Abstract
Objective: The RNA polymerase II mediator complex subunit 12 (MED12) is an important factor for chemotherapy sensitivity. We explored the roles of exosomal transfer of carcinogenic microRNAs (miRNAs) in MED12 regulation and cisplatin resistance of ovarian cancer cells. In this study, the correlation between MED12 expression and cisplatin resistance was analyzed in ovarian cancer cells. The molecular regulation of MED12 by exosomal miR-548aq-3p was investigated by bioinformatics analysis and luciferase reporter assays. Further clinical significance of miR-548aq was assessed with TCGA data. We identified decreased MED12 expression in cisplatin-resistance of ovarian cancer cells. More importantly, coculture with cisplatin-resistant cells attenuated cisplatin sensitivity of parental ovarian cancer cells, as well as reduced MED12 expression to a large extent. Further bioinformatic analysis identified that exosomal miR-548aq-3p was correlated with MED12 transcriptional regulation in ovarian cancer cells. Luciferase reporter assays demonstrated that miR-548aq-3p down-regulated MED12 expression. miR-548aq-3p overexpression enhanced cell survival and proliferation of ovarian cancer cells with cisplatin treatment, while miR-548aq-3p inhibition induced cell apoptosis of cisplatin-resistant cells. Further clinical analysis indicated that miR-548aq was correlated with lower MED12 expression. More importantly, miR-548aq expression was a detrimental factor in the disease progression of ovarian cancer patients. In conclusion, we found that miR-548aq-3p contributed to cisplatin chemotherapy resistance of ovarian cancer cells through MED12 downregulation. Our study supported miR-548aq-3p as a promising therapeutic target for improving chemotherapy sensitivity of ovarian cancer.
Keywords: Epithelial ovarian cancer, chemotherapy resistance, MED12, miR-548aq-3p, cisplatin
Introduction
Ovarian cancer is the major contributor for the morbidity of malignant genital tumors, ranking the second in the death caused from female genital tract cancer [1]. Most ovarian carcinoma patients are diagnosed as advanced and unresectable disease because of its insidious onset [2]. These patients are usually administrated in the range of platinum-based chemotherapy [3], especially for those without homologous recombination defect [4]. However, the majority of epithelial ovarian cancer patients suffered chemoresistance related disease progression after a short-term latency [5]. Detailed mechanism underlying the chemoresistance was still elusive yet presently [6]. Thus, it is of great importance to screen effective biomarkers and targets for improving chemotherapy sensitivity of ovarian cancer.
The RNA polymerase II mediator complex subunit 12 (MED12) is a component of the transcriptional MEDIATOR complex [7]. Previous studies identified that MED12 inhibited epithelial-mesenchymal transition of cancer cells to maintain chemotherapy sensitivity of colon cancer [8]. The physical interaction of MED12 and TGFbR2 is the determinant contributor for the response to ALK and EGFR inhibitors for lung cancer [9]. Decreased MED12 expression predicts poor prognosis of ovarian cancer [10]. However, the functional role of MED12 is still unclear in chemotherapy sensitivity of epithelial ovarian cancer. Further studies of the MED12 regulation mechanism in chemotherapy will provide a novel insight for the drug-resistant ovarian cancer.
Exosomes play crucial roles in achieving survival advantages during malignant progression. Paracrine exosomes release to the extracellular environment from the invagination of late endosomes of cancer cells, which transfer intercellular of molecules such as non-coding RNAs [11]. The post-transcriptional regulation role of specific molecular contributes to the phenotypic reprogramming of recipient cells, including microRNAs (miRNAs) induced chemotherapy resistance [12]. Various molecules are reported to participate in the carcinogenesis and progression of epithelial ovarian cancer, which are potential candidates for the translational application in ovarian cancer treatment [13]. Recent studies indicated that miR-152 interacted with miR-185 to regulate DNA methyltransferase 1 expression, which was involved in the chemotherapy sensitivity of epithelial ovarian cancer [14,15]. Further investigation was still needed for the functional role of exosomal miRNAs in chemotherapy resistance of epithelial ovarian cancer, especially the exosomal miRNAs.
In the present study, we identified decreased MED12 contributed to the chemotherapy-resistance in cocultured ovarian cancer cells. The molecular mechanism was studied for the gene transcriptional regulation by exosomal miR-548aq-3p. We aimed to explore the function and clinical significance of miR-548aq-3p in chemotherapy resistance of ovarian cancer.
Material and methods
Cell culture
Ovarian cancer cell lines (SKOV-3 and A2780) were purchased from the American Type Culture Collection (Manassas, VA). Cells were cultured with DMEM with 10% FBS (Sigma Chemical Co., St. Louis, MO). Cisplatin-resistant ovarian cancer cells were maintained with 10 μM cisplatin treatment. All cells were passaged for less than three months which were cultured from early cryopreservation cells.
Cell transfection
MED12_pLX307 was a gift from William Hahn and Sefi Rosenbluh (Addgene plasmid #98350). MED12 expressing or control vector plasmids (Control) were transfected into cisplatin resistant cells or miR-548aq-3p expressing cells with Lipofectamine 2000 transfection reagent (Invitrogen, Carlbad, CA). Puromycin (Merck, St. Louis, MO) was used for the selection of transfected cells. The miR-548aq-3p mimic, miR-548aq-3p inhibitor (mirVana; has-miR-548aq-3p, MH17925), and their corresponding negative controls (miR-control and control-inh; mirVana; negative control #1) were obtained from Thermo Fisher Scientific (Indianapolis, IN). Lentiviruses expressing miR-548aq-3p were produced by co-transfection of pLenti4-CMV/TO-miR-548aq-3p plasmid (0.4 μg) and packaging plasmids pCMV-VSV-G (0.1 μg) and pCMV-ΔR8.91 (0.3 μg) into 293T cells using TransFectin lipid reagent (Bio-Rad). Ovarian cancer cells were transfected following the manufacturer’s instructions.
Western blot analysis
Whole lysates of the transfected ovarian cancer cells were prepared with RIPA buffer (Beyotime), and then mixed with loading buffer and boiled at 100°C for 10 min. The clarified lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF). Enhanced chemiluminescence were performed for the analysis of the protein bands. The primary antibodies for MED12 (ab70842), Cleaved PARP (ab32064), bcl-2 (ab32124), Bcl-xL (ab32370), and anti-GAPDH (ab181602) were purchased from Abcam (Cambridge, CA).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA extraction of exosomes and microRNAs from ovarian cancer cells was performed with TRIzol (Invitrogen). The reverse-transcription of extracted RNA was performed with AMV reverse transcriptase (Takara, Otsu, Japan) and RevertAid™ H Minus First Strand cDNA Synthesis Kit (Takara). SYBR PrimeScript RT-PCR kit (Takara) was used for the RNA expression levels. GAPDH and U6 were used as endogenous controls for mRNA and miRNA, respectively. The following primers were used: MED12 forward, 5’-GGGATCTTGAGCTACGAACAC-3’, and reverse, 5’-GCAGGCTGGTTATTGAAACCTTG-3’; GAPDH forward, 5’-TGTGGGCATCAATGGATTTGG-3’, and reverse, 5’-ACACCATGTATTCCGGGTCAAT-3’; The miR-548aq-3p specific primers were purchased from miRCURY LNA miRNA PCR Starter kit (YP02100851, QIAGEN).
Cell viability analysis
Prepared ovarian cell lines were cultured with 96-well plates with 3000 cells/well. Five replicates were designed for each group. Indicated treatment was administrated at 37°C. The Cell Counting Kit-8 (Beyotime, Shanghai, China) was used for cell viability analysis following the manufacturer’s instructions. The absorbance at 450 nm was measured with a microplate reader to calculate the relative cell growth.
Annexin V-FITC/PI for cell apoptosis
Cell apoptosis was evaluated with Annexin V-FITC/PI method. The indicated cells were treated with the vehicle or cisplatin. All cells were harvested and resuspended in Becton Dickinson Biosciences IX Annexin Buffer (BD Biosciences, San Jose, CA) and incubated with Annexin V-FITC/PI for 15 minutes at room temperature in the dark. Finally, flow cytometry detection was performed with FACSAria II (Becton Dickinson) and the results were analyzed with the FlowJo software v10.6.1. Each assay was conducted in duplicate, and three independent experiments were performed.
Flow cytometry for cell sorting
CD133+/- cell sorting was performed with FACS Calibur (BD Biosciences, CA). Briefly, A2780/DDP cells were incubated with 10 μl of Alexa Fluor® 647 Anti-CD133 antibody [EPR20980-104] (ab252127) at 4°C for 40 min. The cells were washed with buffer for flow cytometry analysis as manufacturer’s instruction.
Luciferase reporter assay
The interaction between miR548aq-3p and MED12 was analyzed with the TargetScan database (http://www.targetscan.org). The pMIR-REPORT vector with wild-type or mutated 3’-UTR sequences of MED12 were synthesized by Shanghai GenePharma Co. (Shanghai, China). The Dual-Luciferase Reporter assay kit (Promega) was used for the analysis of luciferase activities. Briefly, 293T cells were cultured in six-well plates until approximately 70% confluence. Lipofectamine 2000 was used for the transfection of either wild type or mutant luciferase reporter plasmids and miR-548aq-3p mimics or control. After 48 h culture, luciferase activity was measured and normalized to the Rluc activity.
Dataset selection and differentially expressed genes (DEGs) identification
The data of A2780 cell lines in GSE76449 were selected for the analysis of potential exosomal miRNA which were involved in cisplatin resistance. DEGs were analyzed with GEO2R website (http://www.ncbi. nlm.nih.gov/geo/geo2r), which was performed with the cutoff value of log2 (foldchange) > 2 and adjusted P value < 0.01 [16]. Candidate miRNAs for MED12 down-regulation were analyzed with TargetScan Human 7.2 (www.targetscan.org). The common DEGs from the three cell lines were identified and graphed with the online VENN software (http://www.bioinformatics.com.cn/static/others/jvenn/index.html).
In vivo mouse xenograft
Twelve 6-week-old female BALB/c nude mice were purchased from the Vital River Laboratories Vital River Laboratories (Beijing, China) and housed under specific pathogen-free (SPF) conditions. Mice were injected subcutaneously with 1.0×107 infected A2780 cells (A2780/miR and A2780/miR548aq, n = 6 mice each group). The mice were administered with cisplatin (DDP, 5 mg/kg) three times a week for two weeks from day 12, which the average tumor volumes were 100 mm3. We measured the volumes of the subcutaneous tumors in nude mice once every 3 days. On day 30, the mice were sacrificed to harvest the xenografts. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Center for Experimental Animals of the 960th Hospital of PLA.
Patients and specimens
The retrospective study selected totally 96 ovarian cancer specimens from July 2008 to September 2016. All patients received cisplatin chemotherapy after adnexectomy and pelvic lymphadenectomy in the Affiliated Hospital of Qingdao University. We collected clinical characteristics, operation and other medical information from medical records, telephone, written correspondence and death certificate. Follow-up analysis evaluated objective response rate (ORR) of chemotherapy response according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [17]. Our clinical trial was approved by the Ethics Committee of Affiliated Hospital of Qingdao University.
Immunohistochemical (IHC) staining
We conducted IHC staining with Ventana Discovery XT automated staining system (Ventana Medical Systems, Inc., Tucson, AZ). The procession was same as previous describe [18]. MED12 antibody (ab70842, Abcam, Cambridge, MA) was used as primary antibody. Corresponding control IgG was used as negative control.
Statistical analysis
The results were presented as mean ± standard deviation from at least three independent experiments. Unpaired student’s t-test was used for the analysis of differences between groups with the SPSS 24.0 software (IBM, Chicago, IL). One-way analysis of variance (ANOVA) was used to analyze the correlation among more than two groups. Spearman’s rank test was utilized for analyzing the correlation of miR548aq and clinical parameter. Kaplan-Meier analysis with a log-rank test was used to compare the survival distributions with SPSS or KM-plotter (https://kmplot.com/analysis/) [19]. The P < 0.05 was considered significant.
Results
Cisplatin resistant ovarian cancer cells shows decreased MED12 expression
We first assessed the functional role of MED12 in cisplatin resistance of ovarian cancer cells. Western blot assays indicated decreased MED12 expression in cisplatin resistant ovarian cancer cells than the corresponding parental cells (Figure 1A). Further RT-qPCR analysis also indicated decreased MED12 mRNA expression in cisplatin resistant cells (Figure 1B). Exogenous MED12 overexpression was performed with the cisplatin-resistant cells, which was confirmed with Western blot assays (Figure 1C). Inhibited cell viability was observed in MED12 overexpressing cells with cisplatin treatment compared to control A2780 and SKOV-3 cells (Figure 1D, 1E). The MED12 overexpressing cells were collected with 24 h cisplatin treatment to evaluate the relative levels of cell apoptosis. Increased cell apoptosis was observed in MED12 overexpressing cells with cisplatin treatment compared with the control cells (Figure 1F). Our results supported that MED12 contributed to cisplatin treatment sensitivity of ovarian cancer cells. We sorted cancer stem cells and non-stem cells from the cisplatin-resistant cells according to CD133 expression with flowcytometry (Figure 1G). We observed higher levels of MED12 expression in non-stem cells than the cancer stem cells (Figure 1H). More importantly, both cell subpopulation was treated with cisplatin for 24 h. Higher levels of cell apoptosis was observed in CD133- cells than CD133+ cells (Figure 1I). Taken together, these results suggested cisplatin-resistant cancer cells communicated with the cisplatin-sensitive cells which conferred the non-stem cells of survival advantage with cisplatin treatment.
Figure 1.
Cisplatin resistant ovarian cancer cells shows decreased MED12 expression. (A) Western blot analyses of MED12 in DDP resistant and parental A2780 and SKOV-3 cells. GAPDH was used as an internal control. (B) The mRNA levels of MED12 were measured by RT-qPCR assays in DDP resistant and parental A2780 and SKOV-3 cells. GAPDH was used as an internal control. (C) Exogenous MED12 expression was performed with DDP resistant ovarian cancer cells, which was confirmed by Western Blotting assays. (D and E) Cell viability was compared between MED12 overexpressing A2780/DDP (D) or SKOV3/DDP cells and control cells, which were treated with cisplatin (0-75 μM) for 48 h. (F) Cell apoptosis of the transfected DDP resistant cells were analyzed with the Annexin V/FITC Assays. MED12 overexpressing and control DDP resistant cells were treated with 25 μM cisplatin for 72 h. (G) CD133+/- cells from A2780/DDP were sorted with flow cyotometry, which were cultured for further analysis. (H) Western blot analyses of MED12 in CD133+/- cells as G described. GAPDH was used as an internal control. (I) Cell apoptosis of sorted CD133+/- cells were analyzed with the Annexin V/FITC Assays. The cells were treated with 25 μM cisplatin for 72 h. Student’s t test was used for statistical analysis. *P < 0.01 vs. negative control.
Coculture with cisplatin-resistant cells attenuates cisplatin sensitivity of parental ovarian cancer cells
Further study was performed to explore the potential way to MED12 downregulation in cisplatin resistant cells. We focused on the role of exosome secretion in cisplatin-resistant cells with a coculture system. The parental ovarian cancer cells with 0.4-μm pores were cocultured with cisplatin-resistant cells as indicated. Meanwhile, GW4869 was added in the lower chamber medium to inhibit exosome secretion in the coculture system as another group (Figure 2A). The cocultured cells plated in the upper chamber were collected for subsequent experiments. We observed increased cell viability of the cocultured ovarian cancer cells compared to the parental cells, while GW4869 treatment decreased cell viability of the cocultured cells (Figure 2B, 2C). Further analysis also indicated a decreased apoptosis rate in the cocultured A2780 cells, which was neutralized with GW4869 treatment (Figure 2D, 2E). Notably, decreased MED12 expression was observed in the cocultured cells, and GW4869 treatment rescued MED12 expression (Figure 2F, 2G). Taken together, these results suggested that exosomes secreted from the cisplatin-resistant ovarian cancer cells downregulated MED12 expression to conferred chemoresistance ability to the adjacent cancer cells.
Figure 2.
Coculture with cisplatin-resistant cells attenuates cisplatin sensitivity of parental ovarian cancer cells. (A) Schematic diagram of coculture system of cisplatin resistant cells and parental cells. (B and C) Cell viability was compared between parental ovarian cancer cells (B. A2780, C. SKOV-3), cocultured cells and GW4869 treated cells. All group cells were treated with cisplatin (0-75 μM) for 48 h. (D and E) Cell apoptosis of the cells as indicated as (A) was analyzed with FACS, which was treated with 25 μM cisplatin for 48 h. (F and G) Western blot and RT-qPCR analyses of MED12 expression of the cells as indicated. GAPDH was used as control.
Exosomal miR-548aq-3p inhibits MED12 expression in ovarian cancer cells
To understand the mechanism of exosomes secretion in cisplatin-resistant ovarian cancer cells, we explored A2780 cisplatin resistant cell in GSE76449 for candidate targets in inducing cisplatin resistance (Figure 3A). Then TargetScan Human 7.2 (www.targetscan.org) was used to analyze the candidate miRNAs for MED12 down-regulation. The VENN analysis indicated that only one candidate miRNAs were overlapped, miR-548aq-3p (Figure 3B). We also explored the differently expressed miRNA in cells (Figure 3C). Notably, A2780 cisplatin resistant cells did not show increased miR-548aq-3p expression in cells (P = 0.894). We collected the exosome which was isolated from cisplatin-resistant cells culture medium, and we found that increased miR-548aq-3p expression in the exosome of A2780 and SKOV-3 cisplatin-resistant cells than parental cells (Figure 3D). However, we did not observe significantly increased miR-548aq-3p expression in the secreted exosome of ovarian cancer cells after cisplatin treatment for 24 h (Figure 3E). We designed mutant 3’UTR of MED12 mRNA as indicated for luciferase reporter gene assays (Figure 3F). Our results indicated that miR-548aq-3p negatively regulated MED12 transcriptional activity in A2780 cells, which showed no significant changes in mutant ones (Figure 3G). Furthermore, RT-qPCR analysis showed that miR-548aq-3p inhibition significantly upregulated the expression of MED12 in A2780/DDP cells, while miR-548aq-3p mimics down-regulated MED12 expression in A2780 cells (Figure 3H). Western blot assays also confirmed that miR-548aq-3p inhibited MED12 expression levels in the ovarian cancer cells, while miR-548aq-3p inhibitor restored MED12 expression (Figure 3I).
Figure 3.
miR-548aq-3p inhibits MED12 expression in ovarian cancer cells. (A) Volcano plots for exosomal DEGs in A2780 cisplatin-resistant and parental cells based on GSE76449 datasets. (B) The intersection of the miRNA DEGs and predicted miRNA of MED12 by TargetScan was analyzed with the VENN diagram online tool. (C) Volcano plots for cellular DEGs in A2780 cisplatin-resistant and parental cells based on GSE76449 datasets. (D, E) The miR-548aq-3p expression levels was analyzed in the exosome (D) and cells (E) from the parental and cisplatin resistant ovarian cancer cells. (F) The miR-548aq-3p target site was shown in the sequence of MED12 as TargetScan analysis. The wild-type and mutated sites of MED12 were shown. (G) The luciferase reporter gene assays were performed to measure the activity of MED12 in A2780 cells, which were co-transfected with wild-type or mutated MED12 and miR-548aq-3p or miR-control, respectively. (H) The MED12 mRNA expression in miR548aq-3p inhibitor transfected A2780 and A2780/DDP cells was analyzed with RT-qPCR assays. Student’s t test was used for statistical analysis. *P < 0.01. (I) Western Blots analysis of MED12 in transfected A2780 cancer cells. GAPDH was used as internal control.
Exogenous miR-548aq-3p confers cisplatin resistance of ovarian cancer cells
We further explored the functional role of miR-548aq-3p in ovarian cancer cells. Overexpression of miR-548aq-3p mimics increased the cell proliferation of A2780 and SKOV-3 cells compared to the corresponding control groups (Figure 4A, 4B). More importantly, decreased cell apoptosis was also observed in the miR-548aq-3p transfected cells than the control cells with cisplatin treatment (Figure 4C, 4D). Xenograft analysis was performed with the infected SKOV-3 cells with cisplatin treatment, which indicated increased tumor volumes of exogenous miR-548aq-3p expressing cells compared to control cells (Figure 4E, 4F). Furthermore, we overexpressed MED12 in miR-548aq-3p expressing cells, which was certificated with Western blot (Figure 4G). MED12 overexpression restored cisplatin sensitivity of the miR-548aq-3p expressing cells with decreased cell viability (Figure 4H). Furthermore, flowcytometry analysis also showed that MED12 expression abrogated miR-548aq-3p induced cell survival advantage with cisplatin treatment in ovarian cancer cells (Figure 4I). Our results supported that miR-548aq-3p contributed to MED12 down-regulation induced cisplatin resistance of ovarian cancer cells.
Figure 4.
Exogenous miR-548aq-3p confers cisplatin resistance of ovarian cancer cells. (A, B) A2780 and SKOV-3 cells were transfected with miR-548aq-3p-mimics or scramble control. Cell viability were measured with CCK8 analysis which were treated with 25 μM cisplatin for 72 days. (C) Cell apoptosis of the transfected cells was measured after 72 h cisplatin treatment. (D, E) The xenografts volumes of the miR548aq-3p infected A2780 cells were measured for 30 days (E). The image showed the harvested xenografts of different groups. (F) The miR-548aq-3p infected cells were co-transfected with MED12 or control (control). MED12 expression were certificated with Western Blots analysis. GAPDH was used as internal control. (G, H) Cell viability was analyzed with cisplatin (0-75 μM) treatment for 72 h. (I) Cell apoptosis of the transfected cells as (F) was measured with flow cytometry after 72 h cisplatin treatment. Student’s t test was used for statistical analysis. *P < 0.01 vs. control.
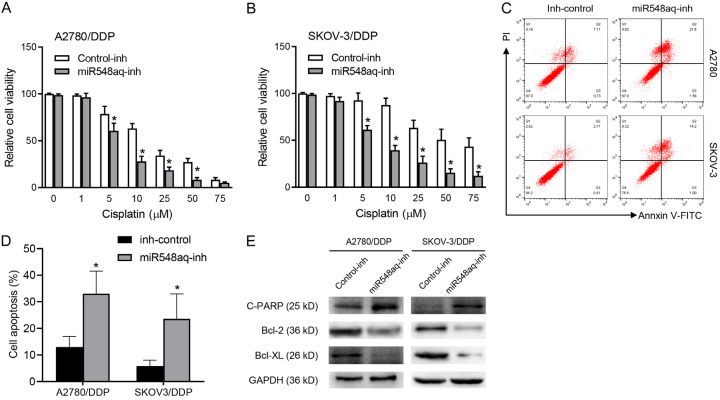
miR-548aq-3p inhibition restores cisplatin sensitivity of ovarian cancer cells
Then, the A2780 and SKOV-3 DDP resistant cells were transfected with the miR-548aq-3p inhibitor or corresponding negative control. Decreased cell viability was observed in miR-548aq-3p inhibitor transfected cells compared to those with the negative control with gradient concentration of cisplatin treatment for 48 h (Figure 5A, 5B). The miR-548aq-3p inhibitor transfected cells were collected within 48 h cisplatin treatment to evaluate the relative levels of cell apoptosis. The extent of cell apoptosis was significantly increased in miR-548aq-3p inhibitor transfected cells than the control ones (Figure 5C, 5D). Further Western blot assays confirmed that increased cleaved PARP expression, decreased bcl-2 and Bcl-XL expression in miR-548aq-3p inhibitor infected cells with cisplatin treatment (Figure 5E). Taken together, our results suggested miR-548aq-3p contributed to the cisplatin resistance of ovarian cancer cells by down-regulating MED12 expression.
Figure 5.
miR-548aq-3p inhibition restores cisplatin sensitivity of ovarian cancer cells. A, B. The cisplatin resistant cells transfected with miR-548aq-3p inh (miR-548aq-3p-inh) or inhibitor control (control). Cell viability was analyzed with cisplatin (0-75 μM) treatment for 72 h. C, D. The cell apoptosis extents of the transfected cisplatin resistant cells were analyzed with FACS. Transfected SKOV-3/DDP cells were treated with 25 μM cisplatin for the 72 h. E. Western blot analyses of cleaved PARP, Bcl-2 and Bcl-XL in the transfected cisplatin resistant cells. GAPDH was used as an internal control. Student’s I test was used for statistical analysis. *P < 0.01 vs. inhibitor control.
miR-548aq predicts disease progression of epithelial ovarian cancer
Firstly, we selected paraffin-embedded specimens from 98 ovarian cancer patients who received cisplatin-based neoadjuvant chemotherapy. IHC staining were performed for the expression levels of MED12, which indicated that MED12 protein was located in the cytoplasm and nuclei (Figure 6A). More importantly, lower percentage of ORR was observed in MED12 negative patients than positive ones (62.00% vs. 82.43%) (Figure 6B). Moreover, negative correlation was observed between MED12 expression and some clinical parameters, including lymph node metastasis and clinical stage (Table 1). We further evaluated the prognostic significance of MED12 expression in ovarian cancer. We observed better PFS and OS in the patients with positive MED12 than negative ones (Figure 6C and 6D).
Figure 6.

miR-548aq predicts disease progression of epithelial ovarian cancer. (A) IHC staining of LOXL2 was performed with CRC specimens. Representative images were provided with positive and negative expression. Bar, 50 μm. (B) The comparation of overall response rate (ORR) was analyzed between MED12-positive patients and negative patients. P < 0.001. (C, D) Kaplan-Meier analysis of progression-free survival (C) and overall survival (D) in cisplatin treated epithelial ovarian cancer patients according to MED12 expression with IHC analysis. (E, F) The correlation of miR-548aq expression and age (E) and histological grade (F) was analyzed with TCGA database. (G) Decreased MED12 mRNA levels were observed in miR-548aq positive ovarian cancer specimens, which was analyzed with TCGA database. (H, I) Kaplan-Meier analysis of progression-free survival (H) and overall survival (I) in cisplatin treated epithelial ovarian cancer patients according to MED12 mRNA expression. (J) Kaplan-Meier analysis of disease-free survival of epithelial ovarian cancer patients according miR-548aq expression.
Table 1.
Relationship between clinical characteristics and MED12 expression
| Characteristic | Number (%) | MED12 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Total | 96 | (50, 52.08%) | (46, 47.92%) | |
| Age, years | ||||
| < 50 | 37 (38.54) | 15 (15.63) | 16 (16.67) | 0.617 |
| ≥ 50 | 59 (61.46) | 35 (36.46) | 30 (31.25) | |
| Tumor size, cm | ||||
| < 10 | 60 (62.50) | 33 (34.38) | 27 (28.13) | 0.460 |
| ≥ 10 | 36 (37.50) | 17 (17.71) | 19 (19.79) | |
| Histological grade | ||||
| I | 43 (44.79) | 12 (12.50) | 16 (16.67) | 0.594 |
| II | 53 (55.21) | 38 (39.58) | 40 (41.67) | |
| Lymph node status | ||||
| 0 | 37 (38.54) | 13 (13.54) | 24 (25.00) | 0.008 |
| ≥ 1 | 59 (61.46) | 37 (38.54) | 22 (22.92) | |
| Clinical stage | ||||
| I-II | 28 (29.17) | 8 (8.33) | 20 (20.83) | 0.003 |
| III-IV | 68 (70.83) | 42 (43.75) | 26 (27.08) | |
| CA125 | ||||
| < 400 | 52 (54.17) | 27 (28.13) | 25 (26.04) | 0.973 |
| ≥ 400 | 44 (45.83) | 23 (23.96) | 21 (21.88) | |
We further accessed the clinical significance of miR-548aq in epithelial ovarian cancer with TCGA data. Totally 374 cases were involved in the analysis. No significant correlation was observed between the miR-548aq expression and age or histological grade (Figure 6E, 6F). However, decreased MED12 expression was observed in the miR-548aq positive specimens than the negative ones (Figure 6G). Moreover, we evaluate the prognostic value of MED12 in ovarian cancer with cisplatin chemotherapy with Kaplan-Meier Plotter (https://kmplot.com/analysis/). We found a favorable PFS in MED12 positive ovarian cancer patients than negative ones (MED12 cutoff value: 754, n = 1259. Figure 6H). More importantly, MED12 positive ovarian cancer patients also showed better OS than negative ones (MED12 cutoff value: 573, n = 1409. Figure 6I). Next, Kaplan-Meier analysis showed that miR-548aq expression predicted lower disease-free survival of epithelial ovarian cancer patients (n = 458. Figure 6J). Taken together, our findings supported that miR-548aq expression predicted disease progression of epithelial ovarian cancer.
Discussion
Platinum-based chemotherapy regimen is the standard treatment for epithelial ovarian cancer, including neoadjuvant chemotherapy prior to debulking surgery and adjuvant chemotherapy after cytoreductive surgery [20,21]. However, disease recurrence after latency periods is observed in a large percentage of the patients [22]. Drug-resistance contributes predominantly to the cancer-related mortality for the ovarian cancer patients [23]. The best candidates for the adjuvant therapeutic setting are currently a lack of consensus [24]. Here in this study, we identified MED12 participated in the cisplatin sensitivity of ovarian cancer. Further study indicated that the transcriptional suppression of MED12 by exosomal miR-548aq-3p contributed to the cisplatin resistance of ovarian cancer cells. miR-548aq-3p expression conferred survival advantage of ovarian cancer cells in cytotoxic environment. These results showed important therapeutic implications of targeting miR-548aq-3p/MED12 for improving chemotherapy sensitivity of ovarian cancer.
MED12 works as a subunit of the Mediator complex to regulate transcriptional activity [25]. Elevated MED12 expression was correlated with chemotherapy sensitivity in breast cancer [26], lung cancer [27], and colon cancer [9,28]. TGFβ-receptor signaling and epithelial-mesenchymal transition was reported to mediate its function in cancer cells [9,29]. Furthermore, MED12 knockdown arrested cell cycle in G0-G1 phase of epithelial ovarian cancer and castration-resistant prostate cancer [10,30]. Decreased MED12 expression was correlated with poor prognosis in patients with NSCLC, breast cancer, ovarian cancer and leukemia to chemotherapeutics [31]. The modulating activity of the CDK8 kinase and interaction with TGF-βR2 signaling, may contribute to therapeutic sensitivity [9,32], including the tyrosine kinase inhibitors and chemotherapy regimens [10]. Besides, post-translational methylation of MED12 by CARM1 renders cells sensitive to 5-fluorouracil, which was correlated with p21/WAF1 regulation [26]. More importantly, MED12 bound to the promoter of EGFR to regulate its expression in epithelial ovarian cancer, which was correlated with therapeutic sensitivity [10]. Consistent with the previous studies, our study indicated MED12 was involved in the chemotherapy sensitivity of platinum-based regimens in ovarian cancer [33]. Restoration of MED12 expression indicated a promising therapeutic strategy for advanced ovarian cancer, especially for the chemotherapy resistant ones.
Differentially expressed exosomal miRNAs was identified in cisplatin-resistant and parental ovarian cancer cells with a microarray analysis dataset (GSE76449) to explore the candidate mechanism which involved in drug-resistance of ovarian cancer. The exosomal miRNAs were screened with log2 (fold change) ≥ 2, and adjusted P value < 0.05. TargetScan Human 7.2 (www.targetscan.org) was used to analyze the candidate miRNAs for MED12 down-regulation. Further VENN analysis identified miR-548aq-3p as an overlapped candidate. Further RT-qPCR verified higher miR-548aq-3p expression levels in the exosomes of cisplatin resistant ovarian cancer cells than parental ones. The exosomes from cisplatin-resistant cells were taken up by parental cells and maintained their effects, such as exosomal miR-429 and miR-205 [12,34]. miR548aq-3p may be appropriate for packaging into exosomes to retain its stability and subsequently play a significant role in cisplatin resistance. Moreover, we also provided evidence that miR-548aq-3p participated in MED12 transcriptional inhibition, which supported miR-548aq-3p as a candidate for MED12 inhibitor. Further investigation of miR-548aq-3p may suggest new therapeutic approaches for epithelial ovarian cancer.
miR-548aq-3p is a novel miRNA with no biological function as yet defined. Recent study indicated that non-invasive far infrared radiation induced miR-548aq-3p down-regulation of endothelial colony forming cells, which contributed to vascular repair in coronary artery disease [35]. To elucidate if miR-548aq-3p is involved in the cisplatin resistance of ovarian cancer cells, we performed miR-548aq-3p overexpression and inhibition experiments in ovarian cancer cells, respectively. Overexpressing miR-548aq-3p showed anti-apoptotic activity with cisplatin treatment, while miR548aq-3p inhibition decreased cell viability and drug resistance with cisplatin treatment. In our study, exosomal miR-548aq-3p expression levels were not significantly changed with short-term cisplatin treatment, whereas cisplatin resistant cells showed decreased MED12 expression and increased exosomal miR-548aq-3p. In consideration of sustained cisplatin treatment enriched cancer stem cells, which maybe contributed predominantly to exosomal miR-548aq-3p secretion. Further investigation is still needed to explore the functional role of miR-548aq-3p in human disease progression.
Accumulating studies supported a crucial role of miRNAs in human malignancies, including tumorigenesis, angiogenesis and metastasis [36,37]. Wide transcriptional changes of miRNAs were also observed during the development and progression of ovarian cancer, which indicated their correlation with clinical stage and histological grade [38]. Moreover, different histological subtype of ovarian tumors also showed distinct miRNA phenotype according to the microarray analyses [39]. Clinical examination for miR-548aq-3p expression will provide valuable information for prognosis and chemotherapy regimens option for the ovarian cancer patients. Moreover, the clinical analysis supported that miR-548aq-3p predicted grim survival estimation in ovarian cancer patients. The ovarian tumors with miR-548aq-3p expression will not benefit from cisplatin-based chemotherapy.
In conclusion, we demonstrated that miR-548aq-3p down-regulated cell apoptotic activity of epithelial ovarian cancer cells via modulating MED12 expression. Further elucidating the mechanisms of miR-548aq-3p/MED12 interaction will provide valuable insight towards chemoresistance, which will also provide novel antitumor strategies for epithelial ovarian cancer.
Acknowledgements
This research was supported by grants from National Natural Science Foundation of China (81972793, 81502283), and Qingdao Outstanding Health Professional Development Fund.
Disclosure of conflict of interest
None.
References
- 1.Boussios S, Abson C, Moschetta M, Rassy E, Karathanasi A, Bhat T, Ghumman F, Sheriff M, Pavlidis N. Poly (ADP-Ribose) polymerase inhibitors: talazoparib in ovarian cancer and beyond. Drugs R D. 2020;20:55–73. doi: 10.1007/s40268-020-00301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 3.Pignata S, Pisano C, Di Napoli M, Cecere SC, Tambaro R, Attademo L. Treatment of recurrent epithelial ovarian cancer. Cancer. 2019;125(Suppl 24):4609–4615. doi: 10.1002/cncr.32500. [DOI] [PubMed] [Google Scholar]
- 4.Boussios S, Karihtala P, Moschetta M, Abson C, Karathanasi A, Zakynthinakis-Kyriakou N, Ryan JE, Sheriff M, Rassy E, Pavlidis N. Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Invest New Drugs. 2020;38:181–193. doi: 10.1007/s10637-019-00867-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Qin K, Zhang W, Yang B, Zhao C, Zhang X, Zhang F, Zhao L, Shan B. Postoperative recurrence of epithelial ovarian cancer patients and chemoresistance related protein analyses. J Ovarian Res. 2019;12:29. doi: 10.1186/s13048-019-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L. Molecular characterization of epithelial ovarian cancer: implications for diagnosis and treatment. Int J Mol Sci. 2016;17:2113. doi: 10.3390/ijms17122113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham JM Jr, Schwartz CE. MED12 related disorders. Am J Med Genet A. 2013;161A:2734–2740. doi: 10.1002/ajmg.a.36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, Van’t Veer L, Tollenaar RA, Jackson DB, Agrawal D, Dai H, Yeatman TJ. EMT is the dominant program in human colon cancer. BMC Med Genomics. 2011;4:9. doi: 10.1186/1755-8794-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Holzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, Groenendijk FH, Mittempergher L, Nijkamp W, Neefjes J, Salazar R, Ten Dijke P, Uramoto H, Tanaka F, Beijersbergen RL, Wessels LF, Bernards R. MED12 controls the response to multiple cancer drugs through regulation of TGF-beta receptor signaling. Cell. 2012;151:937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo XL, Deng CC, Su XD, Wang F, Chen Z, Wu XP, Liang SB, Liu JH, Fu LW. Loss of MED12 induces tumor dormancy in human epithelial ovarian cancer via downregulation of EGFR. Cancer Res. 2018;78:3532–3543. doi: 10.1158/0008-5472.CAN-18-0134. [DOI] [PubMed] [Google Scholar]
- 11.Xavier CPR, Caires HR, Barbosa MAG, Bergantim R, Guimaraes JE, Vasconcelos MH. The role of extracellular vesicles in the hallmarks of cancer and drug resistance. Cells. 2020;9:1141. doi: 10.3390/cells9051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Lin L, Liu Q, Gao W, Chen L, Sha C, Chen Q, Xu W, Li Y, Zhu X. Exosomal transfer of miR-429 confers chemoresistance in epithelial ovarian cancer. Am J Cancer Res. 2021;11:2124–2141. [PMC free article] [PubMed] [Google Scholar]
- 13.Markowska A, Sajdak S. Role of cancer stem cells and microRNA in resistance to chemotherapy in patients with ovarian cancer. Eur J Gynaecol Oncol. 2017;38:181–183. [PubMed] [Google Scholar]
- 14.Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu G, Zhao R, Huang H, Wang X, Qiao Y, Li F, Han D, Wang L, Zhang G, Gao X. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene. 2014;33:378–386. doi: 10.1038/onc.2012.575. [DOI] [PubMed] [Google Scholar]
- 15.Li LW, Xiao HQ, Ma R, Yang M, Li W, Lou G. miR-152 is involved in the proliferation and metastasis of ovarian cancer through repression of ERBB3. Int J Mol Med. 2018;41:1529–1535. doi: 10.3892/ijmm.2017.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis S, Meltzer PS. GEOquery: a bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Shi YL, Zhou K, Wang LL, Yan ZX, Liu YL, Xu LL, Zhao SW, Chu HL, Shi TT, Ma QH, Bi J. PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis. 2018;9:739. doi: 10.1038/s41419-018-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanczky A, Gyorffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J Med Internet Res. 2021;23:e27633. doi: 10.2196/27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28:viii61–viii65. doi: 10.1093/annonc/mdx443. [DOI] [PubMed] [Google Scholar]
- 21.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2016;2016:CD005340. doi: 10.1002/14651858.CD005340.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:1147–1158. doi: 10.1080/14737140.2017.1398088. [DOI] [PubMed] [Google Scholar]
- 23.Di Donato V, Kontopantelis E, Aletti G, Casorelli A, Piacenti I, Bogani G, Lecce F, Benedetti Panici P. Trends in mortality after primary cytoreductive surgery for ovarian cancer: a systematic review and metaregression of randomized clinical trials and observational studies. Ann Surg Oncol. 2017;24:1688–1697. doi: 10.1245/s10434-016-5680-7. [DOI] [PubMed] [Google Scholar]
- 24.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, Dizon DS, Kash JJ, Meyer LA, Moore KN, Olawaiye AB, Oldham J, Salani R, Sparacio D, Tew WP, Vergote I, Edelson MI. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016;34:3460–3473. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, O’Regan R, Xu W. The emerging role of mediator complex subunit 12 in tumorigenesis and response to chemotherapeutics. Cancer. 2020;126:939–948. doi: 10.1002/cncr.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Zeng H, Wang Q, Zhao Z, Boyer TG, Bian X, Xu W. MED12 methylation by CARM1 sensitizes human breast cancer cells to chemotherapy drugs. Sci Adv. 2015;1:e1500463. doi: 10.1126/sciadv.1500463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M, Wang F, Li G, Wang X, Fang X, Jin H, Chen Z, Zhang J, Fu L. MED12 exerts an emerging role in actin-mediated cytokinesis via LIMK2/cofilin pathway in NSCLC. Mol Cancer. 2019;18:93. doi: 10.1186/s12943-019-1020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sooraj D, Sun C, Doan A, Garama DJ, Dannappel MV, Zhu D, Chua HK, Mahara S, Wan Hassan WA, Tay YK, Guanizo A, Croagh D, Prodanovic Z, Gough DJ, Wan C, Firestein R. MED12 and BRD4 cooperate to sustain cancer growth upon loss of mediator kinase. Mol Cell. 2022;82:123–139. e127. doi: 10.1016/j.molcel.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Brunen D, Willems SM, Kellner U, Midgley R, Simon I, Bernards R. TGF-beta: an emerging player in drug resistance. Cell Cycle. 2013;12:2960–2968. doi: 10.4161/cc.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler D, Offermann A, Braun M, Menon R, Syring I, Nowak M, Halbach R, Vogel W, Ruiz C, Zellweger T, Rentsch CA, Svensson M, Andren O, Bubendorf L, Biskup S, Duensing S, Kirfel J, Perner S. MED12 overexpression is a frequent event in castration-resistant prostate cancer. Endocr Relat Cancer. 2014;21:663–675. doi: 10.1530/ERC-14-0171. [DOI] [PubMed] [Google Scholar]
- 31.Dos Anjos Pultz B, da Luz FA, de Faria PR, Oliveira AP, de Araujo RA, Silva MJ. Far beyond the usual biomarkers in breast cancer: a review. J Cancer. 2014;5:559–571. doi: 10.7150/jca.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt F, Leitner A, Kim IV, Ho-Xuan H, Schneider EV, Langhammer F, Weinmann R, Muller MR, Huber R, Meister G, Kuhn CD. A precisely positioned MED12 activation helix stimulates CDK8 kinase activity. Proc Natl Acad Sci U S A. 2020;117:2894–2905. doi: 10.1073/pnas.1917635117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guinney J, Ferte C, Dry J, McEwen R, Manceau G, Kao KJ, Chang KM, Bendtsen C, Hudson K, Huang E, Dougherty B, Ducreux M, Soria JC, Friend S, Derry J, Laurent-Puig P. Modeling RAS phenotype in colorectal cancer uncovers novel molecular traits of RAS dependency and improves prediction of response to targeted agents in patients. Clin Cancer Res. 2014;20:265–272. doi: 10.1158/1078-0432.CCR-13-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, Wu X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai WC, Chiang WH, Wu CH, Li YC, Campbell M, Huang PH, Lin MW, Lin CH, Cheng SM, Chang PC, Cheng CC. miR-548aq-3p is a novel target of far infrared radiation which predicts coronary artery disease endothelial colony forming cell responsiveness. Sci Rep. 2020;10:6805. doi: 10.1038/s41598-020-63311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6:235–246. doi: 10.1158/2159-8290.CD-15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1. [DOI] [PubMed] [Google Scholar]
- 38.Giannopoulou L, Zavridou M, Kasimir-Bauer S, Lianidou ES. Liquid biopsy in ovarian cancer: the potential of circulating miRNAs and exosomes. Transl Res. 2019;205:77–91. doi: 10.1016/j.trsl.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Menard S, Croce CM. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]