Abstract
Statins are cholesterol-lowering agents that act as inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzymeA (HMG CoA) reductase. Recently, statins have received a lot of attention, especially regarding how statins act on the immune system. Here, the clinical impact of statin intake was examined in patients with resected pancreatic cancer, and the underlying mechanisms were investigated in vitro and in vivo. We found that statin intake was associated with favorable prognostic outcomes in patients with resectable pancreatic cancer. Statins, especially lipophilic statins, exert anti-proliferative effects on pancreatic cancer cells in vitro (simvastatin > fluvastatin > atorvastatin > rosuvastatin > pravastatin). Simvastatin had an anti-proliferative effect on pancreatic cancer cells with decreased the yes-associated protein (YAP)/PDZ-binding motif (TAZ) expression by activating the JNK pathway, and simvastatin treatment with oxaliplatin revealed additive anti-growth effects. Furthermore, lipophilic and hydrophilic statins suppressed programmed cell death ligand 1 (PD-L1) expression by downregulating TAZ. Simvastatin treatment with an anti-PD-1 drug (BP0273) provided immediate anti-growth effects compared to controls, such as anti-PD-1 only and simvastatin only, and suppressed progressive disease during the early period of anti-PD-1 treatment in vivo. In conclusion, Statins display two distinct anti-cancer effects (direct anti-growth effect and elimination of immune suppression by downregulating PD-L1 expression) by targeting YAP/TAZ expression.
Keywords: YAP, TAZ, PD-L1, statin, JNK, pancreatic cancer
Introduction
Pancreatic cancer is the fourth most common cause of cancer-related mortality in economically developed countries and is set to become the second most common cause of cancer-related mortality in the next few years [1]. Despite the development of effective chemotherapy regimens, the 5-year overall survival rate for pancreatic cancer still remain less than 10% [1]. Although immune checkpoint inhibitors currently are a major pillar of cancer drug therapy in various cancers [2,3], pancreatic cancer has been reported to have a low frequency of microsatellite instability, low number of lymphocytes, and low expression of neoantigen, so it is believed that immune checkpoint inhibitors do not work well [4-6]. In the field of pancreatic cancer, the development of alternative treatment strategies is expected to fight this deadly disease. Statins are commonly used in patients with metabolic disorders, such as hypercholesterolemia. Statins are cholesterol-lowering agents that are widely prescribed to prevent and manage cardiovascular diseases and act as inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzymeA (HMG CoA) reductase. Interestingly, statins have been reported to have antiangiogenic and anti-proliferative properties, suggesting their possible role as anti-cancer agents [7]. In 1992, Sumi et al. [8] reported that lovastatin inhibited the growth of pancreatic cancer cells in vitro and in vivo. In 2013, Fendrich et al. [9] reported that simvastatin delayed the progression of pancreatic intraepithelial neoplasia and cancer formation, with an accumulation of HMG CoA-reductase in a genetically engineered mouse model of pancreatic cancer. In the clinical setting, reduced cancer-related mortality among statin users compared with those who had never used statins was observed for each of the 13 cancer types, including pancreatic cancer [10]. However, past findings from observational, case-control, cohort studies, and randomized trials in humans have revealed inconsistent results regarding the benefit of statin use on the risk of developing pancreatic cancer and on the survival of patients diagnosed with advanced pancreatic cancer [11-19]. In patients diagnosed with resectable pancreatic cancer, the effect of statins on survival has not yet been fully elucidated.
We previously reported that statin intake was associated with favorable prognostic outcomes in patients with colon cancer [20] and hepatocellular carcinoma [21]. In recent years, statins have received much attention, especially regarding how statins act on the immune system. The programmed death ligand-1/programmed death-1 (PD-L1/PD-1) signaling pathway is an important mechanism of tumor immunosuppression that obstructs the activation of T lymphocytes and enhances the immune tolerance of tumor cells. Statins inhibit geranylgeranylation of small GTPases, resulting in arrested endosomal maturation, prolonged antigen retention, enhanced antigen presentation, and T cell activation [22]. Furthermore, they demonstrated that mevalonate pathway inhibitors, including lipophilic statins, are robust against cancer vaccinations and synergize with anti-PD-1 antibodies in multiple mouse cancer models [22]. Statin therapy could improve the intestinal microbiome and overcome systemic inflammation [23]. The synthesis of cholesterol itself is essential for training myeloid cells, and statins prevent the induction of trained immunity [24]. These findings suggest that statins could be an alternative approach for the immune system by monotherapy or in combination with other anti-cancer agents. However, the mechanism underlying the strengthening of the immune system by statin use has not been fully elucidated.
The Hippo pathway is an evolutionarily conserved regulator of tissue growth and cell fate [23,24]. YAP and TAZ are transcriptional activators pervasively induced in several human solid tumors, and their functions in cancer cells have been the focus of intense investigation [25]. YAP and YAZ have been recognized as signaling keys in the tumor microenvironment, including cancer immune evasion systems [26]. Several studies have indicated that YAP/TAZ is upregulated in pancreatic cancer samples [27-29] and higher YAP expression is associated with poorer survival in patients with pancreatic cancer [30,31]. These findings led us to develop an alternative approach by targeting YAP/TAZ in pancreatic cancer. Previously, we identified that statins inhibited cell proliferation by downregulating TAZ expression rather than YAP expression in hepatocellular carcinoma [21]. Here, we examined whether statins could modulate YAP/TAZ expression with anti-cancer effects, including cancer immune evasion in pancreatic cancer.
Method
Patients
Paraffin-embedded sections were obtained from patients with pancreatic cancer who underwent resection at the Kumamoto University Hospital between January 2004 and December 2018. The study was approved by the medical ethics committee of Kumamoto University, and written informed consent was obtained from all subjects. Among them, 47 patients had taken statins preoperatively and postoperatively, and were categorized into the statin intake group. All 47 patients took statins prior to the operation and continued to take statins for at least 1 year after the operation. The remaining 153 patients who did not take statins were categorized into the statin non-intake group. The type of statins used were rosvastatin (34%), pravastatin (23%), atorvastatin (19%), simvastatin (4%), and others (19%).
Cell culture
Human pancreatic cancer cell lines (MIA PaCa-2, PANC-1, PK-8, and AsPC-1) and mouse pancreatic cell line (Panc02) were used. MIA PaCa-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wako) supplemented with 10% fetal bovine serum (FBS). PANC-1, PK-8, and AsPC-1 cells were cultured in RPMI-1640 medium (Wako Pure Chemical Industries). Cell lines were tested and authenticated using the GenePrint 10 System (Promega).
Western blot analysis
Cells collected from 6-well plates were washed once with phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 1% (v/v) Nonidet™ P-40 (NP40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor). Each protein sample (12 µg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was placed in tris-buffered saline with Tween® 20 (TBST) solution containing the primary antibody, YAP/TAZ (CST, #8418) × 1000, p-p38 (CST, #9211) × 1000, p38 (CST, #8690) × 1000, p-SAPK/JNK (CST, #9251) × 1000, SAPK/JNK (CST, #9252) × 1000, p-Erk1/Erk2 (CST, #4370) × 1000, Erk1/Erk2 (CST, #4695) × 1000, p-Akt (CST, #9271) × 1000, Akt (CST, #9272) × 1000, cleaved Caspase-3 (CST, #9661) × 1000, PD-L1 × 1000, β-actin (CST, #4967) × 1000. Signals were detected by incubation with secondary antibodies labeled using the ECL Detection System (GE Healthcare, Little Chalfont, UK).
TAZ and YAP suppression with siRNA duplex oligonucleotides
Silencing of TAZ and YAP suppression was carried out using siRNA and Stealth RNAi siRNA duplex oligonucleotides (Invitrogen), as previously described [32]. The cells were plated in a 6-well culture plate. Duplex siRNA was transfected using Lipofectamine RNAiMAX Reagent (Invitrogen), according to the manufacturer’s instructions. Whole-cell lysates were prepared 48 h after transfection. Three different siRNAs were designed and purchased for each target gene (Stealth RNAi siRNA; Invitrogen), and the most effective siRNA was used in subsequent experiments. The most effective sequences were siTAZ (WWTR1), 5-CCCAGACAUGAGAUCCAUCACUAAU-3, YAP, 5-GGAAGGAGAUGGAAUGAACAUAGAA-3, and complementary sequences of each oligo. The negative control siRNA used was Stealth RNAi Negative Control Duplexes (Medium GC Duplex; Invitrogen).
TAZ and YAP overexpression using plasmid vector
cDNA corresponding to human YAP1/TAZ was introduced into the pLenti-C-Myc-DDK-IRES-Puro lentiviral gene expression vector (#PS100069; Origene) as previously described [32]. Human YAP1/TAZ cDNA was amplified with forward primers (5-ATGGATCCCGGGCAGCA-3)/(5-ATGAATCCGGCCTCGGC-3) and reverse primers (5’-CTATAACCATGTAAGAAAGCTTTC-3) and (5-TTACAGCCAGGTTAGAAAGG-3) the full sequence of the YAP1/TAZ cDNA was validated by Sanger sequencing.
Growth assay
Cells were seeded in a 96-well plate at a density of 1000 cells per well for MIA PaCa2 and 3000 cells for PK-8, PANC-1, and AsPC-1. The medium in each well was changed daily. Viable cell numbers were measured using a Cell Counting Kit-8 (CCK-8) containing 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8; Dojin Laboratories, Kumamoto, Japan) at 0, 24, 48, and 72 h according to the manufacturer’s instructions. Optical density at 450 nm was measured using an automatic microplate reader (Molecular Devices, Osaka, Japan). Each experiment was performed in triplicates.
Flow cytometric analysis
Harvested cells were washed and incubated in PBS containing 1% FBS containing the below fluorochrome conjugated antibodies in a U-bottom 96-well plate. After washing, the samples were acquired on a BD FACSVerse (TM) (Becton, Dickinson and Company). Data were analyzed using FlowJo software (TreeStar).
In vivo experiment
To assess the effects of statins on tumors in vivo, we transplanted 1.0 × 106 Panc02 cells were subcutaneously transplanted into C57BL/6 mice. Twenty-four mice were randomly divided into four groups (six mice each group). Simvastatin (30 mg/kg) was injected daily into the peritoneum, anti-PD-1 drug (BP0273) 200 μg was injected into the peritoneum on the 3rd, 6th, and 9th days after tumor transplantation. The mice were sacrificed on day 35, the tumors were dissected. Tumor volumes were gauged using the following formula: volume [mm3] = (length [mm]) × (width [mm])2 × 0.52.
Statistical analysis
All experiments were performed in triplicate, and the data shown are representative of the consistently observed results. Data are presented as mean ± SD. Differences between groups with and without statins were tested using the Student’s t-test. Overall survival (OS) and recurrence-free survival (RFS) were examined using the Kaplan-Meier method and compared using the log-rank test. Data were analyzed using JMP (SAS Institute, Tokyo, Japan) and Excel 2016 (Microsoft, Redmond, WA, USA). Statistical significance was set at P < 0.05.
Results
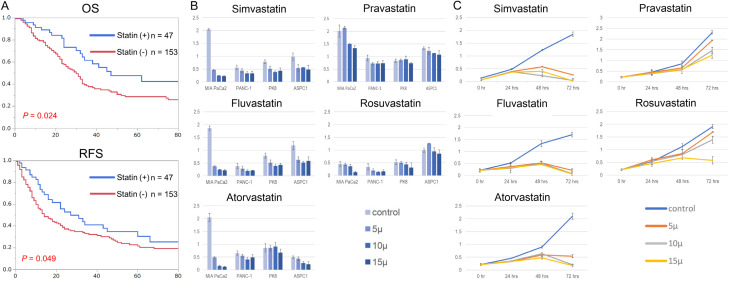
Statin intake is associated with favorable prognostic outcomes in patients with resectable pancreatic cancer, and statin treatment has an anti-proliferative effect on pancreatic cancer cells in vitro
The clinical benefits of statin intake in resectable pancreatic cancer have not been fully elucidated. Except for age, there were significant differences, in the clinicopathological characteristics between the groups with and without statins (Table 1). Interestingly, statin intake provided significantly favorable prognostic outcomes in recurrence-free survival and overall survival in patients who underwent curative resection for pancreatic cancer (Figure 1A; Table 2). To elucidate the underlying mechanism of statin-derived anti-cancer effects on pancreatic cancer, we first examined the growth inhibitory effects on pancreatic cancer cells in vitro using five types of statins (simvastatin, fluvastatin, atorvastatin, rosvastatin, and pravastatin). Llipophilic statins (simvastatin, fluvastatin, and atorvastatin) displayed strong anti-growth effects at 72 h in pancreatic cancer cell lines, whereas those of hydrophilic statins (rosvastatin and pravastatin) were modest (Figure 1B). Furthermore, the anti-proliferative effect of statins in MIA PaCa-2 cells was dose- and time-dependent (Figure 1C). Thus, lipophilic statins have anti-growth effects on pancreatic cancer cells compared to hydrophilic statins (simvastatin > fluvastatin > atorvastatin > rosuvastatin > pravastatin).
Table 1.
Comparison of background characteristics between the groups with and without statins (N = 200)
| Clinical variables | Statin intake (n = 47) | Without statin intake (n = 153) | P-value |
|---|---|---|---|
| Age | 72 (47-90) | 67 (36-86) | 0.010 |
| Gender (Male) | 25 (53%) | 83 (54%) | 0.515 |
| CEA (ng/nL) | 3.0 (0.5-112) | 2.5 (0.2-39.3) | 0.276 |
| CA19-9 (U/L) | 47.5 (0.5-2200) | 64.0 (0.1-4764) | 0.169 |
| Blood loss (g) | 639 (45-13153) | 646 (10-12925) | 0.672 |
| Operation time (min) | 483 (120-791) | 494 (150-1221) | 0.079 |
| T-stage (T3, T4) | 41 (87%) | 129 (84%) | 0.410 |
| Lymph node positive, | 22 (47%) | 85 (56%) | 0.188 |
| Morbidity (CDCs > III) | 13 (28%) | 43 (28%) | 0.556 |
| Adjuvant chemotherapy | 38 (81%) | 126 (82%) | 0.483 |
CDCs, Clavien-Dindo classifications. The T stage was used by the UICC for International Cancer Control 7th edition.
Figure 1.
Statins provide favorable prognostic outcomes in patients with pancreatic cancer and displays the anti-growth effects on pancreatic cancer cells. A. Upper: Kaplan-Meier curve of overall survival (OS) in resected pancreatic cancer patients with and without statin intakes. The median survival of statin users was 45 months while the median survival of non-statin users was 29 months (P = 0.024). Below: Kaplan-Meier curve of recurrence-free survival (RFS) in resected pancreatic cancer patients with and without statin intake. The median relapse-free survival of statin users was 27 months, while that of non-statin users was 14 months (P = 0.049). B. Anti-growth effects on pancreatic cancer cells (MIA PaCa2, PK-8, PANC-1, and AsPC-1) using five types of statins (simvastatin, fuvastatin, atorvastatin, pravastatin, and rosuvastatin) at different concentration (5 μM, 10 μM, and 15 μM). The medium in each well was changed daily. Viable cell numbers were measured at 72 h using a Cell Counting Kit-8 (CCK-8). C. Anti-growth effect of the five types of statins on MIA PaCa2 cells. The medium in each well was changed daily. Viable cell numbers were measured at 24, 48, and 72 h using the Cell Counting Kit-8 (CCK-8).
Table 2.
Univariate and multivariate analyses of overall survival in patients with resected pancreatic cancer (N = 200)
| Clinical variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age > 70 | 1.12 | 0.75-1.66 | 0.56 | |||
| Gender (male) | 1.43 | 0.96-2.14 | 0.08 | |||
| CEA (> 3.4 ng/mL) | 1.54 | 1.01-2.32 | 0.046 | NS | ||
| CA19-9 (> 37 U/L) | 2.29 | 1.48-3.67 | 0.001 | NS | ||
| Blood loss (> 550 g) | 1.51 | 1.04-2.20 | 0.028 | NS | ||
| Operation time (> 700 min) | 1.14 | 0.79-1.65 | 0.47 | |||
| T stage (T3, T4) | 1.99 | 1.17-3.22 | 0.013 | 2.55 | 1.30-5.39 | 0.002 |
| Lymph node positive | 2.23 | 1.54-3.60 | < 0.001 | 1.16 | 1.07-2.53 | 0.009 |
| Morbidity (CDCs > III) | 1.27 | 0.84-2.01 | 0.25 | |||
| Adjuvant chemotherapy | 0.75 | 0.45-1.30 | 0.29 | |||
| Statin intake | 0.61 | 0.37-0.96 | 0.024 | 0.59 | 0.35-0.93 | 0.021 |
NS, not significant; HR, hazard ratio; CDCs, Clavien-Dindo classification. The T stage was used by the UICC for International Cancer Control 7th edition.
Simvastatin attenuated YAP/TAZ expressions by activating JNK pathway in pancreatic cancer cells
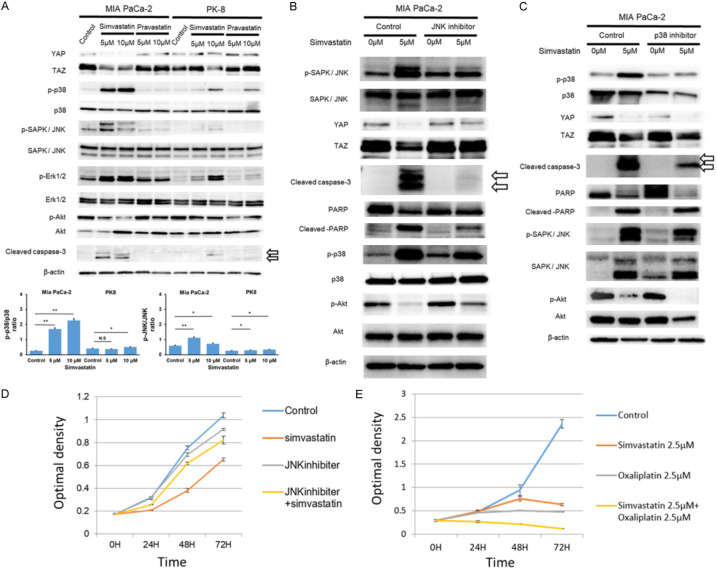
Next, we examined whether statin-induced anti-growth effects depended on YAP/TAZ expression, as previously reported in hepatocellular carcinoma [21]. As shown in Figure 1B, simvastatin displayed strong antigrowth activity in MIA PaCa-2 cells, whereas it was modest in PK-8 cells. Interestingly, simvastatin treatment decreased YAP/TAZ expression in a dose-dependent manner in MIA PaCa-2 cells, but not in PK-8 cells, whereas pravastatin treatment did not result in significant changes in YAP/TAZ expression (Figure 2A). Furthermore, we investigated the phosphorylation of mitogen-activated protein kinase (MAPK) and Akt kinase. Then, of the three MAPKs (p38MAPK, SAPK/JNK, and Erk1/2), the stress-activated p38 MAPKs and JNKs were activated by simvastatin treatment in a dose-dependent manner in MIA PaCa-2 cells rather than in PK-8 cells. Of the three MAPKs, stress-activated p38 MAPKs and JNKs play key roles in balancing cell survival and death in response to both extracellular and intracellular stresses [33]. In contrast, the phosphorylation of Akt decreased in a dose-dependent manner in MIA PaCa-2 cells treated with simvastatin. Furthermore, simvastatin treatment also induced apoptosis, represented by cleaved caspase-3 expression, in a dose-dependent manner, especially in MIA PaCa-2 cells (Figure 2A). These findings suggest that statins exert pro-apoptotic effects on pancreatic cancer cells by suppressing YAP/TAZ expression and activating JNK and p38 MAPKs. To further elucidate the pathway that plays a key role in statin-induced cellular stress and cell death, MIA PaCa-2 cells were treated with simvastatin and inhibitors of JNK and p38. The JNK inhibitor (SP600125, abcom: ab120065) inhibited YAP/TAZ downregulation by simvastatin and suppressed cleaved capase-3 and cleaved PARP, which are indicators of apoptosis (Figure 2B). The decreased expression of phospho-Akt induced by simvastatin was also attenuated by JNK inhibitor treatment (Figure 2B). On the other hand, the p38 inhibitor SB 203580 (ab120162) did not affect YAP/TAZ downregulation by simvastatin and failed to suppress cleaved PARP expression (Figure 2C). Additionally, the p38 inhibitor failed to inhibit the simvastatin-induced decrease in phospho-Akt expression (Figure 2C). Furthermore, a JNK inhibitor (SP600125) attenuated the simvastatin-derived anti-growth effect on MIA-PaCa-2 cells in growth assay (Figure 2D). In the present clinical setting, anti-cancer agents, such as oxaliplatin, are usually used to treat advanced or recurrent pancreatic cancer. We further examined the additive antigrowth effects of simvastatin on oxaliplatin in MIA-PaCa-2 cells. Simvastatin treatment with oxaliplatin enhanced the anti-growth effects in MIA PaCa-2 cells (Figure 2E). Thus, simvastatin exerts anti-cancer effects on pancreatic cancer cells by decreasing YAP/TAZ expression via activation of the JNK pathway.
Figure 2.
Simvastatin downregulates YAP/TAZ expressions via the JNK pathway. A. Simvastatin treatment suppressed YAP/TAZ expressions with stress-activated p38 MAPKs and JNKs compared to controls in pancreatic cancer cell lines (MIA PaCa-2 > PK-8 cells) at 48 h, whereas Pravastatin did not show such the significant downregulation. Medium with statin changed every 24 h. β-actin protein expression served as a loading control. Representative blots are shown. *P < 0.05 and **P < 0.01. B. JNK inhibitor attenuated the YAP/TAZ down-regulation and diminished cleaved Capase-3 and cleaved PARP expressions induced by Simvastatin treatment. JNK inhibitor (SP600125, abcom: ab120065) (30 μM) was added to the medium 2 h before simvastatin treatment. β-actin protein expression was used as the loading control. The representative blots are shown. C. p38 inhibitor addition to simvastatin did not affect YAP/TAZ downregulation by simvastatin and failed to diminished cleaved Capase-3 and cleaved PARP expression. p-38 inhibitor (SB 203580, abcom: ab120162) (20 μM) was added to the medium 2 h before adding simvastatin. β-actin protein expression was used as the loading control. Representative blots are shown. D. JNK inhibitor hindered the simvastatin-derived anti-growth effect in MIA PaCa-2 cells. Medium containing simvastatin (2.5 μM) and JNK inhibitor (30 μM) was changed every 24 h. E. Simvastatin treatment with oxaliplatin enhances the anti-growth effects in MIA-PaCa-2 cells. The medium containing simvastatin (2.5 μM) and oxaliplatin (2.5 μM) was changed every 24 h.
Statins decreases PD-L1 expression in pancreatic cancer cells via TAZ downregulation
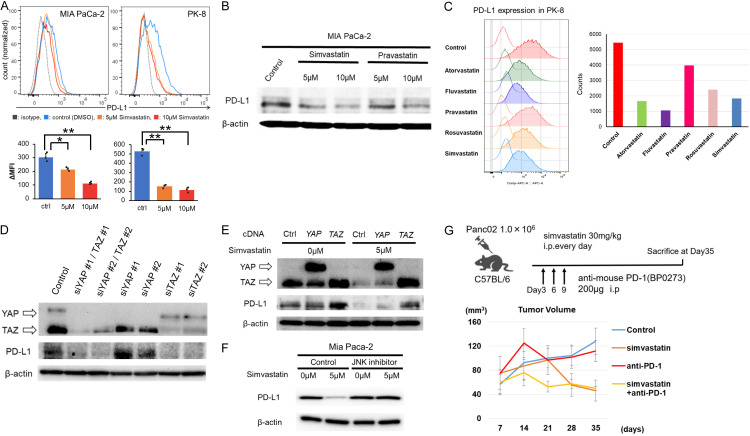
In recent years, statins have received a lot of attention, especially regarding how they act on the immune system [34,35]. The interaction between PD-L1 on cancer cells and PD-1 on lymphocytes stands as the key step in cancer immune escape [36,37]. We examined changes in PD-L1 expression induced by simvastatin in pancreatic cancer cells (MIA PaCa-2). Interestingly, simvastatin treatment reduced PD-L1 expression in pancreatic cancer cell (MIA PaCa-2) by FACS analysis (Figure 3A) Also in western blotting analysis, simvastatin or pravastatin treatment decreased PD-L1 expression (Figure 3B). We further examined the changes in PD-L1 expression in cancer cells (PK-8 cell line) after treatment with the five statins by FACS. Interestingly, lipophilic statins (atorvastatin, fluvastatin, and simvastatin) and hydrophilic statins (pravastatin and rosuvastatin) suppressed PD-L1 expression in cancer cells (Figure 3C). We further investigated the mechanism underlying statin-derived PD-L1 downregulation by focusing on YAP/TAZ expression. The downregulation of YAP/TAZ by siRNA decreased PD-L1 expression (Figure 3D). Interestingly, the downregulation of TAZ expression effectively suppressed PD-L1 expression compared to YAP (Figure 3D). In contrast, the overexpression enhanced PD-L1 expression and attenuated the simvastatin-induced decrease in PD-L1 expression, but not YAP overexpression (Figure 3E). Collectively, statins decreased PD-L1 expression in pancreatic cancer cells via TAZ down-regulation. Furthermore, JNK inhibitor also hindered the PD-L1 down-regulation induced by Simvastatin treatment (Figure 3F). Interestingly, simvastatin treatment in addition to anti-PD-1 drug (BP0273) (200 μg, ip) provided an immediate anti-growth effect compared to controls, such as anti-PD-1 only and simvastatin only, and suppressed progressive disease during the early period after anti-PD-1 treatment in vivo (Figure 3G).
Figure 3.
Statins suppress PD-L1 expression of pancreatic cancer cells by downregulating TAZ expression. A. Simvastatin treatment suppressed PD-L1 expression in MIA PaCa-2 and PK-8 cells by FACS analysis. MFI, median fluorescence intensity. *P < 0.05 and **P < 0.01. B. Simvastatin treatment suppressed PD-L1 expression in MIA PaCa-2 cell by western blot. β-actin protein expression served as a loading control. Representative blots are shown. C. Not only lipophilic statins (atorvastatin, fluvastatin, and simvastatin) but also hydrophilic statins (pravastatin and rosuvastatin) suppressed the PD-L1 expression in PK-8 cell. D. Downregulation of YAP/TAZ using siRNA technique decreased PD-L1 expression in MIA PaCa-2 cell. β-actin protein expression served as a loading control. Representative blots are shown. E. Overexpression of TAZ enhanced PD-L1 expression and attenuated the simvastatin-induced decrease in PD-L1 expression, but not in YAP overexpression. β-actin protein expression served as a loading control. Representative blots are shown. F. JNK inhibitor hindered the PD-L1 down-regulation induced by Simvastatin treatment. β-actin protein expression served as a loading control. Representative blots are shown. G. Simvastatin treatment addition to anti-PD-1 drug displayed immediately anti-growth effect compared to controls such as anti-PD-1 only and simvastatin only, and suppressed progressive disease during early period by anti-PD-1 treatment in vivo.
Discussion
In this study, we found that statins displayed anti-cancer effects by suppressing YAP/TAZ expression via JNK cascade activation and by disturbing cancer immune escape via PD-L1 in cancer cells. First, oral statin intake was significantly associated with favorable prognostic outcomes for overall survival and recurrence-free survival. Second, statins, especially lipophilic statins, exert antigrowth effects via apoptosis by activating the JNK pathway and suppressing YAP/TAZ expression. Notably, YAP/TAZ downregulation and statin-induced apoptosis were attenuated by the JNK inhibitor. Third, statin treatment decreased PD-L1 expression by TAZ (rather than YAP) downregulation in cancer cells. And this statin-induced PD-L1 downregulation was also attenuated by the JNK inhibitor. Thus, the present study elucidated the possible molecular mechanism of statin-induced anti-cancer effects through two distinct mechanisms in pancreatic cancer (Figure 4).
Figure 4.
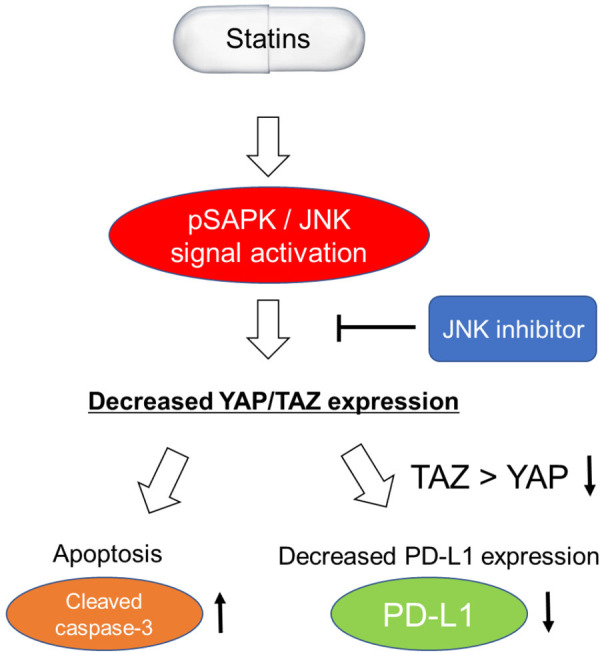
Summary figure for statin-derived anti-cancer effects in this study. Statins suppresses YAP/TAZ expressions via pSAPK/JNK cascade activation. Then, Statins induce apoptosis and PD-L1 downregulation in pancreatic cancer cells. These statin-derived anti-cancer effects are diminished by the JNK inhibitor.
We confirmed that the inhibitory effects of statins on pancreatic cancer cells in vitro were stronger. In the present study, statin intake provided significantly favorable prognostic outcomes in recurrence-free survival and overall survival in patients who underwent curative resection for pancreatic cancer. Only 22% of patients taking statins were taking lipophilic statins, most of which were hydrophilic statins. To confirm the anti-growth effect of statin intake in humans, we investigated the Ki-67 expression in clinical samples. Although statin intake was likely to be associated with low Ki-67 expression levels in pancreatic cancer tissue, the Ki-67 expression levels between patients with and without statin intake did not reach a significant difference (A P-value = 0.08; data not shown). However, statin intake was associated with significant favorable prognostic outcomes. These findings led us to hypothesize that statin intake has not only direct anti-cancer effects but also indirect anti-cancer effects by modulating the immune system. Reports on the effects of statins on the survival of patients diagnosed with resectable pancreatic cancer are still limited [38]. Wu et al. [38] reported that simvastatin use, not lovastatin use, was associated with improved survival and improved overall and disease-free survival among 226 patients undergoing resection for early stage pancreatic cancer. The effect of statins on the survival of patients diagnosed with advanced pancreatic cancer has been inconsistent. Only one RCT on the survival benefit of statin intake is available for advanced pancreatic cancer [39]. In 2014, Hong et al. [39] reported that the addition of low-dose simvastatin to gemcitabine in advanced pancreatic cancer did not provide clinical benefit in a randomized double-blind, placebo-controlled phase II trial. On the other hand, in patients with a diagnosis of pancreatic cancer including the resectable and unresectable condition setting, a recent systematic review and meta-analysis of 14 studies with 33,137 PDAC patients reported that statins use was significantly associated with a reduced risk of death in pancreatic cancer patients [40]. Wang et al. [41] also performed a meta-analysis using five retrospective cohort studies and reported that statin use increased overall survival in patients diagnosed with pancreatic cancer in 2019. In 2019, a pooled analysis of two phase III studies showed that statin use was associated with better overall survival among patients with metastatic pancreatic cancer treated with first-line chemotherapy [42]. Thus, difference from past RCT in 2014, those recent systematic review and meta-analysis suggested that prognostic benefit form statin use on survival of patients diagnosed with pancreatic cancer. We believe that uncovering the underlying molecular mechanism of statin-derived anti-cancer effects will lead to the true clinical benefit of statin intake for cancer patients in the future.
In the present study, statin treatment suppressed YAP/TAZ expression and apoptosis in pancreatic cancer cell lines. The anti-cancer effects of lipophilic statins are stronger than those of hydrophilic statins. Adding simvastatin to an anti-cancer agent (oxaliplatin) provides additive anti-growth effects in pancreatic cancer cells. Yin et al. [43] reported that simvastatin inhibited viability, stemness, tumor growth, and metastasis and enhanced the efficacy of gemcitabine. These changes are associated with modulation of Shh-related gene expression [43]. Importantly, the inhibitory effects of YAP/TAZ were dependent on JNK cascade activation in pancreatic cancer cells. Therefore, the simultaneous use of a certain drug that can inhibit the JNK signaling cascade may attenuate statin-derived anti-growth effects via YAP/TAZ suppression in the clinical setting. Hao et al. [44] reported that lipophilic statins inhibited YAP nuclear localization, co-activator activity, and colony formation in pancreatic cancer cells and prevented the incidence of pancreatic cancer in KrasG12D mice. As another mechanism of statin-derived anti-cancer effects for pancreatic cancer, Kusama et al. [45] reported fluvastatin and lovastatin inhibited the in vitro cancer cell invasion induced by attenuating the EGF-induced translocation of RhoA. Atorvastatin also delayed the progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice [46]. Thus, although the anti-tumor effects of statins have been demonstrated in various experimental studies [47], the underlying mechanism has not been fully elucidated. The present study elucidated a novel mechanism of the statin-derived anti-growth effect with apoptosis by activating the JNK pathway and suppressing YAP/TAZ expression.
As another important anti-cancer effect, statins decreased PD-L1 expression in pancreatic cancer. The PD-L1/PD-1 signaling pathway is an important component of tumor immunosuppression, which can inhibit the activation of T lymphocytes and enhance the immune tolerance of tumor cells, thereby achieving tumor immune escape [48]. PD-L1 shows abnormally high expression in tumor cells, which is considered the main factor responsible for promoting tumor immune escape.
Mevalonic acid metabolism is involved in controlling T-cell activation [49-52]. Statins inhibit geranylgeranylation of small GTPases, resulting in arrested endosomal maturation, prolonged antigen retention, enhanced antigen presentation, and T cell activation. They demonstrated that in multiple mouse cancer models, mevalonate pathway inhibitors are robust against cancer vaccinations and synergize with anti-PD-1 antibody [22]. In colon cancer, systemic administration of statins can elicit effective antitumor immune responses by inducing immunogenic cell death and enhancing dendritic cell-mediated CD8+ T-cell immunity against KRASmut tumors [53]. Thus, there are several reports that statins act on the immune system, but there are limited reports that statins affect changes in PD-L1 expression [54]. Lim et al. reported that statins decrease PD-L1 expression by inhibiting AKT and β-catenin signaling in several types of cancers, except pancreatic cancer [54]. The present study showed that statins suppressed PD-L1 expression by downregulating YAP/TAZ expression (mainly in a TAZ-dependent manner rather than YAP) in pancreatic cancer cells. However, much remains to be discovered about the tumor microenvironment, and the regulation network of PD-L1 by statins remains to be uncovered in detail. The TME was enriched with cholesterol. High cholesterol levels in the tumor microenvironment induce CD8+ T cell exhaustion and upregulate immune checkpoints PD-1, 2B4, TIM-3, and LAG-3 [55]. Furthermore, lowering cholesterol levels in the tumor microenvironment using simvastatin restores the antitumor activity of CD8+ T cells [55]. Further study of the immune response to statins in the tumor microenvironment may provide additional ideas for immunotherapy and a new development direction for effective use in cancer treatment.
In conclusion, this study suggests a novel treatment approach for pancreatic cancer using statins, which provide two distinct anti-cancer effects (direct anti-growth effect and elimination of immune suppression by downregulating PD-L1 expression) by targeting YAP/TAZ expression.
Acknowledgements
We thank H. Nikki March, PhD, from Editage for editing a draft of this manuscript. This work was supported by Japanese Foundation for Multidisciplinary Treatment of Cancer, Japan (to H.H.).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, Lewis SJ, Relton CL, Martin RM. Association between genetically proxied inhibition of HMG-CoA reductase and epithelial ovarian cancer. JAMA. 2020;323:646–655. doi: 10.1001/jama.2020.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumi S, Beauchamp RD, Townsend CM Jr, Uchida T, Murakami M, Rajaraman S, Ishizuka J, Thompson JC. Inhibition of pancreatic adenocarcinoma cell growth by lovastatin. Gastroenterology. 1992;103:982–989. doi: 10.1016/0016-5085(92)90032-t. [DOI] [PubMed] [Google Scholar]
- 9.Fendrich V, Sparn M, Lauth M, Knoop R, Plassmeier L, Bartsch DK, Waldmann J. Simvastatin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Pancreatology. 2013;13:502–507. doi: 10.1016/j.pan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 11.Bonovas S, Filioussi K, Sitaras NM. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am J Gastroenterol. 2008;103:2646–2651. doi: 10.1111/j.1572-0241.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamada T, Khalaf N, Yuan C, Babic A, Morales-Oyarvide V, Qian ZR, Nowak JA, Ng K, Kraft P, Rubinson DA, Stampfer MJ, Giovannucci EL, Fuchs CS, Ogino S, Wolpin BM. Statin use and pancreatic cancer risk in two prospective cohort studies. J Gastroenterol. 2018;53:959–966. doi: 10.1007/s00535-018-1430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkegard J, Lund JL, Mortensen FV, Cronin-Fenton D. Statins and pancreatic cancer risk in patients with chronic pancreatitis: a Danish nationwide population-based cohort study. Int J Cancer. 2020;146:610–616. doi: 10.1002/ijc.32264. [DOI] [PubMed] [Google Scholar]
- 14.Lee HS, Lee SH, Lee HJ, Chung MJ, Park JY, Park SW, Song SY, Bang S. Statin use and its impact on survival in pancreatic cancer patients. Medicine (Baltimore) 2016;95:e3607. doi: 10.1097/MD.0000000000003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooqi MAM, Malhotra N, Mukherjee SD, Sanger S, Dhesy-Thind SK, Ellis P, Leong DP. Statin therapy in the treatment of active cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2018;13:e0209486. doi: 10.1371/journal.pone.0209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbowska E, Swieczkowski D, Gasecka A, Pruc M, Safiejko K, Ladny JR, Kopiec T, Jaguszewski MJ, Filipiak KJ, Rafique Z, Szarpak L. Statins and the risk of pancreatic cancer: a systematic review and meta-analysis of 2,797,186 patients. Cardiol J. 2022 doi: 10.5603/CJ.a2022.0014. [Equb ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archibugi L, Arcidiacono PG, Capurso G. Statin use is associated to a reduced risk of pancreatic cancer: a meta-analysis. Dig Liver Dis. 2019;51:28–37. doi: 10.1016/j.dld.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Liang M, Sun C, Qu G, Shi T, Min M, Wu Y, Sun Y. Statin use and risk of pancreatic cancer: an updated meta-analysis of 26 studies. Pancreas. 2019;48:142–150. doi: 10.1097/MPA.0000000000001226. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Sato Y, Nakatani E, Kaneda H, Yamamoto S, Miyachi Y, Itoh H. Statin exposure and pancreatic cancer incidence: a Japanese regional population-based cohort study, the Shizuoka study. Cancer Prev Res (Phila) 2021;14:863–872. doi: 10.1158/1940-6207.CAPR-21-0123. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa S, Hayashi H, Kinoshita K, Abe M, Kuroki H, Tokunaga R, Tomiyasu S, Tanaka H, Sugita H, Arita T, Yagi Y, Watanabe M, Hirota M, Baba H. Statins inhibit tumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer. 2014;135:2528–2536. doi: 10.1002/ijc.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashi T, Hayashi H, Kitano Y, Yamamura K, Kaida T, Arima K, Taki K, Nakagawa S, Okabe H, Nitta H, Imai K, Hashimoto D, Chikamoto A, Beppu T, Baba H. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33:123. doi: 10.1007/s12032-016-0845-6. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X, Yang Y, Li X, Zhao M, Li L, Zheng M, Han S, Zong Z, Meng X, Deng H, Ye H, Fa Y, Wu H, Oldfield E, Hu X, Liu W, Shi Y, Zhang Y. The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell. 2018;175:1059–1073. e21. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 24.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey KF, Zhang X, Thomas DM. The hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 26.Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19:454–464. doi: 10.1038/s41568-019-0168-y. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morvaridi S, Dhall D, Greene MI, Pandol SJ, Wang Q. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F, Fu K, Chen Y, Solheim J, Singh PK, Song W, Dong J. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6:36019–36031. doi: 10.18632/oncotarget.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozengurt E, Sinnett-Smith J, Eibl G. Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduct Target Ther. 2018;3:11. doi: 10.1038/s41392-017-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM, Yi C. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:1232–1244. doi: 10.1038/onc.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y, Ishimoto T, Kuroki H, Nitta H, Hashimoto D, Chikamoto A, Oki E, Beppu T, Baba H. An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res. 2015;75:4985–4997. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- 33.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 34.Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, Assmann K, Valles-Colomer M, Nguyen TTD, Proost S, Prifti E, Tremaroli V, Pons N, Le Chatelier E, Andreelli F, Bastard JP, Coelho LP, Galleron N, Hansen TH, Hulot JS, Lewinter C, Pedersen HK, Quinquis B, Rouault C, Roume H, Salem JE, Sondertoft NB, Touch S MetaCardis Consortium. Dumas ME, Ehrlich SD, Galan P, Gotze JP, Hansen T, Holst JJ, Kober L, Letunic I, Nielsen J, Oppert JM, Stumvoll M, Vestergaard H, Zucker JD, Bork P, Pedersen O, Backhed F, Clement K, Raes J. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–315. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 35.Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, Netea MG. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172:135–146. e9. doi: 10.1016/j.cell.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 37.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu BU, Chang J, Jeon CY, Pandol SJ, Huang B, Ngor EW, Difronzo AL, Cooper RM. Impact of statin use on survival in patients undergoing resection for early-stage pancreatic cancer. Am J Gastroenterol. 2015;110:1233–1239. doi: 10.1038/ajg.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong JY, Nam EM, Lee J, Park JO, Lee SC, Song SY, Choi SH, Heo JS, Park SH, Lim HY, Kang WK, Park YS. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73:125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 40.Tamburrino D, Crippa S, Partelli S, Archibugi L, Arcidiacono PG, Falconi M, Capurso G. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: a meta-analysis. Dig Liver Dis. 2020;52:392–399. doi: 10.1016/j.dld.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Rodriguez EA, Barkin JS, Donath EM, Pakravan AS. Statin use shows increased overall survival in patients diagnosed with pancreatic cancer: a meta-analysis. Pancreas. 2019;48:e22–e23. doi: 10.1097/MPA.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Rahman O. Statin treatment and outcomes of metastatic pancreatic cancer: a pooled analysis of two phase III studies. Clin Transl Oncol. 2019;21:810–816. doi: 10.1007/s12094-018-1992-3. [DOI] [PubMed] [Google Scholar]
- 43.Yin Y, Liu L, Zhao Z, Yin L, Bauer N, Nwaeburu CC, Gladkich J, Gross W, Hackert T, Sticht C, Gretz N, Strobel O, Herr I. Simvastatin inhibits sonic hedgehog signaling and stemness features of pancreatic cancer. Cancer Lett. 2018;426:14–24. doi: 10.1016/j.canlet.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Hao F, Xu Q, Wang J, Yu S, Chang HH, Sinnett-Smith J, Eibl G, Rozengurt E. Lipophilic statins inhibit YAP nuclear localization, co-activator activity and colony formation in pancreatic cancer cells and prevent the initial stages of pancreatic ductal adenocarcinoma in KrasG12D mice. PLoS One. 2019;14:e0216603. doi: 10.1371/journal.pone.0216603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308–317. doi: 10.1053/gast.2002.31093. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed A, Qian L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951–1962. doi: 10.1002/ijc.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uemura N, Hayashi H, Baba H. Statin as a therapeutic agent in gastroenterological cancer. World J Gastrointest Oncol. 2022;14:110–123. doi: 10.4251/wjgo.v14.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurnher M, Gruenbacher G. T lymphocyte regulation by mevalonate metabolism. Sci Signal. 2015;8:re4. doi: 10.1126/scisignal.2005970. [DOI] [PubMed] [Google Scholar]
- 50.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 52.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nam GH, Kwon M, Jung H, Ko E, Kim SA, Choi Y, Song SJ, Kim S, Lee Y, Kim GB, Han J, Woo J, Cho Y, Jeong C, Park SY, Roberts TM, Cho YB, Kim IS. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J Immunother Cancer. 2021;9:e002474. doi: 10.1136/jitc-2021-002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim WJ, Lee M, Oh Y, Fang XQ, Lee S, Lim CH, Park J, Lim JH. Statins decrease programmed death-ligand 1 (PD-L1) by inhibiting AKT and beta-catenin signaling. Cells. 2021;10:2488. doi: 10.3390/cells10092488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156. e145. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]