Biology 24:1817–1826. [DOI] [PubMed] [Google Scholar]
- Schuwirth N, Borgwardt F, Domisch S, Friedrichs M, Kattwinkel M, Kneis D, Kuemmerlen M, Langhans SD, Martínez-López J, Vermeiren P.. 2019. How to make ecological models useful for environmental management. Ecological Modelling 411:108784. [Google Scholar]
- Simmons BI, Blyth PSA, Blanchard JL, Clegg T, Delmas E, Garnier A, Griffiths CA, Jaco U, Pennekamp F, Petchey OL, et al. 2021. Refocusing multiple stressor research around the targets and scales of ecological impacts. Nature Ecology and Evolution 5:1478–1489. [DOI] [PubMed] [Google Scholar]
- Sofaer HR, Jarnevich CS, Pearse IS.. 2018. The relationship between invader abundance and impact. Ecosphere 9:e02415. [Google Scholar]
- Speißer B, Wilschut RA, van Kleunen M.. 2022. Number of simultaneously acting global change factors affects composition, diversity and productivity of grassland plant communities. Nature Communications 13:7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. 1989. Trade-offs in life-history evolution. Functional Ecology 3:259–268. [Google Scholar]
- Tenhumberg B, Suwa T, Tyre AJ, Russell FL, Louda SM.. 2015. Integral projection models show exotic thistle is more limited than native thistle by ambient competition and herbivory. Ecosphere 6:1–18. [Google Scholar]
- van Tienderen PH. 2000. Elasticities and the link between demographic and evolutionary dynamics. Ecology 81:666–679. [Google Scholar]
- Tӧpper JP, Meineri E, Olsen SL, Rydgren K, Skarpaas O, Vandvik V.. 2018. The devil is in the detail: nonadditive and context-dependent plant population responses to increasing temperature and precipitation. Global Change Biology 24:4657–4666. [DOI] [PubMed] [Google Scholar]
- Turschwell MP, Connolly SR, Schäfer RB, De Laender F, Campbell MD, Mantyka-Pringle C, Jackson MC, Kattwinkel M, Sievers M, Aschauer R, et al. 2022. Interactive effects of multiple stressors vary with consumer interactions, stressor dynamics and magnitude. Ecology Letters 25:1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. 2015. Accelerating extinction risk from climate change. Science 348:571–573. [DOI] [PubMed] [Google Scholar]
- Villellas J, Doak DF, García MB, Morris WF.. 2015. Demographic compensation among populations: what is it, how does it arise and what are its implications? Ecology Letters 18:1139–1152. [DOI] [PubMed] [Google Scholar]
- Wake B. 2019. Experimenting with multistressors. Nature Climate Change 9:357. [Google Scholar]
- Westwood M, Cavender N, Meyer A, Smith P.. 2021. Botanic garden solutions to the plant extinction crisis. Plants People Planet 3:22–32. [Google Scholar]
- Yokomizo H, Possingham HP, Thomas MB, Buckley YM.. 2009. Managing the impact of invasive species: the value of knowing the density-impact curve. Ecological Applications 19:376–386. [DOI] [PubMed] [Google Scholar]
- Zettlemoyer MA. 2022. Monitoring demography of resurrected populations of locally extinct and extant species to investigate drivers of species loss. American Naturalist 200:E36–E51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.