Abstract
Background
Long COVID symptoms occur for a proportion of acute COVID-19 survivors, with reduced risk among the vaccinated and for Omicron compared with Delta variant infections. The health loss attributed to pre-Omicron long COVID has previously been estimated using only a few major symptoms.
Methods
The years lived with disability (YLDs) due to long COVID in Australia during the 2021–22 Omicron BA.1/BA.2 wave were calculated using inputs from previously published case-control, cross-sectional or cohort studies examining the prevalence and duration of individual long COVID symptoms. This estimated health loss was compared with acute SARS-CoV-2 infection YLDs and years of life lost (YLLs) from SARS-CoV-2. The sum of these three components equals COVID-19 disability-adjusted life years (DALYs); this was compared with DALYs from other diseases.
Results
A total of 5200 [95% uncertainty interval (UI) 2200–8300] YLDs were attributable to long COVID and 1800 (95% UI 1100-2600) to acute SARS-CoV-2 infection, suggesting long COVID caused 74% of the overall YLDs from SARS-CoV-2 infections in the BA.1/BA.2 wave. Total DALYs attributable to SARS-CoV-2 were 50 900 (95% UI 21 000-80 900), 2.4% of expected DALYs for all diseases in the same period.
Conclusion
This study provides a comprehensive approach to estimating the morbidity due to long COVID. Improved data on long COVID symptoms will improve the accuracy of these estimates. As data accumulate on SARS-CoV-2 infection sequelae (e.g. increased cardiovascular disease rates), total health loss is likely to be higher than estimated in this study. Nevertheless, this study demonstrates that long COVID requires consideration in pandemic policy planning, given it is responsible for the majority of direct SARS-CoV-2 morbidity, including during an Omicron wave in a highly vaccinated population.
Keywords: COVID-19, long COVID, epidemiology, burden of disease
Key Messages.
Our study is the first to comprehensively estimate long COVID morbidity using its individual symptoms, during Australia’s 2021–22 Omicron wave.
We show that long COVID contributed to almost three-quarters of the non-fatal health loss resulting from Omicron infections in this period.
Long COVID contributes to a substantial proportion of direct COVID-19 morbidity, even in a highly vaccinated population during an Omicron wave. It should therefore be more explicitly considered in future pandemic policy making.
Our method of estimating long COVID morbidity has explicable differences compared with existing long COVID burden of disease approaches and may provide a more accurate estimate of the morbidity attributable to long COVID.
Introduction
A post-acute phase of SARS-CoV-2 infection, commonly termed long COVID, occurs among some individuals following acute infection. Long COVID describes the persistence and/or emergence of a heterogeneous group of symptoms at least 12 weeks after acute infection.1 There is no current consensus on the symptom profile that specifically characterizes long COVID, with a wide range of symptoms being reported across multiple organ systems, including cardiopulmonary, neurological and musculoskeletal systems.2 Some studies report symptom clustering within individuals, but the frequency and significance of this remains unclear and individuals most commonly report experiencing one or two symptoms.3,4 The aetiology of long COVID is proposed to be related to continued immune activation and persistence of the virus in the various organ systems infected during the acute period.5 Numerous risk factors have been identified, including a propensity towards an autoimmune response, female sex, comorbidities such as type 2 diabetes mellitus, and a more severe acute infection.4,6 Importantly, COVID-19 vaccination has been found to reduce the risk of long COVID.7,8
Given ongoing high rates of SARS-CoV-2 transmission globally, it is important to quantify the full health impact of SARS-CoV-2 infection including its longer-term consequences. Recent burden of disease studies have quantified long COVID by treating it as a single outcome, using the Global Burden of Disease (GBD) study health state of ‘post-acute consequences’ from other respiratory illnesses, or chronic fatigue syndrome, to approximate long COVID severity.9–11 Whereas these health states have some overlap with documented long COVID symptoms, they do not acknowledge the full breadth of symptoms linked to long COVID,or the heterogeneity of symptoms reported between individuals.2,9
A lack of high-quality evidence regarding the prevalence and duration of symptoms hampers quantification of long COVID burden. Many studies of long COVID lack an appropriate control group or are subject to other methodological issues. Of particular concern is selection bias from self-selection into studies by those experiencing ongoing symptoms, and loss-to-follow up of those no longer symptomatic. These biases likely inflate estimates of long COVID occurrence. Conversely, studies with insufficient follow-up time may not capture the full burden of long COVID symptoms, which are often relapsing and remitting in nature.2 In addition to these design issues, the majority of published long COVID research has been conducted in cohorts of unvaccinated, pre-Omicron variant infected cases. To accurately quantify the impact of long COVID in highly vaccinated populations during Omicron waves, differences in the risk of long COVID by vaccination and variant need incorporating.
This paper addressed the following research questions: first, what is the extent of the morbidity attributable to long COVID resulting from Omicron variant SARS-CoV-2 infections, quantified ‘bottom-up’ from occurrence rates of each symptom and its severity and duration? Second, what proportion of total years lived with disability (YLDs) and disability-adjusted life years (DALYs) accumulated during the 2021–22 Omicron wave in Australia were due to long COVID, and how does this health loss compare with other major causes of health loss in Australia?
Methods
Morbidity calculations
In this study, the impact of long COVID in the Australian population is measured with a burden of disease approach. Disability-adjusted life years are a standard metric of disease burden and encompass both morbidity (as YLDs), and mortality (as years of life lost [YLL]). YLDs are the focus of this paper, as long COVID is treated as a collection of non-fatal symptoms; however overall DALYs combining both acute and long COVID are also measured.
Long COVID morbidity was calculated as that expected per symptomatic SARS-CoV-2 infected person: where i and j index each possible symptom, and is the prevalence of symptom i minus the sum of the joint occurrence of symptom i with each other symptom (assuming symptom occurrence is independent). The prevalence of each symptom was calculated as a risk difference in symptom frequency between SARS-CoV-2 survivors and SARS-CoV-2-negative controls, extracted from previous research (see Supplementary Tables S1 and S2, available as Supplementary data at IJE online).3,12–14 Only symptoms found to occur more frequently in COVID-19 cases compared with COVID-negative controls, in the controlled literature, were included (presented in Table 1). The severity of each symptom was quantified as a disability weight (DW) taken from the GBD study15 for the matching health state, and best matches otherwise (e.g. DWs of other sensory conditions have been used as proxies for dysosmia and dysgeusia). was calculated as16:
Table 1.
Health states and estimated prevalence of each long COVID symptom among unvaccinated, Omicron-infected cases
| Sequelae | DW (95% CI) | Health state/justification | Prevalence among Omicron cases (unvaccinated)a |
Duration (months)b |
||
|---|---|---|---|---|---|---|
| Community | Hospitalizedc | Community | Hospitalizedc | |||
| Adults | ||||||
| Dysosmia | 0.01 (0.004-0.020) | Health state: hearing loss, mild, and presbyopia. Assumed to be equivalent to mild impairment of other senses | 2.6% | 7.0% | 4 months | 9 months |
| Dysgeusia | 0.01 (0.004-0.020) | Health state: hearing loss, mild, and presbyopia. Assumed to be equivalent to mild impairment of other senses | 2.1% | 6.9% | 4 months | 9 months |
| Fatigue | 0.051 (0.036-0.062)d | Health state: infectious disease, post-acute consequences (adjusted down for depression and pain). No reasonable estimate exists for fatigue in GBD study 2019; however, reasonable estimates exist for muscle/joint pain and depression. Therefore, DW applied in the present study for depression and muscle/joint pain were subtracted from ‘post-acute consequences’ DW [DW = 0.219 (95% CI 0.148-0.308)], which is characterized by weakness/tiredness, depression and body pain | 2.0% | 13.1% | 4 months | 9 months |
| Dyspnoea | 0.019 (0.011-0.033) | Health state: chronic obstructive pulmonary disease (COPD) and other chronic respiratory problems, mild | 1.1% | 10.3% | 4 months | 9 months |
| Chest pain | 0.011 (0.005-0.021) | Health state: abdominopelvic problem, mild. Assumed to be equivalent to gastroesophageal reflux disease (GERD) | 0.5% | 4.1% | 4 months | 9 months |
| Muscle weakness | 0.004 (0.001-0.008) | Health state: anaemia, mild. Mild anaemia characterized by feeling slightly weak/tired | 1.0% | 11.3% | 4 months | 9 months |
| Dizziness | 0.032 (0.021-0.046)d | Health state: vertigo (adjusted to estimate ‘mild’ vertigo health state). Assumed that dizziness is mild form of vertigo—as there is no ‘mild’ vertigo DW, ‘vertigo’ DW ([W = 0.113([95% CI: 0.074-0.158)] was multiplied by 0.29 (average ratio of mild/moderate DWs across all outcomes with mild, moderate and severe ratings in the GBD study 2019) | 0.6% | 4.8% | 4 months | 9 months |
| Muscle/joint pain | 0.023 (0.013-0.037) | Health state: musculoskeletal problems, lower limbs, mild | 0.8% | 6.3% | 4 months | 9 months |
| Headache | 0.037 (0.022-0.057) | Health state: headache, tension type | 0.8% | 4.3% | 4 months | 9 months |
| Numb/tingling limbs | 0.023 (0.013-0.037) | Health state: musculoskeletal problems, lower limbs, mild | 0.8% | 7.0% | 4 months | 9 months |
| Concentration difficultye | 0.069 (0.046-0.099) | Health state: dementia, mild | 1.9% | 10.9% | 4 months | 9 months |
| Memory impairmente | 0.069 (0.046-0.099) | Health state: dementia, mild | 1.4% | 14.3% | 4 months | 9 months |
| Insomnia | 0.03 (0.018-0.046) | Health state: anxiety disorder, mild. Anxiety disorder is characterized by difficulty sleeping and concentrating | 1.3% | 19.4% | 3 months | 3 months |
| Anxiety | 0.03 (0.018-0.046) | Health state: anxiety disorder, mild | N/A | 11.8% | N/A | 3 months |
| Depression | 0.145 (0.099-0.209) | Health state: major depressive disorder, mild | N/A | 23.2% | N/A | 3 months |
| Childrenf | ||||||
| Dysosmia | 0.01 (0.004-0.020) | Health state: hearing loss, mild and presbyopia. Assumed to be equivalent to mild impairment of other senses | 2.0% | 3 months | ||
| Headache | 0.037 (0.022-0.057) | Health state: headache, tension type | 1.3% | 3 months | ||
| Eye soreness | 0.011 (0.005-0.02) | Health state: presbyopia. No health states eye pain exists in GBD study 2019, therefore estimated from presbyopia (characterized by mild near vision loss) | 0.5% | 3 months | ||
| Sore throat | 0.006 (0.002-0.012) | Health state: infectious disease, acute episode, mild. Assumed to be equivalent to mild upper respiratory infection | 0.5% | 3 months | ||
| Cognitive difficulty | 0.045 (0.028-0.066) | Health state: attention deficit hyperactivity disorder (ADHD). ADHD is characterized by difficulty with concentration and memory | 0.8% | 3 months | ||
Health states applied were obtained from the 2019 GBD study.15
CI, confidence interval; DW, disability weight; GBD, Global Burden of Disease; NA, not available.
Multiply by 0.55 (on odds scale) to obtain prevalence among vaccinated.
Duration applied excludes acute morbidity period [1 week for mild (non-hospitalized) adult cases and children, or 2.6 weeks for hospitalized adult cases—see Supplementary Table S4 for acute COVID-19 morbidity details].
Symptom prevalence among initially hospitalized COVID-19 cases does not differ between pre-Omicron and Omicron variants, as outlined in the Methods section.
DW applied is not directly taken from a DW in the 2019 GBD, instead estimated by adjusting existing DWs.
Community and hospitalized subgroups for cognitive symptoms are achieved through weighting of estimates from mild vs severe subgroups as measured by Caspersen et al.6
Children are not separated by severity of acute infection.
It was assumed that only initially symptomatic patients (during the acute illness period) are at risk of long COVID—the risk of long COVID among asymptomatic patients has been found to be low or nil compared with symptomatic patients.17–19 We also assumed that calculations including all three-way (or higher) combinations of symptoms would make negligible difference to morbidity estimates; given that most symptom prevalence estimates are less than 10% (Table 1), and joint prevalence calculations are multiplicative, the joint prevalence of three or more symptoms using this method would be very low. Additionally, it is uncommon to experience more than two symptoms.12
The above ‘base case’ prevalence calculations were for unvaccinated individuals infected with a pre-Omicron variant of SARS-CoV-2, and were split into three subgroups: adult community cases, adult hospitalized cases and children (0–17 years, any acute disease severity).3,12–14 Long COVID symptom prevalence among previously hospitalized adults is approximately twice that among community cases.12 Symptom prevalence is also lower among children compared with adults.14 Duration of each symptom (from 1 week post-infection for mild/moderate cases, and approximately 2.5 weeks post-infection for those hospitalized) was applied based on recent findings by Wulf Hansen et al., who reported a median duration of symptoms for community infections of 4 months and of 8.9 months for previously hospitalized cases.20 Exceptions to this have been applied to psychological symptoms, for which a shorter duration was used, and similarly for children (see Table 1).13,21
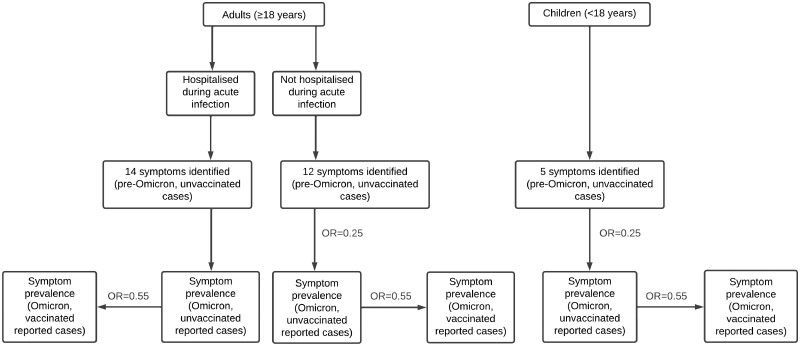
These base case prevalences were then multiplied by an odds ratio (OR) of 0.55 to approximate symptom prevalence among vaccinated cases, based on findings from two studies that found odds of symptoms following the acute infection reduced by 49% and 41%, respectively, for those who had at least two COVID-19 vaccines compared with one/no vaccines.7,8 Vaccination post-infection has been found to have a limited effect on long COVID occurrence—therefore, only vaccination prior to infection was considered.22,23 Next, prevalence estimates were further multiplied by an OR of 0.25 based on an estimate of the reduction in prevalence of any symptoms at least 4 weeks post-infection for Omicron variant compared with Delta variant infections in a vaccinated cohort in the UK.24 It was assumed that this association remains beyond 12 weeks post-infection. The exception to the latter multiplier was adults hospitalized with an Omicron infection (the previously mentioned study was conducted using a cohort of primarily community cases); we assumed that once a case is severe enough to be hospitalized, there is no difference in resulting long COVID compared with pre-Omicron variants. A flow diagram depicting the above method to cross-walk prevalence data from pre-Omicron, unvaccinated cases to Omicron and vaccinated cases is presented in Figure 1.
Figure 1.
Flow diagram—prevalence cross-walk method. OR = 0.25 indicates reduced odds of long COVID among Omicron-infected cases compared with Delta-infections—applied to not hospitalized (i.e. community) adult cases and children. OR = 0.55 indicates reduced odds of long COVD among vaccinated (at least two COVID-19 vaccines) compared with unvaccinated (less than two vaccines) cases—applied to all vaccinated subgroups
Uncertainty was included using a standard deviation (SD) of +/- 20%, applied to base case long COVID morbidity estimates. This method approximated the 95% uncertainty in base case estimates achieved using a more comprehensive approach, where variance in duration, severity and prevalence were each calculated to estimate total variance. For morbidity calculations for vaccinated and Omicron-infected populations, we used an SD of +/- 30% of the expected value to reflect additional uncertainty.
The DW, estimated prevalence and average duration of each identified long COVID symptom among Omicron-infected, unvaccinated cases, along with the uncertainty applied, are presented in Table 1. The prevalence among vaccinated cases is that shown in Table 1 multiplied by 0.55. The estimated prevalence and duration of each symptom among base cases (i.e. unvaccinated, pre-Omicron infections) is presented in Supplementary Table S3, available as Supplementary data at IJE online.
Application to the Omicron wave and health burden comparison
Long COVID YLDs were calculated as the expected long COVID morbidity per symptomatic case multiplied by the total number of symptomatic infections (treated as equivalent to notified cases) during the 4 months of the Omicron BA.1/BA.2 wave in Australia, defined here as 10 December 2021 to 9 April 2022.
The YLDs from acute SARS-CoV-2 infection were estimated based on a previously published method by Blakely et al.,25 updated here to be specific to Omicron-variant infections. Acute COVID-19 morbidity is subdivided into community cases and hospitalized cases that are either ward only or include an intensive care unit (ICU) admission—approximately 8% of cases in hospital have been estimated as requiring ICU admission during the first 4 months of the Omicron wave.26 Symptom duration estimates for hospitalized patients were based on findings from a New South Wales (NSW) study by Tobin et al.,27 which estimated hospital stay duration during December (at the start of the Omicron wave). These duration estimates were weighted across the three age categories presented by Tobin et al.27 (0–39-year-olds, 40–69-year-olds and 70+-year-olds) based on the proportion hospitalized in each age group. A percentage split of 72% mild and 28% moderate acute illness for non-hospitalized cases was based on findings by Menni et al.,28 which indicated that the odds of moderate severity illness were reduced by approximately 44% for Omicron compared with Delta infections. Previous estimates used by Blakely et al.25 for pre-Omicron variant infections used a 50/50 split for the proportions of moderate vs mild community cases. Acute COVID-19 morbidity inputs are presented in Supplementary Table S4, available as Supplementary data at IJE online.
YLLs due to SARS-CoV-2 deaths were estimated using standard burden of disease methods,29 multiplying reported deaths due to COVID-19 by the average remaining life expectancy of those who died [with reference life expectancy by sex and age obtained from the 2019 GBD, retrieved from the Institute for Health Metrics and Evaluation (IHME) results tool].30,31
YLL and YLD calculations were by strata of age (<18-year-olds vs ≥18-year-olds), hospitalization status (as described above) and vaccination—national-level data were not available at the time of writing for vaccination status of COVID-19 cases, therefore data from NSW were applied.32
The total DALYs from SARS-CoV-2 infection in these 4 months were calculated as the sum of the above three components. For the purposes of this paper, we assigned the long COVID YLDs to the SARS-CoV-2 infections occurring in the 4-month window.
For comparison, we took the YLDs and DALYs in Australia for other diseases from GBD 2019 [adjusted to reflect the 2021 population (mid-year)],31,33 dividing by three to make them equivalent to the 4-month duration of the BA.1/BA.2 wave.
Sensitivity analyses
One-way sensitivity analyses were conducted on base case morbidity estimates, separately varying the prevalence, severity and duration components by their 95% uncertainty intervals (UI). Additionally, each base case estimate was varied for the adult cohorts by the symptoms extracted from different source studies. This included varying the prevalence, duration and severity components together by their respective 95% UIs, separately for: physical symptoms obtained from Sørensen et al12; psychological symptoms from Caspersen et al.3; and cognitive symptoms from Magnúsdóttir et al.13
An extreme scenario analysis was also conducted in which the OR for Omicron compared with pre-Omicron infections (OR = 0.25) was applied to the hospitalized patient group, as applied for community cases only in the main analysis.
Results
Some 4.87 million COVID-19 cases were notified in Australia during the first 4 months of the Omicron wave, with approximately 35 500 hospitalizations and 3463 deaths.26,30,34,35
An estimated 61% of notified cases were vaccinated at the time of infection (noting that approximately 90% of 16+-year-olds in the population were fully vaccinated by 10 December 2021).32,36 Reported cases and deaths are shown in Supplementary Tables S5 and S6, available as Supplementary data at IJE online.
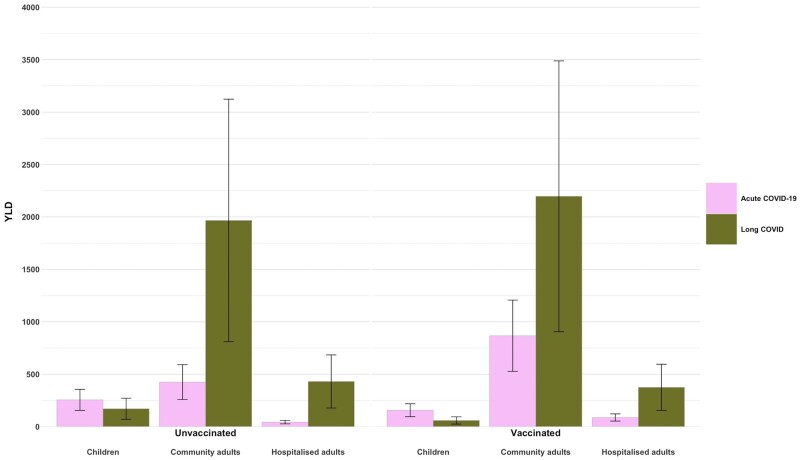
The overall morbidity (i.e. YLDs) resulting from infections in this period, including acute and long COVID, is shown in Figure 2 (with YLDs by subgroup also presented numerically in Table 2). Long COVID accounted for approximately 74% of the overall non-fatal COVID-19 health loss among notified cases during the first 4 months of the Australian Omicron wave, at 5200 YLDs [95% uncertainty interval (UI) 2200-8300]. The majority of long COVID YLDs come from community cases, with the highest number in the vaccinated community adult cases sub-strata (2200 YLDs, 95% UI 900-3500) given that this group represents the greatest proportion (51%) of estimated notified cases (Supplementary Table S5, available as Supplementary data at IJE online). Per-person COVID morbidity estimates, which show the non-fatal health loss due to long COVID per notified COVID-19 case, are shown for each subgroup in Supplementary Table S7, available as Supplementary data at IJE online.
Figure 2.
COVID-19 YLDs resulting directly from Omicron cases during the first 4 months of the Omicron wave, 10 December 2021 to 9 April 2022; 95% uncertainty intervals are shown for long COVID YLD estimates, measured with +/-30% standard deviation. Total morbidity is estimated at 7000 YLDs. YLDs, years lived with disability
Table 2.
COVID-19 YLDs resulting directly from Omicron cases during the first 4 months of the Omicron wave, 10 December 2021 to 9 April 2022
| Population group | YLD (95% UIa) |
||
|---|---|---|---|
| Acute COVID-19 | Long COVID | Total | |
| Hospitalized adults (unvaccinated)b | 43 (26-60) | 431 (178-684) | 498 (205-791) |
| Hospitalized adults (vaccinated)b | 89 (54-124) | 375 (154-595) | 488 (201-774) |
| Non-hospitalized adults (unvaccinated) | 425 (258-591) | 1967 (810-3123) | 2391 (985-3798) |
| Non-hospitalized adults (vaccinated) | 867 (527-1207) | 2197 (905-3488) | 3064 (1262-4865) |
| Children (unvaccinated) | 255 (155-355) | 171 (70-271) | 426 (176-677) |
| Children (vaccinated) | 157 (95-218) | 59 (24-94) | 216 (89-343) |
| Total | 1835 (1116-2555) | 5247 (2162-8333) | 7035 (2898-11 171) |
Numbers presented in the text have been rounded from values in this table.
UI, uncertainty interval; YLD, years lived with disability.
95% UI estimated using +/-30% standard deviation for long COVID and total YLDs, +/-20% standard deviation for acute COVID-19 YLDs.
For acute COVID-19 morbidity, hospitalized group included intensive care unit (ICU) and ward-only calculations.
The overall DALYs resulting from reported COVID-19 infections in the 4-month period was estimated at 50 900 (95% UI 21 000-80 900), of which long COVID contributed 10.3% (compared with the acute COVID-19 morbidity contribution of 3.6%). The remaining DALYs resulted from acute COVID-19 mortality.
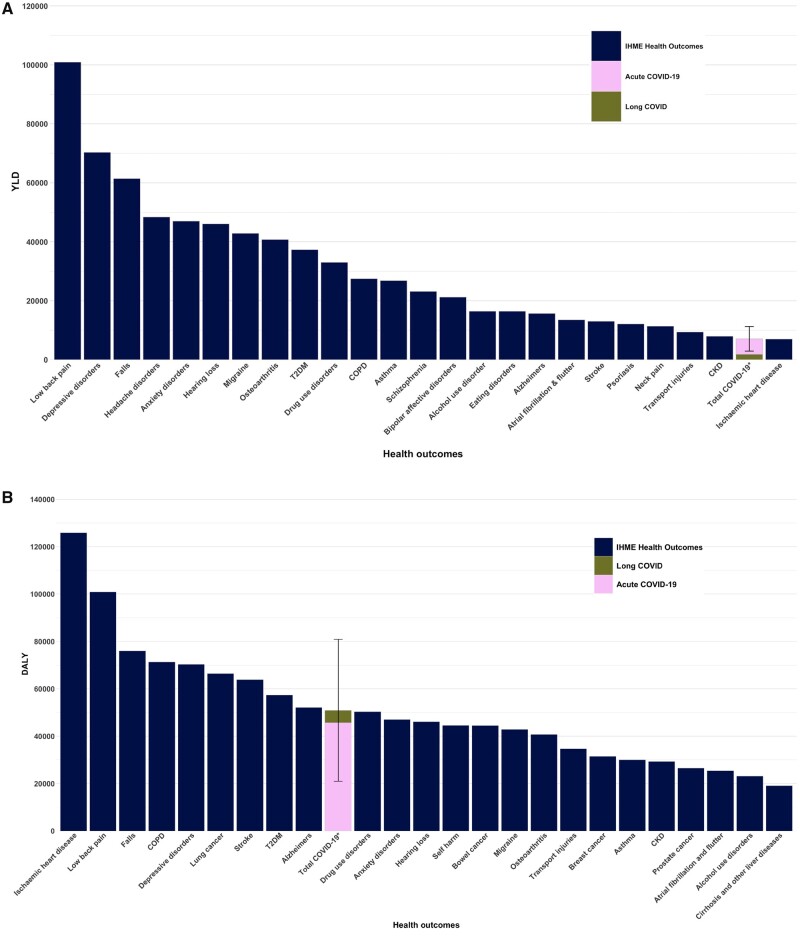
The leading 25 causes of morbidity in Australia ranked by YLDs are presented in Figure 3A, including the estimated YLDs resulting from reported COVID-19 cases during the first 4 months of the Omicron wave. Total COVID-19 morbidity is ranked 24th, comparable to the non-fatal health loss resulting from chronic kidney disease.
Figure 3.
Burden of disease comparison during the first 4 months of the Omicron wave (10 December 2021 to 9 April 2022), Australia. Panel A. Comparison of the YLDs due to COVID-19, separated as long COVID and acute COVID-19, with other outcomes. Total COVID-19 YLDs = 7000 (95% uncertainty interval 2900-11 200). Panel B. Comparison of the DALYs due to COVID-19, separated as long COVID and acute COVID-19, with other outcomes. Total COVID-19 DALYs = 50 900 (95% uncertainty interval 21 000-80 900). Note that COVID-19 YLDs and DALYs include the future morbidity resulting from long COVID for these cases; 95% uncertainty intervals are shown for COVID-19 DALY and YLD estimates (+/-30% standard deviation). YLDs and DALYs for other outcomes are estimated from the 2019 GBD study, updated to the population size as of June 2021, and subsequently divided by three to estimate health loss over a 4-month period.15 COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; T2DM, type 2 diabetes mellitus; YLD, years lived with disability; GBD, Global Burden of Disease; DALYs, disability-adjusted life-years. *Total COVID-19 YLDS/DALYS include acute COVID-19 and long COVID
The leading 25 causes of health loss in Australia ranked by DALYs are presented in Figure 3B, including the estimated DALYs resulting from the first 4 months of the COVID-19 Omicron wave. The DALYs resulting from COVID-19 cases are estimated to be 2.4% of all expected DALY loss in the 4 months, and rank 10th among conditions (between Alzheimer’s and other dementias, and drug use disorders). The 95% UI indicates that the rank could in fact be as high as third (between low back pain and falls) or as low as 24th (below alcohol use disorders).
Discussion
We estimated that long COVID contributed to approximately 74% of the non-fatal health loss (i.e. YLDs) resulting from reported COVID-19 infections in the first 4 months of the Omicron BA.1/BA.2 wave in Australia. Given that morbidity was calculated using notified cases, and some unreported cases will have been symptomatic, this may be an underestimate. The total YLDs for acute and long COVID combined were estimated as comparable to YLDs caused by chronic kidney disease and ischaemic heart disease (Figure 3A). The overall COVID-19 disease burden, YLDs plus years of life lost, was estimated at 50 900 DALYs (95% UI 21 000-80 900) over the initial 4 months of the Omicron wave, the 10th highest cause of DALYs in this 4-month period in Australia and approximately 2.4% of all health loss (Figure 3B).
Our per-person morbidity estimates for long COVID show that for a vaccinated adult case who did not require hospitalization during their acute infection (i.e. the majority of cases in the first 4 months of the Omicron wave in Australia), 0.09% of a healthy year of life is lost due to long COVID (equivalent to one-third of a day of healthy life lost). It is important to note that the morbidity loss for someone actually with long COVID would be much greater than this, as this estimate (and those shown in Supplementary Table S7,) gives the health loss for any surviving notified COVID-19 case.
A strength of our study is allowing for the lower incidence of long COVID among vaccinated people, and with Omicron versus pre-Omicron variants; to our knowledge, previous studies have not made both allowances. Our approach also quantifies long COVID morbidity across multiple patient subgroups and includes a complete symptom profile rather than treating long COVID as a single outcome.
A recent study by Wulf Hanson et al. and Institute for Health Metrics and Evaluation (IHME) collaborators estimated the proportion of SARS-CoV-2 infections developing long COVID, separated into three symptom groups, for SARS-CoV-2 infections in 2020 and 2021.20 Their method involved measuring the prevalence of each symptom individually as well as the prevalence of each symptom pair and all three symptoms together. Using this method, then applying odds ratios for long COVID for Omicron compared with pre-Omicron, and vaccination status, our morbidity estimate observing the equivalent symptom groups is within 20% of the resulting estimate from the IHME method (as shown in Supplementary Table S8, available as Supplementary data at IJE online).20 This indicates that our assumption of independence is valid for burden estimation of long COVID.
Our approach does not provide an overall estimate of long COVID occurrence rate, given that we define long COVID as a heterogeneous group of symptoms, rather than a single outcome. The utility in our approach is in the use of severity and duration estimates specific to each symptom, to more accurately estimate the morbidity attributed to long COVID in a population. The use of single health states, such as ‘post-acute consequences’ (as currently recommended by the European Burden of Disease Network)37 assumes that the average long COVID sufferer has all these symptoms included within the applied health state. It also ignores a large proportion of symptoms that have been identified among long COVID sufferers. In Supplementary Table S9 (available as Supplementary data at IJE online), we compare our morbidity estimate with those from other COVID-19 burden of disease studies that have incorporated long COVID. Long COVID parameters published by the Australian Institute of Health and Welfare (AIHW),10 and adjusted by us to reflect vaccinated Omicron infections, result in an estimate approximately two-thirds of our finding, likely due to the reduced duration applied (91 days) and the use of a single health state (post-acute consequences; DW = 0.219). Three other non-Australian publications, which applied an even shorter duration of 28 days, with a single long COVID health state, produced estimates approximately one-third of our morbidity estimate.38–40 These differences highlight the strength of our paper, which accounts for a wider range of long COVID symptoms and a longer average duration of symptoms reflecting that documented in the existing literature. Our approach also accounts for the difference in risk of long COVID symptoms by acute COVID-19 severity and age, which these publications have not considered. We postulate that our ‘bottom up’ approach provides a more accurate measure of morbidity resulting from long COVID.
Our study has limitations. Long COVID is an area of rapidly emerging and evolving research, currently characterized by limited high-quality literature. As such, there is a high level of uncertainty in our findings (e.g. our ‘cross-walk’ of pre-Omicron unvaccinated data to Omicron vaccinated cases). Additionally, whereas the studies used for our prevalence estimates were included based on an assessment of bias and methodological quality, prevalence may still be overestimated, due to the likely direction of bias to over-estimation in long COVID studies. A recently published prospective cohort study, that controlled for symptoms present prior to COVID-19 diagnosis, found a slightly reduced occurrence of symptoms among COVID-positive cases compared with controls than did our base case analysis.41 Although these estimates were not able to be used in this paper, given that the study did not stratify data by age and severity of acute infection, as more data become available on the prevalence of symptoms among strata Omicron-infected cases, our estimates can be updated. Further stratification of patient groups may also help to improve the accuracy of our current framework, for example by increasing the number of age strata and including sex. There is an increased risk of long COVID among older age groups and females42; however, data on symptom prevalence were not available allow for this in our analysis in addition to the variables we included.
Given the lack of data on the occurrence of long COVID symptoms among previously hospitalized Omicron cases, and the known association between acute COVID-19 severity and long COVID risk,12 we assumed no difference in long COVID morbidity between the hospitalized base case group (i.e. pre-Omicron infections) and Omicron hospitalized cases. In a sensitivity analysis, we tested an ‘extreme’ for this subgroup, applying the odds ratio (OR = 0.25) used for cross-walking of community pre-Omicron to community Omicron cases, to the hospitalized group.24 Whereas this reduced the morbidity for the hospitalized patient group by an average of 81.5% compared with the main analysis, overall long COVID morbidity was only reduced by 12% compared with the main analysis, to 4600 YLDs (95% UI 1900-7200) (see Supplementary Table S10, available as Supplementary data at IJE online). This estimate largely overlaps with that from our primary analysis; however, it will still be important to conduct further research into long COVID among hospitalized Omicron cases in the future, including consideration of those requiring ICU treatment vs ward treatment only (which was not possible in this study, given the lack of data available).
Additional one-way sensitivity analyses, presented as Tornado plots, showed that the majority of the overall uncertainty in long COVID morbidity in our study was due to uncertainty in the morbidity severity estimates for symptoms (i.e. disability weights), as opposed to uncertainty in symptom frequency and duration (Supplementary Figure S1, available as Supplementary data at IJE online). This is of concern, given that the majority of DWs applied were made by estimation from other health states,15 and points to the need for research to better quantify the severity of each long COVID symptom.
It is important to note that many people dying of COVID-19 have comorbidities, so their true YLLs will be less than that measured using standard DALY methods that assume those dying of COVID-19 have the mortality rate specified in the reference life table. It is also important to note that our study focuses on health loss from acute and long COVID; SARS-CoV-2 infection has also been associated with persistent organ damage and an increased risk of chronic conditions including type 2 diabetes mellitus and cardiovascular disease, particularly among those who had a severe acute infection.43–47 Longer-term research will be required in the future to determine the extent to which SARS-CoV-2 infection causes these sequelae, which will lead to the total burden of SARS-CoV-2 being (perhaps considerably) greater than that quantified in this study.
Conclusion
Our approach for estimating the morbidity attributable to long COVID likely provides a more accurate measure of long COVID burden compared with that of existing burden of disease studies. Whilst the prevalence of long COVID symptoms is less now among highly vaccinated populations with Omicron than it was for pre-Omicron variants among unvaccinated populations, long COVID still contributes substantial health loss when summed over all infections. Our findings therefore highlight the need to factor in long COVID-related health loss when making policy decisions.
Ethics approval
This study did not require ethical approval.
Supplementary Material
Acknowledgements
Expert knowledge was kindly provided by Professor John D. Potter (Massey University, Wellington, Fred Hutchinson Cancer Research Centre, Seattle, Washington and University of Washington, Seattle, Washington).
Contributor Information
Samantha Howe, Population Interventions Unit, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Carlton, VIC, Australia.
Joshua Szanyi, Population Interventions Unit, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Carlton, VIC, Australia.
Tony Blakely, Population Interventions Unit, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Carlton, VIC, Australia.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
Concept: S.H., J.S., T.B. Literature review: S.H. Formal analysis: S.H., T.B. Writing original draft: S.H., J.S., T.B. Writing review and editing: S.H., J.S., T.B.
Funding
None.
Conflict of interest
The Population Interventions Unit is expected to receive funding from Moderna Inc. to conduct research on COVID-19 vaccine effectiveness in Victoria, Australia. No other conflicts of interest are declared.
References
- 1. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis HE, Assaf GS, McCorkell L. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caspersen IH, Magnus P, Trogstad L.. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur J Epidemiol 2022;37:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sudre CH, Murray B, Varsavsky T. et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akbarialiabad H, Taghrir MH, Abdollahi A. et al. Long COVID, a comprehensive systematic scoping review. Infection 2021;49:1163–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su Y, Yuan D, Chen DG. et al. ; ISB-Swedish COVID-19 Biobanking Unit. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022;185:881–95.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonelli M, Penfold RS, Merino J. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022;22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Office for National Statistics (ONS). Self-Reported Long COVID After Two Doses of a Coronavirus (COVID-19) Vaccine in the UK: 26 January 2022. ONS. 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk/26january2022 (26 January 2022, date last accessed).
- 9. Smith MP. Estimating total morbidity burden of COVID-19: relative importance of death and disability. J Clin Epidemiol 2022;142:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Australian Institute of Health and Welfare (AIHW). The First Year of COVID-19 in Australia: Direct and Indirect Health Effects. Canberra: AIHW, 2021. [Google Scholar]
- 11. Angeles MR, Wanni Arachchige Dona S, Nguyen HD, Le LK, Hensher M.. Modelling the potential acute and post-acute burden of COVID-19 under the Australian border re-opening plan. BMC Public Health 2022;22:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sørensen AIV, Spiliopoulos L, Bager P. et al. Post-acute symptoms, new onset diagnoses and health problems 6 to 12 months after SARS-CoV-2 infection: a nationwide questionnaire study in the adult Danish population. Nat Commun 2022;13:4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magnúsdóttir I, Lovik A, Unnarsdóttir AB. et al. ; COVIDMENT Collaboration. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health 2022;7:E406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Behnood SA, Shafran R, Bennett SD. et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect 2022;84:158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vos T, Lim SS, Abbafati C. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Baal PHM, Hoeymans N, Hoogenveen RT, de Wit AG, Westert GP.. Disability weights for comorbidity and their influence on Health-adjusted Life Expectancy. PHM 2006;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strahm C, Seneghini M, Güsewell S. et al. Symptoms compatible with long-COVID in healthcare workers with and without SARS-CoV-2 infection - results of a prospective multicenter cohort. Clin Infect Dis 2022;75:e1011–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adler L, Gazit S, Pinto Y. et al. Long-COVID in patients with a history of mild or asymptomatic SARS-CoV-2 infection: a Nationwide Cohort Study. Scand J Prim Health Care 2022;40:342–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hastie CE, Lowe DJ, McAuley A. et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun 2022;13:5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wulf Hanson S, Abbafati C, Aerts JG. et al. ; Global Burden of Disease Long COVID Collaborators. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S.. Long COVID symptoms and duration in SARS-CoV-2 positive children—a nationwide cohort study. Eur J Pediatr 2022;181:1597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayoubkhani D, Bermingham C, Pouwels KB. et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 2022;377:e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wisnivesky JP, Govindarajulu U, Bagiella E. et al. Association of vaccination with the persistence of post-COVID symptoms. J Gen Intern Med 2022;37:1748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ.. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022;399:2263–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blakely T, Thompson J, Bablani L. et al. Association of simulated COVID-19 policy responses for social restrictions and lockdowns with health-adjusted life-years and costs in Victoria, Australia. JAMA Health Forum 2021;2:e211749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. COVID Live. Hospitalizations: Current Cases Admitted to Hospital. 2022. https://covidlive.com.au/report/hospitalized (1 June 2022, date last accessed).
- 27. Tobin RJ, Wood JG, Jayasundara D. et al. Hospital length of stay in a mixed Omicron and Delta epidemic in New South Wales, Australia. medRxiv. doi: 10.1101/2022.03.16.22271361, 19 March 2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 28. Menni C, Valdes AM, Polidori L. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022;399:1618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Australian Institute of Health and Welfare (AIHW). Australian Burden of Disease Study: Impact and Causes of Illness and Death in Australia 2018. Canberra: AIHW, 2021. [Google Scholar]
- 30. COVID-19 National Incident Room Surveillance Team. COVID-19 Australia: epidemiology report 60: reporting period ending 10 April 2022. Commun Dis Intell (2018) 2022;46:1–20. [DOI] [PubMed] [Google Scholar]
- 31. Institute for Health Metrics and Evaluation (IHME). GBD Results. 2022. https://vizhub.healthdata.org/gbd-results/ (2 June 2022, date last accessed).
- 32. New South Wales Ministry of Health. COVID-19 Weekly Surveillance Reports – Archive. 2022. https://www.health.nsw.gov.au/Infectious/covid-19/Pages/weekly-reports-archive.aspx (1 June 2022, date last accessed).
- 33. Australian Bureau of Statistics (ABS). National, State and Territory Population. 2022. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release (14 Nov 2022, date last accessed).
- 34. COVID Live. New Cases: Total Cases and New Last 24hrs. 2022. https://covidlive.com.au/report/cases (1 June 2022, date last accessed).
- 35. Ting I, Workman M, Shatoba K, Hutcheon S, Palmer A; Australian Broadcasting Corporation. Charting the Spread of COVID-19 in Australia. 2020. (updated 25 May 2022). https://www.abc.net.au/news/2020-03-17/coronavirus-cases-data-reveals-how-covid-19-spreads-in-australia/12060704-newcases (1 June 2022, date last accessed).
- 36. Department of Health and Aged Care, Commonwealth of Australia. COVID-19 Vaccine Rollout – 10 December 2021. 2021. https://www.health.gov.au/resources/publications/covid-19-vaccine-rollout-update-10-december-2021 (18 November 2022, date last accessed).
- 37. European Burden of Diseae Network (EBoDN). Burden of Disease of COVID-19: Protocol for Country Studies. 2022. https://www.burden-eu.net/docs/covid19-bod-protocol.pdf (27 September 2022, date last accessed).
- 38. Wyper GMA, Fletcher E, Grant I. et al. Measuring disability-adjusted life years (DALYs) due to COVID-19 in Scotland, 2020. Arch Public Health 2022;180:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cuschieri S, Calleja N, Devleesschauwer B, Wyper GMA.. Estimating the direct Covid-19 disability-adjusted life years impact on the Malta population for the first full year. BMC Public Health 2021;21:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moran DP, Pires SM, Wyper GMA, Devleesschauwer B, Cuschieri S, Kabir Z.. Estimating the direct disability-adjusted life years associated with SARS-CoV-2 (COVID-19) in the Republic of Ireland: the first full year. Int J Public Health 2022;267:1604699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ballering AV, van Zon SKR, Hartman TC, Rosmalen JGM, Lifelines Corona Research Initiative Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022;400:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whitaker M, Elliott J, Chadeau-Hyam M. et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun 2022;13:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ayoubkhani D, Khunti K, Nafilyan V. et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie Y, Xu E, Bowe B, Al-Aly Z.. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Douaud G, Lee S, Alfaro-Almagro F. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022;604:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puntmann VO, Carerj ML, Wieters I. et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dennis A, Wamil M, Alberts J. et al. ; COVERSCAN Study Investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021;11:e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.