Abstract
Background
A later age at natural menopause (ANM) has been linked to several ageing-associated traits including an increased risk of breast and endometrial cancer and a decreased risk of lung cancer, osteoporosis and Alzheimer disease. However, ANM is also related to several proxies for overall health that may confound these associations.
Methods
We investigated the causal association of ANM with these clinical outcomes using Mendelian randomization (MR). Participants and outcomes analysed were restricted to post-menopausal females. We conducted a one-sample MR analysis in both the Women’s Health Initiative and UK Biobank. We further analysed and integrated several additional data sets of post-menopausal women using a two-sample MR design. We used ≤55 genetic variants previously discovered to be associated with ANM as our instrumental variable.
Results
A 5-year increase in ANM was causally associated with a decreased risk of osteoporosis [odds ratio (OR) = 0.80, 95% CI (0.70–0.92)] and fractures (OR = 0.76, 95% CI, 0.62–0.94) as well as an increased risk of lung cancer (OR = 1.35, 95% CI, 1.06–1.71). Other associations including atherosclerosis-related outcomes were null.
Conclusions
Our study confirms that the decline in bone density with menopause causally translates into fractures and osteoporosis. Additionally, this is the first causal epidemiological analysis to our knowledge to find an increased risk of lung cancer with increasing ANM. This finding is consistent with molecular and epidemiological studies suggesting oestrogen-dependent growth of lung tumours.
Keywords: Mendelian randomization, menopause, Women's Health Initiative, UK Biobank, age of natural menopause
Key Messages.
As in prior literature, the age of natural menopause (ANM) was observationally associated with increased risk of breast cancer, endometrial cancer and ovarian cancer, and with a decreased risk of lung cancer, coronary heart disease, ischaemic stroke, fracture, osteoporosis and Alzheimer disease in the Women's Health Initiative and UK Biobank.
However, these associations may be confounded by overall markers of health, such as smoking, so we used a genetic instrument variable to look at the causality of ANM on these adverse outcomes using Mendelian randomization.
A 5-year increase in ANM was causally associated with a decreased risk of fractures and osteoporosis but with an increased risk of lung cancer.
This increase in ANM was not significantly associated with other outcomes; notably, there was no causal association of ANM with coronary heart disease or ischaemic stroke.
Given the increase in lung cancer risk and prior molecular studies linking lung cancer to oestrogen receptor expression, randomized–controlled trials of anti-oestrogen therapies for prevention or treatment of lung cancer should be considered should these results be replicated in additional studies.
Introduction
The age of natural menopause (ANM) has been linked to several ageing-associated traits, including oncologic, cardiovascular, musculoskeletal and neurocognitive-related adverse health outcomes. Among oncologic outcomes, later menopause has been consistently associated with an increased risk of breast1,2 and endometrial cancers3,4 as well as a decreased risk of lung cancer.5,6 Associations with ovarian cancer have been less consistent.7 For ageing-associated traits unrelated to cancer, an older ANM has been consistently associated with a reduced risk of coronary heart disease (CHD) and ischaemic stroke8–11 as well as a higher bone mass and a lower risk of fractures and of osteoporosis defined by the bone mineral density T-score.12,13 Associations with cognition and dementia have been less consistent.14 A strong biological basis exists to explain some of these associations including for breast cancer, endometrial cancer and osteoporosis.15–18 The biological basis for the remaining associations, including a protection against lung cancer and dementia, remains circumstantial.
Residual confounding may be responsible for at least some of the associations between ANM and ageing-associated outcomes. For example, a later ANM has been linked to a lower rate of smoking, higher education, higher income and higher physical activity,8,19 all of which affect the risk of several chronic diseases. Mendelian randomization (MR) represents a well-established approach to guarding against residual confounding.20 A recent large-scale genome-wide association study (GWAS) of ANM in ∼200 000 women leveraged ≤290 genetic variants as instruments to conduct a two-sample MR between ANM and multiple health traits.21 The investigators detected causal associations between later ANM and an increased risk of breast and endometrial cancer, as well as a lower risk of reduced bone mass and fractures.21 Less robust causal associations were detected with ovarian cancer and type 2 diabetes, and no association was detected for CHD.21 An important limitation of this two-sample MR study was the use of publicly available summary statistics for GWAS as convenience data sets. Several of these data sets incorporated a large fraction of men (e.g. CHD) and/or a substantial number of pre-menopausal women (e.g. breast, endometrial, ovarian cancers, multiple cardiometabolic risk factors and fractures) making MR inference much less reliable. Another limitation was the lack of study of the lung cancer outcome.21
Here, we overcome these limitations by first conducting a one-sample MR study within the Women’s Health Initiative (WHI) and UK Biobank (UKB) using individual-level data, restricting to post-menopausal women with documented non-surgical menopause and including the lung cancer outcome. We augment this one-sample analysis with additional publicly available individual-level data sets using a two-sample framework but only after filtering out men as well as women with outcomes of interest occurring before menopause.
Methods
Data sets
A detailed description of the WHI and UKB study designs have previously been published.22–24 The WHI study population consisted of post-menopausal women who had enrolled in the study between 1993 and 1998. The UKB data consisted of women recruited between ages 37 and 73 years who had enrolled between 2006 and 2010. Among the data collected, our study utilized data from questionnaires, anthropometric measurements and outcome data collected from self-report, primary care, hospital records and death records. Data from both sources included post-menopausal women of European ancestry who had undergone natural menopause (Supplementary material, Items 2 and 6, available as Supplementary data at IJE online). We included for replication any available data sets in populations of European ancestry with a reported age of menopause variable and the outcome of interest (Supplementary Table S1 and Supplementary material, Item 6, available as Supplementary data at IJE online) that were downloaded from the National Institutes of Health database of Genotypes and Phenotypes (dbGaP) and from the European Genome-Phenome Archive (EGA).
Definitions of exposure and outcomes
We defined the ANM in the WHI as the self-reported age 1 year past the last menstrual period for those who underwent non-surgical menopause, i.e. excluding women with a history of bilateral oophorectomy. Women who had undergone hysterectomy in the absence of bilateral oophorectomy had an estimated ANM based on their responses to questions about other menopausal symptoms (Supplementary material, Item 2, available as Supplementary data at IJE online). In UKB, we defined ANM from the baseline questionnaire data as the self-reported age that menopause occurred in women without history of bilateral oophorectomy. Other menopause-related variables were not included in the questionnaire and ANM was therefore not available for women who underwent hysterectomy prior to menopause. We additionally excluded those with these surgeries prior to 2 years after the reported age of menopause to mitigate against recall errors. In other data sets downloaded via dbGaP, we used the provided ANM or derived age of ANM from the same surgical criteria, where available. If the menopause phenotype was unavailable in these other data sets, we limited analyses to events occurring past the age of 55 years (cases) or to women over the age of 55 years (controls).
Outcomes in the WHI were extracted for adjudicated cancers (breast, endometrial, ovarian and lung cancers), adjudicated fractures, incident CHD and ischaemic stroke, and self-reported osteoporosis and Alzheimer disease (Supplementary Table S3, available as Supplementary data at IJE online). In UKB, cancers were extracted from the UK cancer registry. Fracture, osteoporosis and Alzheimer disease were extracted from the first-occurrences data, which report the first date each International Classification of Diseases code was found in hospital, primary care or death records, or in self-reported data from the intake survey (with the majority of our outcomes from hospital records). Cardiovascular disease (CVD) outcomes were extracted from a combination of first-occurrences and raw hospital data (Supplementary Table S5, available as Supplementary data at IJE online). We restricted analyses to incident cases.
Covariates
Covariates in observational analysis (Supplementary Tables S2 and S4, available as Supplementary data at IJE online) were age at enrolment, body mass index (BMI), status of oophorectomy and hysterectomy, status of menopause hormone therapy (MHT), smoking status, alcohol consumption, energy expenditure from exercise (WHI only, in weekly kcal/kg) and socio-economic status (for WHI, education and family income; for UKB, Townsend index). We additionally adjusted for parity for oestrogen-related cancers; systolic blood pressure (BP), hypertension and diabetes for CVD outcomes; history of fracture and vitamin D (for WHI, dietary; for UKB, serum measurement) for fracture and osteoporosis outcomes; and baseline diabetes and history of stroke or transient ischaemic attack for Alzheimer disease.
Instrumental variable
Our instrumental variables (IVs) for MR analysis were ≤55 single-nucleotide polymorphisms (SNPs) previously discovered through a GWAS of ∼70 000 women of European ancestry (Supplementary Tables S6 and S7, available as Supplementary data at IJE online)25 independently of all data sets analysed in this study. We included all SNPs with a consistent direction of effect on ANM that were directly measured or were imputed on >90% of samples within each data set. We assessed the strength of these instruments by measuring the F-statistic of the association between a weighted genetic score constructed from these 55 SNPs with ANM in the WHI and the UKB data sets, with weights from the discovery GWAS. We also checked for the association between potential confounders and the IV to test for the second assumption of MR. Where significant associations existed, we conducted sensitivity analyses using multivariable MR to ensure that the third assumption of MR was not violated with a pathway around the exposure via these confounders, thus adjusting for the IV–MHT relationship for all outcomes and additionally for the IV–BP relationship for CHD, stroke and Alzheimer disease outcomes. Importantly, we did not use the larger set of 290 SNPs and insertion/deletions reported in the more recent larger GWAS of ∼200 000 women21 as an instrument, as over half of the women included in that study were UK Biobank participants. Selection of this set of SNPs could result in an overfitting of instruments and exacerbation of weak instrument bias26 in our study, which uses UK Biobank as a primary data set for our one-sample MR.
Statistical analyses
We calculated summary statistics for the exposure, outcomes and covariates for all patients included in observational analyses, as well as for the subset with measured genotypes who were included in MR analyses. Next, we generated observational associations between ANM and each outcome in both the WHI and UKB using logistic regression and adjusting for general and outcome-specific covariates. Observational associations from both cohorts were then combined using a random-effects meta-analysis. Our primary MR analysis was a random-effects inverse-variance weighted (IVW) method. This method combines the causal ratio estimates from each variant according to the variance on those estimates, where the ratio is computed as the outcome–IV association estimate divided by the exposure–IV association estimate. We adjusted each association for age at enrolment. We then combined the IVW results for each outcome in the WHI and UKB through a random-effects meta-analysis. We further conducted two-sample IVW analyses from external publicly available data sets for outcomes where such data sets were available and pre-menopausal women could be reliably excluded, and further combined these results with our one-sample MR analyses through a random-effects meta-analysis. We used an adjusted false discovery rate test to adjust for multiple outcomes testing. We conducted MR sensitivity analyses using weighted median analysis and MR–Egger with subsequent meta-analysis across cohorts for each method to compare effect estimates using MR methodology. Additionally, we used MR-PRESSO to assess for horizontal pleiotropy in the two-sample MR analyses and meta-analysed any resulting outlier-corrected results with the main analyses. Analyses were performed using Stata/SE 13.1 (StataCorp LP, College Station, TX), R (https://cran.r-project.org/) and plink 2.0 (https://www.cog-genomics.org/plink/2.0/).
Results
Menopausal women eligible for the study comprised 106 853 for the WHI observational analysis, 19 543 for the WHI MR analysis and 95 464 for both UKB analyses (Table 1). Mean self-reported ANM was 1 year lower for the WHI (49.3) compared with UKB (50.3). The cohorts were similar in BMI, smoking rates and prevalence of diabetes at enrolment. UKB had substantially lower rates of baseline hysterectomy, largely due to exclusions based on fewer survey questions related to menopause, and substantially lower rates of MHT use, largely related to differences in study design and years of data collection.
Table 1.
Characteristics of the Women’s Health Initiative and UK Biobank participants included in observational and one-sample instrumental variable (IV) analysis
| Women's Health Initiative |
UK Biobank |
||
|---|---|---|---|
| Observational analysis (n = 106 853) | IV analysis (n = 19 543) | Observational and IV analysis (n = 95 464) | |
| Quantitative variables | Mean (SD) or median (first, third quartile) | ||
| Main exposure | |||
| ANM (years) | 49.1 (5.9) | 49.3 (5.9) | 50.3 (4.5) |
| Other | |||
| Age (years) | 63.6 (7.2) | 65.7 (6.9) | 60.6 (5.4) |
| BMI (kg/m2) | 27.5 (5.7) | 27.9 (5.7) | 27 (5.0) |
| Systolic BP (mmHg) | 128.6 (19.2) | 131 (19.5) | 140.5 (20.3) |
| Townsend index | –1.7 (2.8) | ||
| Education | 7.4 (1.8) | 7.2 (1.8) | |
| Family income | 4.3 (1.8) | 4.1 (1.7) | |
| Alcohol (drinks/week) | 2.4 (4.9) | 2.7 (5.3) | 5.4 (6.5) |
| PA (MET h/week) | 9.3 (2.5, 18.8) | 8.3 (2.3, 17.5) | |
| Dietary vitamin D (mcg) | 3.8 (2.4, 5.7) | 3.8 (2.4, 5.7) | |
| Vitamin D (nmol/L) | 50.6 (20.6) | ||
| Number of pregnancies/parity | 2.6 (1.7) | 2.7 (1.7) | 1.9 (1.1) |
| WHO fracture risk score | 9.3 (6.5, 14.3) | 10.6 (7.5, 15.7) | |
| Binary variables | n (%) | ||
| HT trial participant | 8655 (8.1) | 4534 (23.2) | |
| Ca+ & vitamin D trial participant | 11 754 (11.0) | 3733 (19.1) | |
| Hormone use ever | 61 868 (57.9) | 9674 (49.5) | |
| Takes MHT | 4409 (4.6) | ||
| Current smoking | 6945 (6.5) | 1446 (7.4) | 7480 (7.8) |
| Bilateral oophorectomy/oophorectomy history | 6091 (5.7) | 919 (4.7) | 1723 (1.8) |
| Hysterectomy | 31 094 (29.1) | 5257 (26.9) | 2886 (3.0) |
| Hypertension | 23 615 (22.1) | 4788 (24.5) | 45 596 (47.8) |
| Diabetes/T2DM | 3312 (3.1) | 762 (3.9) | 3172 (3.3) |
| Broken bone | 42 955 (40.2) | 8091 (41.4) | |
| Osteoporosis | 8441 (7.9) | 1524 (7.8) | |
| Stroke or transient ischaemic attack | 2885 (2.7) | 625 (3.2) | 1350 (1.4) |
ANM, age of natural menopause; BMI, body mass index; BP, blood pressure; PA, physical activity; Ca+, calcium; WHO, World Health Organization; HT, hormone therapy; MHT, menopausal hormone therapy; T2DM, type 2 diabetes.
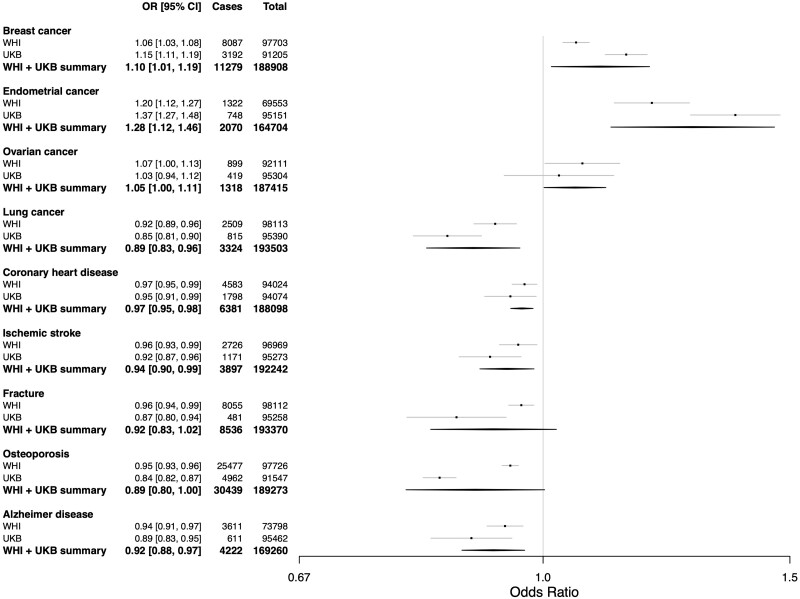
Observational analyses in the WHI and UKB combined linked a 5-year increase in ANM to a higher risk of breast, endometrial and ovarian cancers and a lower rate of lung cancer, CHD, ischaemic stroke and Alzheimer disease (Figure 1 and Supplementary Table S8, available as Supplementary data at IJE online). ANM was also inversely associated with the risk of fracture and osteoporosis with the former having borderline significance (Figure 1 and Supplementary Table S8, available as Supplementary data at IJE online).
Figure 1.
Results from the meta-analysed observational logistic regression analyses of the association between age of natural menopause and outcomes in the Women's Health Initiative and UK Biobank. Odds ratio for disease given a 5-year increase in age of natural menopause. 95% CI, number of individuals who were cases for the disease in each data set and analysis, and total number of individuals (cases + controls) from each data set and analysis are also shown. ANM, age of natural menopause; OR, odds ratio
We found a weighted genetic risk score constructed using the ANM IV SNPs to be strongly associated with an older ANM in both the WHI (F-statistic = 346, R2 = 1.8%) and UKB (F-statistic = 5062, R2 = 5.0%). The same score was not associated with any other baseline characteristics in the WHI except for a nominal association with an indicator for participation in a WHI hormone trial [per 1-SD increase in genetic risk score, odds ratio (OR) = 0.96, 95% CI, 0.93–1.00] and a history of a broken, fractured or crushed bone (OR = 0.97, 95% CI, 0.94–1.00). In UKB, we found the genetic risk score associated only with baseline characteristics of taking MHT (OR = 0.89, 95% CI, 0.86–0.92), age at enrolment (beta = 0.38 years, 95% CI, 0.35–0.42) and systolic BP (beta = 0.53 mmHg, 95% CI, 0.39–0.66) (Supplementary Figures S1 and S2, available as Supplementary data at IJE online).
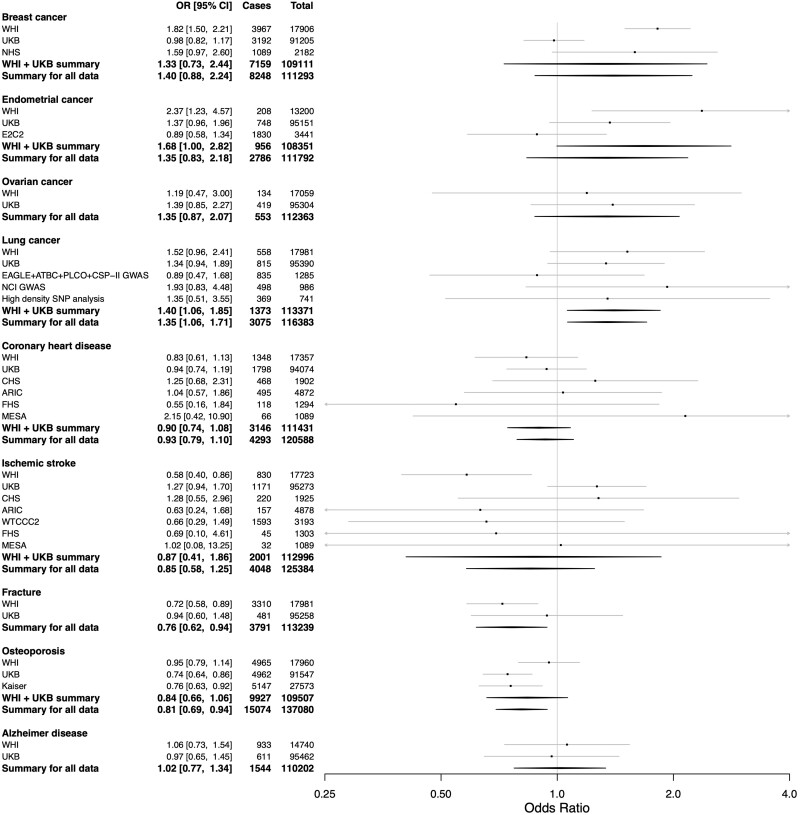
Our main MR results from the meta-analysis of the WHI and UKB with external data sets showed that an increasing ANM was causally associated with an increased risk of lung cancer (OR = 1.35, 95% CI, 1.06–1.71 for each 5-year increase in ANM), in contrast to the protective effect in the observational analysis. The MR results also showed a causally protective effect for fracture (OR = 0.76, 95% CI, 0.62–0.94) and osteoporosis (OR = 0.80, 95% CI, 0.70–0.92) consistently with the protective effects in the observational analysis (Figure 2 and Supplementary Table S9, available as Supplementary data at IJE online). The causal associations for lung cancer, osteoporosis and fractures remained significant for multiple testing with a false discovery rate of 0.05 (Supplementary Table S11, available as Supplementary data at IJE online). Sensitivity analyses showed similar point estimates for weighted median and MR–Egger analyses with generally wider confidence intervals, retaining significance in weighted median analysis for osteoporosis only (OR = 0.80, 95% CI, 0.68–0.94) (Supplementary Table S9, available as Supplementary data at IJE online). ANM was not significantly associated with breast, endometrial or ovarian cancers, CHD, ischaemic stroke or Alzheimer disease in the primary analysis. However, a strong trend towards significance for increased risk was observed for breast cancer in the overall sample as well as for endometrial cancer in our one-sample MR meta-analysis restricted to the WHI and UKB alone (Figure 2 and Supplementary Tables S9 and S10, available as Supplementary data at IJE online). Age at enrolment was already adjusted for in the MR analyses and a multivariable MR adjusting for MHT and, where applicable, BP gave similar results to the main MR results (Supplementary Figure S3, available as Supplementary data at IJE online). Lastly, evaluation for horizontal pleiotropy using MR-PRESSO detected pleiotropy in only a single data set (WTCCC2) for the outcome of ischaemic stroke (Supplementary Table S12, available as Supplementary data at IJE online). We therefore updated the meta-analysis using the MR-PRESSO pleiotropy-corrected result for WTCCC2 (two outlier SNPs removed) and we found no influence on the meta-analysis outcome (Supplementary Table S13, available as Supplementary data at IJE online).
Figure 2.
Results from the inverse-variance weighted Mendelian randomization meta-analysis between the age of natural menopause and outcomes in the Women's Health Initiative, UK Biobank and other data sources. Odds ratio for disease given a 5-year increase in age of natural menopause based on a causal genetic instrument variable analysis. 95% CI, number of individuals who were cases for the disease in each data set and analysis, and total number of individuals (cases + controls) from each data set and analysis are also shown. IVW, inverse-variance weighted; MR, Mendelian randomization; OR, odds ratio; WHI, Women’s Health Initiative; UKB, UK Biobank; NHS, Nurses Health Study; E2C2, Epidemiology of Endometrial Cancer Consortium; High density SNP analysis, High Density SNP Association Analysis of Lung Cancer; NCI GWAS, National Cancer Institute (NCI) Genome Wide Association Study (GWAS) of Lung Cancer in Never Smokers; PLCO+CSP-II GWAS, Prostate, Lung, Colon, Ovary Screening Trial + Cancer Prevention Study II; ARIC, Atherosclerosis Risk in Communities Study; CHS, Cardiovascular Health Study; FHS, Framingham Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; WTCCC2, Wellcome Trust Case Control Consortium 2
Discussion
We estimated causal associations between ANM and several ageing-associated traits using the principal of MR in the WHI, UKB and several other data sets. Our principal findings are a causal decrease in the risk of fractures and osteoporosis, a lack of causal association between ANM and atherosclerotic cardiovascular diseases of CHD and ischaemic stroke, and a causal increase in the risk of lung cancer with an increased ANM. Of these three causal associations, only the findings related to bone health were consistent with findings from the analogous observational analysis. Whereas a lack of a causal protective effect between ANM findings for CHD and stroke may be expected given results of recent randomized–controlled studies (RCTs) of MHT, the causal increased risk for lung cancer has never been previously reported. Our study is the first to our knowledge to study the causal relationship between ANM and many of these adverse outcomes using an MR analysis strictly limited to post-menopausal women. This segmentation is important to ensuring accurate inference in an MR study of adverse health outcomes related to ANM.
The protective effect of older ANM for fractures and osteoporosis is expected given the compelling basic science and clinical evidence linking these health traits. Menopause is accompanied by a well-documented accelerated rate of bone loss whose mechanism is supported by extensive experimental evidence demonstrating the adverse effects of oestrogen deficiency on the basic multicellular units responsible for bone remodeling.18 Current recommendations for the prevention of osteoporosis include screening for post-menopausal women and the World Health Organization fracture risk assessment model considers early menopause to be a risk factor for osteoporosis.27,28 Furthermore, multiple RCTs have shown the value of MHT in the prevention of osteoporosis.29 Whereas this benefit appears to be maximized when MHT is instituted immediately after menopause, it is also observed among women who started MHT at a much later age. Nevertheless, MHT is approved only for the prevention of osteoporosis; bisphosphonates are the current recommendation for the management of osteoporosis in both older men and women given the balance between other beneficial and adverse effects of MHT.27,28
The convincingly absent causal effect of ANM on atherosclerotic cardiovascular diseases despite a strong inverse observational association suggests a substantial role of residual confounding and does not support the hypothesis that adverse changes to lipid profiles attributed specifically to the menopausal transition and independent of age are responsible for a protection from adverse health-related outcomes with an older ANM.30–32 MHT was originally studied for lipid profile improvement with two randomized crossover studies of conjugated estrogens in post-menopausal women with or without hyperlipidemia finding modest reductions in low-density lipoprotein of 15% and 24%, respectively.33,34 However, the WHI trials found that MHT slightly increased the risk of both CHD and stroke, though neither was significant after multiple test correction.29 These null findings may be due to a combination of both positive and negative effects of oral MHT on lipids, coagulation, inflammation and endothelial function.35,36
Our study is the first to our knowledge to implicate a causal association between an older ANM and a higher risk of post-menopausal lung cancer. Our MR analyses for this outcome were directionally opposite to the statistically significant protective effect of increased ANM documented in our observational analyses as well as that in other studies. This notable inconsistency suggests the presence of very substantial confounding that could be driven by factors such as smoking, diet and exercise.8,19 Opposing directions of observational and MR studies are not common but have been documented in other settings including with the moderate use of alcohol.37 Although our study is the first to link lung cancer and ANM through MR, another MR study of lung function similarly found early menopause associated with poorer lung function in observational analysis, but later menopause associated with poorer lung function in genetically instrumented analysis.38 Similarly to other cancers such as breast and endometrial cancers, our findings suggest that a greater length of exposure to oestrogen with a later ANM may promote the transformation of lung cells or the growth and spread of existing subclinical primary lung tumours. In support of this hypothesis are numerous molecular studies of lung cancer which have found that oestrogen receptors (ERs) are present in lung tumours,39 that both ER-alpha and ER-beta expression in cytoplasm are associated with poor lung cancer prognosis (with mixed evidence for nuclear ER-beta)39,40 and that suppression of each of these receptors reduces lung cancer proliferation in vitro.41 Multiple clinical outcome studies among cancer patients also provide persuasive evidence in support of this hypothesis. For example, the use of oestrogen plus progestin was associated with a statistically significant hazard ratio of death from lung cancer in the WHI trials.42 Furthermore, women who smoke are at greater risk than men who smoke and an oestrogen-by-smoking interaction has been hypothesized to explain this trend.39,43 Finally, observational analyses of population cancer data sets have found that anti-oestrogen therapies improve lung cancer-specific survival among patients with lung cancer in the presence or absence of prior history of breast cancer.44,45 If widely replicated, our findings suggest that anti-oestrogen therapies could be repurposed to treat or prevent lung cancers in women at high risk assuming that well powered RCTs are able to prove their benefits in this context.
A principal strength of our study was the MR design we implemented. First, we used two large studies with extensive, reliable and broad ascertainment of health outcomes to conduct a comprehensive MR study involving multiple outcomes relevant to the ANM. The one-sample MR design implemented in the WHI and UKB allowed us to analyse only post-menopausal women. Whereas ANM was not always available in the additional replication cohorts, access to individual-level data allowed two-sample analyses that were still restricted to post-menopausal women. Lastly, we found that the IV we used was largely independent of baseline characteristics that could confound our associations. We adjusted associations for baseline variables found to be nominal associated with our IV or found more significant associations to be inconsequential to the final results as documented through our multivariable MR sensitivity analyses.
The main limitation of our MR study was the potential for inadequate power for some of our outcomes despite the large sample sizes overall. Power in MR studies of binary outcomes is a function of sample size, variance explained of the exposure by the IV, the proportion of cases, the type-1 error rate specified and the true underlying causal OR, which is often not known.46 Further, SNPs in the instrumental variable were well imputed in the main one-sample MR analyses involving the WHI and UKB but were sometimes not as well imputed in other data sets (Supplementary Table S7, available as Supplementary data at IJE online), which could reduce precision for these two-sample MR replication data sets, impacting statistical power. Such a lack of power may be responsible for some of the statistically insignificant associations we observed in our study including for breast and endometrial cancers where substantial basic science, observational and RCT evidence exists implicating increased risk through the effects of prolonged exposure to either endogenous or exogenous estrogens on the cellular transformation of epithelial cells.15–17,29,47 For both breast and endometrial cancers, we are reassured by the fact that our MR results showed either a strong trend towards association overall (breast cancer) or in our main one-sample analysis (endometrial cancer), and that others have found a nominally significant or strongly positive association with larger sample sizes even if pre-menopausal cancers may have been included in the analysis.21,47,48 Our null association of ANM with Alzheimer disease has recently also been documented using MR in an independent study.49 The healthy cohort effect in UKB is also a known weakness that may have limited the number of cases and the generalizability of our findings.50 A weakness in phenotype definition was that osteoporosis and Alzheimer disease were self-reported in the WHI. However, the meta-analysis result still remained positive for osteoporosis and the result for Alzheimer disease was nearly identical to that of UKB where cases were derived from hospital records. These self-reported phenotypes therefore did not change the overall findings. This study included only participants of European ancestry not only because White women were the majority of participants in most of the cohorts we examined, but also because the instruments were discovered through GWAS in predominantly White women. The causal effects of ANM should also be studied in more diverse populations as the diversity of biobank studies increases. Lastly, self-reported race and ethnicity were used for some cohorts, which could affect estimates of SNP effects in these cohorts.
In conclusion, we report for the first time that a later ANM may causally increase the risk of lung cancer despite decreasing the risk of osteoporosis and fractures. Our findings point to the need for further RCTs of anti-oestrogen therapies for the prevention or the treatment of lung cancer among post-menopausal women should our results be replicated in additional population genetic data sets.
Ethics approval
The WHI project was reviewed and approved by the Fred Hutchinson Cancer Research Center (Fred Hutch) IRB in accordance with the US Department of Health and Human Services regulations at 45 CFR 46 (approval number: IR# 3467-EXT). Participants provided written informed consent to participate. Additional consent to review medical records was obtained through signed written consent. Fred Hutch has an approved federalwide assurance (FWA) on file with the Office for Human Research Protections (OHRP) under assurance number 0001920. The UK Biobank data were accessed under Application Number 13721. The Research Ethics Committee reference for UK Biobank is 16/NW/0274. The Stanford IRB reviewed the protocol and determined the research did not include human patients as defined in 45 CFR 46, nor 21 CFR 56. Use of dbGaP and EGA data sets was approved by Stanford University IRB protocol #40313. The dbGaP data were accessed under project #2638. The EGA data were accessed under request ID #3367.
Supplementary Material
Acknowledgements
The WHI programmes are funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004 and 75N92021D00005. We acknowledge the following WHI investigators—programme office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD); clinical coordinating centre: Garnet Anderson, Ross Prentice, Andrea LaCroix and Charles L. Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA); investigators and academic centres: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC), Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA), Rebecca Jackson (The Ohio State University, Columbus, OH), Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ), Jean Wactawski-Wende (University at Buffalo, Buffalo, NY), Marian Limacher (University of Florida, Gainesville/Jacksonville, FL), Jennifer Robinson (University of Iowa, Iowa City/Davenport, IA), Lewis Kuller (University of Pittsburgh, Pittsburgh, PA), Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC), Robert Brunner (University of Nevada, Reno, NV), Karen L. Margolis (University of Minnesota, Minneapolis, MN); WHI memory study: Mark Espeland (Wake Forest University School of Medicine, Winston-Salem, NC).
Conflict of interest
None declared.
Contributor Information
Joanna Lankester, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; VA Palo Alto Health Care System, Palo Alto, CA, USA.
Jin Li, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Thermofisher Scientific, South San Francisco, CA, USA.
Elias Levy Itshak Salfati, Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA, USA.
Marcia L Stefanick, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, CA, USA.
Kei Hang Katie Chan, Departments of Biomedical Sciences and Electrical Engineering, City University of Hong Kong, Kowloon Tong, Hong Kong; Center for Global Cardiometabolic Health, Brown University, Providence, RI, USA; Department of Epidemiology, School of Public Health, Brown University, Providence, RI, USA.
Simin Liu, Department of Epidemiology, School of Public Health, Brown University, Providence, RI, USA; Department of Medicine & Department of Surgery, Alpert School of Medicine, Brown University, Providence, RI, USA.
Carolyn J Crandall, Department of Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Shoa L Clarke, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; VA Palo Alto Health Care System, Palo Alto, CA, USA.
Themistocles L Assimes, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; VA Palo Alto Health Care System, Palo Alto, CA, USA.
Data availability
All data sets used in this study are publicly available to researchers by creating an account for the relevant data sources and following posted instructions for obtaining access. Primary data from the Women's Health Initiative used for this analysis are available through dbGap and the WHI website: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000746.v3.p3; https://www.whi.org. Phenotypic WHI data alone are available through BioLINCC: https://biolincc.nhlbi.nih.gov/home/. Data from UK Biobank can be accessed through the website: https://www.ukbiobank.ac.uk/. Data from other sources are available through the NCBI’s database of Genotypes and Phenotypes (dbGaP) and the European Genomic Archive (EGA): https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000280.v7.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000287.v7.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000893.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000336.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000007.v32.p13; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000753.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000788.v2.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000674.v3.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000209.v13.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000634.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000147.v3.p1; https://ega-archive.org/datasets/EGAD00010000264.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
J.L.2 and T.L.A. conceived of the idea for the paper. J.L.1, J.L.2 and E.L.I.S. curated data for the analyses. J.L.1 and J.L.2 conducted the analyses. J.L.1, J.L.2, S.L.C. and T.L.A. wrote the first draft. All authors contributed to the interpretation of the findings, critically revised the manuscript and approved the final version of the manuscript. J.L.1, S.L.C. and T.L.A. are guarantors of the work.
Funding
J.L.1 was supported by the National Heart, Lung, and Blood Institute through grant number 5F32HL149254-02 and by a Big Data Scientific Training Enhancement Program (BD-STEP) fellowship through the Palo Alto Veteran's Affairs.
References
- 1. Trichopoulos D, MacMahon B, Cole P.. Menopause and breast cancer risk. J Natl Cancer Inst 1972;48:605–13. [PubMed] [Google Scholar]
- 2. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dossus L, Allen N, Kaaks R. et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010;127:442–51. [DOI] [PubMed] [Google Scholar]
- 4. Karageorgi S, Hankinson SE, Kraft P, De Vivo I.. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses' Health Study cohort 1976-2004. Int J Cancer 2010;126:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baik CS, Strauss GM, Speizer FE, Feskanich D.. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev 2010;19:2525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinton LA, Gierach GL, Andaya A. et al. Reproductive and hormonal factors and lung cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer Epidemiol Biomarkers Prev 2011;20:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H.. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019;11:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mondul AM, Rodriguez C, Jacobs EJ, Calle EE.. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–97. [DOI] [PubMed] [Google Scholar]
- 9. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D.. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause 2012;19:1081–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu DS, Chung HF, Dobson A. et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welten S, Onland-Moret NC, Boer JMA, Verschuren WMM, van der Schouw YT.. Age at menopause and risk of ischemic and hemorrhagic stroke. Stroke 2021;52:2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pouilles JM, Tremollieres F, Bonneu M, Ribot C.. Influence of early age at menopause on vertebral bone mass. J Bone Miner Res 1994;9:311–15. [DOI] [PubMed] [Google Scholar]
- 13. Qiu C, Chen H, Wen J. et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013;98:1612–21. [DOI] [PubMed] [Google Scholar]
- 14. Georgakis MK, Kalogirou EI, Diamantaras AA. et al. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta-analysis. Psychoneuroendocrinology 2016;73:224–43. [DOI] [PubMed] [Google Scholar]
- 15. Deblois G, Giguere V.. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer 2013;13:27–36. [DOI] [PubMed] [Google Scholar]
- 16. Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer 2006;6:360–68. [DOI] [PubMed] [Google Scholar]
- 17. Jeon SY, Hwang KA, Choi KC.. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol 2016;158:1–8. [DOI] [PubMed] [Google Scholar]
- 18. Manolagas SC, O'Brien CA, Almeida M.. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013;9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JS, Choe JP, Park JH, Yoo E, Lee JM.. The comparison of physical activity, sedentary behavior, and mental health between early menopausal women and age-matched general middle-aged women. IJERPH 2021;18:7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgess S, Davey Smith G, Davies NM. et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 2019;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruth KS, Day FR, Hussain J. et al. ; 23andMe Research Team. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021;596:393–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson GL, Manson J, Wallace R. et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 23. The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 24. Sudlow C, Gallacher J, Allen N. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Day FR, Ruth KS, Thompson DJ. et al. ; LifeLines Cohort Study. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess S, Thompson SG.. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. Boca Raton: Chapman & Hall/CRC, 2014. [Google Scholar]
- 27. Conley RB, Adib G, Adler RA. et al. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res 2020;35:36–52. [DOI] [PubMed] [Google Scholar]
- 28. Cosman F, de Beur SJ, LeBoff MS. et al. ; National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossouw JE, Anderson GL, Prentice RL. et al. ; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 30. Matthews KA, Crawford SL, Chae CU. et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiacek M, Hagner W, Zubrzycki IZ.. Measures of menopause driven differences in levels of blood lipids, follicle-stimulating hormone, and luteinizing hormone in women aged 35 to 60 years: National Health and Nutrition Examination Survey III and National Health and Nutrition Examination Survey 1999-2002 study. Menopause 2011;18:60–66. [DOI] [PubMed] [Google Scholar]
- 32. Cui Y, Ruan X, Jin J, Jin F, Brucker S, Mueck AO.. The pattern of lipids and lipoproteins during the menopausal transition in Chinese women. Climacteric 2016;19:292–98. [DOI] [PubMed] [Google Scholar]
- 33. Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM.. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med 1991;325:1196–204. [DOI] [PubMed] [Google Scholar]
- 34. Darling GM, Johns JA, McCloud PI, Davis SR.. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N Engl J Med 1997;337:595–601. [DOI] [PubMed] [Google Scholar]
- 35. Rosano GM, Vitale C, Fini M.. Hormone replacement therapy and cardioprotection: what is good and what is bad for the cardiovascular system? Ann N Y Acad Sci 2006;1092:341–48. [DOI] [PubMed] [Google Scholar]
- 36. Shufelt CL, Manson JE.. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J Clin Endocrinol Metab 2021;106:1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holmes MV, Dale CE, Zuccolo L. et al. ; InterAct Consortium. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014;349:g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Plaat DA, Pereira M, Pesce G. et al. Age at menopause and lung function: a Mendelian randomisation study. Eur Respir J 2019;54:1802421. [DOI] [PubMed] [Google Scholar]
- 39. Hsu LH, Chu NM, Kao SH.. Estrogen, estrogen receptor and lung cancer. IJMS 2017;18:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rothenberger NJ, Somasundaram A, Stabile LP.. The role of the estrogen pathway in the tumor microenvironment. IJMS 2018;19:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marquez-Garban DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ.. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007;72:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chlebowski RT, Schwartz AG, Wakelee H. et al. ; Women's Health Initiative Investigators. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuentes N, Silva Rodriguez M, Silveyra P.. Role of sex hormones in lung cancer. Exp Biol Med (Maywood) 2021;246:2098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chu SC, Hsieh CJ, Wang TF, Hong MK, Chu TY.. Antiestrogen use in breast cancer patients reduces the risk of subsequent lung cancer: a population-based study. Cancer Epidemiol 2017;48:22–28. [DOI] [PubMed] [Google Scholar]
- 45. Hsu LH, Feng AC, Kao SH. et al. Second primary lung cancers among breast cancer patients treated with anti-estrogens have a longer cancer-specific survival. Anticancer Res 2015;35:1121–27. [PubMed] [Google Scholar]
- 46. Brion MJ, Shakhbazov K, Visscher PM.. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 2013;42:1497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manson JE, Chlebowski RT, Stefanick ML. et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Mara TA, Glubb DM, Amant F. et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun 2018;9:3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li M, Lin J, Liang S. et al. The role of age at menarche and age at menopause in Alzheimer's disease: evidence from a bidirectional Mendelian randomization study. Aging (Albany NY) 2021;13:19722–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fry A, Littlejohns TJ, Sudlow C. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sets used in this study are publicly available to researchers by creating an account for the relevant data sources and following posted instructions for obtaining access. Primary data from the Women's Health Initiative used for this analysis are available through dbGap and the WHI website: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000746.v3.p3; https://www.whi.org. Phenotypic WHI data alone are available through BioLINCC: https://biolincc.nhlbi.nih.gov/home/. Data from UK Biobank can be accessed through the website: https://www.ukbiobank.ac.uk/. Data from other sources are available through the NCBI’s database of Genotypes and Phenotypes (dbGaP) and the European Genomic Archive (EGA): https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000280.v7.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000287.v7.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000893.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000336.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000007.v32.p13; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000753.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000788.v2.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000674.v3.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000209.v13.p3; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000634.v1.p1; https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000147.v3.p1; https://ega-archive.org/datasets/EGAD00010000264.