Key Features.
The Danish Blood Donor Study (DBDS) was established to study the impact of blood donation on the health of blood donors and to answer broader health-related research questions. The cohort is now a nationwide research resource.
The cohort was initiated in 2010 and by 2015 it covered all blood donation facilities in Denmark.
As of February 2022, DBDS includes 138 491 participants (51% women, age span at inclusion 18–70 years) who have completed a total of 371 589 questionnaires and with 1 091 499 person-years of follow-up. Information on participants from national health registries provides prospective data on hospital contacts, diagnoses, prescriptions, vital status and more.
Upon inclusion, a whole-blood sample is stored for DNA extraction. At every donation, a plasma sample is stored by the blood bank. Participants permit the use of their historical and future plasma samples for research. In total, samples from 2 293 916 donations have been collected thus far and inclusion is ongoing.
Detailed information on the Danish Blood Donor Study is available at [http://www.dbds.dk], or contact the steering committee [info@dbds.dk].
Why was the cohort set up?
The original research question
The Danish Blood Donor Study (DBDS) was established as a platform for the study of health effects of blood donation and as a public health study. Previous results from the Scandinavian Donations and Transfusions Database (SCANDAT, a database including all electronically recorded Danish and Swedish blood donations since the 1980s and 1970s, respectively),1 linked to the national health registers, demonstrated a lower mortality among blood donors when compared with the background population.2 In addition, blood donors donating frequently had a lower mortality than infrequent donors.3 The original research question was thus: is blood donation per se beneficial to the health of the blood donors, or are active blood donors healthier than the rest of the population because of donor selection bias?
As a group, blood donors are healthier than the general population. This phenomenon is known as the Healthy Donor Effect (HDE) and results from a selection bias similar to the Healthy Worker Effect.4,5 Just as the Healthy Worker Effect can mask possible occupational hazards, the HDE may mask deleterious effects of blood donation, inasmuch as only healthy individuals may donate. As blood banks, we are obligated to ensure that donors are in good health both before and after donating blood. We therefore need studies that reveal possible detrimental effects of blood donation, and to further elucidate the HDE.
General health-related research questions
The cohort was also established to assess general health-related research questions. Despite the HDE, some blood donors ultimately fall ill, and with increasing ‘time since last donation’, the mortality among lapsed or deferred donors approaches the mortality of the background population.2,3 Several features add value to the study of blood donors: blood donors typically return to donate blood several times over the span of years. A cohort of blood donors therefore provides a unique opportunity to track changes in health, lifestyle, disease emergence and other exposures/parameters between donations. Hence, large blood donor studies and biobanks with consecutive samples are useful to investigate plasma biomarkers predicting disease. Candidate biomarkers can be measured over time in the same donor and changes recorded. The DBDS has already proved to be a useful platform for the study of general health-related research questions.6–15 Large-scale blood donor studies can thus be leveraged for public health research within biological, psychological and social aspects of general health, as long as the HDE is taken into account.
Given our cohort infrastructure, we are also able to conduct recall by genotype or phenotype studies. The statistical noise from pre-existing disease on dynamic biomarkers is minimized by the fact that blood donors are only allowed to donate if they consider themselves to be in good health and are free of overt disease. For that reason, the cohort is also suitable for the identification of healthy controls for other studies. Furthermore, the high number of blood donations continuously taking place in all parts of the country, even during a pandemic, renders real-time surveillance studies of infectious diseases possible.
The DBDS was initiated in the Capital and Central Denmark Regions in March 2010. From January 2012, the study was expanded to include the Zealand and North Denmark Regions. The Region of Southern Denmark joined the study in June 2015, at which point the cohort became nationwide.
Who is in the cohort?
The DBDS is an ongoing, national prospective cohort of blood donors. Before or during donation, blood donors aged 18 years or above who have previously donated blood at least once receive oral and written information about the study and are invited to join the cohort by signing a consent form. The participants can withdraw consent at any time; see Table 1 for characteristics of participants (at baseline) and non-participants in the study. In the current dataset (28 February 2022), 138 491 participants have completed a total of 371 589 questionnaires (Table 2). To define the population of blood donors not included in DBDS, we identified all donations from non-participants (not yet invited blood donors) from 1 March 2010 to 28 February 2022 from SCANDAT. A single donation from each non-participating donor was sampled randomly as the index donation for that donor, and the age and measurements at that donation were used to characterize the donor. By this method, participants were slightly older, had more donations in the previous 5 years and had similar haemoglobin values compared with non-participants. The number of blood donors who have declined to participate upon invitation is 4.0%.
Table 1.
Characterization of the cohort
| Participants |
Blood donors not participatinga |
|||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Numbers | 70 657 | 67 834 | 186 893 | 146 411 |
| Age (years) | 36 (25–48) | 39 (28–50) | 33 (24–46) | 39 (27–52) |
| Age strata (%) | ||||
| 18–25 | 26.9 | 18.2 | 31.0 | 20.4 |
| 26–35 | 22.3 | 24.7 | 24.0 | 23.0 |
| 36–45 | 21.2 | 22.6 | 18.3 | 18.6 |
| 46–55 | 18.7 | 20.7 | 15.8 | 19.0 |
| 56–70 | 10.8 | 13.8 | 10.8 | 18.9 |
| Haemoglobin (mmol/L) | 137.0 (130.5–141.8) | 151.5 (146.6–157.9) | 135.4 (128.9–141.8) | 151.5 (145–157.9) |
| Full blood donationsb | ||||
| Number of donors | 68 235 | 64 578 | 184 675 | 143 527 |
| Donations during the past year | 2 (2–3) | 2 (2–3) | 2 (1–2) | 2 (1–3) |
| Donations during the past 3 years | 4 (3–6) | 5 (3–8) | 2 (1–4) | 3 (1–6) |
| Donations during the past 5 years | 6 (3–9) | 7 (4–12) | 3 (1–5) | 4 (1–8) |
| Plasma donations | ||||
| Number of donors | 5003 | 6945 | 3614 | 4640 |
| Donations during the past year | 3 (2–4) | 3 (2–5) | 2 (1–3) | 2 (1–3) |
| Donations during the past 3 years | 3 (2–6) | 4 (2–8) | 2 (1–3) | 2 (1–4) |
| Donations during the past 5 years | 3 (2–6) | 4 (2–8) | 2 (1–3) | 2 (1–4) |
| Current smoking status (%) | 15.3 | 14.8 | – | – |
| Weight (kg) | 69 (62–78) | 84 (77–93) | – | – |
| Height (cm) | 169 (165–173) | 182 (178–187) | – | – |
| BMI (kg/m2) | 24 (21.9–27.0) | 25.3 (23.4–27.8) | – | – |
| Short form 12c | ||||
| Physical component score | 57 (55–58) | 57 (55–58) | – | – |
| Mental component score | 55 (50–58) | 56 (53–58) | – | – |
Results are reported as medians (interquartile ranges), numbers, or percentages.
BMI, body mass index; Short form 12, The 12-Item Short Form Health Survey.
Blood donors not participating are defined as donors who had not yet received invitation and participant information. The number of blood donors who have declined to participate upon invitation is 4.0%.
Full blood donors/plasma donors are defined as donors with at least one full blood/plasma donation during the past year before inclusion (including the inclusion donation).
Short form 12: higher scores reflect higher self-reported health-related quality of life.
Table 2.
Samples and data collected
| Phase | Number of samples/measurements | Material |
|---|---|---|
| Baseline samples | 218 149 | Whole blood samples for DNA extraction (stored at -20°C) |
| 223 876 | 1–2 mL EDTA plasma (stored at -20°C) | |
| Sequential samples in total | 1 991 227 | 1–2 mL EDTA plasma (stored at -20°C) |
| Baseline data, 2010–15 | 83 074 | Questionnaire v1
|
| Baseline data, 2015–18 | 52 922 | Questionnaire v2
|
| Baseline data, 2018–20 | 46 513 | Questionnaire v3
|
| Baseline data, 2020- | 30,779 | Questionnaire v4
|
| e-Boks dataa | 62 721 | Questionnaires on migraine and allergy, 2020 |
| 95 580 | Questionnaires on COVID-19, 2020 | |
| Geneticsb | 100 177 | Approximately 650 000 single nucleotide polymorphisms |
| Various biomarkers from baseline samples | 18 447 | Plasma C-reactive protein |
| 14 008 | Plasma suPAR | |
| 8946 | Cytokine auto antibodies | |
| 25 722 | Specific IgEc | |
| 10 006 | MesoScale 54 V plex reference cohort | |
| Every donation | 3 571 852 | Haemoglobin |
| 301 017 | Ferritin | |
| 1 051 801 | Leukocytes | |
| 251 724 | Eosinophils | |
| 286 976 | Neutrophils | |
| 286 985 | Lymphocytes | |
| Microbiology | 13 538 | Nasal swabs cultivated for Staphylococcus aureus stored at −80°C |
| 5550 | Staphylococcus aureus isolates |
Questionnaire data, samples and measurements collected during the years.
EDTA, ethylenediamine tetraacetic acid; suPAR:,soluble urokinase-type plasminogen activator receptor; IgE, immunoglobulin E; ADHD, attention-deficit/hyperactivity disorder.
e-Boks is a public digital mailbox, which all citizens aged 15 years or above use for communication with the Danish public authorities.
Infinium Global Screening Array, Illumina.
Specific IgE for the nine most common respiratory sensitizers, Phadiatop.
To ensure a cost-effective design that allows donors to be enrolled even after their donation has begun, only routine blood samples are used in the study. In Denmark, archive samples (5 mL EDTA gel-separated tubes, various vendors) are routinely collected at every donation. For study participants, 1.2 mL of plasma from all archive samples are reserved for research purposes whether drawn at inclusion, at subsequent donations or from historical samples. Samples used for blood group typing or viral screening are recapped and whole blood is made available for DNA extraction.
How often have they been followed up?
Whenever a participant donates blood, the data from the donation becomes available for the study. The possibility for measuring biomarkers in consecutive samples from the same donor allows for detailed studies of biomarker dynamics. This is, to our knowledge, the most comprehensive platform for collecting sequential samples in any cohort study. By February 2022, a total of 2 293 916 unique plasma samples were available (Table 2).
After enrolment, the participants are followed at subsequent blood donations and through national health registers. The blood bank follow-up allows for additional data collection: the donor may be asked to fill out new questionnaires and biological sampling may take place. The participants are followed through the national registers also after ceasing their blood donor career. Outcomes, exposures and confounding factors can be assessed by register linkage via the unique personal identification number to a collection of nationwide registers regarding sociodemographics (education, employment, income, civil status, vital status, history of emigration) and health (filled prescriptions, general practitioner contacts, inpatient and outpatient hospital contacts, causes of death) as outlined elsewhere.16 Loss to follow-up in the traditional sense does not occur.
What has been measured?
Questionnaires
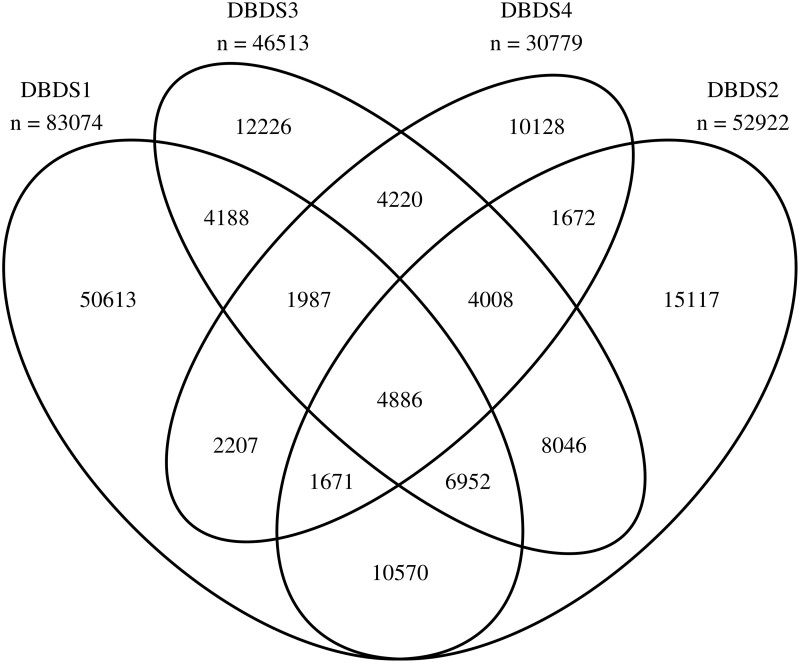
The first questionnaire used in DBDS was a paper-based four-page questionnaire with health-related questions (see overview of questionnaire versions and included items in Table 2 and Venn diagram in Figure 1). In the design of this questionnaire, it was paramount to limit the number of questions to avoid physical bottlenecks in the flow of blood donors passing through the blood bank facility. The questionnaire took an average of 8 min to complete. The questionnaires were scanned, and a software package was used for capturing and storing data. For all questionnaires, data were manually validated by visually comparing the captured data with those of the original questionnaire. Captured data were corrected when in disagreement with the paper form. Subsequent random quality checks comprised 1% of all questionnaires, and the error rate per field was below 0.1%.
Figure 1.
Venn diagram illustrating participants in the Danish Blood Donor Study (DBDS) and their questionnaire responses to the four versions of questionnaires covering a period from 2010 to 2022
In June 2015, the paper questionnaire was replaced by an electronic version that was fully implemented in early 2016. The electronic questionnaire given to participants in three iterations comprises items concerning specific disease entities or traits different from those included in the paper-based version, and is therefore given to both new and previously included participants when attending the blood bank (Table 2). Donors fill out the questionnaire using their own electronic device or by using tablets supplied by the blood bank. The donors sign onto the web-based questionnaire using their unique civil registration number. The questionnaire platform is flexible: it incorporates conditional questions and allows targeted questions for specific subgroups, i.e. specific questions for participants according to age, sex etc. In addition to baseline questionnaires, follow-up questionnaires on migraine and allergy and several COVID-19 specific questionnaires have been sent to participants using the public electronic mailing system, e-Boks. The questionnaire uses the open-source survey software tool LimeSurvey [https://www.limesurvey.org/ver. 2.06+]. Data are automatically encrypted and stored on a secure server.17
Laboratory measurements
Several biomarkers have been measured in baseline and sequential plasma samples (Table 2).7,10,11,13–15,18,19 Additionally, the measurement of 54 immunological biomarkers using a V-PLEX Human Biomarker 54-Plex Kit (MesoScale) in 10 006 donors was recently finalized. Furthermore, genetic markers were assessed in a large fraction of the donors, counting 100 177 participants by February 2022 and growing. The Infinium Global Screening Array (Illumina Inc., USA) was used to genotype more than 650 000 single nucleotide polymorphisms allowing for genome-wide association studies (GWAS).19–22 The genotyping was performed at deCODE genetics (Reykjavik, Iceland). Sequential questionnaire and biomarker data are continually linked to registry data as described (Table 2).
What has it found?
The cohort has already proved useful for both of its primary objectives: the study of donor health and generic health-related research questions. More than 60 peer-reviewed papers have already been published.
Donor health
Briefly, we have confirmed iron deficiency as a major health issue among blood donors and that sex, age and donation frequency are by far the most important predictors of iron deficiency. Specifically, we found that high-frequency blood donors, i.e. donors who have donated nine times or more within the previous 3 years, are very likely to be iron deficient (ferritin below 15 ng/mL; 39% of premenopausal women, 22% of postmenopausal women, 9% of men). Conversely, dietary habits are less strongly associated with the risk of iron deficiency.14 Using genetic data, we showed that single nucleotide polymorphisms associated with haemochromatosis may protect against iron depletion.23 Moreover, 46 novel loci affecting iron homeostasis were identified through a GWAS meta-analysis and are potentially involved in iron homeostasis through mechanisms such as absorption, iron recycling, erythropoiesis and hepcidin regulation.21 We also showed that iron deficiency is not associated with self-reported health.13 However, iron deficiency was the strongest predictor of low haemoglobin among blood donors, and the study supported ferritin monitoring in blood donors as a means to identify donors at risk of developing low haemoglobin.10 In this context, we find that the use of oral iron supplementation is not associated with short-term risk of infections.24 Further, we found that donors who deem themselves to be in good mental health donate more frequently and are less likely to drop out of the donor corps compared with those in worse mental health.25
Generic health research
The study serves as a platform for generic health research. Several projects are in the pipeline and the following list of published results is by no means exhaustive. We have shown that obesity is associated with an increased risk of infections by using the Danish health registers.9 We found that carriers of the C-C chemokine receptor 5-Δ32 deletion are at increased risk of hospitalization due to cardiovascular disease when compared with wild-type homozygotes.7 Additionally, we have reported that low-grade inflammation (defined as a C-reactive protein level between 3 and 10 mg/L) is very prevalent among women taking oral combined contraception, with a prevalence of 30% in this group. In comparison, the prevalence is only 8% in women not taking oral combined contraception and 6% in men.15
DBDS has additionally contributed to other research areas: allergic multimorbidity may represent a primarily specific IgE sensitized phenotype whereas those with single diseases represent more diverse phenotypes. Also, the proportion of young individuals with asthma and allergic rhinitis is still increasing over time in Denmark.11 Restless legs syndrome (RLS) is associated with major comorbidities among blood donors and, using GWAS, we identified three novel RLS-associated variants and eleven genomic loci affecting the levels of soluble urokinase-type plasminogen activator receptor.6,19,22 Self-reported and hospital-diagnosed hyperhidrosis is associated with redeemed prescriptions of antibiotics,8 and we found low adherence to the guideline for the acute treatment of migraine.26 The data can also be used in other ways. We have shown an association between sibship structure and hospitalization for infectious mononucleosis, and then used donors from the DBDS to confirm that the same associations are found when considering general infectious mononucleosis as the outcome.27
Furthermore, the platform has served to explore the Staphylococcus aureus carrier state in the nasal cavity.28 Latest, the infrastructure in the blood banks has contributed to the COVID-19 surveillance in Denmark by facilitating measurements of SARS-CoV-2 antibodies and estimating infection fatality rate among active and retired blood donors.29,30
What are the main strengths and weaknesses?
Strengths
The basic infrastructure needed for establishing this large public health study was readily available in the Danish blood services. Generally, blood banks routinely recruit new donors, handle contact information and obtain informed consent. Donors are accustomed to questionnaires at every donation, blood is already drawn from the donors, systems are in place for the unique labelling of samples and procedures for the correct handling storage, and analysis of samples are well established. Further, donors visit the blood banks as unpaid volunteers to give blood, whereas in other studies, participants must typically be invited, staff must be present solely for the inclusion of participants and a substantial infrastructure must be established for collection and handling of samples and data. In our experience, blood donors are generally willing to participate in research projects, which is reflected in a very high participation rate. Taken together, the DBDS is highly cost-effective compared with other large population studies.
Because of the existing infrastructure, we are also able to include samples taken during prior visits to the blood bank, before the donor joined the study. Data and blood samples from these visits are thus available to research at little to no extra cost. A population study of this magnitude that includes consecutive plasma samples with such short sampling time intervals is unique to the DBDS. This design enables us to study disease pathology and biomarkers that change over time before disease becomes apparent andhence the study portrays a prospective cohort. For example, consider the validation of a new screening candidate for a specific disease. Participants with the disease can be identified in the National Patient Register. The biomarker can be measured in several samples before and as close to the date of the diagnosis as possible. Should the biomarker be positive/observed, earlier samples can be picked and the dynamics of the biomarker over time can be assessed (Figure 2).31 In contrast to blood donor and population studies in other parts of the world, the DBDS benefits from the unique Danish national registers. The registers offer a wide range of opportunities, including the study of generic health questions.
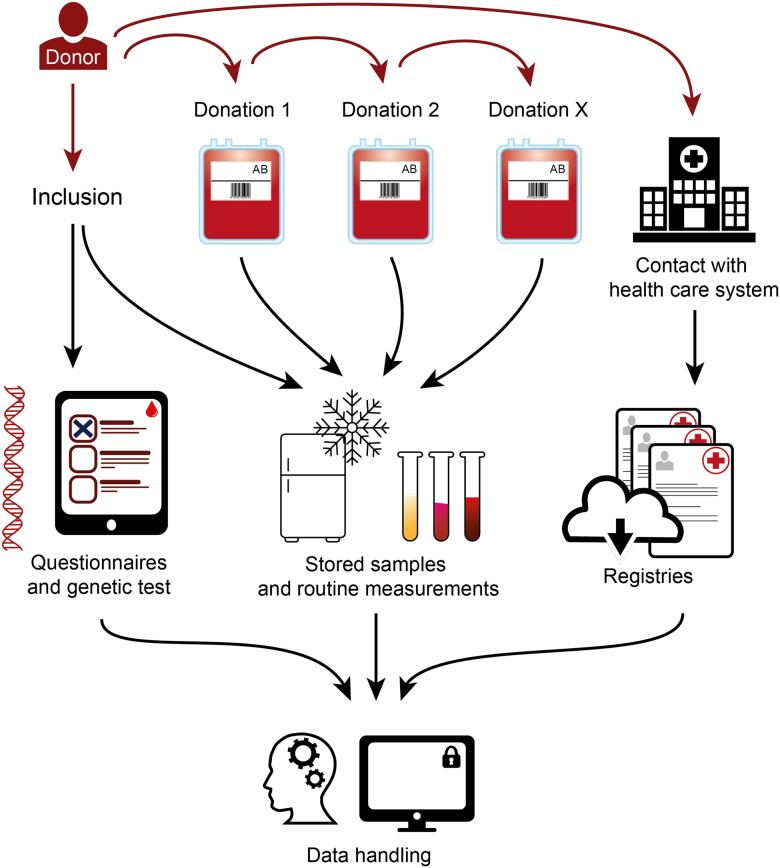
Figure 2.
The overall infrastructure in the Danish Blood Donor Study. Participants are included at an arbitrary donation and may donate several times over the range of years. At each visit a plasma sample is stored. Some participants may develop a disease, which is registered in the National Patient Register. All samples are available for measurements if a biomarker is to be assessed
Weaknesses
Some compromises have been made to increase the participation rate. The questionnaire is not as comprehensive as in many other studies. This is a deliberate choice to minimize the risk of causing delays in the waiting rooms, which would likely reduce the participation rate and increase the level of missing answers.
Only 1.5 mL plasma is collected during each visit. This relatively small sample volume is necessary to facilitate the inclusion of donors already being bled and to reduce costs, since no extra blood samples need be drawn.
Blood donors are healthier than the general population because they are selected and because donors are screened by questionnaires for risk factors associated with transfusion-transmissible infections. Therefore, a blood donor population cannot be used directly to assess the prevalence of health and disease in the general population. Similarly in the study of biomarkers, findings must be verified in other populations. However, the HDE may also be an advantage: in the study of biomarkers and other risk factors for disease, we can assume that the donor was asymptomatic when included. Consequently, it can generally be assumed that if a biomarker is present, it was there before symptoms/the clinical phenotype arose.
Can I get hold of the data? Where can I find out more?
The DBDS is a platform for studies carried out by the Danish blood centres and collaborators. The study is managed by a steering committee who respond to enquiries regarding collaboration. The blood donors participate in the DBDS to increase the scope of their donation, i.e. to help produce valuable research for the benefit of future patients. Additional information can be found at our home page [http://www.dbds.dk]. We invite researchers to collaborate by contacting the steering committee [info@dbds.dk]. Data access requires that projects and applicants obtain permission from the Regional Committees on Health Research Ethics and the Danish Data Protection Agency [http://www.datatilsynet.dk].
Ethics approval
The DBDS was approved by the Central Denmark (1–10-72–95-13) and Zealand (SJ-740) Regional Committees on Health Research Ethics and the Data Protection Agency (P-2019–99). The DBDS GWA study was approved by the Danish National Committee on Health Research Ethics (1700407). SCANDAT was approved by the Data Protection Agency (2008–54-0472). The DBDS was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Acknowledgements
We thank the Danish blood donors for their valuable participation in the Danish Blood Donor Study, and the staff at the blood centres for making this study possible.
Conflict of interest
None declared.
Contributor Information
Christian Erikstrup, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Erik Sørensen, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Kaspar R Nielsen, Department of Clinical Immunology, Aalborg University Hospital, Aalborg, Denmark.
Mie T Bruun, Department of Clinical Immunology, Odense University Hospital, Odense, Denmark.
Mikkel S Petersen, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Klaus Rostgaard, Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark; Danish Cancer Society Research Center, Copenhagen, Denmark.
Lise W Thørner, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Margit Larsen, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Susan Mikkelsen, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Khoa M Dinh, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Michael Schwinn, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Andreas S Rigas, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Maria Didriksen, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Joseph Dowsett, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Jakob H von Stemann, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark.
Thorsten Brodersen, Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark.
Isabella W Paulsen, Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark.
Lotte Hindhede, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark.
Susanne G Sækmose, Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark.
Kathrine A Kaspersen, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark; Danish Big Data Centre for Environment and Health (BERTHA), Aarhus University, Aarhus, Denmark.
Jens K Boldsen, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark; Danish Big Data Centre for Environment and Health (BERTHA), Aarhus University, Aarhus, Denmark.
Bertram Kjerulff, Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark; Danish Big Data Centre for Environment and Health (BERTHA), Aarhus University, Aarhus, Denmark.
Thomas Werge, Institute of Biological Psychiatry, Mental Health Services, Copenhagen University Hospital, Copenhagen, Denmark; LF Center for GeoGenetics, GLOBE Institute, University of Copenhagen, Copenhagen, Denmark; iPSYCH Initiative, Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Søren Brunak, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Karina Banasik, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Thomas F Hansen, Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Danish Headache Center, Department of Neurology, Copenhagen University Hospital, Glostrup, Denmark.
Henrik Ullum, Statens Serum Institut, Copenhagen S, Denmark.
Henrik Hjalgrim, Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark; Danish Cancer Society Research Center, Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Haematology, Copenhagen University Hospital, Copenhagen, Denmark.
Sisse R Ostrowski, Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Ole B Pedersen, Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Data availability
See above ‘Can I get hold of the data?’.
Author contributions
The DBDS cohort is run and the data are collected by the steering committee with teams (C.E., E.S., K.R.N., M.T.B., M.S.P., K.R., L.W.T., M.L., S.M., K.M.D., M.S., A.S.R., M.D, J.D., J.H.S., T.B., I.W.P., L.H., S.G.S., K.A.K., J.K.B., B.K., T.W., S.B., K.B., T.F.H., H.U., H.H., S.R.O. and O.B.P.). O.B.P., H.U., E.S. and C.E. designed this study; S.R.O., O.B.P., E.S. and C.E. manage the study. M.S. and L.H. organized and analysed data. All authors helped interpret findings. C.E. drafted the manuscript. All authors were involved in critically revising the manuscript and approved the final version.
Funding
The initiation of the Danish Blood Donor Study was supported by the Danish Administrative Regions (02/2611) and the Danish Council for Independent Research (09–069412). The study is currently funded by the Danish Administrative Regions and Bio- and Genome Bank Denmark. Funding for specific projects has been received from the Danish Blood Donor Research Foundation, the A. P. Møller Foundation for the Advancement of Medical Science and CHALLENGE grants from the Novo Nordisk Foundation [NNF17OC0027864 (BERTHA) and NNF17OC0027594].
References
- 1. Edgren G, Rostgaard K, Vasan SK. et al. The new Scandinavian Donations and Transfusions database (SCANDAT2): a blood safety resource with added versatility: SCANDAT2 Database. Transfusion 2015;55:1600–06. [DOI] [PubMed] [Google Scholar]
- 2. Edgren G, Tran TN, Hjalgrim H. et al. Improving health profile of blood donors as a consequence of transfusion safety efforts. Transfusion 2007;47:2017–24. [DOI] [PubMed] [Google Scholar]
- 3. Ullum H, Rostgaard K, Kamper-Jørgensen M. et al. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion 2015;55:2479–85. [DOI] [PubMed] [Google Scholar]
- 4. Atsma F, Veldhuizen I, Verbeek A, de Kort W, de Vegt F.. Healthy donor effect: its magnitude in health research among blood donors. Transfusion 2011;51:1820–28. [DOI] [PubMed] [Google Scholar]
- 5. Baillargeon J. Characteristics of the healthy worker effect. Occup Med 2001;16:359–66. [PubMed] [Google Scholar]
- 6. Didriksen M, Allen RP, Burchell BJ. et al. Restless legs syndrome is associated with major comorbidities in a population of Danish blood donors. Sleep Med 2018;45:124–31. [DOI] [PubMed] [Google Scholar]
- 7. Dinh KM, Pedersen OB, Petersen MS. et al. The impact of CCR5-Δ32 deletion on C-reactive protein levels and cardiovascular disease: results from the Danish Blood Donor Study. Atherosclerosis 2015;242:222–25. [DOI] [PubMed] [Google Scholar]
- 8. Henning MAS, Ibler KS, Ostrowski SR. et al. Hyperhidrosis and the risk of being treated for skin infections. J Dermatol Treat 2022;33:2263–69. [DOI] [PubMed] [Google Scholar]
- 9. Kaspersen KA, Pedersen OB, Petersen MS. et al. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology 2015;26:580–89. [DOI] [PubMed] [Google Scholar]
- 10. Kotzé SR, Pedersen OB, Petersen MS. et al. Predictors of hemoglobin in Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2015;55:1303–11. [DOI] [PubMed] [Google Scholar]
- 11. Mikkelsen S, Dinh KM, Boldsen JK. et al. Combinations of self-reported rhinitis, conjunctivitis, and asthma predicts IgE sensitization in more than 25,000 Danes. Clin Transl Allergy 2021;11:e12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedersen OB, Axel S, Rostgaard K. et al. The heritability of blood donation: a population-based nationwide twin study. Transfusion 2015;55:2169–74; quiz 2168. [DOI] [PubMed] [Google Scholar]
- 13. Rigas AS, Pedersen OB, Sørensen CJ. et al. No association between iron status and self-reported health-related quality of life in 16,375 Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2015;55:1752–56. [DOI] [PubMed] [Google Scholar]
- 14. Rigas AS, Sørensen CJ, Pedersen OB. et al. Predictors of iron levels in 14,737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2014;54:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sørensen CJ, Pedersen OB, Petersen MS. et al. Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLoS ONE 2014;9:e88196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt M, Pedersen L, Sørensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–49. [DOI] [PubMed] [Google Scholar]
- 17. Burgdorf KS, Felsted N, Mikkelsen S. et al. Digital questionnaire platform in the Danish Blood Donor Study. Comput Methods Programs Biomed 2016;135:101–04. [DOI] [PubMed] [Google Scholar]
- 18. Mikkelsen S, Boldsen JK, Møller BK. et al. Atopic respiratory diseases and IgE sensitization are associated with leukocyte subset concentrations in 14,440 blood donors. Clin Chim 2021;520:139–46. [DOI] [PubMed] [Google Scholar]
- 19. Dowsett J, Ferkingstad E, Rasmussen LJH. et al. ; DBDS Genomic Consortium. Eleven genomic loci affect plasma levels of chronic inflammation marker soluble urokinase-type plasminogen activator receptor. Commun Biol 2021;4:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen TF, Banasik K, Erikstrup C. et al. DBDS Genomic Cohort, a prospective and comprehensive resource for integrative and temporal analysis of genetic, environmental and lifestyle factors affecting health of blood donors. BMJ Open 2019;9:e028401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bell S, Rigas AS, Magnusson MK. et al. ; DBDS Genomic Consortium. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol 2021;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Didriksen M, Nawaz MS, Dowsett J. et al. Large genome-wide association study identifies three novel risk variants for restless legs syndrome. Commun Biol 2020;3:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sørensen E, Rigas AS, Didriksen M. et al. Genetic factors influencing hemoglobin levels in 15,567 blood donors: results from the Danish Blood Donor Study. Transfusion 2019;59:226–31. [DOI] [PubMed] [Google Scholar]
- 24. Kaspersen KA, Dinh KM, Mikkelsen S. et al. Oral iron supplementation is not associated with short-term risk of infections: results from the Danish Blood Donor Study. Transfusion 2019;59:2030–38. [DOI] [PubMed] [Google Scholar]
- 25. Didriksen M, Thørner LW, Larsen MAH. et al. The impact of health‐related quality of life and depressive symptoms on blood donor career—results from the Danish blood donor study. Transfusion 2021;61:1479–88. [DOI] [PubMed] [Google Scholar]
- 26. Olesen A, Schytz HW, Ostrowski SR. et al. Low adherence to the guideline for the acute treatment of migraine. Sci Rep 2022;12:8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rostgaard K, Nielsen TR, Wohlfahrt J. et al. Sibship structure and risk of infectious mononucleosis: a population-based cohort study. Int J Epidemiol 2014;43:1607–14. [DOI] [PubMed] [Google Scholar]
- 28. Erikstrup LT, Dinh KM, Andersen PS. et al. Cohort description: the Danish Blood Donor Staphylococcus aureus Carriage Study. Clin Epidemiol 2019;11:885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erikstrup C, Hother CE, Pedersen OBV. et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis 2021;72:249–53;ciaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pedersen OB, Nissen J, Dinh KM. et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection fatality rate among elderly Danes: a cross-sectional study on retired blood donors. Clin Infect Dis 2021;73:e2962–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jørgensen S, Paulsen IW, Hansen JW. et al. The value of circulating microRNAs for early diagnosis of B-cell lymphoma: a case-control study on historical samples. Sci Rep 2020;10:9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
See above ‘Can I get hold of the data?’.