Abstract
Del-Nido cardioplegia (DNc) is a single-dose cardioplegia that is widely used in human medicine because of its long duration. In this report, we describe two cases of open-heart surgery with cardiopulmonary bypass (CPB) using DNc. One dog was diagnosed with partial atrioventricular septal defect, and the other dog was diagnosed with myxomatous mitral valve disease stage D. Both dogs were treated with open-heart surgery with DNc to induce temporary cardiac arrest. No complications from DNc were observed, and the patients were discharged. Veterinary heart surgeons should consider DNc as an option for temporary cardiac arrest during open-heart surgery with CPB.
Keywords: Case reports, endocardial cushion defect, mitral valve, cardioplegic solution, cardiopulmonary bypass
INTRODUCTION
Cardioplegia is a pharmacological therapy that induces temporary cardiac arrest and provides a clear surgical field during open-heart surgery [1]. The cardioplegic components contribute to intraoperative myocardial protection from ischemic injury [2]. Therefore, cardioplegia is essential for achieving successful clinical outcomes during open-heart surgery [3].
Since 1951, various types of cardioplegia have been developed to improve the prognosis of open-heart surgery [4]. There have been many reports on the use of different types of cardioplegia in human medicine. However, the use of hyperkalemic cardioplegic solutions under hypothermic conditions is the clinical standard in many human cardiac surgeries worldwide [2].
Del-Nido cardioplegia (DNc) was introduced in neonatal and pediatric cardiac surgery in the early 1990’s [5]. The long duration (up to 3 h) of a single induction dose results in a decrease in surgical interruption; its use has been applied to adult cardiac surgery and has gained increasing acceptance [6]. This report presents two successful open-heart surgery cases for the treatment of partial atrioventricular septal defect and severe mitral valve regurgitation in dogs and assesses the usefulness of single-dose DNc.
CASE PRESENTATION
Case 1
A 2-year-old castrated male Doberman Pinscher weighing 37 kg was referred to our clinic for the treatment of partial atrioventricular septal defect. The patient had experienced mild exercise intolerance since it was 1 year old.
Physical examination revealed a grade 4/6 systolic murmur at the base of the heart. Thoracic radiography revealed severe global cardiomegaly and vascular lung pattern. Echocardiography confirmed a markedly enlarged right heart with a vertebral heart score of 12.9. A large primum defect of the atrial septum, with a maximum diameter of 19.3 mm, was located just above the atrioventricular valve. Left heart volume overload was also confirmed, with a left atrium-to-aortic root ratio (LA/Ao) of 2.08. Color Doppler echocardiography revealed left-to-right blood flow across the atrial septal defect as well as mitral valve regurgitation with a septal mitral valve defect (Fig. 1). The pulmonary-to-systemic blood flow ratio (Qp/Qs) was 2.9, indicating increased right cardiac output with a left-to-right shunt. Therefore, the patient was diagnosed with primum-type atrial septal defect with mitral valve cleft, and surgical treatment was recommended.
Fig. 1. Intraoperative photograph of cases 1 and 2. (A) Repair of the mitral cleft in case 1. (B) Repair of the atrial septal defect in case 1. (C) Implantation of five artificial chordae tendineae in case 2. (D) Trimming of the ruptured chordae tendineae in case 2.
*Autologous pericardial patch
Preanesthetic medications included 0.025 mg/kg atropine (Jeil Atropine Sulfate Injection; Jeil Pharmaceutical Co., Ltd., Korea) 0.3 mg/kg midazolam (Bukwang Midazolam Injection; Bukwang Pharmaceutical Co., Ltd., Korea), 5 μg/kg fentanyl (Myungmoon Fentanyl Citrate Injection; Myungmoon Pharmaceutical Co., Ltd., Korea), and 22 mg/kg of cefazolin (Chong Kun Dang Cefazolin Injection 1 g; Chong Kun Dang Pharmaceutical Corp., Korea), which were injected intravenously. Anesthesia was induced intravenously with 6 mg/kg of propofol (Anepol Injection; Hana Pharmaceutical Co., Ltd., Korea) and maintained with 2.0% isoflurane (Ifran Liguid for Inhalation; Hana Pharmaceutical Co., Ltd., Korea) until cardiopulmonary bypass (CPB) was established.
A roller-type CPB machine (Terumo Advanced Perfusion System 1; Terumo Corp., Japan) and a pediatric oxygenator (CAPIOX RX15; Terumo Corp.) were used for open-heart surgery. The CPB circuit was filled with 20% albumin (100 mL), 20% mannitol (5 mL/kg), 8.4% bicarbonate (1 mL/kg), heparin (500 units), cefazolin (22 mg/kg), and plasma as a volume expander. Whole blood was added after initiating CPB to maintain hematocrit (Hct) 20–25%.
Right thoracotomy was performed in the 4th intercostal space. A partial pericardiectomy was performed, and the excised pericardium was tanned in a 10% glutaraldehyde solution. An 18 Fr arterial cannula was inserted into the ascending aorta. A 20 Fr cannula and a 22 Fr venous cannula were inserted at the cranial and caudal vena cava, respectively; 250 U/kg heparin was administered initially to achieve an activated clotting time of > 350 sec. After initiating CPB, inhalation anesthesia was switched to continuous intravenous infusion of fentanyl and propofol.
A 7 Fr root cannula was inserted into the aortic root to administer DNc. Oxygenated blood from the bypass circuit was added to the DNc solution at a 4:1 ratio (DNc: oxygenated blood). The aorta was cross-clamped and complete cardiac arrest was achieved with 20 mL/kg of 4°C DNc. The primum atrial septal defect (ASD) and mitral cleft were exposed via right atriotomy. The continuous mitral valve defect was repaired with a polypropylene suture (Prolene 6-0; Ethicon, Johnson & Johnson Company, USA). The ASD was then closed with a glutaraldehyde-treated autologous pericardial patch using a simple continuous suture (Fig. 1). The right atrium was closed and rewarming was initiated simultaneously.
The aortic cross-clamp (ACC) was terminated when the body temperature increased to 32°C. Ventricular fibrillation occurred immediately, although sinus rhythm was obtained only once after applying the defibrillator. Subsequently, the bypass flow was reduced to allow cardiac function to return to normal. After termination of CPB, 3 mg/kg protamine (Hanlim Protamine Sulfate Injection; Hanlim Pharmaceutical Co., Ltd., Korea) was administered intravenously over 30 min to reverse the effect of heparin.
During surgery, the heart rate, respiratory rate, rectal temperature, esophageal temperature, arterial oxygen saturation, end-tidal carbon dioxide, and invasive arterial blood pressure were continuously monitored. Simultaneously, arterial blood gas was measured to determine the pH, partial pressure of CO2, partial pressure of oxygen, Hct, and electrolyte balance. Hct was maintained between 20–25% during CPB. The total CPB and ACC times were 133 and 65 min, respectively. During ACC, the mean arterial pressure was 68 mmHg, and the lowest temperature was 29.6°C. The lowest bypass flow rate was 57 mL/kg/min, and the highest flow rate was 110 mL/kg/min.
The dog recovered from anesthesia smoothly and had an uneventful post-operative course. No cardiac murmur was heard immediately after surgery. Post-operative medications, 0.5 mg/kg rivaroxaban (Xarelto; Bayer Yakuhin, Ltd., Japan) once daily, and 0.2 mg/kg of pimobendane (Vetmedin; Boehringer Ingelheim, Germany) twice daily were maintained until 3 months after surgery. The patient was discharged uneventfully 2 weeks after surgery and did not show any clinical signs until post-operative day 246. On thoracic radiography, the vertebral heart scale decreased from 12.9 to 11.4 at 1 month after surgery with no shunt flow (Fig. 2). Echocardiography 3 months after surgery revealed a reduced Qp/Qs from 3.67 to 1.03 and reduced LA/Ao from 2.08 to 1.9. Recent echocardiography values in postoperative day (POD) 246 have shown similar compared to POD 3 months (Table 1).
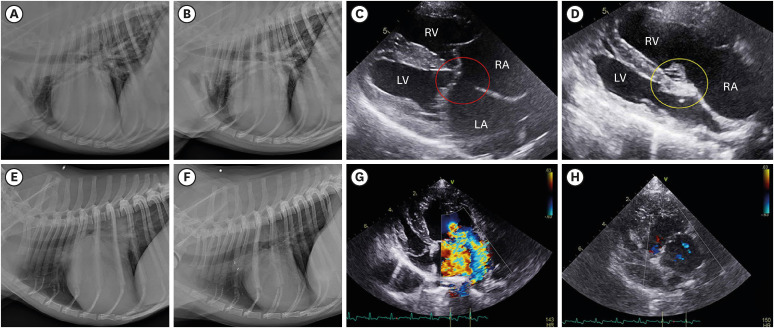
Fig. 2. Radiography and echocardiography examples in cases 1 and 2. (A) Pre-operative and (B) post-operative radiographs at day 42 in case 1. (C) A primum atrial septal defect (red circle) was confirmed between the LA and RA in case 1. (D) Post-operative echocardiography at day 42 in case 1. The defect was closed by autologous pericardial patch (yellow circle) (E) Pre-operative and (F) post-operative radiographs at day 151 in case 2. (G) Severe mitral valve regurgitation was detected on color Doppler mapping in case 2. (H) Case 2 at 151 days after mitral valve repair. Almost all regurgitation disappeared.
RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium.
Table 1. Pre- and postoperative thoracic radiographic and echocardiographic findings.
| Index | Case No. | Pre-operative | 1 mon | 3 mon | Recent value |
|---|---|---|---|---|---|
| VHS | 1 | 12.90 | 11.40 | 11.90 | 11.50 |
| 2 | 11.70 | 10.00 | 9.50 | 8.60 | |
| LA/Ao | 1 | 2.08 | 1.80 | 1.90 | 1.88 |
| 2 | 3.06 | 2.50 | 2.30 | 1.74 | |
| LVIDd (mm) | 1 | 38.80 | 44.50 | 47.30 | 49.86 |
| 2 | 34.80 | 26.10 | 26.90 | 23.80 | |
| LVIDs (mm) | 1 | 30.10 | 28.10 | 33.70 | 35.04 |
| 2 | 14.20 | 15.10 | 18.20 | 11.35 | |
| FS (%) | 1 | 22.30 | 36.89 | 28.71 | 29.73 |
| 2 | 59.31 | 42.29 | 32.30 | 34.43 |
VHS, vertebral heart scale; LA, left atrium; Ao, aorta; LVIDd, left ventricular end-diastolic diameter; LVIDs, left ventricular end-systolic diameter; FS, fractional shortening.
Case 2
A 10-year-old castrated male Shih Tzu dog with a weight of 4.5 kg was referred to us for mitral valve repair. The patient showed syncope and had a history of multiple pulmonary edemata in spite of receiving 0.7 mg/kg of torsemide, 1 mg/kg of spironolactone, and 0.5 mg/kg of pimobendan twice per day.
Physical examination revealed a grade 5/6 systolic murmur at the mitral valve level of the heart. Thoracic radiography revealed severe generalized cardiomegaly, with a vertebral heart scale of 11.7. Echocardiography revealed severe mitral valve prolapse with left-sided cardiac enlargement (Table 1). Based on these findings, the patient was diagnosed with mitral valve insufficiency (American College of Veterinary Internal Medicine stage D) [7].
The anesthesia protocol, CPB setting, and heparin dose for maintaining the activating clotting time were the same as those in case 1. Mitral valve repair was performed as previously described [8]. 8 Fr arterial cannula and a 14 Fr venous cannula were used for CPB, and a 4 Fr root cannula was placed for the administration of cardioplegia. A moderately thickened mitral valve, elongated chordae tendineae, and septal and mural mitral leaflet prolapse were confirmed. Five artificial chordae tendineae (GORE-TEX, CV-6; WL Gore & Associates, Inc., USA) were implanted, and semi-circular annuloplasty was performed using a Hegar dilator (16 mm) as a purse-string suture pattern (Fig. 1). The left atrium was closed and rewarming was initiated immediately. When the temperature was increased to 32°C, the ACC was terminated. Subsequently, the sinus rhythm was confirmed. The patient was hemodynamically stable after weaning from CPB. Protamine was administered intravenously for 30 min to reverse the effect of heparin.
The parameters were monitored in the same manner as in case 1. The total CPB time was 125 min, and the ACC time was 73 min. During ACC, the mean arterial pressure was 87 mmHg and the lowest temperature was 26.7°C. The lowest bypass flow rate was 63 mL/kg/min, and the highest flow rate was 155 mL/kg/min.
The dog recovered uneventfully. No cardiac murmur was heard immediately after surgery. However, acute anuria due to urethral calculus was detected 6 days after mitral valve repair. Emergency surgery was performed successfully. The patient was discharged 2 weeks after the second surgery, and there were no heart-induced clinical signs. Post-operative medications, 0.5 mg/kg of rivaroxaban once daily, 0.3 mg/kg of pimobendane, and 0.5 mg/kg of furosemide (Laxis tablet 40 mg; Handdok Pharmaceutical Corp., Korea) twice daily, were maintained until 3 months after surgery. After 3 months, all drugs except 0.25 mg/kg of pimobendane were stopped and the patient’s body weight increased to 6.7 kg. On radiography and echocardiography, no more mitral regurgitation has shown (Fig. 2) and the vertebral heart scale decreased from 11.7 to 9.5 at 3 months after surgery. Echocardiography revealed a reduced mitral valve annulus from 25.7 to 16.9 mm and reduced LA/Ao from 3.06 to 2.10. Recent follow-up (POD 530) has shown values of advanced reverse cardiac remodeling with no mitral regurgitation (Table 1).
DISCUSSION
In human medicine, a multitude of cardioplegic systems and solutions have been reported for adequate myocardial protection during cardiac surgery. However, a consensus regarding the use of cardioplegia is still lacking in both human and veterinary medicine [2]. Most cardioplegia solutions used in veterinary medicine are multidose ST2 cardioplegia [4].
Both DNc and ST2 cardioplegia are potassium-rich solutions that provide rapid depolarized arrest and reliable recovery [5]. During depolarized arrest, intracellular accumulation of sodium and calcium increases, resulting in a higher risk of reperfusion injury [9].
DNc’s composition, which contain lidocaine and mannitol, can prevent risk during cardiac surgery and reperfusion [1]. Lidocaine blocks fast sodium channels in non-nodal cardiomyocytes [9]. It increases the refractory period during depolarized arrest and prevents sodium inflow into the intracellular space [10] Calcium influx then decreases because of the deactivation of the sodium-calcium exchanger due to low levels of sodium within the cell [4,9]. Mannitol, an osmotic diuretic, has been shown to reduce free radical scavenging, which causes intracellular enzymatic effects and myocardial injury during reperfusion. In addition, mannitol reduces myocardial cell swelling and prevents post-ischemic myocardial impairment [5].
The administration of DNc with oxygenated blood supports aerobic metabolism and provides buffering properties to help correct inner cell acidosis [11]. Previous studies have shown that blood cardioplegia improves coronary perfusion and preserves myocardial metabolism and function [5]. Additionally, the solution buffered by blood may be important for avoiding the calcium paradox [12].
For successful clinical outcomes, the aortic cross-clamp time must be as short as possible [13]. However, multidose cardioplegias have a short duration and should be re-administered. This practical disadvantage leads to prolonged aortic cross clamping. According to a previous study, the use of DNc significantly decreased CPB and aortic cross-clamp duration [14]. Another study showed that the volume of cardioplegia was significantly lower in patients who received DNc, with low defibrillation rates [15]. Therefore, considering the hemodilution of patients and their benefits, we decided to use DNc.
The limitations of this report are the absence of clinical blood test markers for cardiac damage and the small number of cases. In a previous human study, the troponin I response was compared between DNc and ST cardioplegia in pediatric patients. The benefits of DNc were presented in comparison to preventing cardiac index and decreasing morbidity [14].
This case report describes the first use of DNc in veterinary medicine owing to its clinical features presenting a successful outcome. In conclusion, considering the size of the dogs and the duration of the surgery, using single-dose cardioplegia during cardiac surgery in veterinary medicine can have practical benefits by reducing aortic cross-clamp time and improving clinical outcomes. Future studies including a larger number of patients and long-term follow-up periods with clinical measurements are necessary.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.co.kr) for the English language editing.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1C1C1010464).
Conflict of Interest: The authors declare no conflicts of interest and there was no financial support by the manufacturer of the products used in the case report.
- Conceptualization: Kim MH, Yu JH, Kim DH.
- Data curation: Lee WJ, Kim KM, Kim WJ.
- Formal analysis: Lee WJ, Yoon WK, Kim DH.
- Funding acquisition: Kim DH.
- Investigation: Lee WJ, Moon CH, Yoon WK.
- Supervision: Yu JH, Kim DH.
- Writing - original draft: Lee WJ, Moon CH.
- Writing - review & editing: Lee H, Jeong SM, Kim DH.
References
- 1.Misra S, Srinivasan A, Jena SS, Bellapukonda S. Myocardial protection in adult cardiac surgery with del Nido versus blood cardioplegia: a systemic review and meta-analysis. Heart Lung Circ. 2021;30(5):642–655. doi: 10.1016/j.hlc.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Ad N, Holmes SD, Massimiano PS, Rongione AJ, Fornaresio LM, Fitzgerald D. The use of del Nido cardioplegia in adult cardiac surgery: a prospective randomized trial. J Thorac Cardiovasc Surg. 2018;155(3):1011–1018. doi: 10.1016/j.jtcvs.2017.09.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George G, Varsha AV, Philip MA, Vithayathil R, Srinivasan D, Sneha Princy FX, et al. Myocardial protection in cardiac surgery: del Nido versus blood cardioplegia. Ann Card Anaesth. 2020;23(4):477–484. doi: 10.4103/aca.ACA_153_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali JM, Miles LF, Abu-Omar Y, Galhardo C, Falter F. Global cardioplegia practices: Results from the global cardiopulmonary bypass survey. J Extra Corpor Technol. 2018;50(2):83–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol. 2012;44(3):98–103. [PMC free article] [PubMed] [Google Scholar]
- 6.Haider A, Khwaja IA, Khan AH, Yousaf MS, Zaneb H, Qureshi AB, et al. Efficacy of whole-blood del Nido cardioplegia compared with diluted del Nido cardioplegia in coronary artery bypass grafting: a retrospective monocentric analysis of Pakistan. Medicina (Kaunas) 2021;57(9):918. doi: 10.3390/medicina57090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keene BW, Atkins CE, Bonagura JD, Fox PR, Häggström J, Fuentes VL, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33(3):1127–1140. doi: 10.1111/jvim.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WJ, Kim J, Moon CH, Eom T, Son D, Lee S, et al. Successful mitral repair in dogs by mitral annuloplasty using Hegar dilator: two case reports. J Vet Sci. 2022;23(1):e11. doi: 10.4142/jvs.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson GP, Faggian G, Onorati F, Vinten-Johansen J. Hyperkalemic cardioplegia for adult and pediatric surgery: end of an era? Front Physiol. 2013;4(4):228. doi: 10.3389/fphys.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ucak HA, Ucak D. Single-dose del Nido cardioplegia vs. blood cardioplegia in aortic valve replacement surgery. Rev Bras Cir Cardiovasc. 2021;36(2):229–236. doi: 10.21470/1678-9741-2020-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao M, Morita K, Shinohara G, Kunihara T. Modified del Nido cardioplegia and its evaluation in a piglet model. Semin Thorac Cardiovasc Surg. 2021;33(1):84–92. doi: 10.1053/j.semtcvs.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Charette K, Gerrah R, Quaegebeur J, Chen J, Riley D, Mongero L, et al. Single dose myocardial protection technique utilizing del Nido cardioplegia solution during congenital heart surgery procedures. Perfusion. 2012;27(2):98–103. doi: 10.1177/0267659111424788. [DOI] [PubMed] [Google Scholar]
- 13.D’Angelo AM, Nemeth S, Wang C, Kossar AP, Takeda K, Takayama H, et al. Re-dosing of del Nido cardioplegia in adult cardiac surgery requiring prolonged aortic cross-clamp. Interact Cardiovasc Thorac Surg. 2022;34(4):556–563. doi: 10.1093/icvts/ivab310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talwar S, Bhoje A, Sreenivas V, Makhija N, Aarav S, Choudhary SK, et al. Comparison of del Nido and St Thomas cardioplegia solutions in pediatric patients: a prospective randomized clinical trial. Semin Thorac Cardiovasc Surg. 2017;29(3):366–374. doi: 10.1053/j.semtcvs.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Ler A, Sazzad F, Ong GS, Kofidis T. Comparison of outcomes of the use of del Nido and St. Thomas cardioplegia in adult and paediatric cardiac surgery: a systematic review and meta-analysis. Perfusion. 2020;35(8):724–735. doi: 10.1177/0267659120919350. [DOI] [PubMed] [Google Scholar]