Abstract
This review article examines the basic principle underlying trans-arterial chemoembolization (TACE) used for treating unrespectable liver cancer with discussion on the barriers that are present for efficient drug delivery with suggestions on methods that may be used to overcome these barriers and hence enhance the efficacy of the technique. Current drugs used with TACE along with inhibitors of neovascularisation are briefly discussed. It also compares the conventional method of chemoembolization with TACE and rationalizes why there is not much of a difference between the two methods on treatment efficacy. Further it also suggests alternative methods of drug delivery that may be used instead of TACE. Additionally, it discusses the disadvantages on using non degradable microspheres with recommendations for degradable microspheres within 24 hours to overcome rebound neovascularisation owing to hypoxia. Finally, the review examines some of the biomarkers that are used to assess treatment efficacy with indication that non-invasive and sensitive biomarkers should be identified for routine screening and early detection. The review concludes that, if the current barriers present in TACE can be overcome along with the use of degradable microspheres and efficient biomarkers for monitoring efficacy, then a more robust treatment would emerge that may even serve as a cure.
Keywords: Transarterial-chemoembolization, hepatic cellular carcinoma, neo-vascularisation, Sorafenib, biomarkers, microspheres
Introduction
One third of world’s cancer related death has been reported to be caused by hepatic cellular carcinoma (HCC) [1,2]. HCC is distinctively prevalent in South East Asia, amongst which China leads with 55% of the total world cases [3,4] suggesting a clear ethnic difference. This difference may be due to a number of factors such as genetic susceptibility, food habits, exposure to liver toxins, liver infection etc. [5,6]. In California (USA), there is a much higher incidence of HCC in pacific islanders and Latin American [7] and those residing near ethnic neighbourhood indicating that life style factors may be involved. In Europe, there is a wide distribution of HCC with countries in Eastern Europe having a higher incidence. Incidence of HCC in USA has been reported to peak between the age of 50-54 with sex disparity suggesting that estrogens may play a protective role [8,9].
HCC is the end result of chronic liver infection owing to exposure to liver toxins or viral infection such as hepatitis virus B & C (HBV & HCV) [10,11]. High body mass index, fatty diets, alcohol and tobacco use may also predispose subjects to HCC [6,12] whilst a higher incidence also exists in diabetic patients. The pathogenesis by which these agents cause HCC has been reviewed recently by Singh et al. [13]. The selection of treatment modality for HCC depends largely on tumour size, multiplicity and the status of liver [14,15]. Various treatment methods have been developed such as surgical resection, thermo/cryo ablation, radiotherapy, and chemotherapy [16]. For non resectable tumours, trans-arterial chemo-embolisation (TACE) using conventional methods (c-TACE) with drug delivery in an emulsion (lipiodol) along with arterial plugging with gelatine has been frequently used although more recently drug eluting microspheres has become very popular [17,18]. Owing to rebound development of tumour microvasculature after TACE due to hypoxic conditions, anti-angiogenic agents such as tyrosine kinase inhibitor, sorafenib, brivanib, sunitinib, regorafenib etc. are used in therapy to inhibit neo-vascularisation of embolised tumours [19,20].
Drugs commonly used for treating HCC
A selection of chemotherapeutic agents have been used for the treatment of HCC either as single gents or in combination for systemic or loco regional delivery [21]. These agents are either hormonal (ostreotide, tamoxifen), biologic (Thalidomide, interferon), chemotherapy (Sorafenib, 5-fluorouracil, cisplatin, gemcitabine, doxorubicin, capecitabine, mitoxantrone, epirubicin, etopside) or bevacizumab as targeted therapeutic agent [22]. In particular, most of the chemotherapy agents are nucleosides that disrupt the synthesis of DNA and their mode of action along with the other agents have also been fully reviewed recently [23]. Further, the efficacy of these agents either singly or in combination has also been discussed by several authors [21,24]. Since rebound neo-vascular development in tumours is a common occurrence with TACE, methods to suppress this phenomena using vascular epidermal growth factor inhibitors (VEGF) of which amongst the existing tyrosine kinase inhibitors, sorafenib proved superior in several clinical studies owing to its multiplicity in action. Hence, sorafenib or in combination with other chemotoxic agents are used for treating non-resectable liver tumours [25].
Strategic principals of TACE
The fundamental principles underlying TACE is to disrupt (embolize) the proximal blood supply to the tumour with deprivation of nutrients and oxygen supply whilst delivering a sustained supply of chemotherapeutic drugs to the tumour cells. The chemotherapeutic agents are either delivered lodged within the embolus material in drug eluting microspheres (dem-TACE) or suspended in lipiodol emulsion that ascertains a slow and sustained release locally known as conventional TACE (c-TACE). The main advantage being a substantial and sustained exposure of the agents to tumour cells while reducing systemic exposure to a minimum [26]. This principal and efficacy of the system has been summarised in an equation as given by Collins et al. [27] (Equation 1).
 |
RD = Overall selectivity; AUC = area under the curve (total amount of drug delivered); IV = intravenous; CLtotal = total body clearance of drug; Q = blood flow through target organ; E = organ extraction ratio.
TACE method of drug delivery seems to be an ideal method that targets the tumour specifically and hence capable of producing effective tumour regression with minimal systemic exposure. However, the hypoxic environment created by the embolus induces the tumour cells to over express angiogenic factors (VEGF, PDGF, etc) that is responsible for neo-vascularisation and hence rebound development of tumour [28,29], although initial response is regional tumour necrosis. Further, neo-vascularisation (poorly developed and leaky blood vessels) of tumour may also lead to novel blood supply that may in theory enhance clearance of the drugs from the tumour since there is a negative drug gradient present in the new blood vessels [30]. How significant this loss would make to tumour regression may need investigation. This drawback is particularly applicable to very slow drug eluting non-biodegradable microspheres with embolus that are long lasting such as DC beads that do not degrade [31]. This principal has been illustrated in Figure 1. Since, tumoral capillary beds that are developed are leaky; it may also increase the intra tumoral fluid pressure (ITFP) further in the cellular matrix and hence interfere with drug transfer. Hence, further research may be necessary to determine how neo-vascularisation effect drug concentration and drug dispersion in the tumour. In animal models, neo-vascularisation takes place within 3 days [32] and in human it has been reported to be in 36 hours [33].
Figure 1.
It is a diagrammatic representation of enhanced clearance with build-up of Intra-tumoral fluid pressure. Mark 1 Indicates direction of blood flow in the blood vessels; Mark 2 Direction of drug diffusion; Mark 3 drugs escaping to newly formed blood capillaries. ITFP = Intra-tumoral fluid pressure.
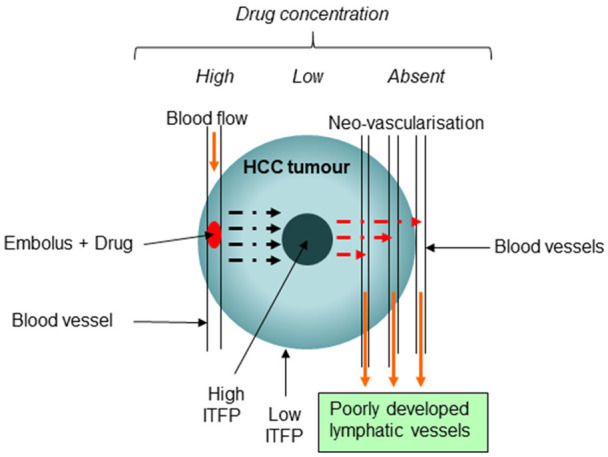
In systemic delivery, the drugs are prone to dilution in the general circulation with side effects on all the organ systems. Hence, the intra venous (IV) dosage is generally calculated at a higher dose to account for the systemic dilution factor. However, in the case of TACE, since drugs are delivered locally to the tumour, the dosage used may be considerably reduced since the dilution factor is minimal [34], with a considerable reduction of systemic exposure and hence the ensuing side effects. This is a major advantage, besides delivering the drugs in situ to the tumour in a sustained manner.
The liver is a highly perfused organ with arterial blood supply accounting for one third of the total blood received whilst venous supply is about two third and hence clearance may be much higher as compared to other organs [35]. Therefore, drug loaded embolizing agents need a much higher content of chemotherapeutic drugs in order to account for the higher clearance [36]. Higher clearance may also predispose to a slight elevated level of chemo-agents systemically and hence side effects when treating HCC using TACE [37], although this elevated level is considerably less compared to systemic delivery.
Marginal differences in efficacy between transarterial embolisation (TAE) and transarterial chemoembolisation (TACE)
The principal underlying TAE is to create a nutritional deficiency as well as a hypoxic environment by introducing an embolus within the microvasculature of the tumour. Cancer cells undergo apoptosis initially owing to deprivation of nutrients and oxygen, although cancer cells rely on aerobic metabolism (Warburg effect) mostly [38]. However, within a very short duration of time, tumour cells undergo changes owing to generous induction of hypoxic factors HIF alpha and beta [39] and become resistant owing to expression of several other survival proteins that induces, anti-apoptosis, replication, angiogenesis and metastasis [40,41], thereby preserving the tumour population. Hence, the end results, although tumour shrinkage is observed initially, residual tumour cells are preserved with subsequent recurrence of the disease.
Introducing a cytotoxic agent to the hypoxic condition should in principal increase the efficacy of the treatment (a double-edged sword) but, many of the cytotoxic agents are more effective in a fast replicating cell population [42]. In a hypoxic situation, tumour cell replication is much slower and hence may not allow the cytotoxic to have its full effect. Hence, several clinical studies have shown that there is not much of a difference in efficacy between TAE and TACE [43,44] since hypoxia may act as a barrier.
Taken together, the introduction of microspheres that disintegrate shortly <24 hrs, with the liberation of its drug load at the tumour site may be a better form of therapy since the rebound hyper-vascularisation may be avoided since neo-vascularisation takes 36 hours in human. In addition, if the chemo-agent delivered by the short lived microspheres can be tethered to a suitable agent that has the potential of binding to the tumour matrix but with ability to release the active agent slowly would be an advantage since the cells will be exposed to the agent continually over a long period of time without long term induced hypoxia.
Clinical evidence for TACE
Currently TACE is recommended as first line therapy for patients categorised as moderate under the Barcelona Liver Cancer Clinic (BLCC) staging system [45]. However, a 2011 Cochrane review analysing 9 trials involving 645 patients showed that there was insufficient evidence to determine whether TACE or TAE showed benefits to survival [46]. Critics of the Cochrane review assert that TACE methods are continually improving, and the Cochrane review was too strict in its inclusion of trials [47]. It will be difficult to conclusively demonstrate whether TACE offers survival benefit due to the substantial time and cost of conducting randomised controlled trials, despite there being still a need to do so. Nonetheless, as it is currently in widespread use across the world, efforts to improve TACE methods such as those described by this paper will still be of practical benefit to clinicians.
Methods to enhance the efficacy of TACE
Currently, a number of chemotherapeutic drugs are available for treating non-resectable HCC tumours, more recently several tyrosine kinase receptors have been tested clinically and of which sorafenib a multi-receptor inhibitor with anti VEGF, PDGF, etc outperformed the others [48,49]. Hence, a combination of anti-tumour drugs and sorafenib are used in treatment in order to derive maximum benefit [50,51]. There are also other new tyrosine kinase inhibitors that can be used as a second line therapy if the tumour is resistant to sorafenib [52]. Further, a number of new tyrosine kinase such as Lenvatinib, imatinib etc have been tested in clinical trials with equal efficacy as sorafenib [53]. The delivery of tyrosine kinase inhibitors and other anti-angiogenic agents, either oral or systemic has shown numerous side effects and patient non-compliance [54,55] and hence the delivery at the tumour site using TACE may be a better option since local delivery may be more effective without undue systemic toxicity with additional benefit of continuous delivery.
When using doxorubicin, mitoxantrone or other drugs which are weak bases, owing to their interaction with the tumoral weakly acidic pH (formation of salt) their uptake through cellular membrane is often reduced [56,57]. Hence, methods to raise the tumoral pH to weakly basic condition may be necessary to enhance the cytotoxic effect of the drug. This may be in the form of additives that would raise the pH of the tumour environment or on the other hand increase the concentration of doxorubicin to account for the poor absorption. This paradigm needs further in vivo and in vitro studies. On the contrary, selection of suitable cytotoxic which are weakly acidic may be more compatible with the tumour acidic environment. On the other hand, alkalization of tumour environment may be another option using proton pump inhibitors [58].
In the case of mucin producing HCC tumours that enable them to form a protective barrier against drug penetration, whilst also enhancing survival pathways [59], suitable agents that break down this barrier may provide better penetration of chemotherapeutic agents thus increasing efficacy. Mucolytics such as N-acetylcysteine, bromelain or other glycolytic and reducing agents [60,61] may be incorporated to disintegrate this barrier and allow better penetration of drugs. Numerous studies on mucin producing cancers showed that the efficacy of cytotoxic was increased in the presence of N-acetyl cysteine and bromelain [62,63], whilst synergistic combinations enabled a dramatic reduction of cytotoxic (paper under review).
Owing to the dense ECM present in the tumour environment, drug transfer is often compromised and hence suitable agents such as bromelain, collagenase etc. and other proteolytic enzymes should be incorporated into the TACE system to breakdown this barrier to enable a better drug transfer [64]. The suppression of collagen I synthesis by losartan in a dose dependent manner has been demonstrated in preclinical models [65]. Further, hyperthermia and ultrasound have also been suggested as a method of softening the dense tumour matrix [66,67].
Since the Intra-Tumoral-Fluid Pressure (ITFP) within the tumour is higher than the surrounding [68], drug passage through the tumour matrix may be difficult resulting in reduced efficacy. Hence, suitable methods such as ultrasound, hyperthermia or a combination of both may enable to reduce the intra tumoral pressure [69,70]. Other methods such as using vasodilators, small molecular weight chemotherapeutics and drugs that increase vascular permeability have been discussed in a recent review [71]. On the other hand, delivery of suitable chemical agents to reduce the ITFP may be necessary to ensure a better absorption of cytotoxic. High ITFP in the tumour is mainly contributed by the stiffening of the cellular matrix along with compromised fluid extraction since the lymphatic out flow is poorly developed. Treatment efficacy is mainly dependent on response to a particular chemotherapeutic agent and at the same time on attaining suitable concentration at the treatment site for sufficient time. Hence, reducing the ITFP or normalising it may greatly enhance drug transfer into cancer cells with greater efficacy. The barriers and possible solutions for increasing the efficacy of TACE may be summarised as shown in Figure 2.
Figure 2.
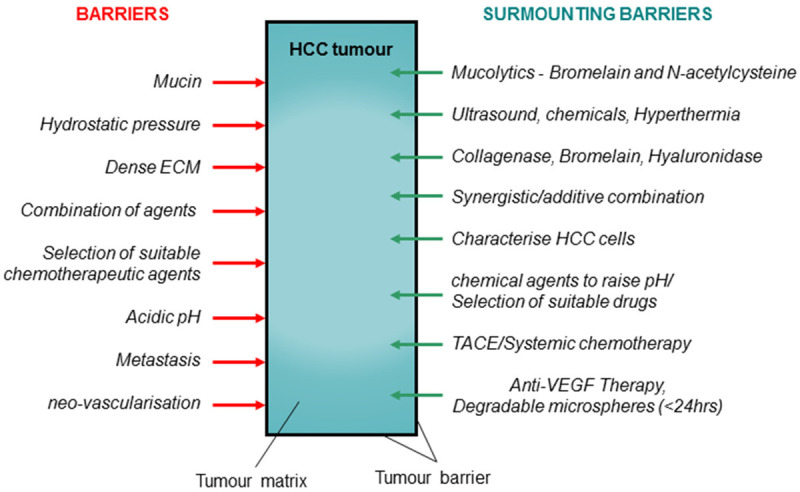
Barriers to drug penetration and efficacy with possible methods to surmount them to increase the efficacy of TACE. ECM: extracellular matrix; HCC: Hepatic cellular carcinoma; TACE: trans-arterial-chemoembolization; VEGF: vascular epidermal growth factors.
Recent classification of HCC has divided them broadly into two groups such as proliferators and non-proliferators that correlate with clinical pathological features, aetiology, and prognosis [72]. Tumours from the proliferator class are highly heterogenous with enhancement replication pathways such as Insulin-like Growth Factor-1 (IGF1), mechanistic target of rapamycin (MTOR) and stem cell feature (NOTCH) [73,74]. Further this class display numerous gene expression associated with tumour recurrence and poor prognosis [75]. Currently, extensive work is undergoing to target some of the new oncogenes that have been identified [76] and with future clinical trials, molecular classification with specific selection of chemotherapeutic drugs may enable a more effective treatment.
New development in drug therapy targeting both the tumour cells as well as angiogenesis using a number of different chemotherapeutic drugs together with tyrosine kinase inhibitors has shown plausible results in increasing patient survival [20,77]. However, at the current therapeutic dosage, these agents have numerous undesirable side effects [78]. Hence, chemotherapy is generally given in four cycles over a month with 7 days rest between cycles. However, if these agents can be combined synergistically, then, the effective dosage of both the agents may be dramatically reduced and hence, therapy may be given more frequently with possible better tumour ablation. Recent in vitro study using doxorubicin + lonafarnib or sorafenib + lonafarnib has shown tremendous synergistic efficacy in tumour cell reduction as compared to doxorubicin + sorafenib [79].
Intra-tumoral drug delivery to overcome the disadvantages posed by TACE
Recent work has indicated that intra-tumoural drug delivery using liposomes conjugated to drugs is an efficient way of delivering chemotherapeutic agents for treating malignant tumours and the various methods that may be employed to surpass some of the barriers for efficient penetration of chemo-agents has been reviewed by Goins et al. [80]. Further, slow releasing paclitaxel containing microspheres have been successfully delivered by intratumoral delivery with great efficacy compared to free drug intra-tumoural delivery in in vitro and in vivo studies [81]. Other studies using intratumoural injection of gels containing losartan microspheres and PLG-g-mPEG-cisplatin nanoparticles showed improved drug penetration, retention and anti-tumor activity [82]. In essence, this delivery method avoids embolisation of the blood vessels and hence may avoid neo-vascularisation and rebound tumour development that is encountered using the classical TACE method. Although, the drugs are delivered intra-tumourally, the drugs still have to overcome some of the barriers that are found within the tumour such as high ITFP, abnormal tumour vasculature, stiff extracellular matrix, poor lymphatics acidic pH etc.
However, one of the major advantage of this method is that the drugs do not have to cross from low to high ITFP as in the TACE method of drug delivery [83], since the tumoral pressure is highest within the central core of the tumour where the drug is delivered and the passage is from high to low pressure (diminishing pressure gradient) at the circumference of the tumour [84] as illustrated in Figure 3.
Figure 3.
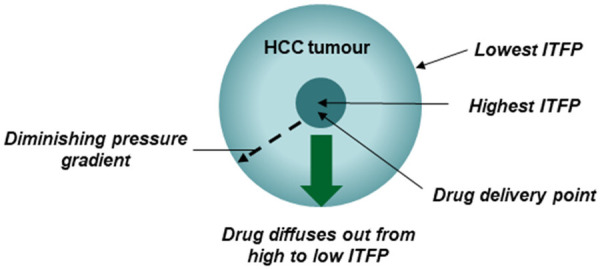
Demonstrates the principals involved in intra-tumoral drug delivery system. ITFP: intratumoural fluid pressure.
Drugs are transported from the intravascular space into the interstitial space in two main ways - diffusion and convection. Diffusion occurs at a faster rate for lower molecular size substances due to faster drift velocities. As derived by Einstein in 1905, the average drift displacement of a suspended sphere in a liquid is inversely proportional to its radius [85]. Therefore, as the molecular size of the drug increases, diffusion becomes less significant, and convection becomes the predominating process for drug transport. The tumours have higher ITFP values centrally with decreasing net fluid pressure away from the tumour, into the intravascular space [86]. This means that drugs have less ability to penetrate high ITFP tumours from the vascular space, especially higher molecular weight drugs that depend on convection as the primary means of drug transport. Drugs with low molecular weight will be less affected by this because they are more easily able to use diffusion to travel into the tumour.
Further, introducing drug carrying microspheres at the central point of tumour will overcome this as the drug will be present at the site of the tumour, in close proximity to tumour cells, hence can be a way to overcome the barrier of high ITFP.
In principal one of the major obstacles in this method of drug delivery is the high ITFP within the central core of the tumour where the drug is delivered and this high ITFP may oppose drug elution from the microspheres. A study shows that drugs are eluted freely and hence the high central ITFP is no obstacle [87]. This may be due to diffusion of drugs from high concentration within the sphere to low concentration (tumour matrix) following Fickian law of diffusion [88].
Further, introducing nano-conjugated drugs or drug carrying microspheres at the central point of tumour where ITFP is greatest may oppose free delivery of medicament. Piercing may in fact lead to efflux of tumoural fluid with metastatic cells leading to cancer spread. Other challenges that are presented to TACE, such as low tumoral pH, poor lymphatic drainage, etc. are also applicable to this method of delivery, although similar remedial techniques may be adopted. Therefore, using the intra-tumoral delivery system (ITD), single drugs such as doxorubicin, mitoxantrone, cisplatin etc. may be an efficient therapy since rebound tumour development owing to neo-angiogenesis that is common in TACE is absent.
Hepatic arterial infusion chemotherapy
Hepatic arterial infusion chemotherapy (HAIC) is the treatment of hepatic tumors using an infusion of chemotherapeutic agents through the hepatic artery and its downstream branches. Currently, while it is not used as standard of care under the AASLD, it is widely used in Japan and endorsed for the treatment of advanced cases of HCC [89]. As opposed to TACE there is no embolization involved, although it shares a similar characteristic with TACE in the locoregional delivery of drug. As such, HAIC displays a pharmacokinetic advantage compared to systemic drug delivery, following Collins’ model described previously. This has been confirmed for the drug (FUdR) (floxuridine), with which tumor concentrations 14 times higher were able to be achieved as measured through radiolabelling [90]. A review by Ensminger (2002) describes how pharmacokinetic as well as pharmacodynamic parameters must be considered when choosing drugs for HAIC [91]. For a drug to be appropriate for HAIC, it must be dosed at a rate that does not saturate the tumor cells, otherwise the incremental benefit of delivering through infusion will be small. Drugs also must have relatively high hepatic extraction ratios otherwise there will be no advantage over intravenous delivery [91].
There are many theoretical advantages with HAIC over TACE. Firstly, higher amounts of drug can be delivered for longer periods of time, as HAIC is not limited by the release kinetics of TACE, whether it be through microspheres, or lipiodol injections. By performing an infusion into the tumor over potentially several hours or even days, we can maintain tumor concentrations at a high equilibrium which is difficult to achieve through TACE. Dynamic control of drug infusion is possible with HAIC through simply adjusting drug concentrations and infusion rates in the implantable port system whereas in TACE, there is no way to control the delivery of drug once injection is complete. Moreover, there will be no rebound angiogenesis using HAIC because there is no embolization, which means drug can be delivered indefinitely whereas with TACE, rebound angiogenesis will quickly cause drug washout through increased tumor clearance of drug.
Experimental evidence that supports the use of HAIC largely comes from high observed rates of tumor response as described through RECIST criteria and mRECIST criteria, as well as a collection of trials comparing HAIC to systemic chemotherapy. Notably, there are no randomised trials comparing TACE with HAIC, representing an area of potential future research. One of the only trials to compare TACE and HAIC came from Kim et al., who compared 36 patients prospectively given HAIC with a retrospectively matched group of patients undergoing TACE with similar patient and tumor characteristics [92]. This study demonstrated a higher rate of objective response according to mRECIST in the HAIC group compared to TACE group (16.7% vs 0%, P = 0.03), as well as higher median survival (193 vs 119 days, P = 0.026). However, the study is limited by the fact that different drugs were used - the HAIC group was treated using 5FU and cisplatin, while the TACE group was treated using doxorubicin. Nevertheless, it is promising evidence that HAIC can be considered as a potential alternative treatment to TACE. Daniels and Wallman also have described significantly lower complication rates through their use of HAIC as opposed to reported complication rates of TACE therapy (1.4% vs 31%) [93]. Another recent trial showed a major survival advantage using HAIC with sorafenib over only sorafenib in a cohort of 247 HCC patients with portal vein invasion randomised to one of two groups (13.37 vs 7.13 months) [94]. The trial also showed a benefit in the time to progression (7 vs 2 months), as well as in response rate (41 vs 2%), however, there were more instances of vomiting (6 vs 1%), neutropenia (10 vs 2%), and thrombocytopenia (13 vs 5%) in the sorafenib and HAIC group. This trial presents substantial evidence that a change in the standard of care is necessary for HCC patients with portal vein invasion and suggests that further research into HAIC in different patient groups is required.
Development of degradable microspheres for TACE
Degradable microspheres are designed to provide embolisation on a transient basis depending on duration of treatment time required after which they disintegrate within the vessels without having any deleterious effect on other organs such as cytotoxicity or subsequent embolization of smaller capillaries with their residual material. Presently, a few different degradable microspheres have been developed using polymerised polylactic glycolic acid (PLGA), PLGA-polyethylene glycol (PEG)-PLGA, carboxymethylcellulose-chitosan (CMC-CCN), chitosan, hydroxyethyl acrylate (HEA) and degradable starch microspheres (DSM) [95].
PLGA microspheres degrades in vivo by hydrolysis of the ester bonds that are found between polylactic acid and polyglycolic, with the former degrading further into lactic acid that is excreted or converted into glucose to form adenosine triphosphate [96] whilst the latter also undergoes further hydrolysis into the monomers which is excreted through the kidneys or used in the tricarboxylic acid cycle [97]. The safety and efficacy of these PLGA microspheres (Occlusin500 from IMBiotechnologies Ltd, Edmonton, AB, Canada) have been successfully tested in sheep model with 150-212 um spheres [98]. Although technically PLGA microspheres degrade in 6 months Occlusin500 took up to 9 months owing to the development fibrous growth within the blood vessel and hence it was disqualified as degradable microspheres since it fell short of the standard set by ISO 10993-1 (international standard for device) which is <30 days [99].
The in vivo degradation of PLGA-PEG-PLGA begins with the degradation of PLGA by similar mechanism as above whilst PEG (polyethylene oxide) is excreted unchanged in urine with limited toxicity [100]. However, if metabolised in the kidneys, PEG could form ethylene glycol metabolite i.e. calcium oxalate and carbon dioxide which may pose toxicity. In vivo, these microspheres were reported to degrade in less than 7 days using 300-500 and 700-900 um spheres in sheep [101]. However, decreased particle size showed more distal occlusion, greater necrosis and lower recanalization rate [102]. Further the microspheres produced less ischemic damage relative to the controls (tris-acryl-gelatine) owing to its short half-life. Given its short embolic life, there will be no fibrotic tissue formation; however, the risk of migration with embolisation in non-target tissues may occur.
The disintegration of CMC-CNN microspheres is determined by the percentage oxidation of carboxymethylcellulose (CMC), 10% oxidised form disintegrates within 14 days whilst 25% oxidised in 30 days [103,104]. In vivo, lysozyme separates the two components by cleaving the Schiff’s base. CMC is non-toxic with limited degradation into glucose by hydrolysis of the 1-4 glycosidic linkages. Chitosan is regarded as non-toxic and it undergoes lyzozymic degradation. These spheres can be produced in a variety of sizes (100-1550 um) [104]. The safety of these microspheres was tested using the renal artery of Rabbit model; however, migration was not addressed [105].
Chitosan microspheres are polymer of glucosamine with N-acetylglucosamine, linked together by 1-4 glycosidic bonds that are hydrolysed by lysozyme into glucosamine [106]. Glucosamine is then converted into glycosaminoglycans, proteoglycans and glycolipids [107] with low systemic toxicity. The safety of chitosan microspheres (150-250 um) were tested in rabbits and the first sign of degradation was observed at 24 weeks (6 months) with complete absence at 32 weeks (8 months). Inflammatory response persisted for 32 weeks, however with low eosinophil count suggesting that allergic reaction may not occur [108].
Degradable starch microspheres (DSM) consists of polymerised partly hydrolysed starch molecules that are linked using glycerol ether groups and are easily degraded by blood α- amylase [109]. EmboCept® S DSM 35/50 (PharmaCept GmbH, Berlin, and Germany) manufactures DSM with an average diameter of 50 um with a half-life being 35-50 minutes both in vitro and in vivo [110]. Hence, repeated treatment is required to achieve optimal results. Degraded smaller fragments of microspheres may lodge non-specifically in other organ system resulting in ischemia and severe pain although this is only very temporary and blood flow is normally resumed in a matter of minutes (10-15 mins) [111]. Recent study has indicated that overall survival of patients can be improved using a combination of cytostatic drugs and DSM when compared to that of IV delivery of cytostatic drugs [112].
Biomarkers to assess the efficacy of TACE treatment
Specific and sensitive tumour biomarkers will enable not only early detection of the disease but also assess treatment efficacy in HCC [13]. Currently, several tumour markers have been identified for the detection of HCC such as α-fetoprotein (AFP), des-γ-carboxyprothrombin (DCP), Glypican-3 (GPC3), Golgi protein-73 (GP73), and circulating mi-RNAs (Figure 4). There are also several reviews addressing other tumour markers for the detection of HCC [113-115].
Figure 4.
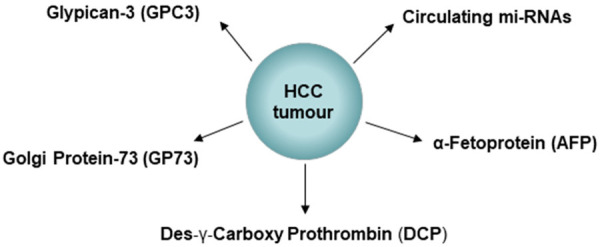
Shows some of the common biomarkers that are detected in HCC for diagnostic and therapeutic purpose.
Glypican-3 (GPC3) is a heparin sulfate proteoglycan and plays a vital role in regulating the growth of cells particularly through the wnt and hedgehog signalling pathway [116]. Numerous studies have found the absence or very low expression of GPC3 in normal liver, focal nodular hyperplasia and hepatocellular adenoma but highly expressed in HCC [117]. Although GPC3 can sometimes be expressed in other cancers such as liposarcoma, lung squamous cell carcinoma and testicular non-seminomatous germ cell tumour, studies have indicated its diagnostic value as a serum marker in HCC [118]. Notably, GPC3 has a higher specificity and sensitivity than human cervical cancer oncogene (HCCR) and Alfa fetoprotein (AFP) in the diagnosis of HCC and the combination of the three markers have indicated a much higher sensitivity to the detection of HCC than any other markers [119].
Alpha-fetoprotein (AFP) is primarily produced in the liver by the foetus during development, however it subsides after birth. The glycosylated form of AFP designated as AFP-L3 is closely associated with HCC and the simultaneous determination of AFP-L3, AFP with p53 antigen or with des-γ-carboxyprothrombin (DCP) has shown great diagnostic accuracy and sensitivity than any of the markers individually [120,121].
Des-γ-carboxyprothrombin (DCP) is a non-functional precursor of prothrombin that is excessively secreted by HCC cells which raises the level of DCP. The level of blood DCP has been well correlated with tumour diameter, disease progression [122]; portal vein invasion with correlation to survival [123]. It has also been suggested that DCP may promote angiogenesis through activation of vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) [124]. Compared to other tumour markers such as AFP, AFP-L3 and Golgi protein 73 (GP73), DCP has 60% sensitivity and 64.5% specificity for early stage diagnosis and 62.5% and 85.5% for all stages [125].
Golgi protein-73 (GP73) is a 73 kDa transmembrane glycoprotein is highly expressed in liver tumours and it promotes HCC cell invasion through the (CREB) mediated pathway [125]. It is a highly sensitive and specific serum biomarker for HCC as indicated by several studies [126] with better scores compared to AFP. Sixty percent of AFP negative HCC patients tested positive for GP73 in a recent study [127] although the sensitivity and diagnostic accuracy was lower. Hence, a combination of AFP and GP73 may serve as a useful diagnostic as well as treatment efficacy markers [128].
Micro RNAs have vital role in the development of HCC since healthy livers express high levels of miRNA 122 compared to HCC [129]. Studies have also shown that miRNA-122 may act as a tumour suppressor [130]. Further miRNA-23a was found to be associated with multiple local hepatic lesions [131] and a cut of value of > 210 miRNA-23a was comparatively more accurate for diagnosis of HCC since it was significantly specific and sensitive as compared to AFP with a cut off value of > 200 ng/ml [132]. Hence, miRNA-23a may have an important role in diagnosis and prognosis. Similarly, miRNA-494 has been proposed for both diagnosis and as a prognostic agent [133].
There are several reviews examining both diagnostic and prognostic biomarkers that can be detected in both histo-pathological and blood samples [13,113-115], with emphasis that a number of biomarkers should be used for diagnostic and prognostic screening since there is a inter patient variation of biomarker expression. Hence, the judicious use of biomarkers on an individual basis may enable a more accurate diagnostic and prognostic evaluation in HCC patients.
Conclusion
TACE appears to be an ideal method for drug delivery, although the use of non-degradable or that degrade very slowly may work against the efficient performance of the cytotoxic drugs since rebound vascular development that supports tumour regrowth takes place within 36 hours in human. The use of oral anti VEGF and tyrosine kinase inhibitors to overcome neovascularisation carry inherent disadvantage such as non-patient compliance owing to severe side effects. Further, these anti angiogenic therapy cannot be used on a long-term basis to overcome neovascularisation since they affect other organ systems [134]. Incorporating the tyrosine kinase inhibitors or anti VEGF therapy within the microspheres may reduce the side effects since the delivery is locoregional with minimal systemic exposure. However, the non-biodegradable spheres create permanent embolism that are very long lasting with lasting neovascularisation. Hence, the use of degradable microspheres may overcome neovascularisation although one major disadvantage is the embolization of non-target organs with degraded fragments, that are however relatively short lived. Other inbuilt barriers within the tumour will still prevail and has to be overcome in order to attain good efficacy. In the case of intra-tumoral drug delivery, although the drug is delivered within the tumour, factors such as low pH, dense tumour matrix and other barriers will still prevail, and they have to be overcome as discussed earlier. After drug delivery by TACE, the efficacy of treatment can be monitored using either dual biomarkers such as AFP-L3 and DCP or the use of several biomarkers that may be specific in certain patients. Since there are several variabilities within the tumour environment that is patient dependant, it would be difficult to attain uniform treatment response amongst patients although certain parameters such as selection of cytotoxic depending on tumour characteristics, type of microspheres, size etc, can be controlled. In an ideal situation where the existing barriers to drug transfer and other inherent disadvantages in microspheres can be overcome, then TACE may serve as an ideal treatment with probable better outcome and even serve as a cure for non-resectable HCC (Figure 5).
Figure 5.
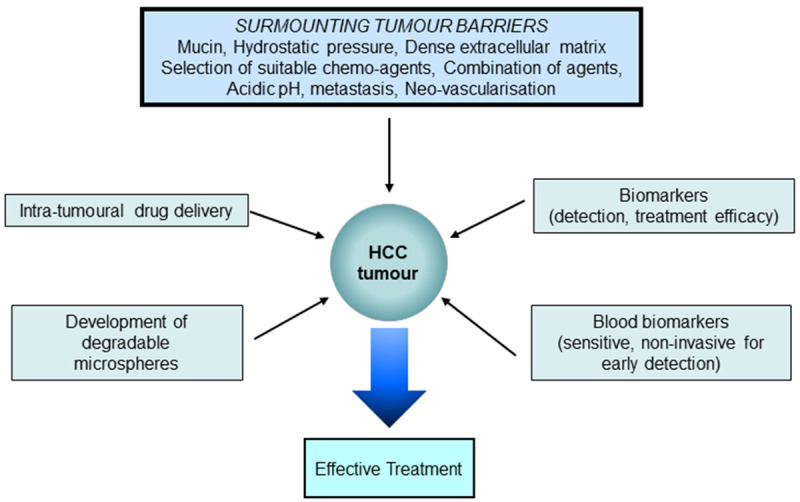
TACE as an ideal treatment for non-resectable HCC.
Disclosure of conflict of interest
None.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The global burden of cancer 2013. JAMA Oncol. 2015;1:505. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37:S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, De Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha J, Chaudhri A, Avirineni A, Pan JJ. Burden of hepatocellular carcinoma among hispanics in South Texas: a systematic review. Biomark Res. 2017;5:15. doi: 10.1186/s40364-017-0096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Xie SH, Hu S, Cheng X, Gao T, Zhang C, Song Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget. 2017;8:68131. doi: 10.18632/oncotarget.19245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladenheim MR, Kim NG, Nguyen P, Le A, Stefanick ML, Garcia G, Nguyen MH. Sex differences in disease presentation, treatment and clinical outcomes of patients with hepatocellular carcinoma: a single-centre cohort study. BMJ Open Gastroenterol. 2016;3:e000107. doi: 10.1136/bmjgast-2016-000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Koh WP, Dan YY, Goh GB, Jin A, Wang R, Yuan JM. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36:893–901. doi: 10.1111/liv.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AK, Kumar R, Pandey AK. Hepatocellular carcinoma: causes, mechanism of progression and biomarkers. Curr Chem Genom Transl Med. 2018;12:9–26. doi: 10.2174/2213988501812010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon H, Choi JE, Lee IJ, Kim TH, Kim SH, Ko YH, Kim HB, Nam BH, Park JW. All-treatment array of hepatocellular carcinoma from initial diagnosis to death: observation of cumulative treatments. J Cancer Res Clin Oncol. 2017;143:2327–2339. doi: 10.1007/s00432-017-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 16.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425–434. doi: 10.3348/kjr.2009.10.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lencioni R, De Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980–985. doi: 10.1007/s00270-011-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez JA, Gish RG. Efficacy of combination treatment modalities for intermediate and advanced hepatocellular carcinoma: intra-arterial therapies, sorafenib and novel small molecules. Transl Cancer Res. 2013;2:460–471. doi: 10.3978/j.issn.2218-676X.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Expe Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol. 2018;48:103–114. doi: 10.1093/jjco/hyx180. [DOI] [PubMed] [Google Scholar]
- 22.Shaaban S, Negm A, Ibrahim EE, Elrazak AA. Chemotherapeutic agents for the treatment of hepatocellular carcinoma: efficacy and mode of action. Oncol Rev. 2014;8:246. doi: 10.4081/oncol.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol. 2018;7:17. doi: 10.1186/s40164-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating GM. Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 2017;12:243–253. doi: 10.1007/s11523-017-0484-7. [DOI] [PubMed] [Google Scholar]
- 25.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging. 2011;36:677–683. doi: 10.1007/s00261-011-9755-4. [DOI] [PubMed] [Google Scholar]
- 27.Collins JM. Pharmacologic rationale for regional drug delivery. J. Clin. Oncol. 1984;2:498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- 28.Dong G, Zheng QD, Ma M, Wu SF, Zhang R, Yao RR, Dong YY, Ma H, Gao DM, Ye SL, Cui JF, Ren ZG, Chen RX. Angiogenesis enhanced by treatment damage to hepatocellular carcinoma through the release of GDF15. Cancer Med. 2018;7:820–830. doi: 10.1002/cam4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8:297. doi: 10.14740/jocmr2496w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welter M, Rieger H. Interstitial fluid flow and drug delivery in vascularized tumors: a computational model. PLoS One. 2013;8:e70395. doi: 10.1371/journal.pone.0070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malagari K, Emmanouil E, Pomoni M, Kelekis D. Chemoembolization with DC Bead™ for the treatment of hepatocellular carcinoma: an update. Hepat Oncol. 2014;1:205–214. doi: 10.2217/hep.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Kobayashi S, Phongkitkarun S, Broemeling LD, Kan Z. Effect of transcatheter hepatic arterial embolization on angiogenesis in an animal model. Invest Radiol. 2006;41:516–521. doi: 10.1097/01.rli.0000209663.00629.8a. [DOI] [PubMed] [Google Scholar]
- 33.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulin M, Delhom E, Pierredon-Foulongne MA, Cercueil JP, Guiu B. Transarterial chemoembolization for hepatocellular carcinoma: an old method, now flavor of the day. Diagn Interv Imaging. 2015;96:607–615. doi: 10.1016/j.diii.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Mehvar R. Clearance concepts: fundamentals and application to pharmacokinetic behavior of drugs. J Pharm Pharm Sci. 2018;21:88s–102s. doi: 10.18433/jpps29896. [DOI] [PubMed] [Google Scholar]
- 36.Vogl TJ, Zangos S, Balzer JO, Nabil M, Rao P, Eichler K, Bechstein WO, Zeuzem S, Abdelkader A. Transarterial chemoembolization (TACE) in hepatocellular carcinoma: technique, indication and results. Rofo. 2007;179:1113–1126. doi: 10.1055/s-2007-963285. [DOI] [PubMed] [Google Scholar]
- 37.Hetta WM, Shebria N. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres. The Egyptian Journal of Radiology and Nuclear Medicine. 2014;45:761–769. [Google Scholar]
- 38.Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C, Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691. doi: 10.18632/oncotarget.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong XX, Qiu XY, Hu DX, Chen XQ. Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma. Mol Pharmacol. 2017;92:246–255. doi: 10.1124/mol.116.107706. [DOI] [PubMed] [Google Scholar]
- 41.Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014;2014:409272. doi: 10.1155/2014/409272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I. Chemotherapy and signaling: how can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther. 2010;10:839–853. doi: 10.4161/cbt.10.9.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: a meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511–518. doi: 10.1177/2050640616673516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0184597. doi: 10.1371/journal.pone.0184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leathers JS, Balderramo D, Prieto J, Diehl F, Gonzalez-Ballerga E, Ferreiro MR, Carrera E, Barreyro F, Diaz-Ferrer J, Singh D, Mattos AZ, Carrilho F, Debes JD. Sorafenib for treatment of hepatocellular carcinoma: a survival analysis from the south american liver research network. J Clin Gastroenterol. 2019;53:464–469. doi: 10.1097/MCG.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 46.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo) embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011:CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray CE Jr, Haskal ZJ, Geschwind JF, Funaki BS. The use of transarterial chemoembolization in the treatment of unresectable hepatocellular carcinoma: a response to the Cochrane Collaboration review of 2011. J Vasc Interv Radiol. 2011;22:1693–6. doi: 10.1016/j.jvir.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziogas IA, Tsoulfas G. Evolving role of Sorafenib in the management of hepatocellular carcinoma. World J Clin Oncol. 2017;8:203–213. doi: 10.5306/wjco.v8.i3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanoff HK, Chang Y, Lund JL, O’Neil BH, Dusetzina SB. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21:1113–1120. doi: 10.1634/theoncologist.2015-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang H, Yang X, Wan X, Lu X, Sang X, Zhao H. Combination treatment including targeted therapy for advanced hepatocellular carcinoma. Oncotarget. 2016;7:71036–71051. doi: 10.18632/oncotarget.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 52.Marino D, Zichi C, Audisio M, Sperti E, Di Maio M. Second-line treatment options in hepatocellular carcinoma. Drugs Context. 2019;8:212577. doi: 10.7573/dic.212577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7:1–19. doi: 10.1159/000487148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganten TM, Stauber RE, Schott E, Malfertheiner P, Buder R, Galle PR, Gohler T, Walther M, Koschny R, Gerken G. Sorafenib in patients with hepatocellular carcinoma-results of the observational INSIGHT study. Clin Cancer Res. 2017;23:5720–5728. doi: 10.1158/1078-0432.CCR-16-0919. [DOI] [PubMed] [Google Scholar]
- 55.Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi: 10.1016/j.ctrv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Vukovic V, Tannock IF. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75:1167–1172. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi H. Cancer chemotherapy specific to acidic nests. Cancers (Basel) 2017;9:36. doi: 10.3390/cancers9040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh M, Fais S, Spugnini EP, Harguindey S, Abu Izneid T, Scacco L, Williams P, Allegrucci C, Rauch C, Omran Z. Proton pump inhibitors for the treatment of cancer in companion animals. J Exp Clin Cancer Res. 2015;34:93. doi: 10.1186/s13046-015-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao CV, Janakiram NB, Mohammed A. Molecular pathways: mucins and drug delivery in cancer. Clin Cancer Res. 2017;23:1373–1378. doi: 10.1158/1078-0432.CCR-16-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pavan R, Jain S, Shraddha , Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol Res Int. 2012;2012:976203. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A. A review on various uses of N-Acetyl cysteine. Cell J. 2017;19:11–17. doi: 10.22074/cellj.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6:350–369. [PMC free article] [PubMed] [Google Scholar]
- 63.Wan VH, Pillai K, Badar S, Akhter J, Morris DL. The effect of hyperthermia (42°C) on the anti-tumoral effect of bromelain, N-acetyl cysteine, chemotherapeutic agents and their combinations-an in vitro evaluation. Journal of Peritoneum (and other serosal surfaces) 2018 [Google Scholar]
- 64.Eikenes L, Bruland ØS, Brekken C, de Lange Davies C. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 65.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koning GA, Eggermont AM, Lindner LH, ten Hagen TL. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res. 2010;27:1750–1754. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phenix CP, Togtema M, Pichardo S, Zehbe I, Curiel L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci. 2014;17:136–153. doi: 10.18433/j3zp5f. [DOI] [PubMed] [Google Scholar]
- 68.Stapleton S, Milosevic M, Tannock IF, Allen C, Jaffray DA. The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J Control Release. 2015;211:163–170. doi: 10.1016/j.jconrel.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659–667. doi: 10.1148/radiol.2352030916. [DOI] [PubMed] [Google Scholar]
- 70.Navalitloha Y, Schwartz ES, Groothuis EN, Allen CV, Levy RM, Groothuis DR. Therapeutic implications of tumor interstitial fluid pressure in subcutaneous RG-2 tumors. Neuro Oncol. 2006;8:227–233. doi: 10.1215/15228517-2006-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baronzio G, Parmar G, Baronzio M. Overview of methods for overcoming hindrance to drug delivery to tumors, with special attention to tumor interstitial fluid. Front Oncol. 2015;5:165. doi: 10.3389/fonc.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Quetglas I, Pinyol R, Dauch D, Torrecilla S, Tovar V, Moeini A, Alsinet C, Portela A, Rodriguez-Carunchio L, Solé M. IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology. 2016;151:1192–1205. doi: 10.1053/j.gastro.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S, Stanger BZ. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. e1667. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nault JC, De Reyniès A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud S, Rousseau F, Azoulay D, Saric J, Blanc JF, Balabaud C, Bioulac-Sage P, Laurent A, Laurent-Puig P, Llovet JM, Zucman-Rossi J. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145:176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 76.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chapiro J, Duran R, Geschwind JF. Combination of intra-arterial therapies and sorafenib: is there a clinical benefit? Radiol Med. 2014;119:476–482. doi: 10.1007/s11547-014-0413-0. [DOI] [PubMed] [Google Scholar]
- 78.Krajewska J, Kukulska A, Paliczka-Cieślik E, Handkiewicz-Junak D, Gawlik T, Olczyk T, Kropińska A, Skoczylas A, Michalik B, Jarząb B. The assessment of side effects of tyrosine kinase inhibitors (TKI) applied in patients with advanced thyroid cancer (TC)-one centre experience. Thyroid Research. 2013 [Google Scholar]
- 79.Wang J, Lian Y, Gu Y, Wang H, Gu L, Huang Y, Zhou L, Huang Y. Synergistic effect of farnesyl transferase inhibitor lonafarnib combined with chemotherapeutic agents against the growth of hepatocellular carcinoma cells. Oncotarget. 2017;8:105047–105060. doi: 10.18632/oncotarget.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goins B, Phillips WT, Bao A. Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opin Drug Deliv. 2016;13:873–889. doi: 10.1517/17425247.2016.1167035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Tissue distribution, metabolism and excretion of paclitaxel in mice. Anticancer Drugs. 1996;7:78–86. doi: 10.1097/00001813-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Yu M, Zhang C, Tang Z, Tang X, Xu H. Intratumoral injection of gels containing losartan microspheres and (PLG-g-mPEG)-cisplatin nanoparticles improves drug penetration, retention and anti-tumor activity. Cancer Lett. 2019;442:396–408. doi: 10.1016/j.canlet.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Nouri YM, Kim JH, Yoon HK, Ko HK, Shin JH, Gwon DI. Update on transarterial chemoembolization with drug-eluting microspheres for hepatocellular carcinoma. Korean J Radiol. 2019;20:34–49. doi: 10.3348/kjr.2018.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao L, Cai S, Cai A, Zhao Y, Xu T, Ma Y, Xu Y, Wang Y, Wang H, Hu Y. The improved antitumor efficacy of continuous intratumoral chemotherapy with cisplatin-loaded implants for the treatment of sarcoma 180 tumor-bearing mice. Drug Deliv. 2019;26:208–215. doi: 10.1080/10717544.2019.1574938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wannier GH. Motion of gaseous ions in a strong electric field. II. Phys Rev. 1952;87:795. [Google Scholar]
- 86.Kim HG, Yu AR, Lee JJ, Lee YJ, Lim SM, Kim JS. Measurement of tumor pressure and strategies of imaging tumor pressure for radioimmunotherapy. Nucl Med Mol Imaging. 2019;53:1–7. doi: 10.1007/s13139-019-00598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren S, Li C, Dai Y, Li N, Wang X, Tian F, Zhou S, Qiu Z, Lu Y, Zhao D, Chen X, Chen D. Comparison of pharmacokinetics, tissue distribution and pharmacodynamics of liposomal and free doxorubicin in tumour-bearing mice following intratumoral injection. J Pharm Pharmacol. 2014;66:1231–1239. doi: 10.1111/jphp.12257. [DOI] [PubMed] [Google Scholar]
- 88.Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7:429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, Kuwahara A, Okusaka T. Current status of hepatocellular carcinoma in Japan. Chin Clin Oncol. 2013;2:40. doi: 10.3978/j.issn.2304-3865.2013.09.01. [DOI] [PubMed] [Google Scholar]
- 90.Sigurdson ER, Ridge JA, Kemeny N, Daly JM. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J. Clin. Oncol. 1987;5:1836–1840. doi: 10.1200/JCO.1987.5.11.1836. [DOI] [PubMed] [Google Scholar]
- 91.Ensminger WD. Intrahepatic arterial infusion of chemotherapy: pharmacologic principles. Semin Oncol. 2002;29:119–125. doi: 10.1053/sonc.2002.31679. [DOI] [PubMed] [Google Scholar]
- 92.Kim HY, Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS, Kim SG, Kim YS, Seo YS, Yim HJ, Um SH. A comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol. 2010;16:355–361. doi: 10.3350/kjhep.2010.16.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daniels JR, Wallman M. Subselective intra-arterial chemotherapy infusion in the treatment of hepatocellular carcinoma. Semin Oncol. 2010;37:83–88. doi: 10.1053/j.seminoncol.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 94.He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doucet J, Kiri L, O’Connell K, Kehoe S, Lewandowski RJ, Liu DM, Abraham RJ, Boyd D. Advances in degradable embolic microspheres: a state of the art review. J Funct Biomater. 2018;9:14. doi: 10.3390/jfb9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boland EL, Shine R, Kelly N, Sweeney CA, McHugh PE. A review of material degradation modelling for the analysis and design of bioabsorbable stents. Ann Biomed Eng. 2016;44:341–356. doi: 10.1007/s10439-015-1413-5. [DOI] [PubMed] [Google Scholar]
- 97.Nagarajan S, Reddy B. Bio-absorbable polymers in implantation-An overview. Chemistry. 2009 [Google Scholar]
- 98.Owen RJ, Nation PN, Polakowski R, Biliske JA, Tiege PB, Griffith IJ. A preclinical study of the safety and efficacy of Occlusin™ 500 artificial embolization device in sheep. Cardiovasc Intervent Radiol. 2012;35:636–644. doi: 10.1007/s00270-011-0218-7. [DOI] [PubMed] [Google Scholar]
- 99.FDA. Use of International Standard ISO10993-1, “Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process”. https://www.fda.gov/media/85865/download. Published 2016. Updated June 16, 2016. Accessed 26 May, 2020.
- 100.Webster R, Elliott V, Park BK, Walker D, Hankin M, Taupin P. PEGylated protein drugs: basic science and clinical applications. Springer; 2009. PEG and PEG conjugates toxicity: towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals; pp. 127–146. [Google Scholar]
- 101.Verret V, Pelage JP, Wassef M, Louguet S, Servais E, Bédouet L, Beaulieu T, Moine L, Laurent A. A novel resorbable embolization microsphere for transient uterine artery occlusion: a comparative study with trisacryl-gelatin microspheres in the sheep model. J Vasc Interv Radiol. 2014;25:1759–1766. doi: 10.1016/j.jvir.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 102.Maeda N, Verret V, Moine L, Bédouet L, Louguet S, Servais E, Osuga K, Tomiyama N, Wassef M, Laurent A. Targeting and recanalization after embolization with calibrated resorbable microspheres versus hand-cut gelatin sponge particles in a porcine kidney model. J Vasc Interv Radiol. 2013;24:1391–1398. doi: 10.1016/j.jvir.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 103.Rosca C, Popa MI, Lisa G, Chitanu GC. Interaction of chitosan with natural or synthetic anionic polyelectrolytes. 1. The chitosan-carboxymethylcellulose complex. Carbohydr Polym. 2005;62:35–41. [Google Scholar]
- 104.Weng L, Le HC, Talaie R, Golzarian J. Bioresorbable hydrogel microspheres for transcatheter embolization: preparation and in vitro evaluation. J Vasc Interv Radiol. 2011;22:1464–1470. e1462. doi: 10.1016/j.jvir.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Weng L, Rusten M, Talaie R, Hairani M, Rosener NK, Golzarian J. Calibrated bioresorbable microspheres: a preliminary study on the level of occlusion and arterial distribution in a rabbit kidney model. J Vasc Interv Radiol. 2013;24:1567–1575. doi: 10.1016/j.jvir.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Yang YM, Hu W, Wang XD, Gu XS. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J Mater Sci Mater Med. 2007;18:2117–2121. doi: 10.1007/s10856-007-3013-x. [DOI] [PubMed] [Google Scholar]
- 108.Kwak BK, Shim HJ, Han SM, Park ES. Chitin-based embolic materials in the renal artery of rabbits: pathologic evaluation of an absorbable particulate agent. Radiology. 2005;236:151–158. doi: 10.1148/radiol.2361040669. [DOI] [PubMed] [Google Scholar]
- 109.Schicho A, Pereira PL, Pützler M, Michalik K, Albrecht T, Nolte-Ernsting C, Stroszczynski C, Wiggermann P. Degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE) in intrahepatic cholangiocellular carcinoma (ICC): results from a national multi-center study on safety and efficacy. Med Sci Monit. 2017;23:796–800. doi: 10.12659/MSM.902901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pieper CC, Meyer C, Vollmar B, Hauenstein K, Schild HH, Wilhelm KE. Temporary arterial embolization of liver parenchyma with degradable starch microspheres (EmboCept®S) in a swine model. Cardiovasc Intervent Radiol. 2015;38:435–441. doi: 10.1007/s00270-014-0966-2. [DOI] [PubMed] [Google Scholar]
- 111.Lote K, Myking AO. Starch microsphere induced small intestinal ischaemia. Blood flow and morphologic investigations of late effects. Acta Radiol Oncol. 1982;21:353–358. doi: 10.3109/02841868209134027. [DOI] [PubMed] [Google Scholar]
- 112.Orlacchio A, Chegai F, Francioso S, Merolla S, Monti S, Angelico M, Tisone G, Mannelli L. Repeated transarterial chemoembolization with degradable starch microspheres (DSMs-TACE) of unresectable hepatocellular carcinoma: a prospective pilot study. Curr Med Imaging Rev. 2018;14:637–645. doi: 10.2174/1573405613666170616123657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Stefano F, Chacon E, Turcios L, Marti F, Gedaly R. Novel biomarkers in hepatocellular carcinoma. Dig Liver Dis. 2018;50:1115–1123. doi: 10.1016/j.dld.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 114.Chauhan R, Lahiri N. Tissue- and serum-associated biomarkers of hepatocellular carcinoma. Biomark Cancer. 2016;8:37–55. doi: 10.4137/BIC.S34413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ocker M. Biomarkers for hepatocellular carcinoma: what’s new on the horizon? World J Gastroenterol. 2018;24:3974–3979. doi: 10.3748/wjg.v24.i35.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588:377–382. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 118.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 119.Qiao SS, Cui ZQ, Gong L, Han H, Chen PC, Guo LM, Yu X, Wei YH, Ha SA, Kim JW, Jin ZT, Li S, Peng JR, Leng XS. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1718–1724. doi: 10.5754/hge11124. [DOI] [PubMed] [Google Scholar]
- 120.Hadziyannis E, Sialevris K, Georgiou A, Koskinas J. Analysis of serum α-fetoprotein-L3% and des-γ carboxyprothrombin markers in cases with misleading hepatocellular carcinoma total α-fetoprotein levels. Oncol Rep. 2013;29:835–839. doi: 10.3892/or.2012.2147. [DOI] [PubMed] [Google Scholar]
- 121.Abdel-Aziz MM, Elshal MF, Abass AT, El-Kafrawy S, Ezzat S, Abdel-Wahab M. Comparison of AFP-L3 and p53 antigen concentration with alpha-fetoprotein as serum markers for hepatocellular carcinoma. Clin Lab. 2016;62:1121–1129. doi: 10.7754/clin.lab.2015.151102. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–2043. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 123.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M. Des-γ-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::aid-cncr1035>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 124.Matsubara M, Shiraha H, Kataoka J, Iwamuro M, Horiguchi S, Nishina S, Takaoka N, Uemura M, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yamamoto K. Des-γ-carboxyl prothrombin is associated with tumor angiogenesis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1602–1608. doi: 10.1111/j.1440-1746.2012.07173.x. [DOI] [PubMed] [Google Scholar]
- 125.Li B, Li B, Guo T, Sun Z, Li X, Li X, Chen L, Zhao J, Mao Y. Artificial neural network models for early diagnosis of hepatocellular carcinoma using serum levels of α-fetoprotein, α-fetoprotein-L3, des-γ-carboxy prothrombin, and Golgi protein 73. Oncotarget. 2017;8:80521. doi: 10.18632/oncotarget.19298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Di Jin JT, Li D, Wang Y, Li L, Hu Z, Zhou Z, Chang X, Qu C, Zhang H. Golgi protein 73 activation of MMP-13 promotes hepatocellular carcinoma cell invasion. Oncotarget. 2015;6:33523. doi: 10.18632/oncotarget.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, Robert A, Verhoef C, IJzermans JN. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Internat. 2013;7:59–64. doi: 10.1007/s12072-012-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2016;9:123. doi: 10.2147/OTT.S90732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48:648–656. doi: 10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 130.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin Y, Wang J, Han J, Luo D, Sun Z. MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/β-cadherin signaling pathway. Exp Cell Res. 2017;360:210–217. doi: 10.1016/j.yexcr.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 132.Mohamed AA, Ali-Eldin ZA, Elbedewy TA, El-Serafy M, Ali-Eldin FA, AbdelAziz H. MicroRNAs and clinical implications in hepatocellular carcinoma. World J Hepatol. 2017;9:1001. doi: 10.4254/wjh.v9.i23.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu K, Liu S, Zhang W, Jia B, Tan L, Jin Z, Liu Y. miR-494 promotes cell proliferation, migration and invasion, and increased sorafenib resistance in hepatocellular carcinoma by targeting PTEN. Oncol Rep. 2015;34:1003–1010. doi: 10.3892/or.2015.4030. [DOI] [PubMed] [Google Scholar]
- 134.Lupo G, Caporarello N, Olivieri M, Cristaldi M, Motta C, Bramanti V, Avola R, Salmeri M, Nicoletti F, Anfuso CD. Anti-angiogenic therapy in cancer: downsides and new pivots for precision medicine. Front Pharmacol. 2017;7:519. doi: 10.3389/fphar.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]


