Abstract
Spasmolytic polypeptide-expressing metaplasia (SPEM) is a trefoil factor 2-expressing metaplasia in the fundic glands that resembles the fundic metaplasia of deep antral glandular cells and arises mainly from transdifferentiation of mature chief cells as well as mucous neck cells or isthmic stem cells. SPEM participates in the regulation of gastric mucosal injury, including focal and diffuse injury. This review focuses on the origin, models, and regulatory mechanisms of SPEM and on its role in the development of gastric mucosal injury. We hope to provide new prospects for the prevention and treatment of gastric mucosal diseases from the perspective of cell differentiation and transformation.
Keywords: Gastric mucosal injury, cellular differentiation, regeneration and repair, preneoplastic metaplasia, dysplasia
Introduction
SPEM is a spasmolytic polypeptide/trefoil factor 2 (TFF2)-expressing metaplasia in the fundic glands that resembles the fundic metaplasia of deep antral glandular cells and has morphological features similar to the abnormal metaplastic cell lineage of Brunner’s glands of the duodenum [1] and the expression of CD44 variant isoform 9 (CD44v9), lectin, mucin 6 (Muc6), GSII-lectin, and HE4, in addition to the characteristic expression of TFF2 [2]. SPEM arises mainly from the transdifferentiation of mature chief cells, which regain the ability to proliferate during acute or chronic injury of the gastric mucosa and then differentiate into metaplastic mucus-secreting SPEM cells [3].
Gastric mucosal injury classified as local and diffuse injury, and the SPEM cell lineage is present regardless of the type of injury [4] (Figure 1). Local injury is a repairable injury that does not alter the pattern of cellular differentiation, and gastric ulcers are the most common type [5]. In gastric ulcer-induced injury, SPEM represents a mucus secretion repair lineage that appears at the ulcer margin and disappears when the mucosa returns to the normal cell lineage [6]. Therefore, SPEM not only recruits repair cells to the site of mucosal injury but also reprograms gastric epithelial cells and increases the protective barrier of the epithelium, which is an important process associated with regeneration and repair after gastric mucosal injury [6,7]. Accumulating studies have shown that SPEM represents an initializing metaplastic response to acute gastric injury and that when injury and chronic inflammation persist, focal injury progresses to diffuse injury and can perpetuate recurrent reprogramming and metaplastic patterns, which are common preneoplastic pathways associated with diffuse injury [2,8,9].
Figure 1.
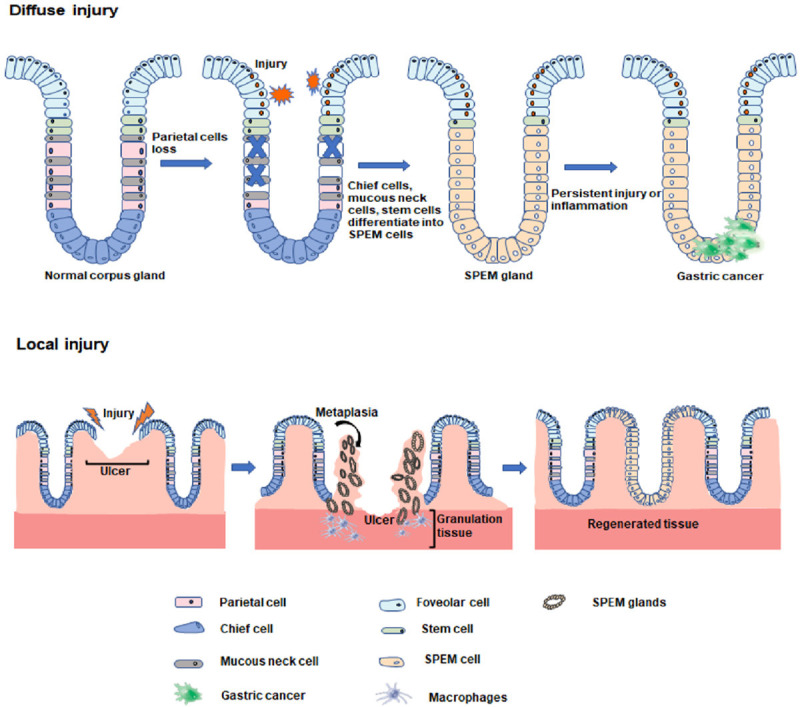
Two phenotypes of SPEM in gastric mucosal disease.
Diffuse injury is a type of chronic injury that alters the pattern of cell differentiation; it is commonly caused by chronic Helicobacter pylori infection or parietal cell loss and is closely related to the development of gastric lesions [10,11]. In diffuse gastric injury, gastric acid-secreting parietal cells undergo atrophy, the number of parietal cells decreases, and zymogen-secreting chief cells also decrease and are replaced by TFF2-expressing metaplasia, resulting in the production of SPEM cell lineages [12]. In the area of the fundic gland, which contains both SPEM and intestinal metaplasia (IM), SPEM appears in the area of the deep fundic gland, while IM can be observed in the lumen of the gland, and IM may have developed from SPEM [13]. The transition from SPEM to IM indicates that metaplasia has passed the transition point of preneoplastic metaplasia and may progress to dysplasia and adenocarcinoma [14,15].
In conclusion, SPEM is an adaptive change and repair mechanism made by gastric epithelial cells in response to injury that regulates gene transcription and changes cell phenotype and tissue structure, thereby producing differentiation and transformation in cells [16]. SPEM represents not only a repair system during focal injury of the gastric mucosa [6] but also the possibility of further development into IM or even gastric cancer during diffuse injury [17]. Focal injury can transform into diffuse injury in the presence of chronic injury and persistent inflammation, but even chronic metaplasia can reverse some types of diffuse injury [9,18]. Therefore, the aim of this paper is to summarize the role of SPEM in gastric mucosal injury diseases and provide a preventive direction for gastric mucosal diseases.
Sources, regulators, and related models of SPEM
Mature chief cells are located at the base of the gland following mitosis, are reprogrammed to transdifferentiate into SPEM cells in response to the repair of gastric injury and are responsible for the secretion of pepsin and other digestive enzymes [3,12,19-21]. Transdifferentiation of chief cells to a SPEM phenotype is a highly elaborate regulatory process not only involving disruption of chief cell secretory architecture and changes in transcription factors [22] but also possibly serving as an acute reparative lesion in injury models, such as those established with DMP-777, L-635, or high-dose tamoxifen (HDT) and models of acid-induced ulcers [23]. However, some scholars have proposed that chief cells are not the only source of SPEM and that SPEM also arises from mucous neck cells or isthmic stem cells. During chronic inflammation, mucous neck cells also exhibit plasticity and contribute to the development of SPEM [3,7,23-25]. Single-cell RNA sequencing analysis of gastric body epithelium showed that both mucous neck cells and chief cells can transform into SPEM in the setting of chronic inflammation, a finding that not only broadens the understanding of the origin of SPEM but also reveals additional epithelial plasticity in metaplasia [26]. To gain insight into the molecular mechanisms driving gastric metaplasia and its progression to tumors, researchers have developed many mouse models that successfully mimic metaplasia before tumorigenesis [27]. In these mouse models, the metaplastic lineage is dominated by SPEM. Based on the induction factors and regulatory mechanism of SPEM, there are several main mouse models: 1) the acute drug-induced SPEM model, 2) the gene and transcriptional manipulation-induced SPEM mouse model [28], and 3) the chronic Helicobacter infection-induced SPEM model. These models are summarized in Table 1.
Table 1.
Pathologic phenotypes of gastric metaplasia in mouse models
| Mouse model | SPEM | SPEM for Proliferation Capacity | Intestinal metaplasia | Foveolar hyperplasia | Inflammatory infiltrate | Invasive glandular production |
|---|---|---|---|---|---|---|
| Acute drug-induced SPEM model | ||||||
| Oral DMP-777 Treatment [29] | Yes | No | No | Yes | No | No |
| Oral high-dose tamoxifen treatmen [25,30] | Yes | No | No | Yes | Yes | No |
| Oral L-635 treatment [32] | Yes | Yes | No | Yes | Yes | No |
| gene and transcriptional manipulation mice induce SPEM model | ||||||
| Slc26a9fl/fl/Atp4b-Cre mice [38] | Yes | Yes | Yes | Yes | Yes | Yes |
| KLF4-deficient mice [39,40] | Yes | No | No | Yes | No | No |
| Runx3 deficient mice [43] | Yes | Yes | No | No | No | No |
| H/K-IFN-γ transgenic mice [47] | Yes | Yes | No | Yes | Yes | Yes |
| H/K-noggin transgenic mice [49] | Yes | No | No | Yes | No | No |
| Amphiregulin-deficient mice [14] | Yes | Yes | Yes | Yes | Yes | Yes |
| Gastrin-deficient mice [31] | Yes | No | No | No | No | No |
| Insulin-gastrin transgenic mice [63] | Yes | Yes | No | Yes | Yes | Yes |
| IL33-deficient mice or IL13-deficient mice [15,83] | No | No | No | No | No | No |
| Claudin 18-deficient mice [111] | Yes | Yes | No | No | Yes | Yes |
| Mist1-Kras mice [9,115] | Yes | Yes | Yes | Yes | Yes | Yes |
| Chronic Helicobacter infection model | ||||||
| H. felis or H. Pylori infection [3] | Yes | Yes | No | No | Yes | Yes |
Acute drug-induced SPEM model
Modeling of the chronic Helicobacter infection model takes more than 6 months and is slow, and asynchronous metaplasia may occur during mass induction. Therefore, researchers developed acute drug-induced models, mainly using DMP-777, high-dose tamoxifen, and L635. DMP-777 is a proton carrier that is specific for the apical acid secretory membrane of parietal cells, leads to the rapid ablation of parietal cells and induces SPEM development, which is reversible and does not produce proliferative SPEM after the cessation of DMP-777 treatment [29]. DMP-777 is also a neutrophil elastase inhibitor that forms a murine gastric SPEM lineage that is inflammation-free [24]. High-dose tamoxifen rapidly induces apoptosis in gastric parietal cells and zymogen-secreting chief cell metaplasia in mice, with scattered proliferative SPEM cells observable within 3 days, and these effects are reversible after tamoxifen discontinuation [25,30]. The loss of oral omeprazole gastric parietal cells was eliminated before tamoxifen or DMP-777 treatment [24,31], but tamoxifen caused more inflammation. L635 is an analog of DMP-777, but the lack of elastase inhibition by L635 leads to a significant inflammatory response, and the inflammatory environment can lead to a shift in SPEM from slow metaplasia to extended proliferative metaplasia [3]. It has been shown that L-635-treated mice develop SPEM lineages with similar phenotypes 6-12 months after H. felis infection [32]. The acute drug-induced SPEM model is less time-consuming, and lesions are reversible compared to other models, which contradicts the concept that metaplasia is irreversible. Therefore, we can hypothesize that the mechanism of drug-induced SPEM may be the response to acute injury and the process of regeneration, and acute drug administration can directly induce rapid parietal cell death, synchronously induce metaplasia, and bypass the chronic immune mechanism, which provides an important experimental basis for the successful and efficient study of specific stages of metaplasia.
Gene and transcriptional manipulation to induce SPEM mouse models
Mouse models of the oxyntic atrophy phenotype can be generated by gene-targeted overexpression or deletion. The Cre-loxP system is an important method for achieving cell- or tissue-specific deletion of the target gene, and a generated transgenic mouse line (Atp4b-Cre) provides a valuable tool for studying gastric parietal cell differentiation, survival, and physiological function [33]. Slc26a9 is strongly expressed in gastric parietal cells in mice and humans and is extremely important for the function of parietal cells [34-37]. Published studies by our group have confirmed that Slc26a9fl/fl/Atp4b-Cre mice generated by parietal cell-specific Slc26a9 knockout have parietal cell loss at 1 month of age, oxyntic atrophy at 2 months of age, and metaplasia (SPEM) and IM with significant expression of spasmolytic peptides at 6 months of age [38], indicating that Slc26a9 is essential for parietal cell function and maintaining gastric cell homeostasis.
Moreover, Kruppel-like factor 4 (KLF4) can regulate cell proliferation and differentiation, and significant oxyntic atrophy, foveal hyperplasia, and TFF2 metaplasia occurs at birth in KLF4-deficient mice [39,40]; this phenotype is similar to that of mice infected with H. felis, but no IM, dysplasia, or invasive lesions occur in the gastric mucosa, a phenotype equivalent to that of benign metaplasia without an inflammatory response [41]. The gastric tumor suppressor Runx3 is highly expressed in chief cells and is an important factor in chief cell differentiation [42]. Runx3-knockout mice mainly exhibit hyperplasia of gastric epithelial cells, loss of chief cells, and significant SPEM lineage populations [43]. Although no glandular dysplasia or invasiveness has been reported, adenocarcinoma can persist following N-methyl-N-nitrosourea treatment [44,45]. H/K-IFN-γ transgenic mice are formed by targeted transfection of mouse IFN-γ into gastric parietal cells using H/K ATPase β-subunit promoter fragments [46]. The gastric mucosa of this mouse model showed significant inflammation, oxyntic atrophy, SPEM, dysplasia at 3 to 5 months of age, cystic gland dilatation with age, occasional breakthrough to the submucosa, and antral polyps or tumors after 12 months [47].
However, in H/K-noggin transgenic mice, in which the H+/K+-ATPase β subunit gene promoter is specifically expressed by parietal cells and regulates noggin expression in the mouse gastric epithelium, noggin expression inhibits bone morphogenetic protein (BMP) signaling in the stomach [48] and has anti-inflammatory effects, and its deletion promotes the development of SPEM [48,49]. In addition, noggin overexpression increases the chronic inflammatory response to H. pylori infection and accelerates the progression of dysplasia [50].
Paracrine factors such as epidermal growth factor receptor (EGFR) ligands, transforming growth factor-α (TGF-α), amphiregulin (AR), and heparin-binding EGF-like growth factor (HB-EGF) are produced by gastric parietal cells and can affect gastric lineage differentiation and transformation [51,52]. Epidermal growth factor (EGF)-like growth factor is induced during acute gastric injury and is involved in the repair of acute gastric mucosal injury [53]. In a rat gastric epithelial cell line (RGM1), EGFR induces the expression of HB-EGF and EGFR tyrosine phosphorylation, followed by a significant increase in HB-EGF and AR transcription in RGM1 cells [53]. However, different EGF receptor ligands have different effects on the development and regulation of SPEM, and TGFα-deficient mice exhibit SPEM similar to wildtype mice [54]. AR-deficient mice spontaneously developed SPEM and exhibited dysplastic changes at 1 year of age [14]. The loss of AR can lead to SPEM acceleration and amplification. In addition, hepatocyte growth factor (HGF) activators (HGFAs) sense mucosal injury and are activated, promote HGF activation and participate in gastric mucosal repair [55-58]. Gastric mucosal lineage changes were observed in HGFA-deficient mice and wild-type mice after DMP-777 treatment, and HGFA promoted foveal cell proliferation and mucosal cell proliferation in acute oxyntic injury without affecting the occurrence of SPEM, but a lack of HGFA signaling delayed the recovery of SPEM cells to normal gastric gland cells [59].
Gastrin secretion, which is increased with parietal cell loss, regulates glandular homeostasis [50,60,61], epithelial cell proliferation and vesicular hyperplasia [62]. Gastrin-knockout mice treated with DMP-777 exhibited accelerated SPEM development [31]. Insulin-gastrin (INS-GAS) transgenic mice also have high circulating levels of gastrin and initially exhibit elevated serum gastrin levels and increased numbers of parietal cells, but increased acid secretion is lost with gastric atrophy as these mice age [63]. This mouse developed SPEM and submucosal lesions at 20 months of age and had accelerated SPEM progression during H. felis infection, further leading to the development of gastric cancer [63].
Chronic H. pylori infection-induced SPEM models
For mice with chronic H. felis or H. pylori infection, parietal cell loss occurs after six months, principal cells differentiate into a highly proliferative SPEM lineage [3], and SPEM-related dysplasia persists for up to 1 year after inflammation, but IM with characteristic goblet cells has not been observed in humans [64-66]. Lesions similar to those of metaplasia due to H. pylori infection in humans can be observed in mice infected with H. felis or H. pylori [63-65,67]. Lineage studies of SLFN4 in H. pylori-infected mice have shown that bone marrow-derived SLFN4+ cells migrate into the stomach via Hh signaling after H. pylori infection, polarize into myeloid-derived suppressor cells (MDSCs) among gastric epithelial cells, and acquire the ability to inhibit T-cell proliferation 4-6 months after H. pylori infection, which is consistent with oxyntic atrophy and SPEM [68]. In the H. pylori-infected human stomach, SLFN12L affects cell migration in a manner similar to SLFN4 in cells of myeloid origin [68]. In addition, SLFN4+ cells in the mouse stomach highly express miR-130b, which is required for MDSC function and is able to regulate suppressive functions of T cells and promote H. pylori-induced metaplasia [69]. Upregulated miR-130b targets Cyld (cylindromatosis gene, encoding a deubiquitinating enzyme), which negatively regulates NF-κB, and the NF-κB signaling pathway is a key pathway in H. pylori-induced gastritis and metaplasia [70]. NF-κB directly binds to the miR-130b promoter to induce its upregulation, thus forming a positive feedback loop, thereby promoting the development of SPEM [69,71].
Highly proliferative SPEM has long been considered a precancerous lesion in gastric carcinogenesis [72], and for further study, many researchers currently use organoids formed in a gastric epithelial cell culture system to characterize changes in epithelial cells after chronic H. pylori infection. In a study by the Wataru Shibata group, chronic inflammation induced by H. pylori infection was found to increase the number of cells expressing tissue stem cell markers and the expression of genes involved in intestinal phenotypes, and the upregulation of these genes and the increase in the number of stem cells were abolished with the eradication of H. pylori, as determined by analysis of the formal classifications [73]. Thus, stemness or metaplastic phenotypes are acquired following chronic inflammation through genetic/epigenetic changes in gastric tissue stem cells [74].
Role of SPEM in gastric mucosal diseases
Role of SPEM in gastric ulcer regeneration and repair (gastric focal injury diseases)
Gastric ulcer repair is an extremely complex process involving tissue re-epithelialization and regeneration, which includes inflammatory infiltration, cell proliferation, and granulation tissue formation [75]. In response to mucosal injury following gastric ulcer development, the glands surrounding the ulcer expand, epidermal growth factor (EGF) expression increases, and granulation tissue begins to proliferate, which is followed by re-epithelialization at the wound base [75-77]. The ulcer-associated cell lineage (UACL) formed here was identified as SPEM, and its marker was CD44v9. SPEM is associated with increased expression of CD44 (particularly CD44v9) on the cell surface and plays an important role in the repair of gastric injury [6,7,78].
CD44-deficient mice have impaired ulcer repair, which is characterized by insufficient epithelial gland regeneration, ulcer persistence, and the loss of epithelial proliferation [7]. CD44v9-expressing gastric xenografts were transplanted into the gastric epithelium of CD44-deficient mice in an orthotopic organoid transplantation model, which resulted in epithelial cell proliferation and regeneration [7]. The appearance of CD44v9 at the ulcer margin is associated with proliferation, as CD44 orchestrates progenitor proliferation within both normal and metaplastic gastric epithelium [14,31,66,79]. In addition, CD44v9 also protects against reactive oxygen species (ROS) during gastric ulcer repair, interacts with the glutamate-cysteine transporter xCT, stabilizes proteins and promotes effective regeneration after injury [80,81].
Mucosal damage repair following gastric ulcer development is closely associated with the development of SPEM, which constitutes a major repair lineage for wound healing following injury [82] and reacts to immune responses following gastric injury [6,7,15,83]. Interleukin 33 (IL-33) is an important driver of SPEM development [83-85] and is a gastric alarmin that acts as a secretory signal [86]. Macrophages infiltrate during acute inflammation and the loss of gastric mucosal parietal cells, and IL-33 is released from gastric foveolar epithelial cells to orchestrate immune defense and repair mechanisms [2]. IL-33 release and signaling lead to upregulation of type II cytokines [2,83,87], including IL-4, IL-5, IL-9, and IL-13. Among these cytokines, IL-13 and IL-4 mediate M2 macrophage activation through the coreceptor IL-4Rα [88]. IL-33 is required for promoting Th2 inflammatory responses and M2a polarization in recruited macrophages and drives the development of SPEM [89,90]. Exogenous treatment of mice with IL33 results in the recruitment of inflammatory cells to gastric epithelial cells, which induces significant inflammatory responses and SPEM development [86,91]. However, IL33KO, ST2KO, and IL13KO mice showed severe parietal cell loss and decreased Mist1 expression after L635 treatment, but chief cells failed to complete transdifferentiation, and parietal cell loss alone could not induce chief cell transdifferentiation [15,83]. Therefore, IL-13 and IL-33 were identified as promoters of SPEM, supporting the central role of cytokines and the immunoregulatory epithelium in the acute injury response, which has an important role in mucosal injury repair after gastric ulcer development [15].
In addition, Sonic Hedgehog (Shh), a secreted protein that regulates gastric ulcer healing, contributes to gastric mucosal recovery after injury [92-94] and plays a very important role in regulating epithelial cell regeneration and differentiation [95]. Shh also regulates immune responses, particularly macrophage recruitment [94]. Shh acts as a macrophage chemotactic agent through a smoothened-dependent mechanism in myeloid lineage cells, leading to expression of the chemokine receptor CCR2 on M2 macrophages [96], thereby linking metaplasia induction and the combined response to injury to the coordination of cytokine signaling by IL-33 and IL-13, which in turn regulates the development of SPEM and is essential for the regeneration and repair of damaged gastric epithelial cells [7].
The role of SPEM in gastric precancerous lesions and gastric cancer (gastric diffuse injury diseases)
The persistence of oxyntic atrophy and inflammation can lead to IM and SPEM [97-100]. IM was initially thought to be a preneoplastic metaplasia leading to intestinal-type gastric cancer [98]. However, recent studies have identified SPEM as a possible preneoplastic metaplasia, and IM can develop a more proliferative phenotype [13]. SPEM can be observed before cancer development and in gastric biopsies from most patients with gastric stump cancer and is strongly associated with early gastric cancer [101,102]. The spread of SPEM from the initially emerging lesser curvature region to the greater curvature was observed as inflammation persisted [66], promoting the development of SPEM in mice and ultimately leading to dysplasia and cancer development [63,66]. Therefore, SPEM represents an important precursor lineage for the development of dysplasia preceding gastric cancer [103]. Understanding the SPEM signaling pathway in gastric cancer provides an important theoretical basis for the prevention and treatment of early gastric cancer.
Slc26a9, which is a member of the Slc26a family of anion transporters [104], is a Cl - uniporter expressed by gastric mucosal cells and glands in mice and humans and plays an important role in parietal cell function and survival [34-37]. Slc26a9fl/fl/Atp4b-Cre mice with specific knockout of the Slc26a9 gene in parietal cells progressively develop parietal cell loss, fundic gland hyperplasia, hypochlorhydria, and hypergastrinemia, and these steps are critical processes in the formation of a precancerous environment [38,105]. Our group confirmed that Slc26a9 deletion can dysregulate stem cell and progenitor cell differentiation and lead to SPEM development, further promoting cell proliferation, inhibiting apoptosis, activating the Wnt pathway, and ultimately leading to the development of spontaneous precancerous lesions and gastric cancer. More importantly, our recent study showed that overexpression of Slc26a9 repaired SPEM (Liu et al., unpublished data), indicating that Slc26a9 plays a key regulatory role in protection of the gastric epithelium. In addition, some mouse models of gastric acid deficiency, such as AE2-/-, nhe4-/-, nhe2-/-, and kcne2-/-, show the loss of parietal cells, and the transformation of the gastric mucosa into SPEM has also been observed in these models [106-109]. However, whether gastric acid deficiency can transform from SPEM into tumors needs to be confirmed by more studies.
Stomach tight junction protein 18 (stCldn18) expression is reduced in gastric cancer, and its loss promotes the development of progressive gastric tumors in mice [110]. Studies in stCldn18-knockout mice showed that SPEM could develop in juvenile mice and that long-term stCldn18 deficiency activated the expression of CXC chemokine ligand 5 and promoted epithelial-mesenchymal transition downstream of the Wnt signaling pathway [111], thereby inducing the formation of gastric tumors [112]. In addition, the Notch and Wnt signaling pathways can synergistically regulate the proliferation and differentiation of gastric stem cells, and the upregulation of Notch is involved in the development of SPEM and gastric cancer in stCldn18-knockout mice [112].
Although mutations in Ras are observed in only approximately 10% of gastric cancers [66,98], increased Kras activity is found in more than 40% of intestinal-type gastric cancers [113,114]. After tamoxifen treatment of Mist1-Kras mice, Kras was activated in gastric chief cells and chief cells differentiated into SPEM, and transformation from SPEM to IM and then to invasive metaplasia was observed [9,115]. Thus, Ras signaling activation is involved in the development of omnidirectional metaplasia. In addition, activation of the Ras/MAPK pathway can lead to rapid gastric mucosal atrophy, foveolar epithelial hyperplasia, and SPEM formation [116]. During this process, the BMP pathway can participate in the development of metaplasia through Smad phosphorylation [48] and synergize with MAPK activation, which in turn regulates the development of SPEM [117,118]. MAP kinase (MEK) is an intermediate link in the Ras/MAPK pathway, and reversal of the SPEM lineage cells to normal gastric mucosal cells has also been observed in mice expressing Kras signaling changes and in H. pylori-infected gerbils treated with MEK inhibitors [9]. Therefore, searching for key targets and inhibitors during the transformation of SPEM into IM and then gastric cancer is the most effective approach to identify strategies to reverse metaplasia and prevent the occurrence of gastric cancer. Many studies have reported proteins that could be targeted to interfere with SPEM progression, such as WAP 4-disulfide core domain protein 2 (WFDC2), also called human epididymis protein 4, a small secreted protein that is upregulated in tissues and gastric juice of premalignant metaplasia and gastric cancer [119,120]. WFDC2 promotes SPEM by upregulating IL33 expression, and Wfdc2 knockdown inhibits the progression of SPEM and precancerous dysplasia [85]. In addition, tristetraprolin (TTP) is an RNA-binding protein encoded by the gene Zfp36 [121] and exhibits decreased expression in gastric cancer samples [122]. TTP regulates the induction of SPEM by abnormal gastric inflammatory lesions, and its overexpression inhibits gastric inflammation and SPEM development [123]. Therefore, therapeutic approaches to increase TTP expression may be effective for the treatment of SPEM-associated gastric neoplastic diseases.
Conclusions
SPEM not only occurs at the edges of gastric ulcers during focal injury and recruits repair cells to the site of mucosal injury, increasing the barrier protection function of the epithelium but also progresses to dysplasia and tumors when diffuse injury and inflammation persists. Therefore, SPEM is not only a cellular system that represents repair during acute gastric mucosal injury but is also a precancerous lesion. In this article, the origin, models, and regulatory pathways of SPEM and its role in gastric mucosal injury were reviewed (Figure 2). We hope to provide basic, systematic and summative knowledge for this field, advocate for more research on the role of SPEM in gastric mucosal diseases, and provide new targets for clinical diagnosis and treatment.
Figure 2.
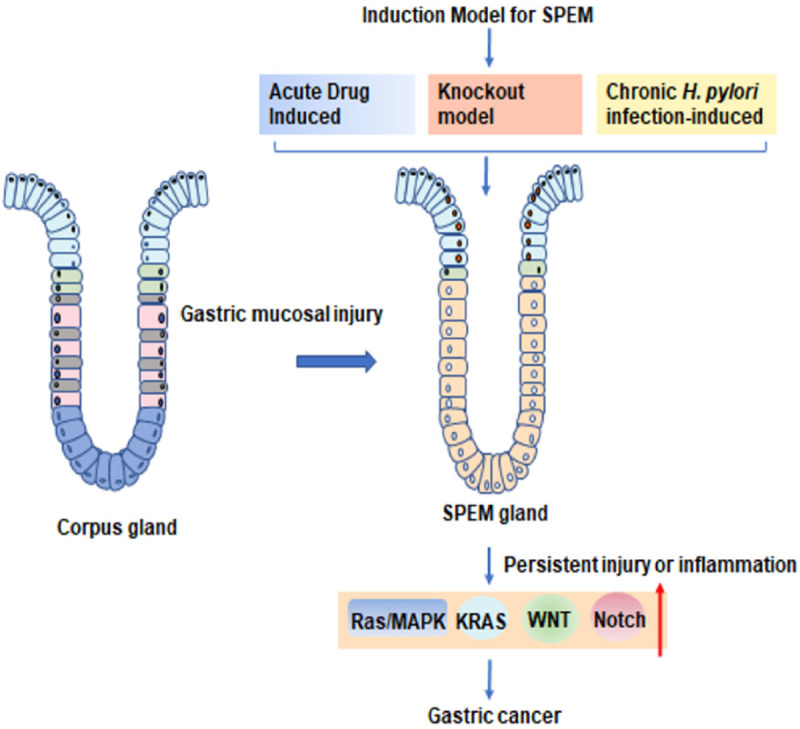
The origin, models, and regulatory pathways of SPEM and its role in gastric mucosal injury (Red up arrow indicates signaling pathway upregulation or activation).
Acknowledgements
We are grateful to GRW, HJ and JXA, who provided suggestions for the article and supported daily experiments. This research was supported by the National Natural Science Foundation of China (82070536 and 81860103 to X.L., 82160505 and 81660098 to T.L., and 82073087 to B.T.), Guizhou Province International Science and Technology Cooperation (Gastroenterology) Base (Qian Ke He Platform Talents-HZJD [2021] 001) to X.L., and Qian Ke He basic research-ZK [2023] major project 059 to X.L.
Disclosure of conflict of interest
None.
Abbreviations
- AR
amphiregulin
- BMP
bone morphogenetic protein
- CD44v9
CD44 variant isoform 9
- Cyld
cylindromatosis gene
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- HGF
hepatocyte growth factor
- HGFAs
hepatocyte growth factor activators
- HB-EGF
heparin-binding EGF-like growth factor
- INS-GAS
insulin-gastrin
- IM
intestinal metaplasia
- IL-33
interleukin 33
- KLF4
Kruppel-like factor 4
- Muc6
mucin 6
- MEK
MAP kinase
- MDSCs
myeloid-derived suppressor cells
- RGM1 cells
rat gastric epithelial cell line
- ROS
reactive oxygen species
- stCldn18
stomach tight junction protein 18
- SPEM
spasmolytic polypeptide-expressing metaplasia
- Shh
sonic hedgehog
- TFF2
trefoil factor 2
- TGF-α
transforming growth factor-α
- UACL
ulcer-associated cell lineage
References
- 1.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol. 2018;596:3861–3867. doi: 10.1113/JP275512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. e2029. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–232. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 6.Engevik AC, Feng R, Choi E, White S, Bertaux-Skeirik N, Li J, Mahe MM, Aihara E, Yang L, DiPasquale B, Oh S, Engevik KA, Giraud AS, Montrose MH, Medvedovic M, Helmrath MA, Goldenring JR, Zavros Y. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertaux-Skeirik N, Wunderlich M, Teal E, Chakrabarti J, Biesiada J, Mahe M, Sundaram N, Gabre J, Hawkins J, Jian G, Engevik AC, Yang L, Wang J, Goldenring JR, Qualls JE, Medvedovic M, Helmrath MA, Diwan T, Mulloy JC, Zavros Y. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol. 2017;242:463–475. doi: 10.1002/path.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR. Expression of activated ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930. e13. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenring JR, Mills JC. Cellular plasticity, reprogramming, and regeneration: metaplasia in the stomach and beyond. Gastroenterology. 2022;162:415–430. doi: 10.1053/j.gastro.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sáenz JB, Vargas N, Mills JC. Tropism for spasmolytic polypeptide-expressing metaplasia allows helicobacter pylori to expand its intragastric niche. Gastroenterology. 2019;156:160–174. e167. doi: 10.1053/j.gastro.2018.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology. 2018;154:839–843. e832. doi: 10.1053/j.gastro.2017.11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. 2210.e2201. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE Jr, Peek RM Jr, Goldenring JR. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146:1727–1738. e1728. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bockerstett KA, Lewis SA, Wolf KJ, Noto CN, Jackson NM, Ford EL, Ahn TH, DiPaolo RJ. Single-cell transcriptional analyses of spasmolytic polypeptide-expressing metaplasia arising from acute drug injury and chronic inflammation in the stomach. Gut. 2020;69:1027–1038. doi: 10.1136/gutjnl-2019-318930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Yasuda T, Choi E, Toyoda T, Roland JT, Uchida E, Yoshida H, Seto Y, Goldenring JR, Nomura S. MEK inhibitor reverses metaplasia and allows re-emergence of normal lineages in helicobacter pylori-infected gerbils. Gastroenterology. 2019;156:577–581. e574. doi: 10.1053/j.gastro.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H, Westphalen CB, Chen X, Takemoto Y, Kim W, Khurana SS, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda AR, Setlik W, Gershon MD, Saha S, Ding L, Shen Z, Fox JG, Friedman RA, Konieczny SF, Worthley DL, Korinek V, Wang TC. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, Goh J, Wong E, Denil SLIJ, Murakami K, Barker N. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19:774–786. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 21.Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, Goldenring JR. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol. 2017;312:G67–G76. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills JC, Sansom OJ. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willet SG, Lewis MA, Miao ZF, Liu D, Radyk MD, Cunningham RL, Burclaff J, Sibbel G, Lo HG, Blanc V, Davidson NO, Wang ZN, Mills JC. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J. 2018;37:e98311. doi: 10.15252/embj.201798311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 25.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bockerstett KA, Lewis SA, Noto CN, Ford EL, Saenz JB, Jackson NM, Ahn TH, Mills JC, DiPaolo RJ. Single-cell transcriptional analyses identify lineage-specific epithelial responses to inflammation and metaplastic development in the gastric corpus. Gastroenterology. 2020;159:2116–2129. e2114. doi: 10.1053/j.gastro.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen CP, Mills JC, Goldenring JR. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol. 2017;3:11–26. doi: 10.1016/j.jcmgh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa M, Nomura S, Car BD, Goldenring JR. Omeprazole treatment ameliorates oxyntic atrophy induced by DMP-777. Dig Dis Sci. 2006;51:431–439. doi: 10.1007/s10620-006-3151-x. [DOI] [PubMed] [Google Scholar]
- 30.Seishima R, Wada T, Tsuchihashi K, Okazaki S, Yoshikawa M, Oshima H, Oshima M, Sato T, Hasegawa H, Kitagawa Y, Goldenring JR, Saya H, Nagano O. Ink4a/Arf-dependent loss of parietal cells induced by oxidative stress promotes CD44-dependent gastric tumorigenesis. Cancer Prev Res (Phila) 2015;8:492–501. doi: 10.1158/1940-6207.CAPR-15-0025-T. [DOI] [PubMed] [Google Scholar]
- 31.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 32.Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Hou N, Sun Y, Teng Y, Yang X. Atp4b promoter directs the expression of Cre recombinase in gastric parietal cells of transgenic mice. J Genet Genomics. 2010;37:647–652. doi: 10.1016/S1673-8527(09)60083-7. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci U S A. 2008;105:17955–17960. doi: 10.1073/pnas.0800616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Li T, Riederer B, Lenzen H, Ludolph L, Yeruva S, Tuo B, Soleimani M, Seidler U. Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3(-) transport and reduces survival in CFTR-deficient mice. Pflugers Arch. 2015;467:1261–1275. doi: 10.1007/s00424-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Henriksnäs J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl-/HCO3- exchange, and is inhibited by NH4+ Am J Physiol Cell Physiol. 2005;289:C493–505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- 37.Chang MH, Plata C, Zandi-Nejad K, Sindić A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9--anion exchanger, channel and Na+ transporter. J Membr Biol. 2009;228:125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Li T, Ma Z, Riederer B, Yuan D, Zhu J, Li Y, An J, Wen G, Jin H, Yang X, Seidler U, Tuo B. SLC26A9 deficiency causes gastric intraepithelial neoplasia in mice and aggressive gastric cancer in humans. Cell Oncol (Dordr) 2022;45:381–398. doi: 10.1007/s13402-022-00672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu LS, Chan CP, Chen CJ, Lin SH, Lai MT, Hsu JD, Yeh KT, Soon MS. Decreased Kruppel-like factor 4 (KLF4) expression may correlate with poor survival in gastric adenocarcinoma. Med Oncol. 2013;30:632. doi: 10.1007/s12032-013-0632-6. [DOI] [PubMed] [Google Scholar]
- 40.Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Krüppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 41.Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, Furth EE, Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Ogasawara N, Tsukamoto T, Mizoshita T, Inada KI, Ban H, Kondo S, Takasu S, Ushijima T, Ito K, Ito Y, Ichinose M, Ogawa T, Joh T, Tatematsu M. RUNX3 expression correlates with chief cell differentiation in human gastric cancers. Histol Histopathol. 2009;24:31–40. doi: 10.14670/HH-24.31. [DOI] [PubMed] [Google Scholar]
- 43.Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K, Ida H, Bouillet P, Strasser A, Bae SC, Ito Y. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito K, Chuang LS, Ito T, Chang TL, Fukamachi H, Salto-Tellez M, Ito Y. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011;140:1536–1546. e1538. doi: 10.1053/j.gastro.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 45.Chuang LS, Ito K, Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer. 2013;132:1260–1271. doi: 10.1002/ijc.27964. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz RG, Gordon JI. Use of transgenic mice to study regulation of gene expression in the parietal cell lineage of gastric units. J Biol Chem. 1993;268:26559–26570. [PubMed] [Google Scholar]
- 47.Syu LJ, El-Zaatari M, Eaton KA, Liu Z, Tetarbe M, Keeley TM, Pero J, Ferris J, Wilbert D, Kaatz A, Zheng X, Qiao X, Grachtchouk M, Gumucio DL, Merchant JL, Samuelson LC, Dlugosz AA. Transgenic expression of interferon-γ in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, Eaton KA, Samuelson LC, Merchant JL, Goldenring JR, Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. e2052. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takabayashi H, Shinohara M, Mao M, Phaosawasdi P, El-Zaatari M, Zhang M, Ji T, Eaton KA, Dang D, Kao J, Todisco A. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406. e397. doi: 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todisco A, Mao M, Keeley TM, Ye W, Samuelson LC, Eaton KA. Regulation of gastric epithelial cell homeostasis by gastrin and bone morphogenetic protein signaling. Physiol Rep. 2015;3:e12501. doi: 10.14814/phy2.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ Jr. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989;84:1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abe S, Sasano H, Katoh K, Ohara S, Arikawa T, Noguchi T, Asaki S, Yasui W, Tahara E, Nagura H, Toyota T. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci. 1997;42:1199–1209. doi: 10.1023/a:1018897922644. [DOI] [PubMed] [Google Scholar]
- 53.Miyazaki Y, Hiraoka S, Tsutsui S, Kitamura S, Shinomura Y, Matsuzawa Y. Epidermal growth factor receptor mediates stress-induced expression of its ligands in rat gastric epithelial cells. Gastroenterology. 2001;120:108–116. doi: 10.1053/gast.2001.20950. [DOI] [PubMed] [Google Scholar]
- 54.Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804–1819. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 55.Kataoka H, Miyata S, Uchinokura S, Itoh H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003;22:223–236. doi: 10.1023/a:1023051500010. [DOI] [PubMed] [Google Scholar]
- 56.Terano A, Hiraishi H, Shimada T, Takahashi M, Yoshiura K, Horie-Sakata K. Cell culture model for antiulcerogenic agents. Microsc Res Tech. 2001;53:389–395. doi: 10.1002/jemt.1107. [DOI] [PubMed] [Google Scholar]
- 57.Miyazawa K, Shimomura T, Naka D, Kitamura N. Proteolytic activation of hepatocyte growth factor in response to tissue injury. J Biol Chem. 1994;269:8966–8970. [PubMed] [Google Scholar]
- 58.Shia S, Stamos J, Kirchhofer D, Fan B, Wu J, Corpuz RT, Santell L, Lazarus RA, Eigenbrot C. Conformational lability in serine protease active sites: structures of hepatocyte growth factor activator (HGFA) alone and with the inhibitory domain from HGFA inhibitor-1B. J Mol Biol. 2005;346:1335–1349. doi: 10.1016/j.jmb.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 59.Yamagata Y, Aikou S, Fukushima T, Kataoka H, Seto Y, Esumi H, Kaminishi M, Goldenring JR, Nomura S. Loss of HGF activator inhibits foveolar hyperplasia induced by oxyntic atrophy without altering gastrin levels. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1254–1261. doi: 10.1152/ajpgi.00107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakajima T, Konda Y, Izumi Y, Kanai M, Hayashi N, Chiba T, Takeuchi T. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G359–366. doi: 10.1152/ajpgi.00117.2001. [DOI] [PubMed] [Google Scholar]
- 61.Fjeldbo CS, Bakke I, Erlandsen SE, Holmseth J, Lægreid A, Sandvik AK, Thommesen L, Bruland T. Gastrin upregulates the prosurvival factor secretory clusterin in adenocarcinoma cells and in oxyntic mucosa of hypergastrinemic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G21–33. doi: 10.1152/ajpgi.00197.2011. [DOI] [PubMed] [Google Scholar]
- 62.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 63.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 64.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 65.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 66.Yoshizawa N, Takenaka Y, Yamaguchi H, Tetsuya T, Tanaka H, Tatematsu M, Nomura S, Goldenring JR, Kaminishi M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 67.Duckworth CA, Burkitt MD, Williams JM, Parsons BN, Tang JMF, Pritchard DM. Murine models of Helicobacter (pylori or felis)-associated gastric cancer. Curr Protoc Pharmacol. 2015;69:14.34.11–14.34.35. doi: 10.1002/0471141755.ph1434s69. [DOI] [PubMed] [Google Scholar]
- 68.Ding L, Hayes MM, Photenhauer A, Eaton KA, Li Q, Ocadiz-Ruiz R, Merchant JL. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126:2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding L, Li Q, Chakrabarti J, Munoz A, Faure-Kumar E, Ocadiz-Ruiz R, Razumilava N, Zhang G, Hayes MH, Sontz RA, Mendoza ZE, Mahurkar S, Greenson JK, Perez-Perez G, Hanh NTH, Zavros Y, Samuelson LC, Iliopoulos D, Merchant JL. MiR130b from Schlafen4(+) MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut. 2020;69:1750–1761. doi: 10.1136/gutjnl-2019-318817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doger FK, Meteoglu I, Ozkara E, Erkul ZK, Okyay P, Yükselen V. Expression of NF-kappaB in Helicobacter pylori infection. Dig Dis Sci. 2006;51:2306–2309. doi: 10.1007/s10620-006-9352-5. [DOI] [PubMed] [Google Scholar]
- 71.Cui X, Kong C, Zhu Y, Zeng Y, Zhang Z, Liu X, Zhan B, Piao C, Jiang Z. miR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-κB and sustains NF-κB activation by decreasing Cylindromatosis expression. Oncotarget. 2016;7:48547–48561. doi: 10.18632/oncotarget.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 73.Shibata W, Sue S, Tsumura S, Ishii Y, Sato T, Kameta E, Sugimori M, Yamada H, Kaneko H, Sasaki T, Ishii T, Tamura T, Kondo M, Maeda S. Helicobacter-induced gastric inflammation alters the properties of gastric tissue stem/progenitor cells. BMC Gastroenterol. 2017;17:145. doi: 10.1186/s12876-017-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 76.Wright NA, Pike CM, Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion. 1990;46(Suppl 2):125–133. doi: 10.1159/000200375. [DOI] [PubMed] [Google Scholar]
- 77.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 78.Wada T, Ishimoto T, Seishima R, Tsuchihashi K, Yoshikawa M, Oshima H, Oshima M, Masuko T, Wright NA, Furuhashi S, Hirashima K, Baba H, Kitagawa Y, Saya H, Nagano O. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nomura S, Settle SH, Leys CM, Means AL, Peek RM Jr, Leach SD, Wright CV, Coffey RJ, Goldenring JR. Evidence for repatterning of the gastric fundic epithelium associated with Ménétrier’s disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 80.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–5198. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 81.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 82.Caldwell B, Meyer AR, Weis JA, Engevik AC, Choi E. Chief cell plasticity is the origin of metaplasia following acute injury in the stomach mucosa. Gut. 2022;71:1068–1077. doi: 10.1136/gutjnl-2021-325310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen CP, Meyer AR, De Salvo C, Choi E, Schlegel C, Petersen A, Engevik AC, Prasad N, Levy SE, Peebles RS, Pizarro TT, Goldenring JR. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. 2018;67:805–817. doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Salvo C, Pastorelli L, Petersen CP, Buttò LF, Buela KA, Omenetti S, Locovei SA, Ray S, Friedman HR, Duijser J, Xin W, Osme A, Cominelli F, Mahabeleshwar GH, Mills JC, Goldenring JR, Pizarro TT. Interleukin 33 triggers early eosinophil-dependent events leading to metaplasia in a chronic model of gastritis-prone mice. Gastroenterology. 2021;160:302–316. e307. doi: 10.1053/j.gastro.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeong H, Lee B, Kim KH, Cho SY, Cho Y, Park J, Lee Y, Oh Y, Hwang BR, Jang AR, Park JH, Park JH, Jeong SH, Lee D, Lee YC, Lim KM, Goldenring JR, Nam KT. WFDC2 promotes spasmolytic polypeptide-expressing metaplasia through the up-regulation of IL33 in response to injury. Gastroenterology. 2021;161:953–967. e915. doi: 10.1053/j.gastro.2021.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buzzelli JN, Chalinor HV, Pavlic DI, Sutton P, Menheniott TR, Giraud AS, Judd LM. IL33 is a stomach alarmin that initiates a skewed Th2 Response to injury and infection. Cell Mol Gastroenterol Hepatol. 2015;1:203–221. e203. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He R, Yin H, Yuan B, Liu T, Luo L, Huang P, Dai L, Zeng K. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol Immunol. 2017;90:42–49. doi: 10.1016/j.molimm.2017.06.249. [DOI] [PubMed] [Google Scholar]
- 88.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 89.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 90.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pastorelli L, De Salvo C, Vecchi M, Pizarro TT. The role of IL-33 in gut mucosal inflammation. Mediators Inflamm. 2013;2013:608187. doi: 10.1155/2013/608187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engevik AC, Feng R, Yang L, Zavros Y. The acid-secreting parietal cell as an endocrine source of Sonic Hedgehog during gastric repair. Endocrinology. 2013;154:4627–4639. doi: 10.1210/en.2013-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–33274. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 94.Xiao C, Feng R, Engevik AC, Martin JR, Tritschler JA, Schumacher M, Koncar R, Roland J, Nam KT, Goldenring JR, Zavros Y. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest. 2013;93:96–111. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–561. 561.e551–558. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chakrabarti J, Dua-Awereh M, Schumacher M, Engevik A, Hawkins J, Helmrath MA, Zavros Y. Sonic Hedgehog acts as a macrophage chemoattractant during regeneration of the gastric epithelium. NPJ Regen Med. 2022;7:3. doi: 10.1038/s41536-021-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hattori T. Development of adenocarcinomas in the stomach. Cancer. 1986;57:1528–1534. doi: 10.1002/1097-0142(19860415)57:8<1528::aid-cncr2820570815>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 98.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 99.Xia HH, Kalantar JS, Talley NJ, Wyatt JM, Adams S, Chueng K, Mitchell HM. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia? Am J Gastroenterol. 2000;95:114–121. doi: 10.1111/j.1572-0241.2000.01609.x. [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Association of spasmolytic polypeptide-expressing metaplasia with carcinogen administration and oxyntic atrophy in rats. Lab Invest. 2002;82:1045–1052. doi: 10.1097/01.lab.0000022225.45996.21. [DOI] [PubMed] [Google Scholar]
- 101.Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 2002;47:573–578. doi: 10.1023/a:1017920220149. [DOI] [PubMed] [Google Scholar]
- 102.Halldórsdóttir AM, Sigurdardóttrir M, Jónasson JG, Oddsdóttir M, Magnússon J, Lee JR, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 103.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 104.Liu X, Li T, Tuo B. Physiological and pathophysiological relevance of the anion transporter Slc26a9 in multiple organs. Front Physiol. 2018;9:1197. doi: 10.3389/fphys.2018.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci. 2010;96:117–131. doi: 10.1016/B978-0-12-381280-3.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem. 2004;279:30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 107.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781–12789. doi: 10.1074/jbc.M414118200. [DOI] [PubMed] [Google Scholar]
- 108.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, Caron TJ, Gad AP, Muthupalani S, Fox JG. Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology. 2018;155:1852–1867. doi: 10.1053/j.gastro.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S. Deficiency of stomach-type claudin-18 in mice induces gastric tumor formation independent of H pylori infection. Cell Mol Gastroenterol Hepatol. 2019;8:119–142. doi: 10.1016/j.jcmgh.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mita H, Toyota M, Aoki F, Akashi H, Maruyama R, Sasaki Y, Suzuki H, Idogawa M, Kashima L, Yanagihara K, Fujita M, Hosokawa M, Kusano M, Sabau SV, Tatsumi H, Imai K, Shinomura Y, Tokino T. A novel method, digital genome scanning detects KRAS gene amplification in gastric cancers: involvement of overexpressed wild-type KRAS in downstream signaling and cancer cell growth. BMC Cancer. 2009;9:198. doi: 10.1186/1471-2407-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holbrook JD, Parker JS, Gallagher KT, Halsey WS, Hughes AM, Weigman VJ, Lebowitz PF, Kumar R. Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine. J Transl Med. 2011;9:119. doi: 10.1186/1479-5876-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 116.Thiem S, Eissmann MF, Elzer J, Jonas A, Putoczki TL, Poh A, Nguyen P, Preaudet A, Flanagan D, Vincan E, Waring P, Buchert M, Jarnicki A, Ernst M. Stomach-specific activation of oncogenic KRAS and STAT3-dependent inflammation cooperatively promote gastric tumorigenesis in a preclinical model. Cancer Res. 2016;76:2277–2287. doi: 10.1158/0008-5472.CAN-15-3089. [DOI] [PubMed] [Google Scholar]
- 117.Bleuming SA, He XC, Kodach LL, Hardwick JC, Koopman FA, Ten Kate FJ, van Deventer SJ, Hommes DW, Peppelenbosch MP, Offerhaus GJ, Li L, van den Brink GR. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 118.Heijmans J, van den Brink GR. Morphogens and the parietal cell: shaping up acid secretion. Gastroenterology. 2010;139:1830–1833. doi: 10.1053/j.gastro.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 119.O’Neal RL, Nam KT, LaFleur BJ, Barlow B, Nozaki K, Lee HJ, Kim WH, Yang HK, Shi C, Maitra A, Montgomery E, Washington MK, El Rifai W, Drapkin RI, Goldenring JR. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Hum Pathol. 2013;44:734–742. doi: 10.1016/j.humpath.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 122.Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006;26:9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Busada JT, Khadka S, Peterson KN, Druffner SR, Stumpo DJ, Zhou L, Oakley RH, Cidlowski JA, Blackshear PJ. Tristetraprolin prevents gastric metaplasia in mice by suppressing pathogenic. Cell Mol Gastroenterol Hepatol. 2021;12:1831–1845. doi: 10.1016/j.jcmgh.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


