Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor expressed in hematopoietic and non-hematopoietic cells. Activation of the AhR by xenobiotics, microbial metabolites, and natural substances induces immunoregulatory responses. Autoimmune pancreatitis (AIP) is a chronic fibroinflammatory disorder of the pancreas driven by autoimmunity. Although AhR activation generally suppresses pathogenic autoimmune responses, the roles played by the AhR in AIP have been poorly defined. In this study, we examined how AhR activation affected the development of experimental AIP caused by the activation of plasmacytoid dendritic cells producing IFN-α and IL-33. Experimental AIP was induced in MRL/MpJ mice by repeated injections of polyinosinic-polycytidylic acid. Activation of the AhR by indole-3-pyruvic acid and indigo naturalis, which were supplemented in the diet, inhibited the development of experimental AIP, and these effects were independent of the activation of plasmacytoid dendritic cells producing IFN-α and IL-33. Interaction of indole-3-pyruvic acid and indigo naturalis with AhRs robustly augmented the production of IL-22 by pancreatic islet α cells. The blockade of IL-22 signaling pathways completely canceled the beneficial effects of AhR ligands on experimental AIP. Serum IL-22 concentrations were elevated in patients with type 1 AIP after the induction of remission with prednisolone. These data suggest that AhR activation suppresses chronic fibroinflammatory reactions that characterize AIP via IL-22 produced by pancreatic islet α cells.
Keywords: aryl hydrocarbon receptor, autoimmune pancreatitis, interleukin 22, Indigo naturalis, indole-3-pyruvic acid, pancreatic islet α cells
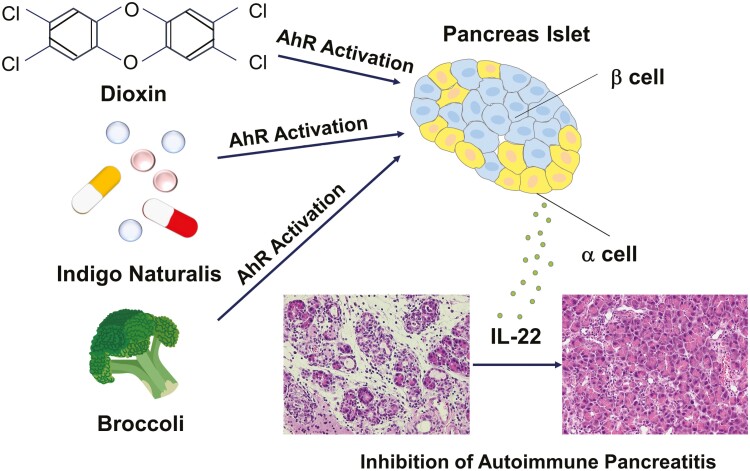
Indigo naturalis and dietary tryptophan rich in broccoli activate aryl hydrocarbon receptor (AhR). AhR activation inhibits the development of experimental autoimmune pancreatitis through IL-22 produced by pancreatic islet alpha cells.
Graphical Abstract
Graphical Abstract.
Introduction
The aryl hydrocarbon receptor (AhR) is a transcription factor activated by a broad range of ligands, including xenobiotics, microbial metabolites, and natural substances [1–6]. The first identified AhR ligand was 2,3,7,8-tetrachlorodibenzo-para-dioxin (TCDD), a toxic environmental contaminant [7]. Dietary tryptophan (Trp) is degraded by the intestinal microbiota into AhR ligands, such as indole-3-pyruvic acid (IPA) [4, 6]. Natural plant compounds, including indigo naturalis (IN), have also been identified as AhR ligands [1–6]. Ligand-dependent activation of the AhR has a wide variety of effects on the human body in terms of metabolism, immunity, and carcinogenesis because AhRs are widely expressed in hematopoietic and non-hematopoietic cells [1–6]. The beneficial roles played by AhR ligands in the immune processes have been demonstrated by recent studies showing that AhR signaling negatively controls autoimmune responses through the induction of regulatory T cells (Tregs) expressing forkhead box P3 (FOXP3) and production of immunoregulatory cytokines such as IL-10 [1–4]. In addition, AhR activation has been shown to induce IL-22 production by innate lymphoid cells (ILCs) and thereby contribute to the maintenance of epithelial cell barrier function [1–6]. Thus, AhR signaling activated by environmental factors suppresses harmful immune reactions, including autoimmunity. This notion is fully supported by clinical trials, in which patients with refractory ulcerative colitis (UC) are successfully treated with IN [8].
Clinicopathological analyses of cases that presented with the enlargement of the pancreas and salivary glands have led physicians to establish a new disease entity called IgG4-related disease (IgG4-RD) [9–11]. IgG4-RD is characterized by elevated serum levels of IgG4, accumulation of IgG4-expressing plasma cells in the affected organs, and multiple organ involvement [9–11]. Autoimmune pancreatitis (AIP) is a pancreatic manifestation of systemic IgG4-RD [12]. Raised awareness and recognition of AIP and IgG4-RD have enabled the establishment of diagnostic criteria for these autoimmune disorders [13, 14]; thus, the number of patients diagnosed with AIP and IgG4-RD is increasing. It should be noted, however, that the immunopathogenesis of AIP and IgG4-RD remains largely unknown. Although an elevated concentration of the IgG4 antibody (Ab) is one of the most prominent features of AIP and IgG4-RD, recent studies have highlighted the pathogenicity of IgG1 rather than IgG4 Ab in these conditions [15–17]. In addition, the IgG4 Ab does not activate complement and Fcγ receptors necessary for the activation of the immune system, suggesting that this IgG subtype plays anti-inflammatory rather than proinflammatory roles [18]. Therefore, elevated IgG4 Ab responses seen in AIP and IgG4-RD may be an epiphenomenon reflecting persistent inflammation. Efficient production of IgG1 and IgG4 Abs requires both adaptive and innate immune responses [11]. Recently, we found that the development of experimental AIP depends on the activation of plasmacytoid dendritic cells (pDCs) producing IFN-α and IL-33 [19–21]. Intestinal dysbiosis potently activates pathogenic pDCs associated with experimental AIP and human IgG4-RD [22–24].
The microbial metabolism of dietary Trp produces AhR ligands [4, 6]. The association between intestinal dysbiosis and the development of AIP led us to hypothesize that intestinal dysbiosis modulates the production of AhR ligands, which could attenuate AIP manifestations. In this study, we provide evidence that AhR activation by various environmental factors, including IN, suppressed the development of experimental AIP.
Materials and Methods
Experimental AIP and activation of AhR
Female, 6-week-old MRL/MpJ mice were purchased from Japan SLC (Hamamatsu, Japan). These mice were treated with repeated intraperitoneal (IP) injections of polyinosinic-polycytidylic acid (poly(I:C), 100 μg, InvivoGen, San Diego, CA) twice a week for a total of 16 times to induce experimental AIP [19, 20, 22]. To activate the AhR, three different AhR ligands were used: IN (Uchidawakanyaku, Ltd, Tokyo, Japan), IPA (Sigma-Aldrich, St. Louis, MO), and TCDD (Sigma-Aldrich). The animal experiments were approved by the Review Board of the Kindai University Faculty of Medicine and adhered to the ARRIVE guidelines.
MRL/MpJ mice were divided into four groups to evaluate the effect of AhR activation on the development of experimental AIP: untreated mice, mice treated with poly(I:C) alone, mice treated with AhR ligands alone, and mice co-treated with poly(I:C) and AhR ligands. Mice that received AhR ligands were treated with 4.0% IN or 0.1% IPA in their diet during the experimental period [25, 26]. The mice received an IP injection of TCDD (0.5 μg) prior to the injection of poly(I:C) [27]. TCDD (50 μg/mL in toluene) was diluted with dimethyl sulfoxide (DMSO) and 10% toluene in DMSO was used as the solvent. In some experiments, mice received an IP injection of an anti-IL-22 Ab (100 μg, eBioscience, San Diego, CA, USA) or control rat IgG (100 μg, Sigma-Aldrich) in combination with IN or IPA in the diet. Injections of the anti-IL-22 or control Abs were performed prior to each poly(I:C) injection.
Pathological analysis
Formalin-fixed pancreatic tissues were deparaffinized and subjected to hematoxylin and eosin (H&E) staining, after which a pathological assessment was performed as previously described [19, 20, 22]. Pancreatic inflammation was scored as follows: 0, pancreas without mononuclear cell infiltration; 1, mononuclear cell aggregation and/or infiltration within the interstitium without parenchymal destruction; 2, focal parenchymal destruction with mononuclear cell infiltration; 3, diffuse parenchymal destruction with preservation of some intact parenchymal areas; and 4, destruction of almost the entire pancreatic tissue except the pancreatic islets, and its replacement with fibrotic or adipose tissue. Pancreatic fibrosis was semi-quantitatively measured using Sirius Red staining as previously described [20, 22].
Flow cytometry analysis
Pancreatic mononuclear cells (PMNCs) were isolated from the pancreas of MRL/MpJ mice as previously described [19, 20, 22]. PMNCs were analyzed through flow cytometry after staining with FITC- or PE-conjugated Abs against B220, pDC antigen-1 (PDCA-1), CD3, CD11b, and CD11c as previously described [19, 20, 22]. All Abs were purchased from eBioscience. Flow cytometry analysis was performed using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) and CFlow Plus software (BD Biosciences).
Intracellular cytokine staining
PMNCs were incubated in the presence of 25 ng/mL phorbol 12-myristate 13-acetate (Merck, Tokyo, Japan), 1 μg/mL ionomycin (Merck), and 10 μg/mL Brefeldin A (Merck) for 5 h. Cells were first blocked with Abs against FcRγIII/II and then stained with CD90.2, CD127, and lineage cocktails, which included Abs against CD3, CD4, CD8a, CD19, B220, CD11b, CD11c, CD49b, FcRI, Ly6C, CD5, and TER-119. Cells were subsequently stained with Abs against GATA3, RORγt, and IL-22 using the FoxP3/Transcription Factor Staining Buffer set (eBioscience). All Abs were purchased from BioLegend Japan (Tokyo, Japan), BD Biosciences, and Invitrogen (Carlsbad, CA). Samples were run on LSRFortessa flow cytometers (BD Biosciences), and the data were analyzed using FlowJo (BD Biosciences).
Cytokine assay and quantitative PCR
Pancreatic concentrations of IFN-α in mice and IL-22 in humans were measured using enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN, USA), as previously described [20, 21]. Pancreatic concentrations of IL-10, IL-13, IL-17, IL-22, and IL-33 in mouse samples were measured using ELISA kits from eBioscience, as previously described [28, 29]. Briefly, pancreatic tissues were sliced into small pieces and then homogenized. Protein extraction was performed using a Nuclear Extract kit (Active Motif, San Diego, CA), according to the manufacturer’s instructions. After the protein concentrations were determined, isolated protein extracts were subjected to ELISA.
In some experiments, mRNAs were isolated from the pancreas and subjected to quantitative PCR (qPCR) analysis to determine the expression of regenerating islet-derived protein (REG) 3β, REG3γ, and cytochrome p450 family 1A1 (CYP1A1) [30, 31]. Briefly, the mRNA was isolated using a RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and then transcribed into cDNA using a Transcriptor First Strand cDNA Synthesis Kit (Roche, Tokyo, Japan) according to the manufacturer’s instructions. qPCR analyses were performed using FastStart Universal SYBR Green Master Rox (Roche). The primer sequences used were as follows: REG3γ, forward: AGAGAACAGGAAACAGCTACCAATA, reverse: CCCAGTTAAAGTAATTCATCACGTC; REG3β, forward: GTGGTAACAGTGGCCAATATGTATG, reverse: ATTCGTCTCCCAGTTGATGTAATTC; Cyp1a1, forward:
CATCACAGACAGCCTCATTGAGC
, reverse: CTCCACGAGATAGCAGTTGTGAC.The mRNA expression of GAPDH was used as a reference.
Immunohistochemical and immunofluorescence analyses
Deparaffinized pancreatic sections were incubated with mouse or rabbit anti-amylase Ab (Sigma-Aldrich), mouse anti-pan cytokeratin (CK) Ab (Sigma-Aldrich), rabbit anti-SRY-box9 (SOX9) Ab (CST, Danvers, MA), rabbit Ab against phosphorylated signal transducer and activator of transcription 3 (p-STAT3, CST), rabbit anti-insulin Ab (CST), rabbit anti-glucagon Ab (CST), rabbit anti-CD3 Ab (Abcam), and rat anti-IL-22 Ab (eBioscience). Alexa Fluor 488- or 546-conjugated anti-rat, anti-mouse, and anti-rabbit IgG were used as secondary Abs (Invitrogen). The cell nuclei were counterstained with 4ʹ,6-diamidino-2-phenylindole (DAPI). Immunofluorescence images were captured using an immunofluorescence microscope (Keyence Biozero 8100, Keyence, Osaka, Japan).
In some experiments, deparaffinized pancreatic sections were incubated with rabbit anti-AhR Ab (Novus Biologicals, Centennial, CO). The protein expression of the AhR was visualized using a DAKO-Envison+ Kit (DAKO JAPAN, Tokyo, Japan) according to the manufacturer’s instructions [29].
Human samples
Twelve patients with chronic alcoholic pancreatitis, twenty-one patients with AIP/IgG4-RD, and eight healthy control individuals were enrolled in this study, as previously described [32]. These patients met the diagnostic criteria for definite type 1 AIP and/or IgG4-RD, or chronic pancreatitis (CP) [13, 14, 33]. Serum samples were obtained at the time of diagnosis of AIP, IgG4-RD, or CP. The study protocol conformed to the ethical guidelines for human clinical research established by the Japanese Ministry of Health, Labor, and Welfare. This human study was approved by the Ethics Committee of the Kindai University Faculty of Medicine. Written informed consent was obtained from all patients and healthy controls at enrollment. Oral prednisolone (PSL) was administered to induce remission in 12 patients with AIP/IgG4-RD, as previously described [32]. Successful induction of remission was verified using positron emission tomography/computed tomography.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA), as previously reported [32]. The Mann–Whitney U test, a nonparametric version of the unpaired t test, was used to evaluate the differences between two groups. The Kruskal–Wallis test, a nonparametric alternative of one-way analysis of variance (ANOVA), was used to evaluate the differences between multiple comparisons. For post hoc analysis, the Bonferroni-corrected Mann–Whitney U test was performed for comparisons between two groups. The Pearson’s correlation coefficient was calculated in the correlation analyses. Effects were considered statistically significant at P < 0.05.
Results
Activation of AhR by IN inhibits the development of experimental AIP
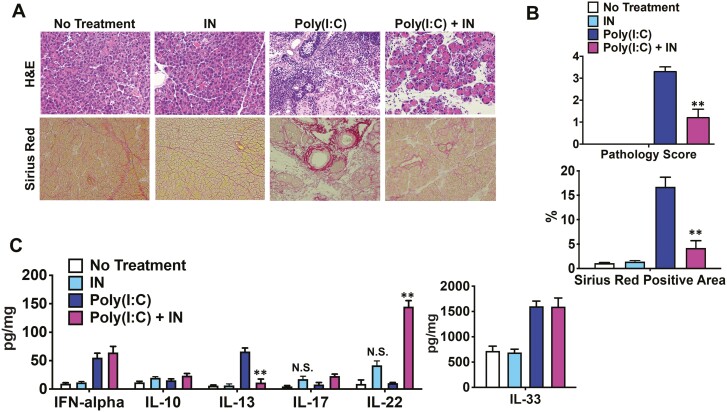
The possible role of AhR signaling in the development of AIP and IgG4-RD has not been previously investigated. In our initial experiments, we sought to determine whether IN, an herbal component utilized in Chinese traditional medicine known to activate AhR and recently shown to treat UC [8, 34], suppressed the fibroinflammatory manifestations of pancreatitis in an experimental murine model of AIP. For this purpose, we assessed the effect of oral administration of IN on the development of pancreatitis in MRL/MpJ mice after repeated IP injections of poly(I:C). Consistent with previous reports [11, 19, 20], repeated IP injections of poly(I:C) into MRL/MpJ mice triggered severe AIP characterized by the destruction of the pancreatic acinar architecture, infiltration of immune cells, and fibrosis (Fig. 1A). However, as reflected in the histopathologic scoring, the development of AIP was markedly suppressed (but not completely prevented) in mice co-treated with poly(I:C) and dietary IN (4.0%) (Fig. 1A, B). A comparable ameliorative effect of IN feeding on poly(I:C)-induced fibrosis was revealed by Sirius Red histological staining: in this case, the extent of fibrosis prevention in IN-treated mice was even slightly higher than the decrease in the pathology score (Fig. 1A, B).
Figure 1.
Activation of the aryl hydrocarbon receptor by indigo naturalis inhibits the development of experimental autoimmune pancreatitis. Four groups of MRL/MpJ mice were used: untreated mice (n = 5), mice treated with 4% indigo naturalis (IN) in the diet (n = 5), mice treated with repeated intraperitoneal injections of polyinosinic-polycytidylic acid (poly(I:C), n = 5), and mice co-treated with poly(I:C), and IN (n = 5). Injections of poly(I:C) (100 μg) were performed twice a week for a total of 16 times, and the mice were sacrificed 3 h after the final injection. (A) Representative images of pancreatic sections stained with hematoxylin and eosin (H&E) or Sirius Red are shown (magnification ×400). (B) Pathological scores for autoimmune pancreatitis and areas occupied by pancreatic fibrosis derived from the analyses of H&E and Sirius Red staining, respectively, are illustrated. The areas positive for Sirius Red staining were semi-quantitatively measured. (C) Concentrations of IFN-α, IL-10, IL-13, IL-17, IL-22, and IL-33 in pancreatic lysates determined by enzyme-linked immunosorbent assays. Results are shown as the mean + standard error. **P < 0.01, as compared with values in mice treated with poly(I:C) alone. NS, not significant, as compared with values in untreated mice
To ascertain whether dietary IN activates AhR, the expression of AhR-target genes was assessed through qPCR. The expression of Cyp1a1, one of the representative AhR target genes [1–6], was significantly higher in the pancreas of mice treated with dietary IN irrespective of poly(I:C) injection (Supplementary Fig. 1A). Next, we determined the localization of AhR in the pancreas. AhR expression was predominantly observed in islet cells rather than in acinar cells in both inflamed and uninflamed pancreatic tissue in mice treated with or without dietary IN (Supplementary Fig. 1B). In addition, pancreatic immune cells were positive for AhR staining in the inflamed pancreas of poly(I:C)-treated mice (Supplementary Fig. 1B). These data suggest that dietary IN inhibits the development of AIP through the activation of AhR.
IN-mediated inhibition of experimental AIP is independent of pDC activation
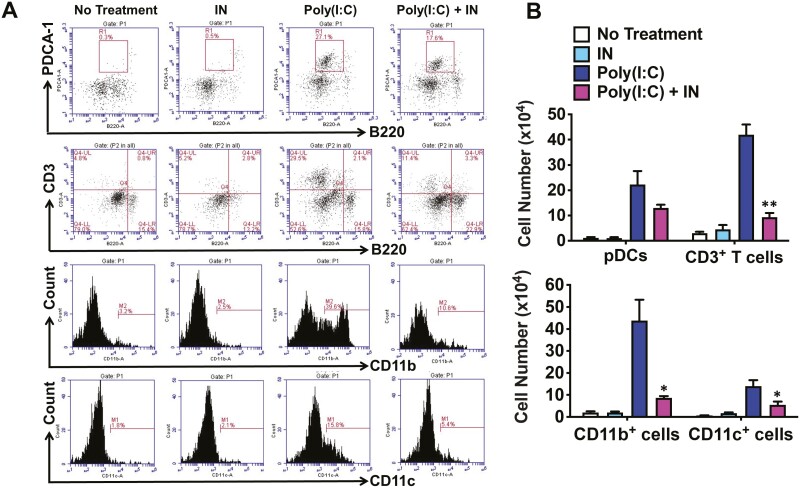
In previous studies, we showed that the development of pancreatitis in MRL/MpJ mice after poly(I:C) injection is critically dependent on the activation of pDCs [11, 19, 20]. Therefore, we determined the number of pDCs in the pancreatic tissue of MRL/MpJ mice with induced pancreatitis with and without dietary administration of IN. We used flow cytometry assessment of the percentage of PDCA-1+B220low cells and calculated total mononuclear cell counts within the pancreatic tissue. We observed that the percentage and number of pancreatic pDCs increased dramatically in mice treated with poly(I:C) compared to those in mice that were not injected with poly(I:C), regardless of the IN-treatment status. Furthermore, the numbers of pDCs in mice treated with poly(I:C) alone were not statistically different from those in mice co-treated with poly(I:C) and IN (Fig. 2). This lack of effect of IN on the activation of pDCs was supported by the observation that pancreatic concentrations of the proinflammatory and profibrotic cytokines IFN-α and IL-33, shown previously to be produced by pDCs responsible for pancreatic inflammation in the MRL/MpJ model [11, 19, 20], were increased to the same extent in poly(I:C)-injected mice treated with or without dietary IN (Fig. 1C). Thus, the activation of AhR by IN suppressed the development of experimental AIP in a pDC-independent manner.
Figure 2.
Accumulation of immune cells in the pancreas of mice treated with indigo naturalis. Four groups of MRL/MpJ mice were used, as described in Figure 1. The numbers of pancreatic immune cells were determined by flow cytometry. Plasmacytoid dendritic cells (pDCs) were defined as PDCA-1+B220low cells. Results are shown as the mean + standard error. *P < 0.05, **P < 0.01, as compared with values in mice treated with poly(I:C) alone
Inhibition of experimental AIP by IN is associated with enhanced production of IL-22
Given that dietary IN suppressed AIP in the MRL/MpJ mouse model of IgG4-RD, we next determined whether such suppression was mediated by changes in various cellular and cytokine components that usually accompany pancreatitis. By using flow cytometry and pancreatic cell counting analysis in mouse samples from all treatment groups (as described above), we found that unlike in the case of pDCs, percentages and/or numbers of other pancreatic inflammation-associated cellular components, such as CD11b+ myeloid cells and CD3+ T cells, were markedly reduced in the pancreas of mice co-treated with poly(I:C) and IN compared with those in mice treated with poly(I:C) alone (Fig. 2). In addition, treatment with IN decreased the production of IL-13, a prototypical profibrogenic cytokine, in mice that received poly(I:C). In contrast, the concentrations of IL-17 and IL-22, cytokines known to be induced by AhR activation [35, 36], were increased in mice co-treated with poly(I:C) and IN compared with those in mice treated with poly(I:C) alone. Furthermore, the secretion of these cytokines was increased in mice treated with IN in the absence of poly(I:C) treatment although not statistically significant (Fig. 1C). Finally, although AhR activation was also shown to enhance IL-10 production [4, 5], the pancreatic expression of IL-10 was comparable between IN-treated and untreated mice. Taken together, these data suggested that dietary IN inhibited AIP manifestations such as cellular infiltration and profibrotic cytokine production in the MRL/MpJ model of IgG4-RD. However, our observations also showed that at the same time, treatment with IN induced the production of cytokines such as IL-22, which can have an anti-inflammatory effect.
STAT3 is a crucial transcription factor in the IL-22-mediated signaling pathway [37]. Consistent with the enhanced production of IL-22 in mice co-treated with poly(I:C) and IN, marked nuclear translocation of STAT3 was observed in the pancreas of these mice (Supplementary Fig. 2). The number of acinar cells expressing amylase and characterized by the nuclear translocation of STAT3 was markedly increased in the pancreas of mice co-treated with poly(I:C) and IN compared with that in mice treated with poly(I:C) alone. Thus, activation of the AhR by IN inhibited the development of experimental AIP, which was accompanied by the augmentation of IL-22-mediated signaling pathway activity.
Activation of the AhR by IPA inhibits the development of experimental AIP
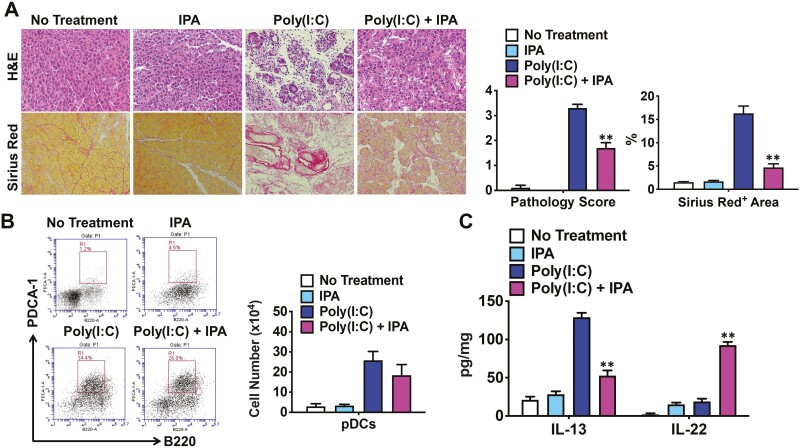
Having found that IN suppressed the development of experimental AIP, we next examined whether another AhR ligand would have a similar effect. IPA, a microbial metabolite derived from dietary Trp, has been shown to activate the AhR [38]. MRL/MpJ mice received a diet containing 0.1% IPA. As shown in Fig. 3A, co-administration of poly(I:C) and IPA inhibited the development of experimental AIP, as evaluated by pathological scoring. In addition, Sirius Red staining revealed markedly decreased areas of pancreatic fibrosis in mice co-treated with poly(I:C) and IPA, as compared with those in mice that received poly(I:C) alone (Fig. 3A). Consistent with the results in the IN-treated mice, administration of IPA did not significantly affect the number of pancreatic pDCs in mice treated with poly(I:C) (Fig. 3B). Pancreatic cytokine analysis showed that co-administration of poly(I:C) and IPA upregulated and downregulated IL-22 and IL-13 concentrations, respectively, whereas IL-33 production by pDCs was comparable in these groups of mice (Fig. 3C, data not shown). Taken together, these data suggested that AhR activation by IPA suppressed the development of experimental AIP.
Figure 3.
Activation of the aryl hydrocarbon receptor by indole-3-pyruvic acid inhibits the development of experimental autoimmune pancreatitis. Four groups of MRL/MpJ mice were used: untreated mice (n = 5), mice treated with 0.1% indole-3-pyruvic acid (IPA) in the diet (n = 5), mice treated with repeated intraperitoneal injections of polyinosinic-polycytidylic acid (poly(I:C), n = 5), and mice co-treated with poly(I:C) and IPA (n = 5). Injections of poly(I:C) (100 μg) were performed twice a week for a total of 16 times, and the mice were sacrificed 3 h after the final injection. (A) Representative images of pancreatic sections stained with hematoxylin and eosin (H&E) or Sirius Red are shown in the left panel (magnification ×400). Pathological scores for autoimmune pancreatitis and areas occupied by pancreatic fibrosis derived from the analyses of H&E and Sirius Red staining, respectively, are illustrated in the right panel. The areas positive for Sirius Red staining were semi-quantitatively measured. (B) Flow cytometry analyses of the percentages and numbers of pancreatic plasmacytoid dendritic cells (pDCs), defined as PDCA-1+B220low cells. (C) Concentrations of IL-13 and IL-22 in pancreatic lysates determined by enzyme-linked immunosorbent assays. Results are shown as the mean + standard error. **P < 0.01, as compared with values in mice treated with poly(I:C) alone
Administration of TCDD, a prototypical dioxin chemical, suppresses the development of experimental AIP
Finally, we tested the effect of AhR activation by TCDD, a prototypical dioxin compound, on the development of experimental AIP [4, 27]. Mice were treated with an IP injection of TCDD or poly(I:C) alone or co-treated with poly(I:C) and TCDD. As shown in Supplementary Fig. 3A, IP injections of TCDD suppressed the development of experimental AIP, as evaluated by pathology scores. Pancreatic fibrosis was markedly reduced in mice co-treated with poly(I:C) and TCDD as compared with that in mice treated with poly(I:C) alone (Supplementary Fig. 3A). Consistent with the results obtained in mice treated with IN or IPA, TCDD administration did not change the pancreatic accumulation of pDCs in mice treated with poly(I:C) (Supplementary Fig. 3B).
To exclude the possibility that the solvent used in the preparation of TCDD might have had some influence on the sensitivity of the mice to experimental AIP, mice were co-injected with the solvent (10% toluene/90% DMSO) and poly(I:C). As shown in Supplementary Fig. 4, injection with the solvent did not inhibit the development of AIP induced by poly(I:C).
The profiles of pancreatic cytokines revealed enhanced production of IL-22 and IL-17 in mice co-treated with poly(I:C) and TCDD, whereas the concentrations of IFN-α or IL-33 were not affected by TCDD administration (Supplementary Fig. 5). These findings were consistent with the lack of significant alterations in the pancreatic accumulation of pDCs. Furthermore, TCDD administration decreased IL-13 production in mice treated with poly(I:C).
The increase in IL-17 was higher in mice treated with TCDD than in those treated with IN, whereas the increase in IL-22 was lower in mice treated with TCDD than in those treated with IN or IPA. These findings suggest that the activation of the AhR and/or signaling pathways downstream of the AhR might lead to different cytokine profiles depending on the ligands used. Nevertheless, the results of these experiments with TCDD as an AhR ligand were in line with the results obtained with IN and IPA: the suppression of experimental AIP by the activation of the AhR with structurally different ligands was associated with enhanced production of IL-22.
Suppression of experimental AIP by AhR activation is IL-22 dependent
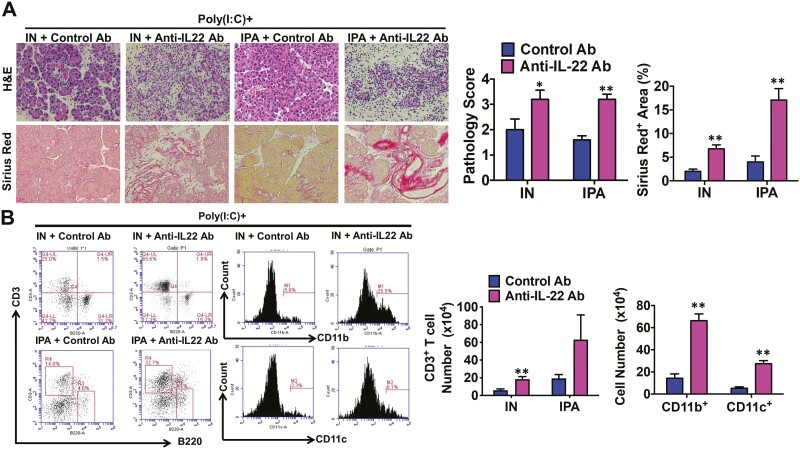
IL-22 is a critical regulator of immune homeostasis and is involved in the tissue regeneration process [39]. Therefore, we hypothesized that IL-22 mediates the protective effect of AhR activation on experimental AIP. To test this hypothesis, mice were treated with repeated IP injections of poly(I:C) and provided with IN or IPA in the diet, as described in Figs. 1 and 3. The mice were also treated with a neutralizing Ab against IL-22 or a control Ab. As shown in Fig. 4A, activation of the AhR by IN or IPA inhibited the development of experimental AIP, but these preventive effects were almost completely lost in mice treated with anti-IL-22 Ab. The areas with pancreatic fibrosis, as assessed through Sirius Red staining, were significantly greater in mice treated with anti-IL-22 Ab than in those treated with control Ab (Fig. 4A). Flow cytometry analysis also showed that treatment with anti-IL-22 Ab increased the accumulation of CD3+ T cells, CD11b+ myeloid cells, and CD11c+ DCs in the pancreas (Fig. 4B). In contrast to the effects of the anti-IL-22 Ab, the administration of the anti-IL-17 Ab did not alter the severity of experimental AIP in mice co-treated with poly(I:C) and IN (data not shown). These data suggest that AhR activation by IN or IPA suppressed experimental AIP through IL-22-mediated signaling pathways.
Figure 4.
Suppression of experimental autoimmune pancreatitis by indigo naturalis or indole-3-pyruvic acid depends on IL-22. Four groups of mice that received intraperitoneal injections of polyinosinic-polycytidylic acid (poly(I:C)) were used (n = 5 per group). Two groups were also treated with 4% indigo naturalis (IN), and two other groups received 0.1% indole-3-pyruvic acid (IPA) in the diet. Finally, one group on the same diet received an intraperitoneal injection of an anti-IL-22 Ab (n = 5), whereas the other group on the same diet was treated with a control Ab (n = 5). Injections of poly(I:C) (100 μg) were performed twice a week for a total of 16 times, and the mice were sacrificed 3 h after the final injection. (A) Representative images of pancreatic sections stained with hematoxylin and eosin (H&E) or Sirius Red are shown in the left panel (magnification ×400). Pathological scores for autoimmune pancreatitis and areas occupied by pancreatic fibrosis derived from the analyses of H&E and Sirius Red staining, respectively, are illustrated in the right panel. (B) Flow cytometry analyses of the percentages and numbers of pancreatic CD3+ T, CD11b+, and CD11c+ cells. Data on CD11b+ and CD11c+ cells were from mice treated with poly(I:C) and/or IN. Results are shown as the mean + standard error. *P < 0.05, **P < 0.01, as compared with values in mice treated with a control Ab
IL-22 has been shown to inhibit the development of experimental acute pancreatitis through the induction of antimicrobial REG3 peptide expression [40]. In addition, REG3 expression induced by IL-22 has been shown to contribute to the maintenance of intestinal epithelial integrity [41]. No significant differences were observed in the pancreatic mRNA expression levels of REG3β and REG3γ in mice treated with both IN and poly(I:C) and mice treated with poly(I:C) alone (Supplementary Fig. 6).
AhR activation by IN or IPA maintains homeostasis of pancreatic acinar cells via IL-22
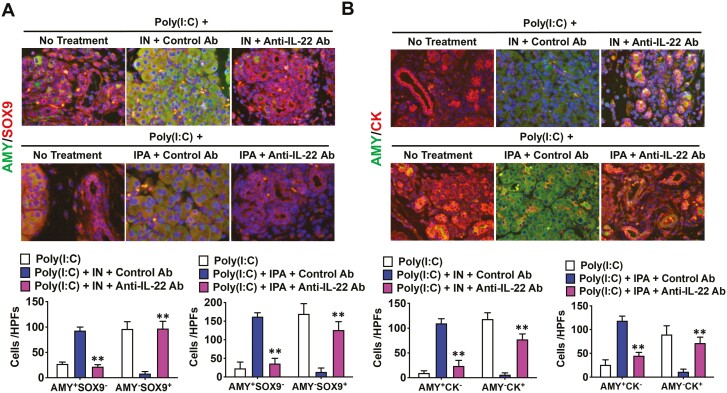
Non-hematopoietic epithelial cells are a primary target of IL-22 [42]. AIP progression is characterized by the loss of acinar tissue and its replacement by the acinar-to-ductal metaplasia (ADM) [43, 44]. Given that IL-22 is a critical cytokine for tissue regeneration, it may contribute to the maintenance of pancreatic acinar architecture. Next, we examined whether induced IL-22 following AhR activation with IN or IPA would inhibit ADM [43, 44]. ADM is defined as the appearance of amylase−SOX9+ cells or amylase−CK+ cells. As shown in Fig. 5, the number of cells with characteristic ADM features was much higher in the pancreas of mice treated with anti-IL-22 Ab, poly(I:C), and IN than in those treated with control Ab, poly(I:C), and IN. Consistent with the results obtained in mice treated with IN, AhR activation by IPA also inhibited the development of ADM. The number of cells exhibiting ADM was much higher in the pancreas of mice treated with anti-IL-22 Ab, poly(I:C), and IPA than in those treated with control Ab, poly(I:C), and IPA (Fig. 5). Thus, AhR activation by IN or IPA inhibited the development of ADM and maintained the normal pancreatic acinar architecture.
Figure 5.
Activation of the aryl hydrocarbon receptor inhibits the development of acinar-to-ductal metaplasia. Six groups of mice that received intraperitoneal injections of polyinosinic-polycytidylic acid (poly(I:C)) were used (n = 5 per group). Two groups were also treated with 4% indigo naturalis (IN), and another two groups received 0.1% indole-3-pyruvic acid (IPA) in the diet. These four groups of mice were also treated with intraperitoneal injections of an anti-IL-22 Ab (n = 5) or a control Ab (n = 5) in combination with IN/poly(I:C) or IPA/poly(I:C). Two groups did not receive any treatment other than poly(I:C) and were used as positive controls for the experiments with IN and IPA. Injections of poly(I:C) (100 μg) were performed twice a week for a total of 16 times, and the mice were sacrificed 3 h after the final injection. Acinar-to-ductal metaplasia (ADM) was visualized by the presence of (A) amylase−SRY-box9 (SOX9)+ (AMY−SOX9+) cells or (B) amylase-cytokeratin+ (AMY−CK+) cells by immunofluorescence analyses (magnification ×1,200). AMY+SOX9−, AMY−SOX9+, AMY+CK−, and AMY−CK+ cells were semi-quantitatively counted. Cell nuclei were counterstained with DAPI. Results are shown as the mean + standard error. **P < 0.01, as compared with values in mice treated with a control Ab
Pancreatic α produce IL-22 in response to AhR activation by IN or IPA
IL-22 is produced by a variety of immune cells, including T helper type 2 (TH2) cells, TH17 cells, as well as innate lymphoid type 2 (ILC2) and type 3 cells (ILC3) [42]. Next, we determined the type of immune cells that produced IL-22 in our AIP model. For this purpose, intracellular cytokine staining was performed. Pancreatic immune cells were isolated from mice treated with poly(I:C) alone or co-treated with poly(I:C) and IPA. As shown in Supplementary Fig. 7, the fractions of cells positive for IL-22 were comparable in TH2, TH17, ILC2, and ILC3 cells in the two groups of mice. Thus, immune cells were not the major sources of increased IL-22 in mice treated with IPA. Consistent with the flow-cytometric data, CD3+ T cells were negative for IL-22 staining in the pancreas of mice treated with both IN and poly(I:C) (Supplementary Fig. 8A).
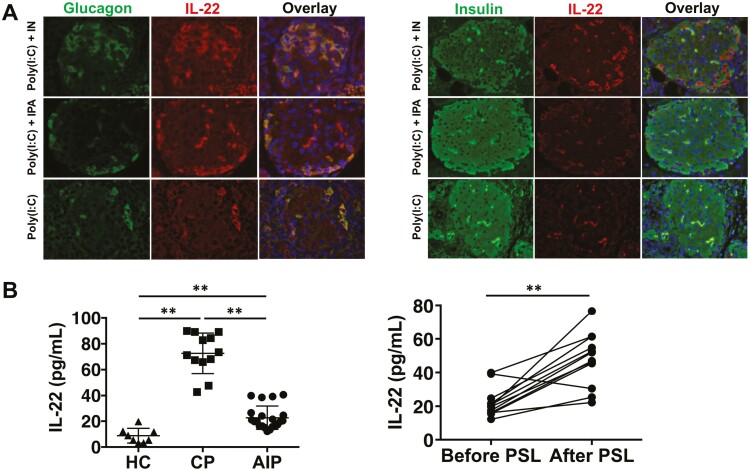
We then determined the types of cells producing IL-22 using immunofluorescence analysis. IL-22 expression was not detected in acinar cells expressing amylase (Supplementary Fig. 8B) or β cells expressing insulin (Fig. 6A). Interestingly, IL-22 expression was observed in α cells expressing glucagon in the pancreas of mice treated with poly(I:C) in combination with IN or IPA (Fig. 6A). The number of α cells positive for IL-22 staining was greater in mice treated with poly(I:C) and IN or IPA than in those treated with poly(I:C) alone (Fig. 6A). Thus, these data suggested that AhR activation by IN or IPA suppressed the development of experimental AIP through augmenting IL-22 production by α cells.
Figure 6.
IL-22 is produced by pancreatic α cells in response to aryl hydrocarbon receptor activation by indigo naturalis or indole-3-pyruvic acid. Mice were co-treated with repeated intraperitoneal injections of polyinosinic-polycytidylic acid (poly(I:C)) twice a week for 8 weeks and received indigo naturalis (IN) or indole-3-pyruvic acid (IPA) in the diet. (A) Co-localization of IL-22+ and glucagon+ α cells or insulin+ β cells in the pancreas of mice co-treated with poly(I:C) and IN or IPA. Cell nuclei were counterstained with DAPI. Green fluorescence, glucagon or insulin; red fluorescence, IL-22 (magnification, left panel; ×1,200, right panel ×800). (B) Left panel: serum IL-22 concentrations in patients with autoimmune pancreatitis (AIP, n = 21), chronic pancreatitis (CP, n = 12), and in healthy controls (HC, n = 8) were enrolled. Right panel: serum IL-22 concentrations in patients with AIP before and after induction of remission by prednisolone (PSL). **P < 0.01
Serum concentration of IL-22 is upregulated in patients with AIP after induction of remission by prednisolone
Finally, we addressed the clinical relevance of IL-22 in patients with AIP. We used serum samples from patients with AIP, those with CP, and healthy controls, as previously described [32]. Serum concentrations of IL-22 were higher in patients with AIP or CP than those in healthy controls. Importantly, the serum concentration of IL-22 was lower in patients with AIP than in those with CP (Fig. 6B). The serum concentration of IL-22 did not correlate with that of IgG or IgG4 in patients with AIP (data not shown). Although serum concentrations of IFN-α and IL-33 were identified as biomarkers for AIP diagnosis and disease activity monitoring [32], the serum concentration of IL-22 did not correlate with that of IFN-α or IL-33 (data not shown). Notably, the serum concentration of IL-22 was markedly increased in patients with AIP after successful induction of remission (Fig. 6B). Thus, IL-22 induced by AhR activation might also play a protective role in human AIP.
Discussion
The AhR is a ligand-activated transcription factor that plays critical roles in immunity and tissue homeostasis [3–5]. A variety of diverse compounds from the environment, diet, and microbiome activate the AhR [3–5]. Although AhR activation can both positively and negatively regulate immune responses, recent studies have highlighted the immunosuppressive function of AhR ligands [3–5]. In fact, recent clinical trials addressing the effectiveness of IN in patients with UC revealed that the rates of clinical response and remission in patients treated with IN were higher than those in placebo-treated patients [8, 34]. Thus, AhR activation by IN has been shown to regulate autoimmune responses. However, the effects of AhR activation on AIP have not been examined in detail. Here, we provide evidence that AhR activation inhibits the development of experimental AIP by using three different types of AhR ligands, IN, IPA, and TCDD. Although the development of experimental AIP requires the activation of pDCs producing IFN-α and IL-33, as was shown in our previous report [19, 20], AhR activation inhibited the development of AIP independent of pDC activation. Instead, AhR activation by IN or IPA suppressed chronic fibroinflammatory responses through signaling pathways mediated by IL-22 derived from pancreatic α cells. Thus, we clearly showed that AhR activation inhibited the development of AIP by upregulating IL-22 production. Furthermore, attenuation of experimental AIP development by AhR activation with IN and IPA was not observed in mice treated with anti-IL-22 Ab. The AhR was predominantly expressed in islet cells in both inflamed and uninflamed pancreatic tissue and the mRNA expression of Cyp1a1, one of the representative AhR-target genes, was markedly elevated in the pancreas of mice treated with dietary IN (Supplementary Fig. 1). In addition, islet α cells were positive for IL-22 staining, whereas CD3+ T cells or acinar cells were negative for IL-22 staining (Fig. 6 and Supplementary Fig. 8). These data strongly suggest that dietary IN directly activates the AhR expressed in islet α cells to induce the production of IL-22, which can suppress AIP. Given that flow-cytometric analysis revealed no significant differences in the production of IL-22 by various types of immune cells, it is likely that IL-22 derived from islet α cells plays critical roles in the suppression of AIP. However, the contribution of IL-22 derived from immune cells cannot be completely excluded since the quantitative measurement of α cell-derived IL-22 is technically challenging.
Regarding the molecular mechanisms by which IL-22 could inhibit chronic fibroinflammatory responses in AIP, we found that this cytokine, which is induced by AhR activation, reduced the degree of ADM, in which amylase+ acinar cells are replaced by amylase−SOX9+ cells or amylase−CK+ cells [43, 44]. This idea is supported by the finding that the blockade of IL-22 signaling pathways by the anti-IL-22 Ab markedly increased the number of cells displaying ADM in mice co-treated with poly(I:C) and IN or IPA, as compared to that in mice treated with the control Ab. These experiments strongly suggested that IL-22, induced by the AhR ligands IN or IPA, contributed to the maintenance of pancreatic acinar architecture. AhR activation is involved in the differentiation of Tregs expressing FOXP3 [3, 4]. However, it is less likely that these cells mediated the suppression of experimental AIP because AhR activation by IN, IPA, or TCDD did not increase the number of FOXP3+ Tregs in mice treated with poly(I:C) (data not shown). Therefore, AhR activation by IN or IPA inhibited the development of experimental AIP through the upregulation of IL-22 production, but not via the induction of FOXP3+ Treg proliferation.
Consistent with our results in a mouse model of experimental AIP, AhR activation protected mice from experimental acute pancreatitis by inducing IL-22 production [35]. Xue et al. reported that CD4+ T cells are the major producers of IL-22 in the pancreas and that IL-22 interacts with the IL-22 receptor expressed in pancreatic acinar cells to maintain pancreatic acinar architecture [35]. In line with these data, IL-22-overexpressing mice were resistant to the induction of acute and chronic types of pancreatitis by repeated injection of cerulein [45]. Thus, these studies support the idea that AhR activation inhibits the development of both AIP and CP through the induction of IL-22 production. In contrast, another report provided evidence that IL-22 plays a pathogenic role in the development of experimental CP [46]. AhR activation by TCDD exacerbated chronic fibroinflammatory responses characterizing CP, despite the increased expression of IL-22 in pancreatic CD4+ T cells. Neutralization of IL-22 attenuated both inflammation and fibrosis in mice treated with TCDD and cerulein, suggesting that the exacerbation of CP by TCDD was mediated by IL-22 [46]. These conflicting reports indicate that the effect of AhR activation on the development of CP is complex. In contrast to the case of experimental CP, pancreatic α cells are a major cellular source of IL-22 in experimental AIP. Extensive intracellular cytokine staining revealed no significant differences in the percentage of IL-22 production in ILC2, ILC3, or TH17 cells. Therefore, it is possible that differences in cellular sources of IL-22 determine the effect of this cytokine on the development of chronic inflammation.
As shown in our previous studies [11, 19, 20], experimental AIP induced by poly(I:C) requires activation of pDCs followed by robust production of IFN-α. This idea is supported by the finding that the depletion of pDCs or neutralization of IFN-α-mediated signaling pathways inhibited the development of experimental AIP [11, 19, 20]. In contrast to these previous findings, AhR activation by IN, IPA, or TCDD suppressed the chronic fibroinflammatory responses in a pDC- and IFN-α-independent manner because no significant alterations in pancreatic pDC numbers or IFN-α concentrations were observed in mice treated with poly(I:C) and those co-treated with poly(I:C) and AhR ligands. As for the molecular mechanisms underlying the suppression of the development of experimental AIP by AhR activation, we identified the protective roles played by IL-22 derived from pancreatic islet α cells. The appearance of ADM features was markedly suppressed by oral administration of AhR ligands independent of pDC activation. Therefore, it is likely that IL-22 plays a crucial role in the maintenance of pancreatic acinar architecture, although it remains unknown why IL-22 does not downregulate pDC activation. Consistent with this idea, IL-22 has been shown to protect acinar cells against injury and inflammation in experimental models of cerulein-induced pancreatitis [40, 45, 47].
In patients with AIP, PSL-mediated induction of remission was accompanied by increased concentrations of serum IL-22. The molecular mechanisms accounting for the increased IL-22 responses in patients with remittent AIP are currently unknown. Direct activation of the AhR by PSL is unlikely since PSL has not been regarded as an AhR ligand [1–6]. Thus, PSL might increase IL-22 responses in patients with remittent AIP through the resolution of inflammation and reduction of proinflammatory cytokine responses. This idea is supported by our preliminary data showing that pancreatic concentrations of IL-22 were greater in mice co-treated with poly(I:C) and dietary IN than in those treated with dexamethasone and poly(I:C) (60.3 ± 7.0 pg/mg versus 29.5 ± 5.7 pg/mg).
We previously reported that alterations in the intestinal microbiota composition are associated with the development of experimental AIP and human IgG4-RD [22–24]. Thus, the development of AIP is partially dependent on the immune responses against intestinal microflora. It is well established that AhR ligands are produced by intestinal bacteria from dietary Trp [3, 48–50]. For example, Lactobacillus species such as Lactobacillus reuteri and Lactobacillus bulgaricus convert Trp into AhR ligands. Importantly, colonization of the gastrointestinal tract by the bacteria producing AhR ligands leads to increased production of IL-22, which helps to maintain intestinal homeostasis [3, 48–50]. Stimulation of IL-22 production by the bacteria that produce AhR ligands attenuates inflammation of the gastrointestinal and other organs [3, 48–50]. Therefore, colonization by bacteria producing AhR ligands might suppress the development of AIP and IgG4-RD. However, we failed to identify changes in the proportions of intestinal bacteria producing AhR ligands in the feces of mice or patients with AIP [22, 23].
Naganuma et al. performed a multicenter randomized controlled trial addressing the efficacy and safety of IN in patients with UC [8, 34]. They reported that the proportion of patients in clinical remission was significantly higher in the IN-treated group than in the placebo group. Moreover, the proportion of patients displaying mucosal healing was much higher in the IN-treated group than in the placebo group, owing to increased IL-22 production [8, 34]. In this study, we found that the inhibition of experimental AIP by IN depended on IL-22 production. Thus, UC and AIP share IL-22 as an immunoregulatory cytokine, strongly suggesting that patients with AIP can be treated with IN similar to those with UC, although the effectiveness of IN in AIP awaits future clinical trials. In addition, we have to be cautious regarding the clinical application of IN in patients with AIP because the clinical trial for patients with UC was terminated due to the occurrence of pulmonary arterial hypertension [51].
In conclusion, AhR activation by IPA or IN suppressed the development of experimental AIP. The suppressive effect of AhR activation was likely mediated by IL-22 derived from pancreatic islet α cells. Serum IL-22 concentrations were elevated after the induction of remission by PSL in patients with AIP. These data suggest that similar to patients with UC, patients with AIP might be treated with AhR ligands.
Supplementary Material
Acknowledgements
The authors would like to thank Prof. Takanori Kanai, Dr. Toshiaki Teratani, and Dr. Yusuke Yoshimatsu for instructions on how to create and administer indigo naturalis, and Ms. Yukiko Ueno for secretarial support.
Abbreviations
- Ab
antibody
- ADM
acinar-to-ductal metaplasia
- AhR
aryl hydrocarbon receptor
- AIP
autoimmune pancreatitis
- CK
cytokeratin
- CP
chronic pancreatitis
- CYP1A1
cytochrome p450 family 1A1
- DAPI
4ʹ,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- FOXP3
forkhead box P3
- H&E
hematoxylin and eosin
- IgG4-RD
IgG4-related disease
- ILC
innate lymphoid cell
- IN
indigo naturalis
- IP
intraperitoneal
- IPA
indole-3-pyruvic acid
- pDC
plasmacytoid dendritic cell
- PDCA-1
pDC antigen-1
- PMNC
pancreatic mononuclear cell
- PSL
prednisolone
- Poly(I:C)
polyinosinic-polycytidylic acid
- Quantitative PCR
qPCR
- REG3
regenerating islet-derived protein 3
- SOX9
SRY-box 9
- STAT3
signal transducer and activator of transcription 3
- TCDD
2;3;7;8-tetrachlorodibenzo-para-dioxin
- TH2
T helper type 2
- Treg
regulatory T cell
- Trp
tryptophan
- UC
ulcerative colitis
Contributor Information
Ken Kamata, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Akane Hara, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Kosuke Minaga, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Tomoe Yoshikawa, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Masayuki Kurimoto, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Ikue Sekai, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Natsuki Okai, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Naoya Omaru, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Yasuhiro Masuta, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Yasuo Otsuka, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Ryutaro Takada, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Shiki Takamura, Department of Immunology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Masatoshi Kudo, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan.
Warren Strober, Mucosal Immunity Section, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Tomohiro Watanabe, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Osaka, Japan; Mucosal Immunity Section, Laboratory of Host Defenses, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Data availability
Data obtained in this study may be shared with other researchers upon reasonable request to the corresponding author.
Author contributions
Ken Kamata: Conceptualization, Methodology, Resources, Writing - Original Draft; Akane Hara, Kosuke Minaga, Tomoe Yoshikawa, Masayuki Kurimoto, Ikue Sekai, Natsuki Okai, Naoya Omaru, Yasuhiro Masuta, Yasuo Otsuka, Ryutaro Takada, Shiki Takamura: Investigation, Formal Analysis; Masatoshi Kudo: Resources; Warren Strober: Resources, Writing - Review & Editing; Tomohiro Watanabe: Conceptualization, Methodology, Investigation, Formal Analysis; Resources, Writing - Original Draft; Writing - Review & Editing.
Funding
This work was supported by Grants-in-Aid for Scientific Research (21K15987, 22K07996, and 22K08090) from the Japan Society for the Promotion of Science, Takeda Science Foundation, Smoking Research Foundation, Yakult Bio-Science Foundation, SENSHIN Medical Research Foundation, and a 2022 Kindai University Research Enhancement Grant (KD2208).
Conflict of interest
The authors have no potential conflicts of interest to declare.
Ethics Approval
This human study was approved by the Ethics Committee of the Kindai University Faculty of Medicine. The animal experiments were approved by the Review Board of the Kindai University Faculty of Medicine and adhered to the ARRIVE guidelines.
Patient consent
Written informed consent was obtained from all patients and healthy controls at enrollment, according to the Declaration of Helsinki.
References
- 1. Yue T, Sun F, Yang C, Wang F, Luo J, Yang P, et al. The AHR signaling attenuates autoimmune responses during the development of type 1 diabetes. Front Immunol 2020, 11, 1510. doi: 10.3389/fimmu.2020.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bungsu I, Kifli N, Ahmad SR, Ghani H, Cunningham AC.. Herbal plants: the role of AhR in mediating immunomodulation. Front Immunol 2021, 12, 697663. doi: 10.3389/fimmu.2021.697663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothhammer V, Quintana FJ.. The Aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 2019, 19, 184–97. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 4. Lamas B, Natividad JM, Sokol H.. Aryl sHydrocarbon receptor and intestinal immunity. Mucosal Immunol 2018, 11, 1024–38. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 5. Shinde R, McGaha TL.. The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol 2018, 39, 1005–20. doi: 10.1016/j.it.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutiérrez-Vázquez C, Quintana FJ.. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poland A, Knutson JC.. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 1982, 22, 517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 8. Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 2018, 154, 935–47. [DOI] [PubMed] [Google Scholar]
- 9. Stone JH, Zen Y, Deshpande V.. IgG4-related disease. N Engl J Med 2012, 366, 539–51. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 10. Kamisawa T, Zen Y, Pillai S, Stone JH.. IgG4-related disease. Lancet 2015, 385, 1460–71. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe T, Minaga K, Kamata K, Kudo M, Strober W.. Mechanistic insights into autoimmune pancreatitis and igg4-related disease. Trends Immunol 2018, 39, 874–89. doi: 10.1016/j.it.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 12. Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T.. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut 2013, 62, 1373–80. doi: 10.1136/gutjnl-2012-304224. [DOI] [PubMed] [Google Scholar]
- 13. Kawa S, Kamisawa T, Notohara K, Fujinaga Y, Inoue D, Koyama T, et al. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas 2020, 49, e13–4. doi: 10.1097/MPA.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Umehara H, Okazaki K, Nakamura T, Satoh-Nakamura T, Nakajima A, Kawano M, et al. Current approach to the diagnosis of IgG4-related disease - combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol 2017, 27, 381–91. doi: 10.1080/14397595.2017.1290911. [DOI] [PubMed] [Google Scholar]
- 15. Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2018, 67, 728–35. doi: 10.1136/gutjnl-2017-314548. [DOI] [PubMed] [Google Scholar]
- 16. Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med 2018, 10, eaaq0997. doi: 10.1126/scitranslmed.aaq0997. [DOI] [PubMed] [Google Scholar]
- 17. Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–32. doi: 10.1136/gutjnl-2015-310336. [DOI] [PubMed] [Google Scholar]
- 18. Aalberse RC, Stapel SO, Schuurman J, Rispens T.. Immunoglobulin G4: an odd antibody. Clin Exp Allergy 2009, 39, 469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 19. Arai Y, Yamashita K, Kuriyama K, Shiokawa M, Kodama Y, Sakurai T, et al. Plasmacytoid dendritic cell activation and IFN-alpha production are prominent features of murine autoimmune pancreatitis and human IgG4-related autoimmune pancreatitis. J Immunol 2015, 195, 3033–44. doi: 10.4049/jimmunol.1500971. [DOI] [PubMed] [Google Scholar]
- 20. Watanabe T, Yamashita K, Arai Y, Minaga K, Kamata K, Nagai T, et al. Chronic fibro-inflammatory responses in autoimmune pancreatitis depend on IFN-alpha and IL-33 produced by plasmacytoid dendritic cells. J Immunol 2017, 198, 3886–96. doi: 10.4049/jimmunol.1700060. [DOI] [PubMed] [Google Scholar]
- 21. Minaga K, Watanabe T, Arai Y, Shiokawa M, Hara A, Yoshikawa T, et al. Activation of interferon regulatory factor 7 in plasmacytoid dendritic cells promotes experimental autoimmune pancreatitis. J Gastroenterol 2020, 55, 565–76. doi: 10.1007/s00535-020-01662-2. [DOI] [PubMed] [Google Scholar]
- 22. Kamata K, Watanabe T, Minaga K, Hara A, Yoshikawa T, Okamoto A, et al. Intestinal dysbiosis mediates experimental autoimmune pancreatitis via activation of plasmacytoid dendritic cells. Int Immunol 2019, 31, 795–809. doi: 10.1093/intimm/dxz050. [DOI] [PubMed] [Google Scholar]
- 23. Kamata K, Watanabe T, Minaga K, Hara A, Sekai I, Otsuka Y, et al. Gut microbiome alterations in type 1 autoimmune pancreatitis after induction of remission by prednisolone. Clin Exp Immunol 2020, 202, 308–20. doi: 10.1111/cei.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshikawa T, Minaga K, Hara A, Sekai I, Kurimoto M, Masuta Y, et al. Disruption of the intestinal barrier exacerbates experimental autoimmune pancreatitis by promoting the translocation of Staphylococcus sciuri into the pancreas. Int Immunol 2022, 34, 621–34. doi: 10.1093/intimm/dxac039. [DOI] [PubMed] [Google Scholar]
- 25. Yoshimatsu Y, Sujino T, Miyamoto K, Harada Y, Tanemoto S, Ono K, et al. Aryl hydrocarbon receptor signals in epithelial cells govern the recruitment and location of helios(+) Tregs in the gut. Cell Rep 2022, 39, 110773. doi: 10.1016/j.celrep.2022.110773. [DOI] [PubMed] [Google Scholar]
- 26. Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y.. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J Immunol 2018, 201, 3683–93. doi: 10.4049/jimmunol.1701734. [DOI] [PubMed] [Google Scholar]
- 27. Neamah WH, Singh NP, Alghetaa H, Abdulla OA, Chatterjee S, Busbee PB, et al. AhR activation leads to massive mobilization of myeloid-derived suppressor cells with immunosuppressive activity through regulation of CXCR2 and MicroRNA miR-150-5p and miR-543-3p that target anti-inflammatory genes. J Immunol 2019, 203, 1830–44. doi: 10.4049/jimmunol.1900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chung H, Watanabe T, Kudo M, Chiba T.. Hepatitis C virus core protein induces homotolerance and cross-tolerance to toll-like receptor ligands by activation of toll-like receptor 2. J Infect Dis 2010, 202, 853–61. doi: 10.1086/655812. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe T, Sadakane Y, Yagama N, Sakurai T, Ezoe H, Kudo M, et al. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce il-33-dependent chronic pancreatitis. Mucosal Immunol 2016, 9, 1234–49. doi: 10.1038/mi.2015.144. [DOI] [PubMed] [Google Scholar]
- 30. Watanabe T, Minaga K, Kamata K, Sakurai T, Komeda Y, Nagai T, et al. RICK/RIP2 is a NOD2-independent nodal point of gut inflammation. Int Immunol 2019, 31, 669–83. doi: 10.1093/intimm/dxz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asano N, Imatani A, Watanabe T, Fushiya J, Kondo Y, Jin X, et al. Cdx2 Expression and intestinal metaplasia induced by H. pylori infection of gastric cells is regulated by NOD1-mediated innate immune responses. Cancer Res 2016, 76, 1135–45. doi: 10.1158/0008-5472.CAN-15-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minaga K, Watanabe T, Hara A, Kamata K, Omoto S, Nakai A, et al. Identification of serum IFN-alpha and IL-33 as novel biomarkers for type 1 autoimmune pancreatitis and IgG4-related disease. Sci Rep 2020, 10, 14879. doi: 10.1038/s41598-020-71848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimosegawa T, Kataoka K, Kamisawa T, Miyakawa H, Ohara H, Ito T, et al. The revised Japanese Clinical Diagnostic Criteria for chronic pancreatitis. J Gastroenterol 2010, 45, 584–91. doi: 10.1007/s00535-010-0242-4. [DOI] [PubMed] [Google Scholar]
- 34. Naganuma M, Sugimoto S, Fukuda T, Mitsuyama K, Kobayashi T, Yoshimura N, et al. Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: a post hoc analysis from the INDIGO study. J Gastroenterol 2020, 55, 169–80. [DOI] [PubMed] [Google Scholar]
- 35. Xue J, Nguyen DT, Habtezion A.. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology 2012, 143, 1670–80. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen PM, Putoczki TL, Ernst M.. STAT3-activating cytokines: a therapeutic opportunity for inflammatory bowel disease?. J Interferon Cytokine Res 2015, 35, 340–50. doi: 10.1089/jir.2014.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 2016, 22, 598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keir M, Yi Y, Lu T, Ghilardi N.. The role of IL-22 in intestinal health and disease. J Exp Med 2020, 217, e20192195. doi: 10.1084/jem.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huan C, Kim D, Ou P, Alfonso A, Stanek A.. Mechanisms of interleukin-22’s beneficial effects in acute pancreatitis. World J Gastrointest Pathophysiol 2016, 7, 108–16. doi: 10.4291/wjgp.v7.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 Links IL-22 Signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009, 206, 1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dudakov JA, Hanash AM, van den Brink MRM, van den Brink MR.. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015, 33, 747–85. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wanner-Seleznik GM, Reding T, Chen R, Gupta AK, Lenggenhager D, Browning J, et al. Amelioration of murine autoimmune pancreatitis by targeted LTbetaR inhibition and anti-CD20 treatment. Immunohorizons 2020, 4, 688–700. doi: 10.4049/immunohorizons.2000079. [DOI] [PubMed] [Google Scholar]
- 44. Quilichini E, Fabre M, Dirami T, Stedman A, De Vas M, Ozguc O, et al. Pancreatic ductal deletion of Hnf1b disrupts exocrine homeostasis, leads to pancreatitis, and facilitates tumorigenesis. Cell Mol Gastroenterol Hepatol 2019, 8, 487–511. doi: 10.1016/j.jcmgh.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci 2012, 8, 249–57. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, et al. Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology 2016, 151, 1206–17. doi: 10.1053/j.gastro.2016.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Wong K, Ouyang W, Rutz S.. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol 2019, 11, a028548. doi: 10.1101/cshperspect.a028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 49. Pernomian L, Duarte-Silva M, de Barros Cardoso CR.. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol 2020, 59, 382–90. doi: 10.1007/s12016-020-08789-3. [DOI] [PubMed] [Google Scholar]
- 50. Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 2018, 28, 737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 51. Naganuma M, Sugimoto S, Suzuki H, Matsuno Y, Araki T, Shimizu H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol 2019, 54, 891–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data obtained in this study may be shared with other researchers upon reasonable request to the corresponding author.