Abstract
Background
Whether changes in stroke mortality are affected by age distribution and birth cohorts, and if the decline in stroke mortality exhibits heterogeneity by stroke type, remains uncertain.
Methods
We undertook a sequential time series analysis to examine stroke mortality trends in the USA among people aged 18–84 years between 1975 and 2019 (n = 4 332 220). Trends were examined for overall stroke and by ischaemic and haemorrhagic subtypes. Mortality data were extracted from the US death files, and age-sex population data were extracted from US census. Age-standardized stroke mortality rates and incidence rate ratio (IRR) with 95% confidence interval [CI] were derived from Poisson regression models.
Results
Age-standardized stroke mortality declined for females from 87.5 in 1975 to 30.9 per 100 000 in 2019 (IRR 0.27, 95% CI 0.26, 0.27; average annual decline -2.78%, 95% CI -2.79, -2.78). Among males, age-standardized mortality rate declined from 112.1 in 1975 to 38.7 per 100 000 in 2019 (RR 0.26, 95% CI 0.26, 0.27; average annual decline -2.80%, 95% CI -2.81, -2.79). Stroke mortality increased sharply with advancing age. Decline in stroke mortality was steeper for ischaemic than haemorrhagic strokes.
Conclusions
Stroke mortality rates have substantially declined, more so for ischaemic than haemorrhagic strokes.
Keywords: Stroke, haemorrhagic stroke, ischaemic stroke, mortality, sex, age-period-cohort analysis
Key Messages.
With dramatic shifts in the age distribution of the population, whether declines in stroke mortality rates are affected by the underlying age distribution, and if the decline in stroke mortality exhibits heterogeneity in patterns by stroke type, age and sex, remains uncertain.
Although stroke mortality rates have declined, the decline has been steeper for ischaemic than haemorrhagic stroke.
This study underscores opportunities to develop public health campaigns and interventions to reduce untoward behaviours that negatively affect stroke and metabolic health.
Introduction
Cerebrovascular accident, or stroke, afflicts almost 800 000 people annually in the USA.1 Age-standardized stroke mortality declined from 42.0 per 10 000 people in 1990 to 28.7 per 10 000 people in 2017—a relative decline of 32% – yet stroke accounted for 5.2% of all deaths in the USA in 2017,2 making it the most burdensome neurological disorder.
Stoke is categorized into two main subtypes, ischaemic and haemorrhagic strokes. Ischaemic strokes, which comprise 80% to 85% of all strokes, are attributed to embolism, thrombosis and systemic hypoperfusion. Haemorrhagic strokes occur in the presence of a ruptured blood vessel(s) and include intracerebral and subarachnoid haemorrhages. Each subtype has distinct aetiologies and epidemiological risk factors, including differences based on non-modifiable (age, sex, race/ethnicity) and modifiable risk factors.1 Hyperlipidaemia, diabetes mellitus, comorbid cardiac conditions and physical inactivity are associated with ischaemic but not haemorrhagic strokes.3,4 This classification of strokes also appears to have differences in both sex and age at onset.5
What accounts for declining stroke mortality rates is largely unknown. With the dramatic shifts in the age distribution of the American population, whether declines in stroke mortality rates are affected by the underlying age distribution, and if the decline in stroke mortality exhibits heterogeneity in patterns by stroke type, age and sex, remains uncertain. How these factors contribute to trends in stroke mortality based on stroke subtypes is also uncertain.
We examined three closely related factors that provide insight into trends in stroke mortality: age, year of death (period) and birth year (cohort). The changing age distribution, as well as stroke-related mortality, are rooted in both biological determinants and environmental factors. Therefore, we examined population trends in overall female and male stroke mortality rates and by stroke subtype in the USA over the past four decades.
Methods
Data source and study design
We undertook a sequential time series analysis, using data from two sources. First, we extracted data on US deaths from vital statistics death files, a nationwide, population-based death registry, from 1975 through 2019 (latest year available). These data are derived from standard death certificates in the 50 states and the District of Columbia and forwarded to the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention, and include over 99% of deaths in the USA. Second, for data on the population, we accessed the annual (1975–2019) US census mid-year population estimates by age and sex from the US Census Bureau.6 This manuscript has been structured to follow the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. All data used in this study are publicly available and de-identified; therefore, we did not seek approval from an institutional review board.
Stroke mortality data
We restricted the analysis to people who were aged 18 to 84 years with stroke listed as the primary cause of death. We excluded people aged ≥85 years because the US census data do not consistently report yearly population counts at or beyond age 85 years. In addition, we examined mortality trends based on ischaemic and haemorrhagic stroke types. We also examined deaths from embolic and thrombotic stroke as the sub-classifications of ischaemic stroke; and subarachnoid haemorrhage and intracerebral haemorrhage as the sub-classifications of haemorrhagic stroke. The primary cause of death was based on the International Classification of Disease (ICD), eighth revision (1975–78), ICD-9 (1979-1998) and ICD-10 (1999-2019) (Supplementary Table S1, available as Supplementary data at IJE online).
Statistical analysis
Sex-specific, age-standardized stroke mortality rates (per 100 000 population) with 95% confidence interval (CI) were estimated for overall stroke mortality and by stroke type. Age-standardization was based on age distribution of the mid-year US population (direct standardization method) for females and males. Sex-specific temporal changes in stroke mortality from 1975 to 2019 overall and by stroke type were examined by calculating the incidence rate ratio (IRR) with 95% CI.
Prior to implementing an age-period-cohort (APC) modelling approach, we examined the observed stroke mortality rates in a series of four plots: (i) mortality rates by age, with observations within each period connected, depicting cross-sectional age-specific rates; (ii) mortality rates by age, with observations within each birth cohort connected, depicting longitudinal age-specific mortality rates; (iii) mortality rates by period, with observations within each age-class connected; and (iv) mortality rates by birth cohort, with observations within each age-class connected. Mortality patterns from these plots guided the formalization of the APC modelling framework.
An APC analysis was performed by modelling trends in stroke mortality rates in relation to age, year of death (period) and year of birth (cohort). The numbers of stroke deaths were cross-classified by age, period and cohort (all in single years), with the mid-year census population as an offset variable in a Poisson regression model. Since APC models have an inherent identifiability problem (age = cohort-period), modelling was implemented after the imposition of a pre-specified set of constraints. Modelling began with stroke mortality rates as a function of age, followed by an overall linear trend in rates, which reflects the sum of the linear component of period and cohort effects (also referred to as the ‘drift’ parameter, representing the average annual change in rates). Deviations from linearity which can be uniquely attributed to the period and cohort effects were then assessed (also referred to as the ‘curvature’ effect). These parameter estimates can be interpreted as the direction and magnitude of the change in the linear trend by period and cohort. All models were determined a priori and sequentially fit beginning with age only, then adding the drift parameter and subsequently adding the period and cohort. Age, period and cohort were modelled as flexible non-linear smooth functions based on natural splines each with 10 knots. Given the large size of the study with wide ranges of age, period and birth cohorts, 10 knots were used to balance bias-efficiency trade-off and to identify important curvature effects.
The APC analysis was performed in R implemented in the Epi package7 in the RStudio (version 1.2).
Patient and public involvement
No patient nor the public was involved in the design, analysis, interpretation and reporting of results in this study.
Results
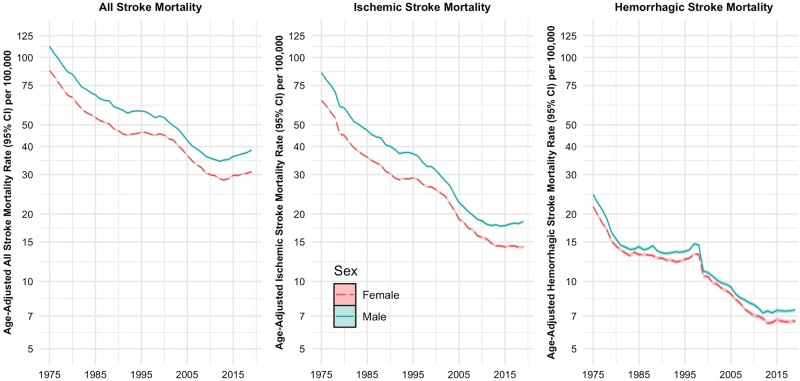
During the 45-year period, the total number of stroke deaths among people aged 18 to 84 years decreased from 2 152 881 in 1975 to 1 907 378 in 2019. The age-standardized stroke mortality rates declined among females [87.5, 95% confidence interval (CI) 86.8, 88.1 in 1975 to 30.9, 95% CI 30.6, 31.2 per 100 000 in 2019) and males (112.1, 95% CI 111.3, 113.0 in 1975 to 38.7, 95% CI 38.3, 39.0 per 100 000 in 2019) (Table 1). Also, both ischaemic and haemorrhagic stroke mortality rates declined for females and males during the study period (Table 1 and Figure 1). Mortality rates from subarachnoid and intracerebral haemorrhages also declined among females and males.
Table 1.
Age-standardized mortality rates from any stroke and ischaemic and haemorrhagic stroke types in females and males in 1975 and 2019 in the USA
| Number of stroke deaths |
Age-standardized mortality rate (95% CI) per 100 000 population |
Incidence rate ratioa (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| 1975–2019 | 1975 | 2019 | 1975–2019 | 1975 | 2019 | ||
| Females | |||||||
| Any stroke | 2 289 470 | 75 947 | 41 798 | 44.4 (44.4, 44.5) | 87.5 (86.8, 88.1) | 30.9 (30.6, 31.2) | 0.27 (0.26, 0.27) |
| Ischaemic stroke | 1 369 772 | 44 031 | 19 334 | 26.3 (26.2, 26.3) | 50.2 (49.7, 50.6) | 14.2 (14.0, 14.4) | 0.21 (0.20, 0.21) |
| Haemorrhagic stroke | 533 668 | 17 862 | 8843 | 10.8 (10.7, 10.8) | 21.6 (21.2, 21.9) | 6.7 (6.5, 6.8) | 0.35 (0.34, 0.36) |
| Subarachnoid haemorrhage | 165 451 | 4860 | 2965 | 3.5 (3.4, 3.5) | 6.2 (6.0, 6.3) | 2.3 (2.2, 2.4) | 0.32 (0.31, 0.33) |
| Intracerebral haemorrhage | 341 719 | 11 903 | 5878 | 6.8 (6.8, 6.8) | 14.1 (13.8, 14.4) | 4.4 (4.3, 4.5) | 0.24 (0.23, 0.25) |
| Males | |||||||
| Any stroke | 2 042 751 | 67 770 | 43 639 | 53.3 (53.3, 53.4) | 112.1 (111.3, 113.0) | 38.7 (38.3, 39.0) | 0.26 (0.26, 0.27) |
| Ischaemic stroke | 1 220 040 | 40 613 | 20 854 | 32.3 (32.2, 32.3) | 68.0 (67.3, 68.6) | 18.5 (18.3, 18.8) | 0.21 (0.20, 0.21) |
| Haemorrhagic stroke | 460 967 | 15 608 | 8588 | 11.5 (11.5, 11.6) | 24.4 (24.0, 24.8) | 7.5 (7.4, 7.6) | 0.36 (0.35, 0.37) |
| Subarachnoid haemorrhage | 98 045 | 3275 | 1873 | 2.3 (2.3, 2.3) | 4.8 (4.7, 5.0) | 1.6 (1.5, 1.7) | 0.28 (0.26, 0.29) |
| Intracerebral haemorrhage | 334 481 | 10 738 | 6715 | 8.4 (8.4, 8.5) | 17.0 (16.7, 17.4) | 5.9 (5.7, 6.0) | 0.27 (0.26, 0.28) |
Female population at risk: 4 607 238 284 in 1975–2019; 76 552 203 in 1975; and 126 466 141 in 2019. Male population at risk: 4 349 115 179 in 1975–2019; 70 575 827 in 1975; and 121 029 013 in 2019. Standardization was based on the women’s and men’s age distribution of the annual mid-year US population.
Incidence rate ratios contrast age-standardized stroke mortality rates in 1975 versus 2019 (reference).
Figure 1.
Age-standardized overall stroke and ischaemic and haemorrhagic stroke mortality rates (with 95% confidence intervals) among females and males: USA, 1975 to 2019
Stroke mortality trends
Stroke mortality rates among females and males consistently increased for every fifth period by age groups between 20–24 and 80–84 years (Supplementary Table S2, available as Supplementary data at IJE online). Supplementary Figure S1 (available as Supplementary data at IJE online) shows the age-specific mortality rates overall and among ischaemic and haemorrhagic strokes by period. Mortality rates declined in general, but rates were higher among males than females. Supplementary Figure S2 (available as Supplementary data at IJE online) shows the age-specific mortality rates overall and among ischaemic and haemorrhagic strokes by birth cohort. Once again, age-specific stroke mortality rates declined with successive birth cohorts.
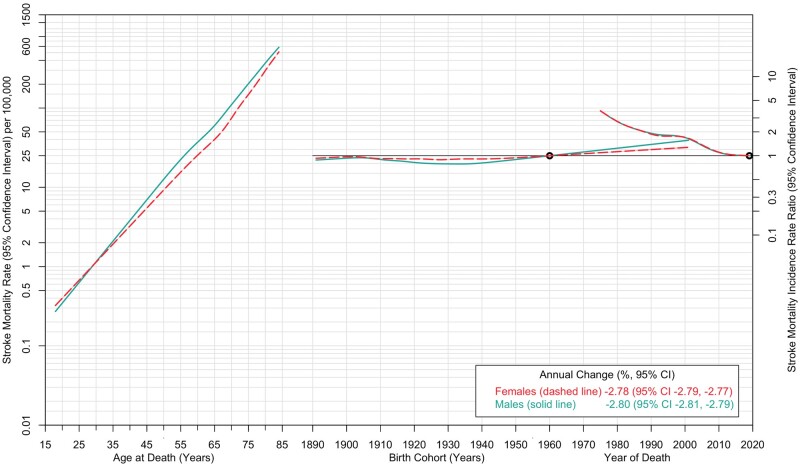
We fit an APC model to examine trends in overall stroke mortality rates (Figure 2). Mortality rates showed a steep linear increase by age, with lower rates among males compared with females prior to age 30 and higher rates thereafter. Sharp declines in stroke mortality rates were evident by period. The average age-adjusted annual decline in overall stroke mortality rates, reflecting a period change, was 2.78% and 2.80% among females and males, respectively.
Figure 2.
Age-period-cohort trends in overall stroke mortality rates among females and males: USA, 1975 to 2019. Stroke mortality rates (per 100 000 population) are shown in relation to age (for the reference period, 2019) on the left axis. The stroke mortality incidence rate ratio (95% confidence interval) is shown in relation to birth cohort (with the 1960 birth year as the reference) and period (with 2019 as the reference) on the right axis. The shaded bands denote 95% confidence intervals
Ischaemic and haemorrhagic stroke mortality trends
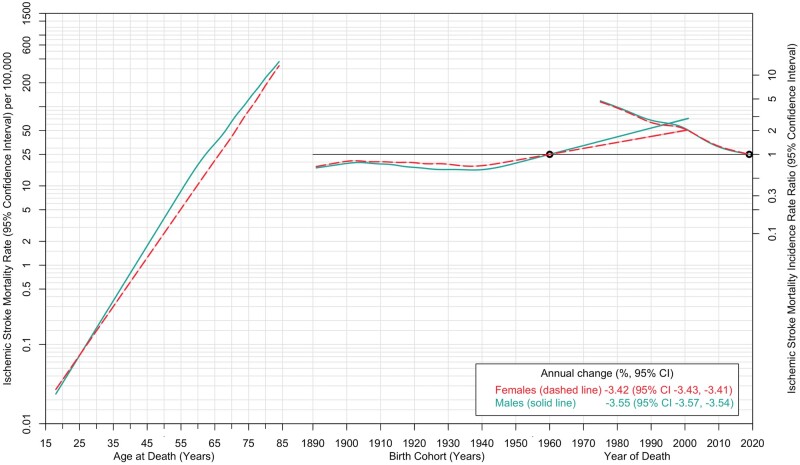
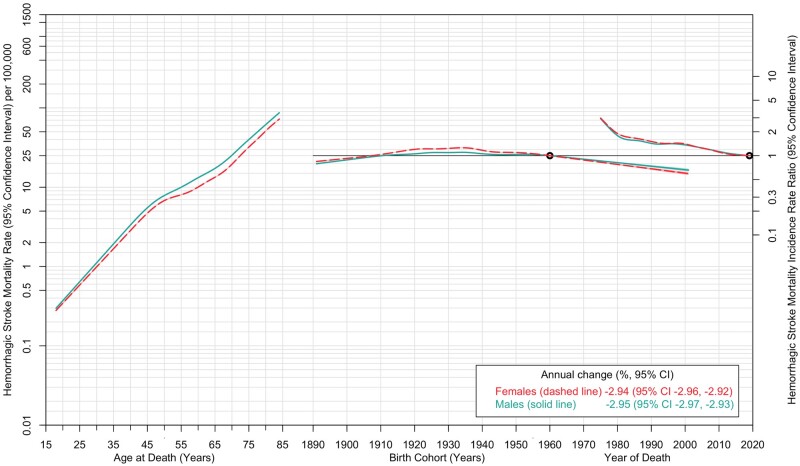
Mortality rates from ischaemic and haemorrhagic strokes declined between 1975 and 2010 and leveled off thereafter (Figure 1). Mortality rates from haemorrhagic stroke sharply declined between 1975 and 1979. Age-specific mortality rates in 2019 indicated a sharp linear increase, among both females and males (Supplementary Figure S3, available as Supplementary data at IJE online). Across every fifth-year age category, both ischaemic and haemorrhagic stroke mortality rates increased by period (Supplementary Tables S2 and S3, available as Supplementary data at IJE online). The APC analysis by stroke type showed that mortality rates from ischaemic stroke were strongly associated with age (Figure 3). Compared with the reference birth cohort (1960), mortality rates from ischaemic stroke showed an increase by birth cohort, with the those born in 1990–2000 demonstrating substantially increased IRR. A decline in ischaemic stroke mortality was observed by period. The analysis for mortality rates due to haemorrhagic stroke also demonstrated a strong age effect, with a sharp linear increase with age up to 50 years and less so at later years (Figure 4). Like ischaemic strokes, there were declining trends in rates of haemorrhagic stroke mortality by period, but in contrast there was no cohort effect. Trends in subarachnoid and intracerebral haemorrhagic stroke mortality are described in Supplementary Figure S4 (available as Supplementary data at IJE online), and the corresponding age, period and cohort trends for these subtypes are shown in Supplementary Figures S5 and S6 (available as Supplementary data at IJE online). A summary of the findings of this study is provided in the graphical abstract (Supplementary Figure S7, available as Supplementary data at IJE online).
Figure 3.
Age-period-cohort trends in ischaemic stroke mortality rates among females and males: USA, 1975 to 2019. Ischaemic stroke mortality rates (per 100 000 population) are shown in relation to age (for the reference period, 2019) on the left axis. The stroke mortality incidence rate ratio (95% confidence interval) is shown in relation to birth cohort (with the 1960 maternal birth year as the reference) and period (with 2019 as the reference) on the right axis. The shaded bands denote 95% confidence intervals
Figure 4.
Age-period-cohort trends in haemorrhagic stroke mortality rates among females and males: USA, 1975 to 2019. Haemorrhagic stroke mortality rates (per 100 000 population) are shown in relation to age (for the reference period 2019) on the left axis. The stroke mortality incidence rate ratio (95% confidence interval) is shown in relation to birth cohort (with the 1960 birth year as the reference) and period (with 2019 as the reference) on the right axis. The shaded bands denote 95% confidence intervals
Discussion
Despite the strong temporal decline in stroke mortality rates observed from 1975 to 2019, stroke is ranked as the fourth leading cause of death in the USA.8 There were two key findings in our study, which is one of the largest, population-based studies of stroke mortality trends in the US. Overall stroke mortality rates declined for both females and males, and the sex disparity in mortality rates has narrowed for ischaemic stroke, but widened for haemorrhagic stroke. We observed that mortality rates from ischaemic stroke, but not haemorrhagic stroke, showed an increase among those born in more recent birth cohorts.
The sex disparity in stroke deaths, which narrowed for ischaemic strokes, may be because older females have a greater prevalence of, and worse outcome after, ischaemic stroke than do males and younger females.9 In addition certain risk factors for stroke, such as hypertension and atrial fibrillation, tend to occur more often in older women,10 and other risk factors, such as diabetes mellitus and smoking, adversely affect females more than males.11 Females are also more likely to live alone prior to a stroke, which may affect stroke survival.12
We observed declines in stroke mortality overall and for stroke types, with a decline that was greater for ischaemic than haemorrhagic strokes. Although we did not examine non-fatal stroke complications, the Greater Cincinnati/Northern Kentucky Stroke Study showed that incidence rates of ischaemic, but not haemorrhagic, stroke declined between 1993 and 2015 in both sexes.13 The Framingham Heart study, with 68 years of follow-up, found that the incidence of intracerebral haemorrhagic stroke increased in the oldest patients.14 The reduction in ischaemic stroke mortality may be the result of increased use of aspirin and antithrombotic therapy.15 The better treatment of common causes of embolic stroke, including atrial fibrillation and comorbid cardiac conditions,16 may have also contributed to these trends. Mortality rates from haemorrhagic strokes declined sharply between 1975 and 1979 (Figure 1). Whereas reasons for this decline are not clear, we speculate that pharmacotherapeutic treatment for hypertension (a strong risk factor for haemorrhagic strokes1) plateaued after most people were treated to better blood pressure targets based on early studies in the 1960s and 1970s. Treatment for hypertension in the early years were the first concerted effort for most patients, although blood pressure goals dropped further in subsequent decades.17
A Global Burden of Disease study18 showed that stroke mortality rates in the USA dropped from 42.0 (95% CI 41.4, 42.9) in 1990 to 28.6 (95% CI 27.6, 29.5) per 100 000 in 2017, a relative decline of 32.0% (95% CI 29.8, 34.5). The study also showed that disability-adjusted life-years declined by 24.0% (95% CI 21.3, 26.9). Findings from the original and offspring data of the Framingham Study19 (9152 participants; follow-ups from 1950 to 1977, 1978 to 1989 and 1990 to 2004) showed that the age-adjusted annual incidence of stroke in people aged 55 to 94 years decreased from 6.2 to 5.1 per 1000 person-years for females and 7.6 to 5.3 per 1000 person-years for males. In the prospective Atherosclerosis Risk in Communities cohort20 (14 357 participants), stroke mortality decreased [hazard ratio (HR) 0.80, 95% CI 0.66, 0.98]. The decrease in stroke mortality was most pronounced among those aged <65 years (HR 0.65, 95% CI 0.46, 0.93).
Although females suffer higher rates of comorbidity and stroke severity than males,21 mortality rates from stroke are higher among males than females, an observation also seen in previous studies.22 It has been postulated that hormone effects contribute to better stroke protection among females.23 However, a study from the California Kaiser Permanente Medical Care Program showed that the odds ratio of ischaemic and haemorrhagic strokes among current users of oral contraceptives (compared with former users and women who had never used such drugs) were 1.18 (95% CI 0.54, 2.59) and 1.14 (95% CI 0.60, 2.16), respectively, after adjustment for other risk factors for stroke.24 Given these findings, it is unlikely that the hormone effect may account for the observed trends.
We observed a birth cohort effect in mortality rates from ischaemic, but not haemorrhagic, strokes; IRR for ischaemic stroke mortality were higher in the more recent birth cohorts compared with those born in 1960. Birth cohort is a marker of environmental and sociopolitical factors that impact on health outcomes. Notable factors such as famine, war, pandemics, pollution, climate change, behaviour (e.g. tobacco use) and diet (particularly high sodium intake25), have varied effects on people born at different periods. A similar cohort effect for ischaemic stroke complications with increased risks among those born after 1954 has also been reported.26 It has been speculated that the increasing prevalence of diabetes and obesity since the mid-1950s may have played a role in shaping mortality risks from ischaemic stroke. In contrast, birth cohorts exerted no effect on mortality rates for haemorrhagic stroke. This could be the result of an increase in treatment of hypertension, a major risk factor for haemorrhagic stroke.27 This also suggests that environmental factors may contribute less to this risk.
Several modifiable risk factors for stroke, including hypertension, high cholesterol, diabetes mellitus, overweight and obesity, as well as heart disease, are likely to have affected trends in stroke mortality.28 Smoking remains an important risk factor for stroke incidence and stroke mortality.1,29 The increased stroke risk is also evident for those with very low smoking exposure: the relative risk of stroke for females and males who smoked on average one cigarette per day was 1.46 (95% CI 1.20, 1.78) and 1.30 (95% CI 1.11, 1.53), respectively.29 Studies have also documented reduced stroke risks within 2–4 years among those who quit smoking.
Limitations of the study
Although we evaluated age, period and birth cohort trends in stroke mortality, we were unable to consider other important factors, including smoking, hypertension, dyslipidaemia and sodium intake, due to unavailability of data. Smoking is a recognized causal risk factor for stroke, and smoking cessation can reduce stroke risk, so further examination of how persistent smoking and quit patterns affect stroke mortality may shed important insights. Public health awareness regarding timely identification of the first symptoms of stroke and improvements in neurological management are likely to have affected stroke mortality rates. In particular, the use of antihypertensive medications, statins and aspirin may have affected the trends we report. Whereas these medications may reduce the burden of non-fatal stoke and its sequelae, they are unlikely to shift the trends in stroke mortality. In addition we report trends in stroke mortality, but how rates of non-fatal stroke have changed remains uncertain and is a topic worthy of investigation. We employed US census data wherein people were included each year of their lives. In some ways, this design is analogous to a longitudinal cohort where people enter the cohort at age 18 years, exit at 85 years (dead or alive) and are followed up every year in between. Nonetheless, given the retrospective nature of the study, the findings must be cautiously interpreted.
We are uncertain how the varying classification of stroke by type and subtype may have changed over time based on the utilized ICD codes.30 A study31 comparing ICD coding for stroke based on administrative data compared with data extracted from hospital medical records (gold standard) reported a kappa coefficient of 0.79 (95% CI 0.68, 0.90) using all discharge stroke diagnoses. The sensitivity, specificity and positive predictive values were superior for ischaemic stroke (86%, 95% and 90%, respectively; kappa 0.82, 95% CI 0.65, 0.98) and subarachnoid haemorrhage (98%, 92%, and 96%, respectively; kappa 0.87, 95% CI 0.71, 1.00) than intracerebral haemorrhage (82%, 93% and 80%, respectively; kappa 0.74, 95% CI 0.58, 0.90).31 These findings suggest the potential for minor misclassification of stroke, but not serious enough to wipe away the strong trends noted in this study.
Strengths of the study
The study’s greatest strengths are related to its size (4.3 million stroke deaths and almost 9 billion persons followed along the life course) and methodological rigor, which allowed us to identify subtle, yet clinically important, stroke mortality trends. Identification of deaths was restricted to stroke as the primary cause of death; this avoids confounding by other cardiovascular disease causes where stroke may constitute a secondary diagnosis. The findings are also generalizable to the US population. It is unlikely that a larger study could be designed to evaluate these trends. The study underscores the importance of tailored approaches for the prevention and treatment of these distinct clinical stroke types.
Conclusions
This population-based study in the USA shows that stroke mortality declined over the 45-year period, with greater reduction in ischaemic than haemorrhagic stroke mortality. Sharp increases in mortality with increasing age were observed. Although males were more likely to die from stroke than females, this sex difference narrowed for ischaemic stroke. The contribution of predisposing risk factors to declines in stroke mortality underscores opportunities to develop public health campaigns and interventions to reduce untoward behaviours that negatively affect stroke and metabolic health.
Ethics approval
Since all data used in this study were fully de-identified and publicly available, we did not require ethics approval.
Supplementary Material
Acknowledgements
We thank Nikki Brandt for generating the ‘infographic’ Supplementary Figure S7 (available as Supplementary data at IJE online). This manuscript was presented at the 2021 virtual American Heart Association annual meeting in the ‘Recent Advances in Stroke and Brain Health’ session.
Conflict of interest
None declared.
Contributor Information
Cande V Ananth, Division of Epidemiology and Biostatistics, Department of Obstetrics, Gynecology, and Reproductive Sciences, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA; Cardiovascular Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA; Department of Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA; Department of Biostatistics and Epidemiology, Rutgers School of Public Health, Piscataway, NJ, USA; Environmental and Occupational Health Sciences Institute, Rutgers Robert Wood Johnson Medical School, Piscataway, NJ, USA.
Justin S Brandt, Division of Maternal-Fetal Medicine, Department of Obstetrics, Gynecology, and Reproductive Sciences, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Katherine M Keyes, Department of Epidemiology, Joseph L. Mailman School of Public Health, Columbia University, New York, NY, USA.
Hillary L Graham, Division of Epidemiology and Biostatistics, Department of Obstetrics, Gynecology, and Reproductive Sciences, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
John B Kostis, Cardiovascular Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA; Department of Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
William J Kostis, Cardiovascular Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA; Department of Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Data availability
All data used in this study can be accessed from the Centers for Disease Control and Prevention [http://www.cdc.gov/] or from the National Bureau of Economics Research [https://www.nber.org].
Supplementary data
Supplementary data are available at IJE online.
Author contributions
C.V.A. conceptualized and designed the study; acquired and assembled the data; analysed and interpreted the data. C.V.A. and J.S.B. drafted the manuscript. All authors offered intellectual suggestions and critically revised the manuscript to its final form. All authors have given final approval and agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Funding
C.A., H.G. and W.K. are supported, in part, by the National Heart, Lung, and Blood Institute (grant R01-HL150065). C.A. is additionally supported, in part, by the National Institute of Environmental Health Sciences (grant R01-ES033190).
References
- 1. Boehme AK, Esenwa C, Elkind MS.. Stroke risk factors, genetics, and prevention. Circ Res 2017;120:472–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep 2019;68:1–77. [PubMed] [Google Scholar]
- 3. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Prospective studies collaboration. Lancet 1995;346:1647–53. [PubMed] [Google Scholar]
- 4. O'Donnell MJ, Xavier D, Liu L. et al. ; INTERSTROKE investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–23. [DOI] [PubMed] [Google Scholar]
- 5. Virani SS, Alonso A, Aparicio HJ. et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
- 6. United States Census Bureau. Age and Sex Tables. https://www.census.gov/topics/population/age-and-sex/data/tables.html (10 February 2021, date last accessed).
- 7. Carstensen B, Plummer M, Laara E, Hills M.. Epi: a Package for Statistical Analysis in Epidemiology (R Package Version 1.1.34). 2012. http://CRAN.R-project.org/package=Epi (20 April 2021, date last accessed).
- 8. Lackland DT, Roccella EJ, Deutsch AF. et al. ; Council on Functional Genomics and Translational Biology. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 2014;45:315–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bots SH, Peters SAE, Woodward M.. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health 2017;2:e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chugh SS, Havmoeller R, Narayanan K. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huxley RR, Woodward M.. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297–305. [DOI] [PubMed] [Google Scholar]
- 12. Branyan TE, Sohrabji F.. Sex differences in stroke co-morbidities. Exp Neurol 2020;332:113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madsen TE, Khoury JC, Leppert M. et al. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke 2020;51:1070–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lioutas VA, Beiser AS, Aparicio HJ. et al. Assessment of incidence and risk factors of intracerebral hemorrhage among participants in the Framingham Heart Study between 1948 and 2016. JAMA Neurol 2020;77:1252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perera KS, Ng KKH, Nayar S. et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS trial. JAMA Neurol 2020;77:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Marchis GM, Sposato LA, Kuhne M. et al. New avenues for optimal treatment of atrial fibrillation and stroke prevention. Stroke 2021;52:1490–99. [DOI] [PubMed] [Google Scholar]
- 17. Vasan RS, Beiser A, Seshadri S. et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA 2002;287:1003–10. [DOI] [PubMed] [Google Scholar]
- 18. Feigin VL, Vos T, Alahdab F. et al. ; GBD 2017 US Neurological Disorders Collaborators. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol 2021;78:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carandang R, Seshadri S, Beiser A. et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006;296:2939–46. [DOI] [PubMed] [Google Scholar]
- 20. Koton S, Schneider AL, Rosamond WD. et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–68. [DOI] [PubMed] [Google Scholar]
- 21. Gall SL, Donnan G, Dewey HM. et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology 2010;74:975–81. [DOI] [PubMed] [Google Scholar]
- 22. Reeves MJ, Bushnell CD, Howard G. et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kittner SJ, Stern BJ, Feeser BR. et al. Pregnancy and the risk of stroke. N Engl J Med 1996;335:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petitti DB, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK.. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996;335:8–15. [DOI] [PubMed] [Google Scholar]
- 25. Cao J, Eshak ES, Liu K, Gero K, Liu Z, Yu C.. Age-period-cohort analysis of stroke mortality attributable to high sodium intake in China and Japan. Stroke 2019;50:1648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swerdel JN, Rhoads GG, Cheng JQ. et al. Ischemic stroke rate increases in young adults: evidence for a generational effect? J Am Heart Assoc 2016;5:e004245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diener HC, Hankey GJ.. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC focus seminar. J Am Coll Cardiol 2020;75:1804–18. [DOI] [PubMed] [Google Scholar]
- 28. Hopstock LA, Morseth B, Cook S. et al. Treatment target achievement after myocardial infarction and ischaemic stroke: cardiovascular risk factors, medication use, and lifestyle: the Tromso Study 2015-16. Eur J Prev Cardiol 2022;29:362–70. [DOI] [PubMed] [Google Scholar]
- 29. Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D.. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018;360:j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischaemic stroke: effect of modifier codes. Stroke 1998;29:1602–604. [DOI] [PubMed] [Google Scholar]
- 31. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study can be accessed from the Centers for Disease Control and Prevention [http://www.cdc.gov/] or from the National Bureau of Economics Research [https://www.nber.org].