Abstract
Primary Sjögren’s syndrome (pSS) is a chronic inflammatory autoimmune disease, which mainly damages patients’ exocrine glands. Sensitive early diagnostic indicators and effective treatments for pSS are lacking. Using machine learning methods to find diagnostic markers and effective therapeutic ways for pSS is of great significance. In our study, first, 1643 differentially expressed genes (DEGs; 737 were upregulated and 906 were downregulated) were ultimately screened out and analyzed by Gene Ontology and Kyoto Encyclopedia of Genes and Genomes based on the datasets from the Gene Expression Omnibus. Then, support vector machine, least absolute shrinkage and selection operator regression, random forest, and weighted correlation network analysis were used to screen out feature genes from DEGs. Subsequently, the intersection of the feature genes was taken to screen 10 genes as hub genes. Meanwhile, the analysis of the diagnostic efficiency of 10 hub genes showed their good diagnostic value for pSS, which was validated through immunohistochemistry on the paraffin sections of the labial gland. Subsequently, a multi-factor regulatory network and correlation analysis of hub genes were performed, and the results showed that ELAVL1 and IGF1R were positively correlated with each other but both negatively correlated with the other seven hub genes. Moreover, several meaningful results were detected through the immune infiltration landscape. Finally, we used molecular docking to screen potential therapeutic compounds of pSS based on the hub genes. We found that the small molecules DB08006, DB08036, and DB15308 had good docking scores with ELAVL1 and IGF1R simultaneously. Our study might provide effective diagnostic biomarkers and new therapeutic ideas for pSS.
Keywords: diagnosis, machine learning, molecular docking, Sjögren’s syndrome
Primary Sjögren's syndrome(pSS) is a chronic inflammatory autoimmune disease, sensitive early diagnostic indicators and effective treatments for pSS are lacking. The goal of our study was to screen out the effective markers and related potential therapeutic agents for pSS by analyzing the experimental dataset from multiple data platforms and validating through the validation dataset combined with experiments. This might provide effective diagnostic biomarkers and new therapeutic ideas for treating pSS.
Graphical Abstract
Graphical Abstract.
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic autoimmune disease with an unknown etiology and a prevalence ranging from 0.03% to 4.80% globally. It occurs approximately 9–20 times more frequently in women than in men. pSS is mainly characterized by exocrine gland involvement, such as the lacrimal and salivary glands, and can manifest as dry mouth and eyes [1]. Meanwhile, multiple organs and systems can be involved, leading to interstitial lung disease, nervous system disease, renal tubular acidosis, and blood system disease [2, 3]. Currently, pSS is diagnosed based on a combination of clinical, serologic, and histological indicators, which are usually manifested only in the advanced stages of the disease (e.g. intolerable dry mouth and eyes mentioned in the 2012 ACR criteria; severely impaired glandular function can occur). Therefore, early diagnosis of pSS is challenging [4, 5]. As an indispensable way used to diagnose pSS, labial minor salivary gland (LSG) biopsy plays a significant role in the established American-European Consensus Group classification and the proposed American College of Rheumatology (ACR)/European League Against Rheumatism(EULAR) criteria, which also has a disadvantage in terms of the resulting traumatic damage [6, 7]. Furthermore, SSA and SSB are currently used as important serological markers for diagnosing pSS. However, they are inadequate for diagnosing early-stage and serologically negative pSS. Therefore, finding new and feasible biomarkers for pSS diagnosis is of great significance.
No substantial change in the treatment of glandular symptoms and systemic organ complications of pSS has been reported in recent years. Patients with diverse pSS symptoms often require different therapeutic approaches, including symptomatic treatment based on Sjögren’s symptomatology and various immunosuppressive treatments targeting systemic immune impairment. The EULAR recently issued recommendations for treating pSS based on findings from recent studies, some of which have shown effectiveness in treating patients with pSS [8]. The development of biotechnology-based targeted therapies that can interfere with or block the key points of inflammatory signaling pathways can bring new approaches for treating pSS [9]. New targeted therapeutic agents and efficacy indicators in combination with patient’s gene phenotypes may help improve the diagnosis and treatment of pSS, which is a milestone worth looking forward to [10].
As a branch of artificial intelligence, machine learning is a technology that can automatically build data models and deal with complex relationships among diverse data, thus helping find diagnostic predictors through Big Data analytics [11]. Furthermore, machine learning can build risk models based on Big Data by learning data obtained from existing medical tests or surveys of patients, which can be used to predict and diagnose diseases and evaluate disease prognosis by finding potential indicators with a great impact on diseases [12, 13]. At present, machine learning has been widely applied in the field of clinical medicine, such as the establishing a cardiovascular risk prediction system [14], finding predictors to predict the incidence trend of type 2 diabetes mellitus [15], generating an acute kidney injury risk model [16], and establishing tumor pathological diagnosis and early prevention model [17]. More important, machine learning has entered a new stage in exploring the therapeutic drugs of rheumatology. In addition, the machine learning method can be used to identify the effects of potential drug candidates so as to provide guidance for the clinical treatment of diseases [18]. However, thus far, reports on the application of machine learning in predicting pSS, especially in drug screening for pSS therapy, are few.
In this study, machine learning was used to screen sensitive and specific predictors of pSS, and potential therapeutic compounds for pSS were discovered through molecular docking. First, differentially expressed genes(DEGs) were screened based on pSS-related data in the Gene Expression Omnibus (GEO) database, and functional and pathway enrichment analysis of DEGs was conducted. Based on DEGs, four methods, including support vector machine (SVM), random forest, least absolute shrinkage and selection operator (LASSO) regression, and weighted correlation network analysis(WGCNA) co-expression network, were used to screen the feature genes. Subsequently, a multi-factor regulatory network was constructed, and immune infiltration analysis was conducted based on the hub genes. Finally, the potential therapeutic compounds of the disease were screened based on the hub genes by molecular docking. Our findings may bring a new direction and understanding to the diagnosis and treatment of pSS.
Methods
Data sources
GSE66795 and GSE84844 were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) (Table 1), in which the gene expression data were from whole blood in pSS patients and health voluntary participants. The data were preprocessed as follows: First, the probes were mapped to genes according to annotation files, and no-load probes were removed. If multiple probes corresponded to the same gene, the maximum value was selected as the expression level of the gene. During hierarchical clustering pretreatment, it was found that some samples (20 cases) were clustered into the control group. Therefore, these samples were excluded from the analysis.
Table 1:
Profile dataset of RNA expression for Sjogren’s syndrome
DEG analysis
The “Lima package” in R was used for DEG analysis.Log2 foldchange (logFC) >0 and p.adj <0.05 were set as thresholds to screen DEGs.
Gene enrichment analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on 1643 DEGs obtained from the GSE66795 dataset using “enrichGO” and “enrichKEGG” in the “clusterprofiler” of the R package.
SVM training
For the DEG expression data screened from the GSE66795 dataset, “the rfe function” in “caret package” was used to conduct SVM linear training, and the feature number was screened from 1 to 200.
LASSO regression analysis
The “glmnet function” in the R package was used to model the dichotomous LASSO regression on DEGs, and “cv.glmnet function” was used for cross-validation. Lambda.min was used as the best lambda parameter.
Screening of DEGs using random forest
Random forest was used for further screening of DEGs, and carat and random forest were used for analysis. The final model was constructed according to the mtry and ntree parameters with the best accuracy rate in the model.
Construction of WGCNA co-expression network
The expression levels of DEGs were screened out, and the WGCNA package was used to construct the co-expression network of all gene expression matrices. First, the change trend of the scale-free fitting index and average connectivity of the network were calculated when the softpower changed from 1 to 60. The scale-free fitting index > 0.8 was considered as the threshold, and the softpower was set as three to meet the scale-free network. Finally, the co-expression network was constructed, and the diagnostic modules were identified. The minimum number of genes in the module was 30, and the modules with a correlation coefficient >0.7 were merged.
Receiver operating characteristic curves
The expression data of hub genes were filtered from the standardized expression matrix of the dataset, and the linear combination was fitted using the generalize linear model(GLM) function. Then, the receiver operating characteristic analysis curve was calculated and visualized using the pROC package according to the grouping information.
Extraction of interaction relationship among miRNA, lncRNA, transcription factor (TF), and mRNA to construct a multi-factor regulatory network
MiRNA–mRNA interactions were obtained from the starBase database (http://starbase.sysu.edu.cn/) to screen the miRNAs correlated with the feature genes of pSS. The numbers of CLIP ≥ 5, degradome ≥ 0, cancer type ≥ 5, and prediction procedure ≥ 3 were separated into three groups to filter the miRNA–mRNA interactions. Meanwhile, mRNA–lncRNA and human TF–mRNA interactions were downloaded from the RAID database (http://www.rna-society.org/raid/index.html) and the Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining v2 database (https://www.grnpedia.org/trrust/). Finally, Cytoscape was used to plot the interaction network.
Correlation analysis of infiltrating immune cells
The CIBERSORT algorithm was used to evaluate the distribution of immune cells in different samples. The prcomp function was used to reduce the dimensionality of samples using principal component analysis (PCA). The fviz-pca-ind function in the factoextra package was used for visualization. The corr. test function was used to calculate the Pearson correlation between the hub gene expression level and the proportion of immune cells in the psych package, and the Wilcoxon-test was used to calculate the difference in the distribution of immune cells among groups.
Molecular docking and drug screening
The corresponding compound structure information was downloaded from the DrugBank database, and 5462 small-molecule compounds were screened out according to Lipinski’s rule (hydrogen bond acceptor ≤ 10, hydrogen bond donor ≤ 5, rotatable bond ≤ 10, logarithm of lipid/water partition coefficient ≤ 5, molecular weight 180–480, and polar surface area ≤ 140). The protein date bank (PDB) structure file of hub genes was retrieved from the PDB website. The whole molecule was taken as the docking box range due to the lack of ligands, and other relevant parameters of autodock-vina were then set. Autodock-vina was used for docking, and pymol was used to draw the docking conformation diagram. Ligplus was used to perform interaction force analysis.
Patients and tissue samples
The paraffin-embedded slices of 85 patients with pSS diagnosed by pathology in The First Affiliated Hospital of Henan University of Science and Technology and normal labial gland tissues of 20 patients undergoing stomatological surgery were collected. Patients with pSS were diagnosed and classified according to the 2002 American-European Consensus Group (AECG) pSS classification criteria [19]. All the 85 patients with pSS were diagnosed as pSS for the first time and not treated with anti-rheumatic drugs and glucocorticoids before enrollment, including 9 males and 76 females. According to the grading criteria of labial gland pathology, there were 8 cases of grade II, 48 cases of grade III, and 29 cases of grade IV. Detailed information on pSS patients is provided in Table 2. Written informed consent was provided by the participants. The present study was approved by the ethics committee of The First Affiliated Hospital of Henan University of Science and Technology (approval no. 2021-130).
Table 2:
General clinical characteristics of patients with pSS
| Grade II | Grade III | Grade IV | ||
|---|---|---|---|---|
| Gender | Male (n) | 0 | 5 | 4 |
| Female (n) | 8 | 43 | 25 | |
| Age (year) | 50.8 ± 6.7 | 50.65 ± 7.1 | 51.4 ± 5.3 | |
| Duration of disease (month) | 26 ± 14 | 35 ± 12 | 38 ± 17 | |
| IgG (g/l) | 21.6 ± 5.7 | 22.1 ± 4.8 | 22.9 ± 8.2 | |
| Anti-SSA + (n) | 7 | 43 | 25 | |
| Anti-SSB + (n) | 2 | 15 | 10 | |
| Schirmer’s test (mm/5 min) | 3.5 ± 2.6 | 2.8 ± 2.3 | 2.2 ± 2.1 | |
| Unstimulated whole salivary flow(ml/15 min) | 1.27 ± 0.04 | 0.83 ± 0.03 | 0.55 ± 0.02 | |
Immunohistochemistry(IHC)
The paraffin-embedded sections were dewaxed, hydrated, and rinsed with water. The tissues were repaired using the corresponding antigen (Tris–EDTA thermal repair) according to the requirements of the first antibody. Peroxidase blocking reagent (H2O2) was added to the sections, incubated at room temperature for 10 min, and rinsed with phosphate-buffered saline (PBS) three times, 3 min each time. After removing PBS, primary antibodies [concentrations: EZH2 (1:500), ELAVL1 (1:300), and IGF1R (1:400)] were added to the sections dropwise, and the samples were incubated overnight at 4°C. After PBS was removed, the secondary antibody was added to the sections, and the samples were incubated at room temperature for 15 min. The samples were washed with PBS three times, 3 min each time. After PBS was removed, freshly prepared DAB chromogenic agent was added to the sections for chromogenic development for about 5 min. The color development was terminated by washing with water, and the sections were stained with hematoxylin, differentiated with 1% hydrochloric acid–ethanol, dehydrated with gradient ethanol series, made transparent with xylene, and sealed with neutral gum. The experiments were repeated three times.
Statistical analysis
The DEGs were analyzed using the limma package in R by the differential expression analysis method. The statistical test data of the expression of hub genes were analyzed using the t-test, and the data on immune cell infiltration were analyzed using the Wilcoxon test with P < 0.5 considered to be statistically significant.
Results
DEGs and enrichment analysis
In the GSE66795 dataset, 737 genes were up-regulated and 906 genes were down-regulated in the pSS group compared with the control group, which are shown in Fig. 1A, Supplementary Table S1). In the GSE84844 dataset, 3338 genes were up-regulated and 4400 genes were down-regulated. Meanwhile, the top 50 DEGs are displayed in Fig. 1B according to the LogFC value. Furthermore, the clusterProfiler package was used to perform GO and KEGG enrichment analyses on 1643 DEGs obtained by differential analysis. These genes were found to be closely related to the immune response and function of cells, such as regulation of innate immune response, response to viruses, regulation of response to biotic stimuli, defense response to symbionts, defense response to viruses, and transcription coregulator activity (Fig. 1C–F and Supplementary Table S2).
Figure 1:
Differential expression genes (DEGs) and functional enrichment analysis of DEGs in GSE66795 dataset. (A) Volcano map of DEGs. (B) Heat map of top 50 DEGs. (C) GO enrichment analysis of DEGs (BP). (D) GO enrichment analysis of DEGs (MF). (E) GO enrichment analysis of DEGs (CC). (F) KEGG enrichment analysis of DEGs
Feature genes of pSS were screened based on DEGs9
First, the feature genes of pSS were screened based on DEGs from the GSE66795 dataset through SVM. Our results showed that the training reached the best level when 125 feature genes were included, and the top 125 genes were selected as SVM feature genes (Fig. 2A). Second, the genes with the top 100 scores of MeanDecreaseGini were selected as feature genes by the random forest method based on the DEGs in GSE66795 (Fig. 2B–D). Then, 52 feature genes were screened out using LASSO (Fig. 2E and F). Finally, WGCNA was used to construct the co-expression network for all gene expression matrices in the GSE66795 dataset. Finally, the genes were classified into 25 modules; the Salmon module had the strongest positive correlation with pSS, including 239 genes (Fig. 3A–E).
Figure 2:
Feature genes were screened based on DEGs. (A) Training changes of SVM accuracy and the number of genes. (B–D) Random forest analysis of DEGs. (B) Relationship between the accuracy of the random forest model and the number change of variables (mtry). (C) Relationship between the error rate of the random forest model and the number of decision trees. (D) Importance ranking of top 30 genes screened by the random forest. Average accuracy decreased in importance and Gini index. (E, F) Parameter optimization of DEGs by LASSO regression modeling. (E) Change in regression error. (F) Vertical axis: the change in LASSO regression coefficient of genes, and horizontal axis: logarithm of LASSO penalty term λ
Figure 3:
Identification of gene co-expression modules in the GSE66795 dataset. (A) Change in scale-free fitting index with soft threshold parameter. (B) Average network connectivity varies with the soft threshold parameter. (C) Heat map of the correlation between the module and disease traits. (D) Clustering dendrogram of genes. (E) Correlations of genes in Salmon module with modules and disease traits
Hub genes were obtained based on the intersection of feature genes
The intersection of feature genes obtained by the aforementioned four methods was taken. The intersection number of genes was 13 (without ELAVL1). Furthermore, the genes that appeared twice or more in SVM, RF, and WGCNA were also considered as candidate genes, and then 92 genes were obtained(Fig. 4A). Their interaction network was queried in the STRING database (https://cn.string-db.org/) (node genes without interaction relationship were removed) (Fig. 4C). The Cytohubba’s closeness algorithm was used to reserve the top 10 genes, which were used as hub genes (Fig. 4B and Supplementary Table S3). Finally, EZH2, DUSP5, HIST1H4H, C1QB, MS4A4A, C3AR1, ELAVL1, IGF1R, PARP3, and C2 were screened as hub genes. Subsequently, we analyzed the diagnostic efficiency of these 10 genes and their linear combination, and our results showed that they all had good diagnostic efficiency (Fig. 4D). As shown in Fig. 4D, the AUC values about the diagnostic efficiency of 10 hub genes from large to small were as follows: MS4A4A = 0.888, DUSP5 = 0.854, HIST1H4H = 0.832, C2 = 0.829, PARP3 = 0.819, C3AR1 = 0.816, EZH2 = 0.795, C1QB = 0.782, ELAVL1 = 0.719, and IGF1R = 0.689. The AUC values of genes were positively correlated with the diagnostic efficiency, and the higher the AUC value, the higher the diagnostic efficiency of genes in pSS. A combination of 10 hub genes showed the best diagnostic efficiency compared with any other single gene with the best AUC value (AUC = 0.979), implying that the comprehensive detection of hub genes could be used as a possible method for diagnosing pSS.
Figure 4:
Hub genes of pSS screening. (A) Venn diagram of SVM, RF, and WGCNA screening results. (B) Interaction network of 92 candidate genes shown by the STRING database. (C) Closeness algorithm of cytohubba was used to retain the top 10 hub genes. (D) The ROC diagnostic curve analysis of hub genes and their combination.
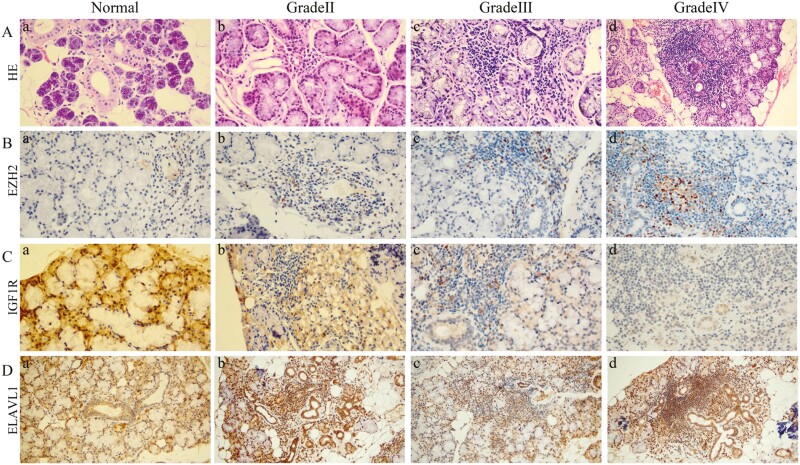
The paraffin-embedded sections of the labial gland from 85 patients with pSS and 20 healthy individuals were analyzed by immunohistochemistry and compared with the corresponding HE-stained sections to verify the diagnostic value of hub genes (Fig. 5). First, according to the diagnostic criteria for pSS, HE staining of normal labial gland tissue and pSS grades II to IV labial gland tissue was shown as a control (Fig. 5A). Our results showed that EZH2 was positively expressed in the labial gland lymphocytes of pSS patients and increased with disease grade, while it was negatively expressed in normal labial gland tissues. The IHC results were consistent with hematoxylin-eosin (HE) staining results (Fig. 5B). However, in contrast to EZH2, IGF1R was diffusely expressed in acinus of normal labial glandular tissue and was significantly stronger than that of pSS. Moreover, the expression of IGF1R decreased with the increase of pSS grading (Fig. 5C). Finally, ELAVL1 was found to be diffusely expressed in labial gland tissue and higher than that in normal labial gland tissues. However, there was no difference among different grades of pSS (Fig. 5D).
Figure 5:
IHC was used to verify the diagnostic value of hub genes in pSS. (A) Representative HE-stained sections of the labial gland in patients with pSS (a. normal; b. grade II; c. grade III; d. grade IV). (B) Representative IHC-stained sections showing EZH2 expression in the labial gland (a. normal; b. grade II; c. grade III; d. grade IV). (C) Representative IHC-stained sections showing IGF1R expression in the labial gland (a. normal; b. grade II; c. grade III; d. grade IV). (D) Representative IHC-stained sections showing ELAVL1 expression in the labial gland (a. normal; b. grade II; c. grade III; d. grade IV)
Expression of hub genes in patients with pSS and normal controls
The differential expression of hub genes in the training GSE66795 dataset and validation GSE84844 dataset was statistically analyzed. Our results showed that ELAVL1 and IGF1R were significantly down-regulated and EZH2, DUSP5, HIST1H4H, C1QB, and MS4A4A were significantly up-regulated in patients with pSS compared with normal controls in both datasets (P < 0.05, Fig. 6A and B). However, the expression of PARP3 in patients with pSS was significantly higher than that in normal controls in the training GSE66795 dataset (P < 0.05), whereas the expression of PARP3 in patients with pSS was lower than that in normal controls in the validation GSE84844 dataset but did not reach statistical significance (P > 0.05). Furthermore, the expression of C3AR1 and C2 in patients with pSS was significantly higher than that in normal controls in the training GSE66795 dataset (P < 0.05), whereas the expression of C3AR1 and C2 in pSS was higher in the validation GSE84844 dataset but did not reach statistical significance (P > 0.05, Fig. 6A and B).
Figure 6:
Differential expression analysis of hub genes in pSS. (A) Differential expression of hub genes in pSS and normal controls in the GSE66795 dataset (*P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant). (B) Differential expression of hub genes in pSS and normal controls in the GSE84844 dataset ( *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant). (C) Multifactorial regulatory network of hub genes. (D) Heat map of hub gene correlations (the colored bar on the right side represents the Pearson coefficient)
Next, the multi-factor regulatory network (ncRNA/TF) was constructed based on hub genes (Fig. 6C). All the interaction pairs directly related to hub genes were derived to construct a multi-factor regulatory network, including mRNA, lncRNA, miRNA, and TF. As shown in Fig. 4C, among 10 hub genes, EZH2, DUSP5, IGF1R, and ELAVL1 had complicated regulatory relationships. Among these, EZH2 interacted with IGF1R through BRCA1, TP53, and CCAT1. In addition, EZH2 also interacted with IGF1R through hSA-let-7I-5p, hSA-let-7G-5p, and hSA-let-7B-5P. Meanwhile, EZH2 also regulated DUSP5 through AFAP1-AS1. At the same time, we found that IGF1R could regulate HIST1H4H and PARP3 through FENDRR. IGF1R also interacted with DUSP5 through lncRNA CRNDE. The regulatory network of ELAVL1 was relatively simple, while C3AR1 only had a mutual regulatory relationship with lncRNA DANCR (Fig. 6C). Furthermore, the clusterProfiler package was used to perform GO and KEGG enrichment analyses on the target genes of hub genes, and our results showed that the target genes of EZH2 were closely related to miRNAs in cancer and human T-cell leukemia virus 1 infection (Supplementary Fig. S1). However, the target genes of IGF1R were closely related to the transcriptional misregulation in cancer and nuclear chromatin (Supplementary Fig. S2).
Furthermore, the Pearson correlation coefficient was used to evaluate the correlation between the 10 hub genes in the GSE66795 dataset, and the results were as follows: EZH2 expression was negatively correlated with IGF1R and the difference was statistically significant (P < 0.05), while EZH2 expression was negatively correlated with ELAVL1, but the difference was not statistically significant (P > 0.05). ELAVL1 was positively correlated with IGF1R (P > 0.05), and both genes were negatively correlated with EZH2, DUSP5, HIST1H4H, C3AR1, C1QB, MS4A4A, and C2 (Fig. 6D).
Immune infiltration landscape and correlation analysis between hub genes and immune infiltrating cells
The CIBERSORT algorithm was used to calculate the distribution of each type of immune cell in the GSE66795 dataset. According to the results, the “prcomp function” was used to reduce the dimension of samples by PCA, and the “fviz_pca_ind function” in the factoextra package was used for visualization (Fig. 7A). The “corr.test function” in the psych package was used for correlation analysis, and the Wilcoxon test was used for difference analysis (Fig. 7B). The results showed that the cell proportion of CD4+ naive T cells in pSS group was significantly smaller than control group (P < 0.05), while the cell proportion of CD4+ memory activated T cells, monocytes, and activated myeloid dendritic cells (DCs) were larger compared with normal controls (P < 0.05) (Fig. 7A–C and Supplementary Table S4). Subsequently, the correlation between hub genes and the distribution of various immune cells was calculated according to the distribution of each type of immune cell in the GSE66795 dataset. ELAVL1 and IGF1R were positively correlated with CD8+ T cells and neutrophils, respectively. EZH2, DUSP5, C3AR1, HIST1H4H, PARP3, C2, and most of C1QB and MS4A4A were positively correlated with CD4+ memory activated T cells and myeloid-activated DCs (Fig. 8 and Supplementary Table S5).
Figure 7:
Analysis of immune cell infiltration in the GSE66795 dataset. (A) Principal component analysis of immune cell distribution. (B) Correlation heat map of immune cell distribution. (C) Differences in immune cell infiltration between patients with pSS and normal controls in the GSE66795 dataset (*P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant)
Figure 8:
Correlation between hub genes and immune cell distribution. The black sphere on the right side represents correlation; the colored bar on the right side represents the P value. (A) EZH2. (B) IGF1R. (C) DUSP5. (D) HIST1H4H. (E) PARP3. (F) ELAVL1. (G) C3AR1. (H) C1QB. (I) MS4A4A. (J) C2
Potential therapeutic compounds screened based on hub genes through molecular docking
The corresponding compound structure information was downloaded from the DrugBank database and screened according to Lipinski’s rule (hydrogen bond acceptor ≤ 10, hydrogen bond donor ≤ 5, rotatable bond ≤ 10, logarithm of lipid/water partition coefficient ≤ 5, molecular weight 180–480, and polar surface area ≤ 140). Finally, 5462 small-molecule compounds were obtained. The PDB structure files of 10 hub genes were retrieved from the PDB website. Then, the relevant data of ELAVL1, IGF1R, and PARP3 were found, and the corresponding structure files 4ED5, 3LW0, and 4L7N were downloaded. Meanwhile, the docking box range was determined according to the ligand position. Autodock-vina was used for docking and pymol was used to draw the docking conformation map, and Ligplus was used for interaction force analysis. Affinity < −7 was set as a threshold to screen, and our results showed that DB08006, DB08036, and DB15308 had good docking scores with ELAVL1 and IGF1R (Fig. 9 and Supplementary Fig. S3). Furthermore, Genz-10850, Olaparib, and loprazolam had good docking scores with PARP3 (Supplementary Fig. S4, Table 3, and Supplementary Table S6). The aforementioned findings might provide new ideas for selecting pSS treatment drugs.
Figure 9:
Conformation and force analysis of ELAVL1 docking with small-molecule compounds. (A) DB08006, (B) DB08036, and (C) DB15308. The upper part: docking conformation and hydrogen bond shown by pymol. The cyan color shows a small molecule, the yellow dotted line shows a hydrogen bond, and the blue color shows an amino acid residue forming a hydrogen bond with a small molecule. Bottom part: Ligplus force analysis. A small molecule in the middle, surrounded by relevant protein amino acid residues; the green dotted line shows a hydrogen bond, and green color shows an amino acid residue forming a hydrogen bond with a small molecule
Table 3:
Docking score of small molecule and Hub gene.
| Protein | DrugBank_ID | Name | Affinity (kcal/mol) |
|---|---|---|---|
| ELAVL1 | DB08006 | N-Anthracen-2-yl-5-methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-amine | −8.5 |
| DB08036 | 6,7,12,13-Tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-5-one | −8.5 | |
| DB15308 | Ridinilazole | −8.5 | |
| IGF1R | DB08006 | N-Anthracen-2-yl-5-methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-amine | −9.2 |
| DB08036 | 6,7,12,13-Tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-5-one | −9.6 | |
| DB15308 | Ridinilazole | −9.6 | |
| PARP3 | DB04289 | Genz-10850 | −12.9 |
| DB09074 | Olaparib | −12.8 | |
| DB13643 | Loprazolam | −12.6 |
Discussion
As a common autoimmune disease, pSS is characterized by the involvement of lymphocytes in the induced exocrine gland inflammation, such as salivary and lacrimal glands, whose symptoms differ greatly and range from common sicca symptoms to systemic symptoms [20–23]. The wide variation in clinical manifestations and biological phenotypes among patients with pSS makes early diagnosis difficult, and the lack of standardized criteria used for the diagnosis and classification of the disease leads to difficulty in epidemiologic studies. Several years ago, Segal reported that it took 7 years for a patient with pSS to be diagnosed from the onset of symptoms because of the lack of effective approaches for early diagnosis [24]. Furthermore, although diagnostic approaches such as LSG, SSA, SSB, and disordered saliva secretion have been widely used for pSS diagnosis and classification, and some molecular biomarkers of pSS were detected in some studies, their sensitivity still needs further improvement [25–30]. Previous studies found that the genes involved in the pathogenesis of pSS included HLA-DRA, CCL19, CXCL9, and CD3D [31–34]. Meanwhile, the gene enrichment analysis identified related gene pathways, including immune system processes, T-cell activation, B-cell activation, and response to viruses [35–42]. In our study, we combined two public datasets to screen the potential biomarkers of pSS. In the GSE66795 dataset containing 111 pSS and 29 control samples, 737 genes were upregulated and 906 genes were downregulated. Previously reported biomarkers, such as HLA-DRA, CCL19, CXCL9, and CD3D, were also included in the DEGs found in our study. Our results from functional enrichment analysis showed that 1643 DEGs were closely related to the immune response and function of cells, such as regulation of innate immune response, response to viruses, regulation of response to biotic stimuli, which was consistent with the previously reported results.
A study conducted by Li et al reported that the hub genes of pSS based on bioinformatics analysis, including MS4A1, CD19, TCL1A, CCL19, CXCL9, CD3G, and CD3D, were selected as potential biomarkers, and the results showed that they all had good AUC values (MS4A1: 0.849; CD19: 0.723; TCL1A: 0.654; CCL19: 0.9; CXCL9: 0.958; CD3G: 0.707; and CD3D: 0.855) [43]. In our study, EZH2, DUSP5, HIST1H4H, C1QB, MS4A4A, C3AR1, ELAVL1, IGF1R, PARP3, and C2 were screened as hub genes. Sui et al. found that MS4A4A regulated the expression of arginase 1 in macrophages under IL-4 stimulation. Furthermore, MS4A4A regulated eosinophil infiltration during lung allergic inflammation induced by the intranasal administration of house dust mites [44]. Zahra et al. found that HIST1H4 was involved in the pathogenesis of HELLP syndrome and was closely related to autoimmunity as one of the hub genes with the highest degree of connectivity [45]. Yue reported that the number of autoantibodies against C3AR1 decreased in pSS [46]. Moreover, our results showed that 10 hub genes all had good diagnostic efficiency. The AUC value of genes was positively correlated with the diagnostic efficiency, and the higher the AUC value, the higher the diagnostic efficiency of genes in pSS. A combination of 10 hub genes showed the best diagnostic efficiency compared with any other single gene, implying that the comprehensive detection of hub genes could be used as a possible method for diagnosing pSS. Therefore, the aforementioned results demonstrated that the hub genes screened by our machine learning approaches might have good diagnostic potential for pSS and thus might become the diagnostic biomarkers for pSS in the future. EZH2, as an enzyme subunit of multi-combination inhibition complex 2, catalyzes the trimethylation of histone H3 lysine 27 (H3K27me3) and causes epigenetic gene silencing and transcriptional inhibition [47]. EZH2 has been shown to play an important role in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus. Consistent with our results, He et al. found that EZH2 was abnormally overexpressed in CD4+ T cells of pSS patients and was positively correlated with B cell overactivation markers and the circulating Th cell population. Moreover, EZH2 promoted the activation and proliferation of CD4+ T cells and promoted Tfh differentiation by enhancing STAT3 phosphorylation in pSS [48]. Skarlis et al. found that IGF1R variation increased the susceptibility to seropositive primary SS and that the inhibition of IGF1R mRNA and protein expression in salivary gland tissues might be related to the increase in apoptosis and the subsequent activation of the inflammatory body pathway, thus promoting the occurrence and progression of pSS [49]. ELAVL1 has been found to regulate the mRNA expression of pro-inflammatory cytokines (tumor necrosis factor-α, interleukin-6, transforming growth factor-13, and interferon-γ), the pro-inflammatory mediator inducible nitric oxide synthase (iNOS), and chemokine monocyte chemotactic protein-1(MCP-I), which may be involved in the pathogenesis of pSS [50]. In our study, the expression of EZH2, IGF1R, and ELAVL1 was analyzed by immunohistochemistry on the paraffin-embedded sections of labial gland biopsies from 85 patients with pSS and 20 healthy controls and compared with the corresponding HE-stained sections. The results showed that the expression of EZH2 was proportional to the level of pSS and was consistent with HE-staining. The expression of IGF1R was inversely proportional to the pSS grade. As predicted, these results confirmed the potential value of hub genes in diagnosing pSS. However, it needs to be further verified.
The immunological abnormalities of pSS are characterized by the massive infiltration of exocrine T and B cells and the production of autoantibodies such as anti-SSA and anti-SSB antibodies. Studies have shown that innate immune abnormalities, B-cell activation, and abnormal T-cell expression play a major role in the early occurrence and development of pSS [51]. DCs are the most functional antigen-presenting cells in innate immunity, which can activate primary T cells and work as a bridge between innate and adaptive immunity. Plasmacytoid DCs (pDCs) have a variety of immune functions and play an important role in inducing and regulating T-cell immune function. Abnormal pDC numbers and functions have been gradually recognized in the pathogenesis of pSS [51, 52]. Studies found that the number of pDCs significantly decreased in the peripheral blood of patients with pSS compared with healthy controls, and the expression of its activation marker CD40 increased. Activated pDCs were found in salivary gland tissues, indicating that pDCs in the peripheral blood were activated and transferred to salivary gland tissues [53, 54]. Moreover, B cell activation is considered the main pathogenic mechanism of pSS. Hypergammaglobulinemia, germinal center formation, and autoantibody production are associated with increased B-cell activation [55]. The activated B cells can produce IL-1, IL-6, TNF-α, and other cytokines to further regulate the activation of B and T cells. IL-10 stimulates the transformation of memory B cells into plasma cells in tissues, and the increase in the number of B cells in monocytes infiltrating salivary gland tissues of patients with pSS indicates the existence of local immune response [56]. Previous studies showed that T cells played an important role in developing pSS, and the number of activated T cells increased in the peripheral blood of patients with pSS. Further analysis found that the proportion of activated CD8+ T cells in the peripheral blood of patients with pSS was significantly higher than that of CD4+ T cells, suggesting that activated CD8+ T cells might play a role in the pathogenesis of the disease [57]. As important immune cells in the human immune system, CD4+ T cells, also known as helper T cells (Th), play an important role in cellular immune response and immune regulation. Studies showed that Th1/Th2 cell imbalance was involved in the pathogenesis of pSS [57]. In addition, studies suggested that Th17 and Treg inhibited each other and the imbalance of Th17/Treg might cause autoimmune diseases, thus playing an important role in the pathogenesis of pSS. Treg cells have immunosuppressive effects, can effectively inhibit CD4+ T and CD8+ T lymphocytes, and play an important role in maintaining immune balance and avoiding the occurrence of autoimmune diseases [58, 59]. Our study applied CIBERSORT for assessing the immune infiltration process within pSS to more specifically examine the effects exerted by the infiltration of immune cells in pSS. Our results showed that CD4+ naive T cells, CD4+ memory-activated T cells, monocytes, and myeloid-activated DCs had significant differences in their distribution in patients with pSS. Furthermore, we found that ELAVL1 and IGF1R were positively correlated with CD8+ T cells and neutrophils, respectively. However, EZH2, DUSP5, C3AR1, HIST1H4H, PARP3, C2, C1QB, and MS4A4A were positively correlated with CD4+ memory activated T cells and myeloid-activated DCs. The aforementioned results implied that CD4+ T cells, CD8+ T cells, and myeloid-activated DCs might participate in the occurrence and progression of pSS through hub genes such as ELAVL1, IGF1R, and EZH2.
To date, no treatment for pSS has been proven to be effective. However, great advances in the theory of disease pathology and biological mechanisms, as well as the establishment of targeted therapies, especially T- and B-cell-related genes, may open new horizons for treating patients with pSS. For example, various ways have been developed to suppress B cells, including targeting cytokines (e.g. anti-IL-6 receptor antibodies), direct targeting of B cells (e.g. anti-CD20, anti-BAFF, or anti-BAFF receptor antibodies), inhibiting co-stimuli (e.g. anti-CD40 antibodies), and targeting of small molecules (e.g. kinases). A new strategy of rituximab combined with belimumab in treating pSS is currently under exploration, which is supported by multiple lines of evidence [60]. A phase IIa study evaluating the anti-BAFFR antibody ianalumab (VAY736) in patients with pSS was conducted, and the results suggested a partial efficacy [61]. The aforementioned results indicated that targeting BAFFR and BAFF-BAFFR pathway inhibition might become a promising way of treating pSS. In our study, a molecular docking screen was used to select potential therapeutic compounds for pSS based on hub genes. Our results demonstrated that three small-molecule compounds, such as ridinilazole, had good docking scores with ELAVL1 and IGF1R simultaneously. Furthermore, the other three compounds, Genz-10850, olaparib, and loprazolam, had good docking scores with PARP3. These results suggested that patients with high expression of ELAVL1, IGF1R, and PARP3 could be treated with drugs containing corresponding compounds, achieving promising results. However, we must clearly realize that this is only a theoretical design, and the real results need to be verified by further research and practice.
The goal of our study was to screen out the effective markers and related potential therapeutic agents for pSS by analyzing the experimental dataset from multiple data platforms and validating through the validation dataset combined with experiments. However, the validation experiment of our study was only based on a limited number of samples, and no relevant experimental data were available for the therapeutic agents analyzed in the study, which need further verification in the future.
In conclusion, our bioanalysis of the genes and clinical data of pSS might provide effective diagnostic biomarkers and new therapeutic ideas for treating pSS.
Supplementary Material
Acknowledgements
We sincerely thank Dr C. Q., and W.Z. for their help in the experimental verification process. Also, we are particularly grateful to International Science Editing (https://www.internationalscienceediting.com) for their language polishing.
Abbreviations
- ACR
American College of Rheumatology
- DEG
differentially expressed gene
- EULAR
European League Against Rheumatism
- GEO
Gene Expression Omnibus
- GLM
generalize linear model
- GO
gene ontology
- HE
hematoxylin-eosin
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LASSO
least absolute shrinkage and selection operator
- LSG
labial minor salivary gland
- MCP-I
monocyte chemotactic protein-1
- PCA
principal component analysis
- PDB
protein date bank
- pDC
plasmacytoid DC
- pSS
primary Sjögren’s syndrome
- SVM
support vector machine
- TF
transcription factor
- WGCNA
weighted correlation network analysis.
Contributor Information
Liqing Zhou, Department of Rheumatology and Immunology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Haojie Wang, Department of Central Laboratory, Zhengzhou University, Luoyang Central Hospital, Luoyang, China.
He Zhang, Department of Rheumatology and Immunology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Fei Wang, Department of Rheumatology and Immunology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Wenjing Wang, Department of Rheumatology and Immunology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Qiong Cao, Department of Pathology, The Third Affiliated Hospital of Henan University of Science and Technology, Luoyang, China.
Zhihao Wei, Department of Pathology, Yiluo Hospital of Luoyang, The Teaching Hospital of Henan University of Science and Technology, Luoyang, China.
Haitao Zhou, Department of Central Laboratory, Zhengzhou University, Luoyang Central Hospital, Luoyang, China.
Shiyong Xin, Department of Urology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Jianguo Zhang, Department of Urology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Xiaofei Shi, Department of Rheumatology and Immunology, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Conflict of Interests
The authors declare no conflicts of interest.
Funding
This study was supported by the Medical Science and Technology project of Henan Province in 2020 (No. LHGJ20200582) and 2019 (No. LHGJ201905670).
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Author Contributions
X.S. and J.Z. devised the research methodology. L.Z. and H.W. completed the analysis part and drafted the manuscript. H.Z., F.W., and W.W. assisted in the collection of labial gland tissue and clinical data. S.X., Q.C., and Z.W. performed the validation experiments. H.Z. analyzed the experimental date. All authors approved the final version of the manuscript to be released and agreed to be responsible for all aspects of the work.
References
- 1. Miyamoto ST, Valim V, Fisher BA.. Health-related quality of life and costs in Sjögrenʹs syndrome. Rheumatology (Oxford) 2019,. 15, key370. doi:10.1093/rheumatology/key370. [DOI] [PubMed] [Google Scholar]
- 2. Jaskólska M, Chylińska M, Masiak A, Nowicka-Sauer K, Siemiński M, Ziętkiewicz M, et al. Peripheral neuropathy and health-related quality of life in patients with primary Sjögrenʹs syndrome: a preliminary report. Rheumatol Int 2020, 40, 1267–74. doi: 10.1007/s00296-020-04543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos-Casals M, Brito-Zerón P, Solans R, Camps M-T, Casanovas A, Sopeña B, et al.; SS Study Group. Systemic involvement in primary Sjögrenʹs syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEAS-SS Registry). Rheumatology (Oxford) 2014, 53, 321–31. doi: 10.1093/rheumatology/ket349. [DOI] [PubMed] [Google Scholar]
- 4. Shen L, He J, Kramer JM, et al. Sjögren’s syndrome: animal models, etiology, pathogenesis, clinical subtypes, and diagnosis. J Immunol Res 2019, 2019, 1–3. doi: 10.1155/2019/8101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noaiseh G, Baer AN.. Toward better outcomes in Sjögren’s syndrome: the promise of a stratified medicine approach. Best Pract Res Clin Rheumatol 2020, 34, 101475. doi: 10.1016/j.berh.2019.101475. [DOI] [PubMed] [Google Scholar]
- 6. Fox RI. Standardisation of labial salivary gland biopsies in Sjogren’s syndrome: importance for the practicing rheumatologist. Ann Rheumatic Dis 2017, 76, 1159–60. doi: 10.1136/annrheumdis-2016-210851. [DOI] [PubMed] [Google Scholar]
- 7. Fisher BA, Brown RM, Bowman SJ, Barone F.. A review of salivary gland histopathology in primary Sjogren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheumatic Dis 2015, 74, 1645–50. doi: 10.1136/annrheumdis-2015-207499. [DOI] [PubMed] [Google Scholar]
- 8. Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, et al.; EULAR-Sjögren Syndrome Task Force Group. EULAR recommendations for the management of Sjögrenʹs syndrome with topical and systemic therapies. Ann Rheum Dis 2020, 79, 3–18. doi: 10.1136/annrheumdis-2019-216114. [DOI] [PubMed] [Google Scholar]
- 9. Gwenny M V, Sarah P, Hendrika B, Frans GMK.. Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat Rev Rheumatol 2021, 17, 333–48. doi: 10.1038/s41584-021-00605-2. Epub 2021 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang B, Chen SJ, Li Y, Xuan JX, Liu Y, Shi GX.. Targeted therapy for primary Sjögren’s syndrome: where are we now? BioDrugs 2021; 35(6): 593-610. doi: 10.1007/s40259-021-00505-7. Epub 2021 Nov 3. [DOI] [PubMed] [Google Scholar]
- 11. Deo RC. Machine learning in medicine. Circulation 2015, 132, 1920–30. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joe GG, Shaun MK, Lewis M, David TJ.. 2A guide to machine learning for biologists. Nat Rev Mol Cell Biol. 2022; 23 (1): 40-55. doi: 10.1038/s41580-021-00407-0. Epub 2021 Sep 13. [DOI] [PubMed] [Google Scholar]
- 13. Kee YN, Ing WK.. Big data and machine learning algorithms for health-care delivery. Lancet Oncol 2019, 20, 293. doi: 10.1016/S1470-2045(19)30294-3. [DOI] [PubMed] [Google Scholar]
- 14. Ambale-Venkatesh,Yang X,Wu CO, et al. Cardiovascular event prediction by machine learning: the multi-ethnic study of atherosclerosis. Circ Res 2017, 121, 1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee BJ, Kim JY.. Identification of type 2 diabetes risk factors using phenotypes consisting of anthropometry and triglycerides based on machine learning. IEEE J Biomed Health Inf 2016, 20, 39–46. doi: 10.1109/JBHI.2015.2396520. [DOI] [PubMed] [Google Scholar]
- 16. Kate RJ, Perez RM, Mazumdar D, Pasupathy KS, Nilakantan V.Prediction and detection models for acute kidney injury in hospitalized older adults. BMC Med Inform Decis Mak 2016, 16, 39. doi: 10.1186/s12911-016-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang MW, Chen CW, Lin WC, Ke S-W, Tsai C-F.SVM and SVM ensembles in breast cancer prediction. PLoS One 2017, 12, e0161501. doi: 10.1371/journal.pone.0161501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang KX, Xiao C, Lucas M G, Cathy W C, Greg G, Sun JM.. Machine learning applications for therapeutic tasks with genomics data. Patterns (NY) 2021. ;2(10):100328.doi: 10.1016/j.patter.2021.100328. eCollection 2021 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander E L, Carsons SE, et al.; European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American—European Consensus Group. Ann Rheum Dis 2002, 61, 554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel R, Shahane A.. The epidemiology of Sjogren’s syndrome. Clin Epidemiol 2014, 6, 247–55. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mariette X, Criswell LA.. Primary Sjögren’s syndrome. N Engl J Med 2018, 378, 931–9. doi: 10.1056/NEJMcp1702514. [DOI] [PubMed] [Google Scholar]
- 22. Masaki Y, Sugai S.. Lymphoproliferative disorders in Sjögren’s syndrome. Autoimmun Rev 2004, 3, 175–82. doi: 10.1016/S1568-9972(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 23. Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, Bosch X.. Primary Sjogren syndrome. BMJ 2012, 344, e3821. doi: 10.1136/bmj.e3821. [DOI] [PubMed] [Google Scholar]
- 24. Segal B, Bowman SJ, Fox PC, Vivino FB, Mclean L.. Primary Sjgren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 2009, 7, 46. doi: 10.1186/1477-7525-7-46---34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang P, Zhu W, Lan T, Tao Q.. Detection of salivary protein biomarkers of saliva secretion disorder in a primary Sjögren syndrome murine model. J Pharm Biomed Anal 2018, 154, 252–62. doi: 10.1016/j.jpba.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 26. Oyelakin A, Horeth E, Song E, Min S, Che M, Marzullo B, et al. Transcriptomic and network analysis of minor salivary glands of patients with primary Sjögren’s syndrome. Front Immunol 2021. Jan 8, 11, 606268. doi: 10.3389/fifimmu.2020.606268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Xu P, Wang X, Zhang Z, Zhao W, Li Z, et al. Identification of differentially expressed genes in primary Sjögren’s syndrome. J Cell Biochem 2019, 120, 17368–77. doi: 10.1002/jcb.29001. [DOI] [PubMed] [Google Scholar]
- 28. Shi H, Cao N, Pu Y, Xie L, Zheng L, Yu C.. Long non-coding RNA expression profile in minor salivary gland of primary Sjögren’s syndrome. Arthritis Res Ther 2016, 18, 109. doi: 10.1186/s13075-016-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aqrawi LA, Galtung HK, Vestad B, Øvstebø R, Thiede B, Rusthen S, et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther 2017, 19, 14. doi: 10.1186/s13075-017-1228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inamo J, Suzuki K, Takeshita M, Kassai Y, Takiguchi M, Kurisu R, et al. Identification of novel genes associated with dysregulation of B cells in patients with primary Sjögren’s syndrome. Arthritis Res Ther 2020, 22, 153. doi: 10.1186/s13075-020-02248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Z, Li F, Pan A, Xue H, Jiang S, Zhu C, et al. CCL19 elevated/expression during the disease process of primary Sjögren’s syndrome. Front Immunol 2019, 10, 795. doi: 10.3389/fimmu.2019.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adnan E, Matsumoto T, Ishizaki J, Onishi S, Suemori K, Yasukawa M, et al. Human tolerogenic dendritic cells generated with protein kinase C inhibitor are optimal for functional regulatory T cell induction—a comparative study. Clin Immunol (Orlando Fla) 2016, 173, 96–108. doi: 10.1016/j.clim.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 33. Aota K, Yamanoi T, Kani K, Nakashiro K, Ishimaru N, Azuma M.. Inverse correlation between the number of CXCR3 macrophages and the severity of inflammatory lesions in Sjögren’s syndrome salivary glands: a pilot study. J Oral Pathol Med: Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol 2018, 47, 710–8. doi: 10.1111/jop.12756. [DOI] [PubMed] [Google Scholar]
- 34. Hjelmervik T, Petersen K, Jonassen I, Jonsson R, Bolstad A.. Gene expression profifiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum 2005, 52, 1534–44. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 35. Martin-Gutierrez L, Peng J, Thompson N, Robinson G, Naja M, Peckham H, et al. Two shared immune cell signatures stratify patients with Sjögren’s syndrome and systemic lupus erythematosus with potential therapeutic implications. Arthritis Rheumatol (Hoboken NJ) 2021. doi:10.1002/art.41708. [DOI] [PubMed] [Google Scholar]
- 36. Pontarini E, Verstappen G, Grigoriadou S, Kroese F, Bootsma H, Bombardieri M.. Blocking T cell co-stimulation in primary Sjögren’s syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers. Clin Exp Rheumatol 2020, 4, 222–7. [PubMed] [Google Scholar]
- 37. Wang Y, Chen S, Chen J, Xie X, Gao S, Zhang C, et al. Germline genetic patterns underlying familial rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren’s syndrome highlight t cell-initiated autoimmunity. Ann Rheumatic Dis 2020, 79, 268–75. doi:10.1136/annrheumdis-2019-215533. [DOI] [PubMed] [Google Scholar]
- 38. Stergiou I, Papageorgiou A, Chatzis L, Tzioufas A, Voulgarelis M, Goules A.. T cell lymphoma in the setting of Sjögren’s syndrome: T cells gone bad? Report of five cases from a single centre cohort. Clin Exp Rheumatol 2020, 4, 125–9. [PubMed] [Google Scholar]
- 39. Wang H, Yang F, Luo Z.. An experimental study of the intrinsic stability of random forest variable importance measures. BMC Bioinf 2016, 17, 60. doi: 10.1186/s12859-016-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B (Methodol) 1996, 58, 267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- 41. Suykens JAK, Vandewalle J.. Least squares support vector machine classifiers. Neural Process Lett 1999, 9, 293–300. doi: 10.1023/A:1018628609742. [DOI] [Google Scholar]
- 42. Zhang B, Horvath S.. A General framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 2005, 4, Article17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 43. Li N, Li L, Wu M, Li Y, Yang J, Wu Y, et al. Integrated bioinformatics and validation reveal potential biomarkers associated with progression of primary Sjögren’s syndrome. Front Immunol 2021, 12, 697157. doi: 10.3389/fimmu.2021.697157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44..Sui YQ, Zeng WW.. MS4A4A regulates arginase 1 induction during macrophage polarization and lung inflammation in mice. Eur J Immunol 2020, 50, 1602-1605. doi: 10.1002/eji.202048585. Epub 2020 Jul 29. [DOI] [PubMed] [Google Scholar]
- 45. Zahra A, Reza M, Mohsen M, Zohre SN, et al. Bioinformatics analysis of microarray data to identify hub genes, as diagnostic biomarker of HELLP syndrome: System biology approach. J Obstet Gynaecol Res 2022. doi: 10.1111/jog.15363. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 46. . Yue XY, Deng FY, Chen J, et al. Autoantibodies against C5aR1, C3aR1, CXCR3, and CXCR4 are decreased in primary Sjogren’s syndrome. Mol Immunol 2021;131:112-120. doi: 10.1016/j.molimm.2020.12.027. Epub 2021 Jan 11. [DOI] [PubMed] [Google Scholar]
- 47. Margueron R, Reinberg D.. The polycomb complex Prc2 and its mark in life. Nature 2011, 469, 343–9. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He C, Yang Y, Chen Z, Liu S, Lyu T, Zeng L, et al. EZH2 promotes T follicular helper cell differentiation through enhancing STAT3 phosphorylation in patients with primary Sjögren’s syndrome. Front Immunol 2022, 13, 922871. doi: 10.3389/fimmu.2022.922871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Skarlis C, Marketos N, Nezos A, Papanikolaou A, Voulgarelis M, Koutsilieris M, et al. +3179G/A insulin-like growth factor-1 receptor polymorphism: a novel susceptibility contributor in anti-Ro/SSA positive patients with Sjögren’s syndrome: potential clinical and pathogenetic implications. J. Clin. Med 2021, 10, 3960. doi: 10.3390/jcm10173960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. .Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N, et al. Human antigen R contributes to hepatic stellate cell activation and liver 6bmsis. Hepatology. 2012, 56, 1870–82. doi: 10.1002/hep.25828. Epub 2012 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Juliana IK, Johanna KS, Gunnel N.. Epigenetic alterations in primary Sjögren’s syndrome-an overview. Clin Immunol 2018, 196, 12–20. doi:10.1016/j.clim.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 52. Goules AV, Tzioufas AG.. Primary Sjögren’s syndrome: clinical phenotypes, outcome and the development of biomarkers. Immunol Res 2017, 65, 331–44. doi: 10.1007/s12026-016-8844-4. [DOI] [PubMed] [Google Scholar]
- 53. Rizzo C, Barbera L L, Pizzo M L, et al. Invariant NKT cells and rheumatic disease: focus on primary Sjögren syndrome. Int J Mol Sci 2019, 20, 5435. doi:10.3390/ijms20215435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Albin B, Elina RA, Juliana I-K, et al. Protein and DNA methylation-based scores as surrogate markers for interferon system activation in patients with primary Sjögren’s syndrome. RMD Open 2020, 6, e000995. doi:10.1136/rmdopen-2019-000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kroese FG, Abbdulahad WH, Haacke E, et al. B-cell hyperactivity in primary Sjögren’s syndrome. Expert Rev Clin Immunol 2014, 10, 483–99. doi:10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 56. Ibrahem HM. B cell dysregulation in primary Sjögren’s syn- drome: a review. Jpn Dent Sci Rev 2019, 55, 139–44. doi: 10.1016/j.jdsr.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Witas R, Gupta S, Nguyen CQ.. Contributions of major cell populations to Sjögren’s syndrome. J Clin Med 2020, 9, 3057. doi: 10.3390/jcm9093057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin X, Rui K, Deng J, et al. Th17 cells play a critical role in the development of experimental Sjögren’s syndrome. Ann Rheum Dis 2015, 74, 1302–10. [DOI] [PubMed] [Google Scholar]
- 59. Alunno A, Bistoni O, Caterbi S, Bartoloni E, Cafaro G, Gerli R.Serum interleukin-17 in primary Sjögren’s syndrome: association with disease duration and parotid gland swelling. Clin Exp Rheumatol 2015, 33, 129–34. [PubMed] [Google Scholar]
- 60. US National Library of Medicine. Safety and efficacy study of subcutaneous belimumab and intravenous rituximab co-administration in Subjects with primary Sjögren’s syndrome[EB/OL]. 2020. https://clinicaltrials.gov/ct2/show/NCT02631538 (1 April 2022, date last accessed).
- 61. Dörner T, Posch MG, Li Y, Petricoul O, Cabanski M, Milojevic JM, et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis 2019, 78, 641–7. doi: 10.1136/annrheumdis-2018-214720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.