Abstract
Background
during pain assessment in persons unable to self-report, such as people living with dementia, vocalisations are commonly used as pain indicators. However, there is a lack of evidence from clinical practice regarding their diagnostic value and relationship with pain. We aimed to explore vocalisations and pain in people with dementia undergoing pain assessments in clinical practice settings.
Methods
a total of 22,194 pain assessments were reviewed in people with dementia (n = 3,144) from 34 different Australian aged care homes and two dementia specific programs. Pain assessments were conducted by 389 purposely trained health care professionals and cares using PainChek pain assessment tool. Vocalised expressions were determined based on nine vocalisation features included in the tool. Linear mixed models were used to examine the relationship of pain scores with vocalisation features. Using a single pain assessment for each of the 3,144 people with dementia, additional data analysis was conducted via Receiver Operator Characteristic (ROC) analysis and Principal Component Analysis.
Results
vocalisation scores increased with increasing pain intensity. High pain scores were more likely with the presence of sighing and screaming (8 times). The presence of vocalisation features varied depending on the intensity of pain. The ROC optimal criterion for the voice domain yielded a cut-off score of ≥2.0 with a Youden index of 0.637. The corresponding sensitivity and specificity were 79.7% [confidence interval (CI): 76.8–82.4%] and 84.0% (CI: 82.5–85.5%), respectively.
Conclusion
we describe vocalisation features during presence of different levels of pain in people with dementia unable to self-report, therefore providing evidence in regard to their diagnostic value in clinical practice.
Keywords: vocalisation features, voice, pain assessment, pain levels, PainChek, older people
Key Points
Vocalisations increase in higher pain intensities.
This study provides evidence regarding the diagnostic value of vocalisations during assessment of pain.
Our findings raise the possibility of digital phenotyping of vocalisations as a clinically relevant biomarker.
Introduction
In population groups with communication difficulties such as those living with moderate and severe dementia, vocalised and verbal disruptive behaviours occur commonly and are an important source of patient distress and caregiver burden [1]. They can also be troublesome to family and caregivers as well as cause reactive vocalisations in other patients [1, 2]. The American Geriatrics Society (AGS) suggests vocalisations are one of the key domains to consider when assessing pain in older adults with communication difficulties such as those living with dementia [3]. As a result, a number of vocalised expressions of pain have been proposed by a variety of observational pain assessment scales [4].
Currently, vocalised features of people with dementia are subjectively rated on observational pain scales [5–7] and surprisingly, there is no clear characterisation of these features in relation to the pain experience. As such, available scales vary in their content of vocalised expressions, ranging between non-verbal utterances, verbal utterances and breaths, and also vary in relation to differences between ordinal and binary assessment [6–17]. In addition, the AGS domains have been developed based on consensus rather than empirical results, therefore leaving open the possibility that pain assessment could be further explored through a more specific subset of these domains [18].
To date, no study has explored the relationship between vocalised expressions, pain and other non-vocalised pain indicators in adults with pain in large and clinical settings. In addition, less attention has been given to exploring vocalisations in the context of dementia, in comparison to other pain-related features such as facial expressions. Furthermore, although the literature suggests that voice parameters change in patients experiencing pain [19–21], there is a paucity of studies exploring more subtle characteristics of vocalisation in patients with pain in general.
Here we aimed to explore vocalised expressions of people with moderate to severe dementia undergoing pain assessments in clinical practice settings with the view of providing empirical evidence related to a subset of reported pain-indicative vocalised expressions. In particular, we aimed to identify which vocalisation features are present or absent during different levels of pain and the association of these features in relation to high pain scores. In addition, we also aimed explore the discriminating power of vocalisations for categorising pain. We leverage a unique large database of pain assessments collected using a technology-guided pain assessment tool known as PainChek® in clinical practice [22–24].
Methods
This was a 2-year retrospective study carried out in 34 Australian residential aged care homes (RACHs) and by two national dementia-specific behaviour support programs over the period of September 2017 to March 2019. A total of 22,194 pain assessments conducted by trained clinical staff during this time were reviewed. Pain assessments were completed using the PainChek® pain assessment tool, a six-domain point-of-care (POC) medical device that uses facial recognition and analysis technology to identify facial action units (smallest building blocks of facial expressions) indicative of pain [17, 25, 26]. This is done in real time using artificial intelligence (AI)-powered algorithms. Initially, the user assesses subject’s face via the Face domain (Domain 1; 9 features) and then digitally combines the resultant scores with those of five other domains: Voice (9 features), Movement (7 features), Behaviour (7 features), Activity (4 features) and Body (6 features). Each feature observed is given a score of 1, with the maximum score being 42. A final total pain score and severity (which includes voice score) is calculated automatically by totalling features recorded in each of the six domains. The total calculated pain score and severity belong to one of four categories: no pain (score: 0–6), mild pain (7–11), moderate pain (12–15) and severe pain (16–42).
The Voice domain assesses nine vocalisation features: noisy pain sounds (e.g., ‘ouch’, ‘ah’, ‘mm’), requesting help repeatedly, groaning, moaning, crying, screaming, loud talk, howling and sighing. These features, as in other non-facial domains, are assessed by trained assessors and manually entered into a digital checklist in the PainChek app at POC. Although completing the pain assessment, the PainChek user can click the information button adjacent to each vocal feature to find out a description of that feature. More information about the PainChek tool can be accessed elsewhere [27].
The study was approved (HR10/2014) by the Human Research Ethics Committee of Curtin University (Bentley, WA, Australia). Permission was also granted by PainChek Ltd (Sydney, NSW, Australia) to provide the dataset. The data comes from pain assessments conducted on POC smart devices that are automatically synchronised to a cloud repository database. This existing database is accessible via web administration portal (PainChek portal) allowing aggregation of deidentified data for the purposes of research and analysis as per the terms of the PainChek Application service agreement with the aged care provider.
Pain assessments and data collection
PainChek pain assessments were completed by 389 trained users (i.e., consultants, nurses, allied health professionals and care support staff) working in 34 different Australian RACHs across various Australian states and territories (Australian Capital Territory, Victoria, New South Wales, Queensland, South Australia and Western Australia) and two national dementia-specific care programs. Pain assessments were completed as part of routine patient care procedures. Prior to using the PainChek App, users received either face-to-face or online training which lasted between 1.5–2 h. This training was essential to ensure that users were competent in using the tool and to meet the regulatory standards of quality and safety. Training received by PainChek users, in addition to ensuring competency in using the PainChek tool also included information on challenges of pain assessment in dementia, pain behaviours in people with dementia, compromised ability to self-report as well as pain triggers in people with dementia. This training guided PainChek users to first assess the capacity for self-report as a means of assessing pain and only proceed with pain assessment using the PainChek tool if this ability to self-report is compromised in the person with dementia. The actual recording of completed pain assessments is a part of a workflow platform that includes the PainChek App (i.e. the pain assessment tool) and the PainChek Portal. The PainChek Portal is a central web-based repository that allows the aggregation, storage and retrieval of electronic records of all pain assessment data from the PainChek App. The deidentified data which were provided by PainChek Ltd for the purposes of this study included: demographics of users and patients, chronological logs of pain assessments, pain scores, pain intensity categories and the features recorded in each of the six PainChek domains for each assessment.
Data analysis
We used SPSS version 27 (IBM Corp. 2020) for the data analysis unless stated otherwise. For all statistical tests, P < 0.05 was adopted to assess statistical significance, with confidence intervals (CIs) reported as appropriate. Key demographics and proportion of total assessments conducted were described using frequency (f) and percent (%), mean (M) and standard deviation (SD).
A Voice domain score was computed by summing the Voice domain items and described (right skewed Kolmogorov–Smirnov) using M, SD, median (Md) and 25–75% interquartile range (IQR). Each Voice domain item was described using f and % for the total sample, four pain categories (none [pain score 0–6], mild [7–11], moderate [12–15] and severe [16+]), and for the dichotomised pain category (low [none and mild], high [moderate and severe]).
Total pain score which includes the voice score (right skewed Kolmogorov–Smirnov) was described for each Voice domain item using M, SD, Md and IQR. Cohen’s d effect size with 95% CIs and Common Language Effect Size (CLES) for non-parametric data was computed and interpreted as small (0.2), medium (0.5) and large (0.8) [27].
Linear mixed models (LMMs) were used to examine the relationship of total pain score with Voice domain score and demographic patient items. LMMs are flexible models that account for correlated errors associated with repeated, continuous and correlated observations and account for missing data. Two models were examined: one with Voice domain score (covariate) and the other with each Voice domain item (factor). The LMM examined pain scores as a continuous outcome, including fixed effects such as age (covariate), sex and potential confounders aged care home and assessor role (factors). The patient identifier was set as a random effect with a variance components covariance matrix. A restricted maximum likelihood method of estimation was selected. Model residuals were inspected and where violations were noted, a log transformed dependent variable was used to resolve the violation. In addition, an interaction effect between age care home/program and assessor role was examined with model fit assessed by Akaike’s Information Criterion (AIC), with lower AIC indicating an improved model fit. A separate comparison analysis was conducted with the outcome total pain score (minus voice score) and the results did not change.
The likelihood of a high pain category compared with low pain was examined using a binary logistic generalised estimated equation (GEE) with logit link function. Fixed effects included age (covariate), sex and voice domain items (factors), and patient identifier as the repeated subject. Similarly, a negative binomial with log link GEE was used to examine the four-category pain score. The odds ratio (OR) and Wald CI are reported.
For the subset of 3,144 primary cases, a Receiver Operator Characteristic (ROC) analysis with sensitivity (true positive rate) and 100-specificity (false positive rate) for the Voice domain score was conducted using NCSS (v21.0.12021) [28]. Youden index was used to determine the diagnostic accuracy across potential cut-off points (sensitivity + specificity − 1). Scores can range from 0 to 1, with higher scores representing the optimal cut-off point. Pain condition was determined based on pain score categories of low pain (no pain or mild pain) and high pain (moderate or severe pain). In addition, a Principal Component Analysis (PCA) for low and high pain was conducted for the Voice domain items using Eigenvalues >1 for extraction and employed the direct Oblimin rotation method. Both Kaiser–Meyer–Olkin measure of sampling adequacy (0.686) and Bartlett’s test of sphericity (P < 0.001) assumptions for factorability were met, with a Monte Carlo PCA for parallel analysis conducted (variables = 9, subjects = 2,500, replications = 100) [29] to confirm factor structure.
Results
Sample demographics and user data
A total of 3,144 patients with dementia and cognitive impairment had 22,194 pain assessments conducted by trained users during various activities of daily living. Patients were aged 44–106 years (Mage 83.3 years [9.0]) with slightly more females (59.0%). The average number of pain assessments completed per patient was 7.1 (SD = 35.7), with most (60.8%) assessments conducted for females. Total pain scores ranged from 0 to 35. Most assessments were conducted by nurses (44.5%) and care support staff (20.1%). Supplementary file, available in Age and Ageing online, provides further demographic details. Full demographic characteristics are described elsewhere [30].
Association between the presence/absence of vocalisation features and pain scores/pain intensities
Pain scores for the presence or absence of Voice domain items and according to pain intensity are described in Table 1. For all Voice domain features, the presence of the vocalisation was associated with a higher median pain score with large effect sizes. Absent vocalisation was associated with a median pain score of 4.0 for all nine pain-related features of the Voice domain. Median pain scores, when the vocalisations were present, ranged from 9.0 to 13.0, with the highest scores observed when screaming (Md = 13.0) and/or howling (Md = 12.0) were present. When comparing pain intensity, during severe pain (n = 580), noisy pain sounds were the most frequent vocalisation (62.4%), followed by sighing (57.8%), groaning (52.8%) and moaning (52.4%). In lower pain intensities (i.e., mild pain episodes n = 3,865), sighing was the most common vocalisation (28.2%) followed by noisy pain sounds (18.9%) and moaning (18.0%), whereas howling was the least common (2.5%). Noteworthy for high pain, emotive vocalisations, namely crying (19.4%), screaming (18.3%) and howling (9.1%) were least frequently reported.
Table 1.
Voice domain items ‘present’ described for total sample, pain score, four pain categories and low/high pain.
| Domain Item | Total | Pain score | Effect size | Pain categories (intensities) | Pain dichotomiseda | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f (%) | M (SD) [Md, IQR] | f (%) | f (%) | |||||||||
| Absent | Present | CLES | Cohen’s d | None | Mild | Moderate | Severe | Low | High | |||
| n | 22,194 | d | 95% CI | 16,617 | 3,865 | 1,132 | 580 | 20,482 | 1,712 | |||
| Noisy pain sounds | 1,843 (8.3) | 4.4 (3.3) [4.0, 2.0–6.0] |
11.5 (4.9) [11.0, 8.0–15.0] |
0.93 | 2.05 | 2.00–2.10 | 272 (1.6) | 730 (18.9) | 479 (42.3) | 362 (62.4) | 1,002 (4.9) | 841 (49.1) |
| Requesting help repeatedly | 1,534 (6.9) | 4.6 (3.6) [4.0, 2.0–6.0] |
10.3 (4.9) [9.0, 6.0–13.3] |
0.86 | 1.54 | 1.49–1.59 | 401 (2.4) | 575 (14.9) | 323 (28.5) | 235 (40.5) | 976 (4.8) | 558 (32.6) |
| Groaning | 1,551 (7.0) | 4.5 (3.5) [4.0, 2.0–6.0] |
11.3 (5.0) [11.0, 8.0–14.0] |
0.91 | 1.88 | 1.82–1.93 | 265 (1.6) | 590 (15.3) | 390 (34.5) | 306 (52.8) | 855 (4.2) | 696 (40.7) |
| Moaning | 1,821 (8.2) | 4.4 (3.5) [4.0, 2.0–6.0] |
10.6 (5.1) [10.0, 7.0–14.0] |
0.89 | 1.70 | 1.65–1.75 | 418 (2.5) | 697 (18.0) | 402 (35.5) | 304 (52.4) | 1,115 (5.4) | 706 (41.2) |
| Crying | 727 (3.3) | 4.7 (3.8) [4.0, 2.0–6.0] |
11.5 (5.3) [11.0, 7.0–15.0] |
0.89 | 1.76 | 1.69–1.84 | 142 (0.9) | 253 (6.5) | 169 (14.9) | 163 (28.1) | 395 (1.9) | 332 (19.4) |
| Screaming | 524 (2.4) | 4.8 (3.7) [4.0, 2.0–6.0] |
13.2 (5.5) [13.0, 9.0–17.0] |
0.94 | 2.24 | 2.15–2.33 | 55 (0.3) | 155 (4.0) | 139 (12.3) | 175 (30.2) | 210 (1.0) | 314 (18.3) |
| Loudtalk | 1,737 (7.8) | 4.5 (3.6) [4.0, 2.0–6.0] |
9.9 (5.2) [9.0, 6.0–13.0] |
0.85 | 1.44 | 1.39–1.49 | 539 (3.2) | 612 (15.8) | 331 (29.2) | 255 (44.0) | 1,151 (5.6) | 586 (34.2) |
| Howling | 277 (1.2) | 4.8 (3.9) [4.0, 2.0–6.0] |
13.2 (5.5) [12.0, 9.0–17.0] |
0.94 | 2.14 | 2.02–2.26 | 24 (0.1) | 98 (2.5) | 65 (5.7) | 90 (15.5) | 122 (0.6) | 155 (9.1) |
| Sighing | 2,883 (13.0) | 4.3 (3.4) [4.0, 2.0–6.0] |
9.5 (4.7) [9.0, 6.0–12.0] |
0.85 | 1.45 | 1.41–1.49 | 902 (5.4) | 1,089 (28.2) | 557 (49.2) | 335 (57.8) | 1,991 (9.7) | 892 (52.1) |
Note. f, frequency; %, percent; M, mean; SD, standard deviation; Md, median; CLES, common language effect size.
aLow = no or mild pain, and high = moderate or severe pain.
Gender and age
Gender was significantly associated with pain score (P < 0.001), with females recording slightly lower pain scores than males (β = −0.45, SE = 0.11, CI: −0.67 to −0.23). Age was not significantly associated with pain score (P = 0.494).
Assessor role and aged care home/program
LMM confirmed an adjustment for confounding by assessor role and aged care home/program was necessary. For assessor role, the ‘consultant’ category had the highest estimated marginal mean score (14.9, SE = 0.7, CI: 13.6–16.3) compared with other categories, with ‘care support employee’ category scoring the lowest (13.4, SE = 0.3, CI: 12.7–14.0). Likewise, the assessment of pain varied across the sample from a low estimated marginal mean of 12.5 (SE = 1.2, CI: 10.1–14.9) up to a high of 19.2 (SE = 3.1, CI: 13.1–25.3). Overall, the tests of fixed effects from the LMM showed that the number of Voice domain features was significantly associated with pain score (P < 0.001) (Figure 1).
Figure 1.
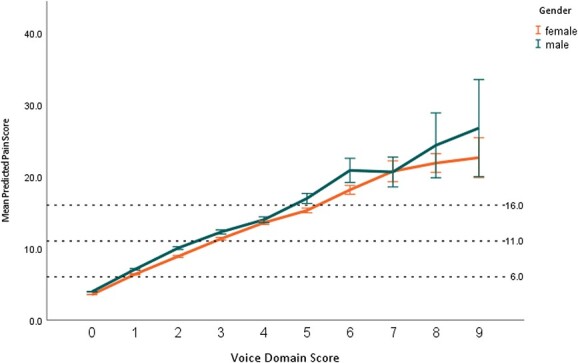
Predicted pain scores for number of Voice domain features present, separated for gender.
Presence of vocalisation features and predictability of high pain scores
The binary logistic GEE odds of reporting a high pain score are reported in Table 2. The presence of sighing and screaming was associated with the highest odds of a high pain score (8 times), followed by noisy pain sounds and loudtalk (five times), then crying (4 times). The remaining domain features increased the odds of a high pain score around three times. The likelihood of a high pain score was higher in males (almost 2 times), but no significant difference was noted for age (P = 0.414). Similarly, using a negative binomial with log link GEE model, all Voice domain features were significantly associated with the four-category pain intensity (Table 2), with sighing, loudtalk and noisy pain sounds having higher additive effects.
Table 2.
Generalised estimating equation predictability of high pain scores when Voice domain item was present.
| Model | Exp (β) | 95% CI Exp (β) | P-value | |
| Pain: low/high | Lower | Upper | ||
| Intercept | 0.0 | 0.0 | 0.2 | <.001* |
| Gender (male)a | 1.8 | 1.3 | 2.6 | .001 |
| Age | 1.0 | 1.0 | 1.0 | .414 |
| Noisy pain soundsb | 5.0 | 4.0 | 6.3 | <.001* |
| Requesting help repeatedlyb | 3.3 | 2.3 | 4.9 | <.001* |
| Groaningb | 3.3 | 2.7 | 4.1 | <.001* |
| Moaningb | 2.8 | 2.2 | 3.7 | <.001* |
| Cryingb | 4.1 | 2.6 | 6.4 | <.001* |
| Screamingb | 7.7 | 5.3 | 10.6 | <.001* |
| Loudtalkb | 5.0 | 3.6 | 7.1 | <.001* |
| Howlingb | 2.9 | 1.8 | 4.5 | <.001* |
| Sighingb | 8.1 | 6.4 | 10.4 | <.001* |
| Model | β (SE) | 95% CI (β) | P-value | |
| Pain: no, low, moderate, severe | Lower | Upper | ||
| Intercept | −1.2 (0.4) | −2.1 | −0.4 | .005 |
| Gender (male)a | 0.3 (0.1) | 0.1 | 0.4 | .008 |
| Age | −0.0 (0.0) | −0.0 | 0.0 | .033 |
| Noisy pain soundsb | 0.8 (0.0) | 0.8 | 0.9 | <.001* |
| Requesting help repeatedlyb | 0.7 (0.1) | 0.6 | 0.8 | <.001* |
| Groaningb | 0.6 (0.0) | 0.5 | 0.8 | <.001* |
| Moaningb | 0.6 (0.1) | 0.5 | 0.7 | <.001* |
| Cryingb | 0.6 (0.1) | 0.5 | 0.7 | <.001* |
| Screamingb | 0.7 (0.1) | 0.6 | 0.8 | <.001* |
| Loudtalkb | 0.9 (0.1) | 0.8 | 1.0 | <.001* |
| Howlingb | 0.3 (0.1) | 0.2 | 0.5 | <.001 |
| Sighingb | 1.2 (0.1) | 1.1 | 1.3 | <.001* |
Note. Pain (low/high) model reports a binary logistic with OR (Exp [β]) presented; Pain (no, low, moderate, severe) reports a negative binomial with log link with beta estimate (β) and standard error (SE) presented.
aCompared to male.
bCompared to absent.
*Statistically significant at P < 0.000001.
Discriminating power of Voice domain score for categorising low and high pain groups
Of the subset sample of 3,144 initial pain assessments for each patient analysed, a total of 827 (26.3%) had high pain and 2,317 (73.7%) had low pain episodes. The ROC area under the curve (0.884, SE = 0.007, 95% CI: 0.871–0.896, z = 58.7, P < 0.000001) indicated that the criterion variable Voice domain score was able to distinguish between the low and high pain groups. The optimal criterion Voice domain score was ≥2.0 with a Youden index of 0.637. Corresponding sensitivity was 79.7% (CI: 76.8–82.4%) and specificity was 84.0% (CI: 82.5–85.5%). The ROC analysis, Youden Index, counts and classification proportions across cut-off values are presented in Table 3.
Table 3.
Voice domain score ROC analysis summary of Youden index, counts and classification proportions
| Correctly classified | Incorrectly classified | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off Value | Youden Index | True Positive (n) |
False Positive (n) |
False Negative (n) |
True Negative (n) |
Proportion | 95% CI Lower | 95% CI Upper | Proportion | 95% CI Lower | 95% CI Upper |
| ≥0.0 | 0.00 | 827 | 2,317 | 0 | 0 | 0.26 | 0.25 | 0.28 | 0.74 | 0.72 | 0.75 |
| ≥1.0 | 0.51 | 792 | 1,034 | 35 | 1,283 | 0.66 | 0.64 | 0.68 | 0.34 | 0.32 | 0.36 |
| ≥2.0 | 0.64 | 659 | 370 | 168 | 1,947 | 0.83 | 0.82 | 0.84 | 0.17 | 0.16 | 0.18 |
| ≥3.0 | 0.47 | 434 | 116 | 393 | 2,201 | 0.84 | 0.82 | 0.85 | 0.16 | 0.15 | 0.18 |
| ≥4.0 | 0.28 | 243 | 27 | 584 | 2,290 | 0.81 | 0.79 | 0.82 | 0.19 | 0.18 | 0.21 |
| ≥5.0 | 0.16 | 130 | 3 | 697 | 2,314 | 0.78 | 0.76 | 0.79 | 0.22 | 0.21 | 0.24 |
| ≥6.0 | 0.07 | 59 | 1 | 768 | 2,316 | 0.76 | 0.74 | 0.77 | 0.24 | 0.23 | 0.26 |
| ≥7.0 | 0.03 | 25 | 0 | 802 | 2,317 | 0.74 | 0.73 | 0.76 | 0.26 | 0.24 | 0.27 |
| ≥8.0 | 0.02 | 14 | 0 | 813 | 2,317 | 0.74 | 0.73 | 0.76 | 0.26 | 0.24 | 0.27 |
| ≥9.0 | 0.01 | 5 | 0 | 822 | 2,317 | 0.74 | 0.72 | 0.75 | 0.26 | 0.25 | 0.28 |
Note. Grey shaded row indicates the optimal criteria based on the highest Youden Index value of 0.637. CI, confidence intervals.
PCA revealed the presence of three components within the Voice domain with eigenvalues exceeding 1, explaining 23.8, 15.6 and 11.1% of the variance, respectively. The scree plot and parallel analysis suggested an optimal two-factor structure. For both the three-factor and two-factor PCA models, groaning (0.763, 0.737), moaning (0.732, 0.728) and noisy sounds (0.638, 0.621) loaded together, respectively. The remaining domain features loaded to form the second component in the two-factor model, whereas for the three-factor model crying (0.750), requesting help (0.602), howling (0.598) and screaming (0.375) loaded together; and loudtalk (0.771), sighing (−0.754) and screaming (0.539) loaded together. Voice domain scores ranged from 0–9 (M = 0.6, SD = 1.11, Md = 0.0, IQR = 0.0–1.0). Figure 2 provides further details on Voice domain scores for (a) dichotomised pain intensities, i.e., low and high and (b) four pain intensities (no, mild, moderate and severe pain).
Figure 2.
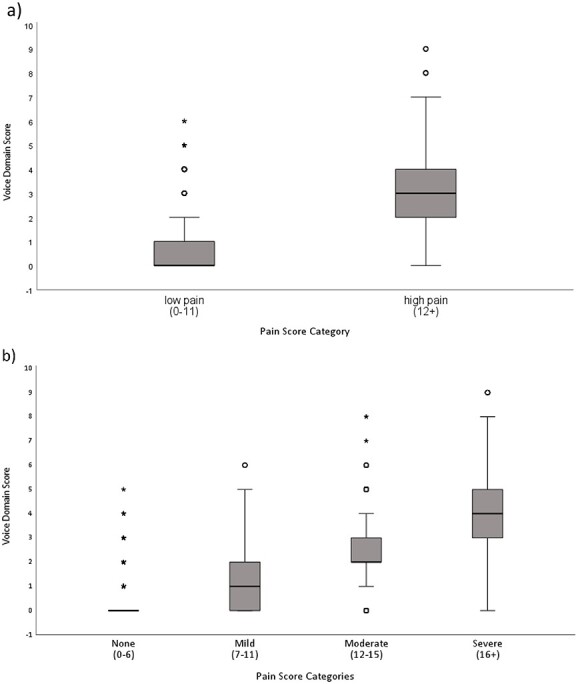
Boxplot depicting voice domain score across pain intensity categories. a) Two category pain plot. Note: Voice domain scores for low and high pain ranged from 0–6 (M = 0.4, SD = 0.8, Md = 0.0, IQR 0.0–1.0) and 0–9 (M = 3.0, SD = 1.6, Md = 3.0, IQR 2.0–4.0), respectively. b) Four category pain plot. Note: Voice domain scores ranged from 0–5 (M = 0.2 SD = 0.5 Md = 0.0 IQR 0.0–0.0) for no pain, 0–6 (M = 1.2 SD = 1.2 Md = 1.0 IQR 0.0–2.0) mild pain, 0–8 (M = 2.5 SD = 1.4 Md = 4.0 IQR 2.0–3.0) moderate pain, and 0–9 (M = 3.8 SD = 1.7 Md = 4.0 IQR 3.0–5.0) for severe pain.
Discussion
In this study, we described pain-specific vocal expressions of people living with advanced dementia during assessment of their pain. As such, it becomes the first study to do so by analysing the data from a large database of over 20,000 pain assessments conducted in clinical settings.
Our findings suggest that the presence of vocalisation is associated with higher median pain scores, therefore providing evidence to associations of vocalisation behaviours not only in relation to pain presence but its intensity as well. In addition, analysis of identified vocalisations during different pain categories revealed specific patterns that were more prevalent dependent on whether subjects experienced severe pain or mild pain. These results support existing evidence that has suggested that there is a change in voice parameters in patients experiencing pain [19–21].
Furthermore, our study suggests that vocalised expressions of pain are graded behaviours rather than discrete. This is congruent with several studies in other population groups (e.g., infants) [31, 32].
Recently, Veldwijk-Rouwenhorst et al. found that higher frequencies of vocalisations characterised by vocal behaviours such as higher levels of screaming, were correlated with higher levels of antipsychotic use, as well as aberrant motor behaviours, anxiety, night-time behaviours and euphoria in residents with dementia [33]. A strong association between screaming and pain intensity found in our study provides evidence to support this. In this context, it is also worth mentioning that presence of pain has been previously shown to be associated with higher severity of neuropsychiatric behaviours in people with dementia [34].
This study has a number of strengths which are primarily related to the large and representative database that stems from clinical practice. The database is automatically compiled after pain assessments are completed using a POC tool and the data then are transmitted via cloud computing to support documentation processes. In addition, the study benefited from the consistency in the pain assessment process, enabled by a validated pain assessment tool that was used consistently across assessments and different sites. Trained assessors ensured competency in the pain assessment process. The study also has a number of limitations including the fact that different types of dementia were not accounted for, and the data were not labelled to account for the degree or severity of cognitive impairment. Pain experience can be affected by these aspects especially considering that various dementia types involve different neural processing mechanisms and brain regions which as a result may affect the pathways through which pain is processed [35]. In addition, we did not account for potential confounding effects of medications and other medical conditions including the impact of non-pain-related impact of neuropsychiatric symptoms. Further research is needed to study the implications of these factors in the context of pain and vocalisations. Also, further research exploring the relationship of individual and combined vocalisation behaviours with other pain behaviours would be beneficial to phenotype the multidimensionally aspects of pain experience.
Nonetheless, our findings contribute to the existing literature by providing new insights related to vocalisation behaviours and the presence and intensity of pain, therefore supporting their diagnostic value. Furthermore, considering the relationships between neuropsychiatric symptoms, vocalisation behaviours and pain, findings of this study could inform clinical practice and therefore have implications in relation to a timelier assessment of pain and its intensity, and subsequent reduction of pain-related complications such as neuropsychiatric symptoms. Furthermore, our findings raise the possibility of digital phenotyping of vocalisations emerging as a broader clinically relevant biomarker of clinical evaluation of later stage dementia. The need for mechanistic phenotyping and therefore individualisation of pain management in dementia has been recently raised by Collins et al., whereas Soiza as well as Close in separate editorials highlighted the value of harnessing big data to inform clinical practice [36–38]. In this regard, digital phenotyping of vocalisations, enabled by big data availability and analysis could assist with identification of previously unrecognised patterns of pain experience by the person with dementia. This has potential to contribute towards individualisation and improvement of pain management.
Conclusions
Our findings provide evidence from clinical practice that contributes to further insights into the occurrence and relationship between vocalised behaviours and the presence and intensity of pain in people living with dementia who are unable to self-report. As such, the study confirms the diagnostic value of vocalised behaviours in assessing pain in non-verbal people with dementia. Our findings suggest that the identification of increased vocalised behaviours should prompt clinicians to consider the presence of significant pain, and therefore complete a formal multidimensional pain assessment using a validated multidimensional pain assessment tool to confirm the intensity of pain and therefore direct appropriate treatment. The above considerations as well as the opportunity for digital phenotyping of vocalisations can provide valuable clinical information that may contribute towards individualised pain assessment and management in people living with dementia.
Supplementary Material
Acknowledgements
We thank everyone involved in the study.
Contributor Information
Kreshnik Hoti, Faculty of Medicine, University of Pristina, Prishtina, Kosovo; Curtin Medical School, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia.
Mustafa Atee, Curtin Medical School, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia; The Dementia Centre, HammondCare, Osborne Park, WA, Australia; Sydney Pharmacy School, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia; Centre for Research in Aged Care, School of Nursing and Midwifery, Edith Cowan University, Joondalup, WA, Australia.
Paola Chivers, Institute for Health Research, The University of Notre Dame Australia, Fremantle, WA, Australia; School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia.
Ipsit Vahia, McLean Hospital, Belmont, MA, USA; Harvard Medical School, Boston, MA, USA.
Jeffrey Hughes, Curtin Medical School, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia.
Declaration of Conflicts of Interest
K.H., M.A., and J.H. are co-inventors of the original PainChek instrument (branded ePAT at the time), which was acquired and subsequently commercialised by PainChek Ltd. They are shareholders of PainChek Ltd. K.H. is employed as a consultant by PainChek, while also serving as a Professor at the University of Prishtina, Kosovo and is University Associate at the Curtin Medical School, Curtin University, WA, Australia. M.A. previously held the position of a Senior Research Scientist (October 2018–May 2020) at PainChek Ltd, and currently serving the position of Research and Practice Lead at The Dementia Centre, HammondCare. J.H. currently holds the position of Chief Scientific Officer at PainChek Ltd, while serving as an Emeritus Professor at Curtin Medical School. The co-inventors had authored a patent titled ‘A pain assessment method and system; PCT/AU2015/000501’ which was assigned to PainChek Ltd and who have, to date, received granted patents in the jurisdictions of China, Japan, and the United States. P.C. was paid as an independent consultant to complete the data analysis for the project. P.C. is the Principal Consultant of DATaR Consulting providing independent biostatistical services, while also serving as Associate Professor at the University of Notre Dame Australia and adjunct at Edith Cowan University in Western Australia.
Declaration of Sources of Funding
PainChek Ltd, P.C., was paid as an independent consultant to complete the data analysis for the project. Financial sponsor played no other role in the study.
Data Availability Statement
Data which is deidentified and does not violate confidentiality can be made available following approval from PainChek Ltd, upon reasonable request. This data cannot be shared with a third party and can be used for research purposes only, not for product related work.
References
- 1. Cohen-Mansfield J, Werner P. Typology of disruptive vocalizations in older persons suffering from dementia. Int J Geriatr Psychiatry 1997; 12: 1079–91. [DOI] [PubMed] [Google Scholar]
- 2. Sefcik JS, Ersek M, Hartnett SC, Cacchione PZ. Integrative review: persistent vocalizations among nursing home residents with dementia. Int Psychogeriatr 2019; 31: 667–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Geriatrics Society (AGS) . Panel on persistent pain in older persons: the management of persistent pain in older persons. J Am Geriatr Soc 2002; 50: 205–S224. [DOI] [PubMed] [Google Scholar]
- 4. Zwakhalen S, Hamers J, Abu-Saad H, Berger M. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr 2006; 6: 3. 10.1186/1471-2318-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lautenbacher S, Salinas-Ranneberg M, Niebuhr O, Kunz M. Phonetic characteristics of vocalizations during pain. Pain Rep 2017; 2: e597. 10.1097/PR9.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abbey J, Piller N, De Bellis A et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004; 10: 6–13. [DOI] [PubMed] [Google Scholar]
- 7. Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc 2003; 4: 9–15. [DOI] [PubMed] [Google Scholar]
- 8. Lefebvre-Chapiro S. The DOLOPLUS 2 scale—evaluating pain in the elderly. Eur J Palliat Care 2001; 8: 191–4. [Google Scholar]
- 9. Warden V, Volicer L, Hurley AC, Rogers EN. Pain assessment in advanced dementia. Gerontologist 2001; 41: 146. [Google Scholar]
- 10. Morello R, Jean A, Alix M, Sellin-Peres D, Fermanian J. A scale to measure pain in non-verbally communicating older patients: the EPCA-2 study of its psychometric properties. Pain 2007; 133: 87–98. [DOI] [PubMed] [Google Scholar]
- 11. Cohen-Mansfield J. Pain assessment in noncommunicative elderly persons—PAINE. Clin J Pain 2006; 22: 569–75. [DOI] [PubMed] [Google Scholar]
- 12. Snow AL, Weber JB, O’Malley KJ et al. NOPPAIN: a nursing assistant-administered pain assessment instrument for use in dementia. Dement Geriatr Cogn Disord 2004; 17: 240–6. [DOI] [PubMed] [Google Scholar]
- 13. Fuchs-Lacelle S, Hadjistavropoulos T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs 2004; 5: 37–49. [DOI] [PubMed] [Google Scholar]
- 14. Villanueva MR, Smith TL, Erickson JS, Lee AC, Singer CM. Pain assessment for the dementing elderly (PADE): reliability and validity of a new measure. J Am Med Dir Assoc 2003; 4: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Snow AL, Ljunggren AE. Mobilization-observation-behaviour-intensity-dementia pain scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. J Pain Symptom Manage 2007; 34: 67–80. [DOI] [PubMed] [Google Scholar]
- 16. Corbett A, Achterberg W, Husebo B et al. An international road map to improve pain assessment in people with impaired cognition: the development of the pain assessment in impaired cognition (PAIC) meta-tool. BMC Neurol 2014; 14: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atee M, Hoti K, Parsons R, Hughes JD. Pain assessment in dementia: evaluation of a point-of-care technological solution. J Alzheimers Dis 2017; 60: 137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ammaturo DA, Hadjistavropoulos T, Williams J. Pain in dementia: use of observational pain assessment tools by people who are not health professionals. Pain Med 2017; 18: 1895–907. [DOI] [PubMed] [Google Scholar]
- 19. Oshrat Y, Bloch A, Lerner A, Cohen A, Avigal M, Zeilig G. Speechprosody as a biosignal for physical pain detection. In: Conf Proc 8th Speech Prosody, May 31–June 3, 2016. Boston, MA: International Speech Communication Association, p. 420–4. https://www.isca-speech.org/archive_v0/SpeechProsody_2016/pdfs/11.pdf. [Google Scholar]
- 20. Crombez G, Eccleston C, Baeyens F, Eelen P. The disruptive nature of pain: an experimental investigation. Behav Res Ther 1996; 34: 911–8. [DOI] [PubMed] [Google Scholar]
- 21. Reinersmanna A, Haarmeyer GS, Blankenburg M et al. Left is where the L is right. Significantly delayed reaction time in limb laterality recognition in both CRPS and phantom limb pain patients. Neurosci Lett 2010; 486: 240–5. [DOI] [PubMed] [Google Scholar]
- 22. Atee M, Hoti K, Hughes JD. Psychometric evaluation of the electronic Pain Assessment Tool (ePAT): an innovative instrument for individuals with moderate to severe dementia. Dement Geriatr Cogn Disord 2018; 44: 256–67. [DOI] [PubMed] [Google Scholar]
- 23. Hoti K, Atee M, Hughes JD. Clinimetric properties of the electronic Pain Assessment Tool (ePAT) for aged-care residents with moderate to severe dementia. J Pain Res 2018; 11: 1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atee M, Hoti K, Parsons R, Hughes JD. A novel pain assessment tool incorporating automated facial analysis: interrater reliability in advanced dementia. Clin Interv Aging 2018; 13: 1245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atee M, Hoti K, Hughes JD. A technical note on the PainChek™ system: a web portal and mobile medical device for assessing pain in people with dementia. Front Aging Neurosci 2018; 10: 117. 10.3389/fnagi.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ekman P, Friesen WV, Hager JC, eds. Facial Action Coding System. Salt Lake City: Research Nexus, Network Research Information, 2002. [Google Scholar]
- 27. Lenhard W, Lenhard A. Calculation of Effect Sizes. https://www.psychometrica.de/effect_size.html. Dettelbach (Germany): Psychometrica, 2016. 10.13140/RG.2.2.17823.92329 [DOI] [Google Scholar]
- 28. NCSS . Statistical Software (2021). Kaysville, UT: NCSS, LLC, 2021. ncss.com/software/ncss. [Google Scholar]
- 29. Watkins MW. Determining parallel analysis criteria. J Mod Appl Stat Methods 2006; 5: 344–6. [Google Scholar]
- 30. Atee M, Hoti K, Chivers P, Hughes JD. Faces of pain in dementia: learnings from a real-world study using a technology-enabled pain assessment tool. Frontiers in pain. Research 2022; 3: 3. 10.3389/fpain.2022.827551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellieni CV, Sisto R, Cordelli DM, Buonocore G. Cry features reflect pain intensity in term newborns: an alarm threshold. Pediatr Res 2004; 55: 142–6. [DOI] [PubMed] [Google Scholar]
- 32. Sauter DA, Eisner F, Calder AJ, Scott SK. Perceptual cues in nonverbal vocal expressions of emotion. Quarter J Exp Psychol 2010; 63: 2251–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veldwijk-Rouwenhorst AE, Zuidema SU, Smalbrugge M et al. Very frequent physical aggression and vocalizations in nursing home residents with dementia. Aging Ment Health 2020; 25: 1442–51. [DOI] [PubMed] [Google Scholar]
- 34. Atee M, Morris T, Macfarlane S, Cunningham C. Pain in dementia: prevalence and association with neuropsychiatric behaviors. J Pain Symptom Manage 2021; 61: 1215–26. [DOI] [PubMed] [Google Scholar]
- 35. Scherder EJ, Schwaab DF. Pain in dementia. In: Cervero F, Jensen TS, eds. Handbook of Clinical Neurology. Amsterdam: Elsevier, 2006; 817–35. [DOI] [PubMed] [Google Scholar]
- 36. Collins JT, Harwood RH, Cowley A et al. Chronic pain in people living with dementia: challenges to recognising and managing pain, and personalising intervention by phenotype. Age Ageing 2023; 52: afac306. 10.1093/ageing/afac306. [DOI] [PubMed] [Google Scholar]
- 37. Soiza RL. Editor’s view. Age Ageing 2023; 52: afad029 10.1093/ageing/afad029. [DOI] [Google Scholar]
- 38. Close JC. Big data—big opportunity. Age Ageing 2023; 52: afac262. 10.1093/ageing/afac262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data which is deidentified and does not violate confidentiality can be made available following approval from PainChek Ltd, upon reasonable request. This data cannot be shared with a third party and can be used for research purposes only, not for product related work.


