Abstract
Background
Antibiotic resistance is a significant public health concern around the globe. Antimicrobial peptides exhibit broad-spectrum and efficient antibacterial activity with an added advantage of low drug resistance. The higher water content and 3D network structure of the hydrogels are beneficial for maintaining antimicrobial peptide activity and help to prevent degradation. The antimicrobial peptide released from hydrogels also hasten the local wound healing by promoting epithelial tissue regeneration and granulation tissue formation.
Objective
This study aimed at developing sodium alginate based hydrogel loaded with a novel antimicrobial peptide Chol-37(F34-R) and to investigate the characteristics in vitro and in vivo as an alternative antibacterial wound dressing to treat infectious wounds.
Methods
Hydrogels were developed and optimized by varying the concentrations of crosslinkers and subjected to various characterization tests like cross-sectional morphology, swelling index, percent water contents, water retention ratio, drug release and antibacterial activity in vitro, and Pseudomonas aeruginosa infected wound mice model in vivo.
Results
The results indicated that the hydrogel C proved superior in terms of cross-sectional morphology having uniformly sized interconnected pores, a good swelling index, with the capacity to retain a higher quantity of water. Furthermore, the optimized hydrogel has been found to exert a significant antimicrobial activity against bacteria and was also found to prevent bacterial infiltration into the wound site due to forming an impermeable barrier between the wound bed and external environment. The optimized hydrogel was found to significantly hasten skin regeneration in animal models when compared to other treatments in addition to strong inhibitory effect on the release of pro-inflammatory cytokines (interleukin-1β and tumor necrosis factor-α).
Conclusions
Our results suggest that sodium alginate -based hydrogels loaded with Chol-37(F34-R) hold the potential to be used as an alternative to conventional antibiotics in treating infectious skin wounds.
Keywords: Cathelicidin, antibacterial agent, dressings, trauma, skin
INTRODUCTION
The skin is an excellent barrier that protects the body from bacterial invasion, but it is also the more prone to damage being largest human organ. Wound infection with bacteria, in particular, not only inhibits wound healing but can also develop into sepsis, which poses a serious threat to human health [1]. Pseudomonas aeruginosa is a conditional pathogenic bacterium in human and animal with immunocompromised, extensive burns, surgical trauma, and long-term use of immunosuppressive agents. It causes several acute and chronic infections such as ventilator-associated pneumonia, meningitis, abscess, infections of skin and soft tissues, bacteraemia corneal infections [2]. The detection rate of the bacterium in surgical trauma infection is the highest. Since their discovery in 20th century, antibiotics had been widely used in human and animal ailments caused by susceptible bacteria [3]. Due to the abusive use of antibiotics, drug-resistant bacterial infections may resurface and hence endanger public health [4]. Antibiotic resistance of P. aeruginosa is 10% of all hospital-acquired infections worldwide, resulting in high morbidity and mortality [5]. The development of new antimicrobials with potent antibacterial activity and less resistance to replace conventional antibiotics is the key problem to be solved. The antimicrobial peptides (AMPs) are small molecular metabolites with a broad-spectrum antibacterial activity and play an essential role in the innate immune defensive system [6]. AMPs have low drug resistance and high potential for development and can be widely used as alternative antibiotic medicine in food, medicine, and animals [7].
Porcine myeloid antimicrobial peptides (PMAPs) are the cathelicidin family of AMPs derived from porcine bone marrow cells with stable α-helix structures and have been reported to possess high antibacterial, antifungal, antitumor, and antiparasitic activity [8]. PMAPs hold enormous promise to develop stable, safe, and effective antimicrobial drugs. In our previous study, Chol-37(F34-R), a novel AMP synthesized using PMAP-37 as a template, demonstrated higher antibacterial activity, effective anti-biofilm activity, low toxicity, and no hemolytic activity [9]. Furthermore, Chol-37(F34-R) can promote wound healing and abscess reduction in animal trauma and ameliorate pathological injuries in the peritonitis model of Staphylococcus aureus ATCC 25923 infections [9]. Most AMPs are sensitive to the complex microbial environment at the wound, and were degraded quickly after topical application, reducing their antibacterial activity and wound healing effects [10]. Thus, it is necessary to develop a topically applied dosage form that can improve stability and continuous delivery of AMPs, allowing the drug to exert antibacterial and wound healing effects for a more extended period.
An effective wound dressing should have high swelling, high water retention, and a significant antibacterial activity. In addition, it shall help clear wound debris, provide a moist environment at wound site, exert the bactericidal effect, and prevent bacterial infiltration from the external environment, which are detrimental attributes required of a wound healing platform [11]. Hydrogels are 3D polymeric cross-linked networks that have the inherent ability to retain a higher water content without diminishing the structural integrity. Both natural and synthetic polymers can be used for hydrogels fabrication, but the polymer should be biocompatible, non-immunogenic, biodegradable, no toxicity, and bears less production cost. The hydrogels can swell when in contact with water without getting dissolved, a property similar to that found in biological tissues, making them ideal platforms to be used for drug delivery and wound dressings [12]. Antibacterial agents have been included in many biomaterials, such as SA, gelatin, hyaluronic acid, cellulose, chitosan, polyethylene glycol, polyvinyl alcohol, and others. So far, several wound dressings have been produced [13]. The higher water content and 3D network structure are deemed favorable to maintain AMPs activity and help prevent the degradation and degeneration [14]. The AMPs released from hydrogels also hasten the local wound healing by promoting epithelial tissue regeneration and granulation tissue formation [15].
Sodium alginate (SA) is a natural polysaccharide polymer readily undergoes cross linking through the carboxyl moieties when exposed to Ca2+ and other divalent cations and form hydrogel under simple and mild conditions. SA had been widely employed in the field of biology, medicine, and chemistry due to the good biocompatibility, low cost of acquisition, and degradability [16]. Calcium chloride has been used as a calcium donor for Ca2+-donating compound in most traditional SA hydrogels, which leads to the formation of non-homogenous 3D structure due to the rapid cross-linking reaction [17]. In contrast, CaCO3 as a calcium donor for Ca2+-donating compound not only ensures gentle release of Ca2+ by regulating the pH of the solution with water-soluble gluconolactone (GDL), but also generates a stable, consistent, and well cross linked structure with SA. To the best of our knowledge, no studies have investigated the developing and optimizing an antibacterial hydrogel by combining Chol-37(F34-R) as an antibacterial drug and SA hydrogel as a drug carrier. Thus, this study investigated the basic properties and antibacterial activity of the SA based-hydrogels loaded with Chol-37(F34-R) and the therapeutic efficiency in a wound model of P. aeruginosa GIM1.551 infection.
MATERIALS AND METHODS
Materials
Chol-37(F34-R) (≥ 95%, Shanghai Apeptide Biological Technology Co., Ltd, China), Pasteurella suis PM-29, S. aureus ATCC 25923, Staphylococcus epidermidis LY8, and P. aeruginosa GIM1.551 were obtained from our laboratory were used. Formaldehyde (≥ 95%), agar (≥ 99%), serum (≥ 99%), SA (≥ 99%), CaCO3 (≥ 99%), GDL (≥ 95%), deionized water (≥ 99%), compound polymyxin B ointment (CPBO, ≥ 95%), ceftiofur sodium (CS, ≥ 98%), Specific pathogen-free BALB/c mice (Henan Province Experimental Animal Center).
Hydrogel formation and loading of AMPs
SA hydrogel synthesis
SA hydrogel was produced using the in-situ release method [18]. In-situ release method generally employs CaCO3 with GDL as calcium source, and GDL controls the release of calcium ions. GDL would slowly release H+ during the process of dissolution, and H+ could decompose CaCO3 to release calcium ions, avoiding the gel inhomogeneity caused by the rapid reaction of calcium alginate. An accurately weighed 0.05 g of SA was thoroughly mixed with 3 mL of deionized water to make an aqueous solution of SA. An appropriate amount of CaCO3 was added to 1 mL of deionized water and mixed to produce a CaCO3 suspension. Similarly, an appropriate amount of GDL was added to 1 mL of deionized water separately and thoroughly mixed to obtain GDL solution. All solutions were then thoroughly mixed for 30 sec with a vortex mixer, and allowed to form a gel under ambient conditions. Three different hydrogels were prepared keeping SA concentration to 1 wt.%, while the molar ratio of Ca2+ to -COOH (f) and the molar ratio of GDL to Ca2+ (n) of the three hydrogels are as follows: Hydrogel A: f = 0.4, n = 0.8; Hydrogel B: f = 0.3, n = 0.8; Hydrogel C: f = 0.4, n = 0.4.
Preparation of SA hydrogel with AMPs
The preparation and application of antibacterial hydrogel are shown in Fig. 1. AMPs are loaded into SA hydrogel by the in-situ loading method [19]. In-situ loading means that a drug is co mixed with a drug delivery formulation at the time of its preparation into a formulation that is a solid or semi-solid depot, to which the drug is loaded. Briefly, the appropriate amount of AMPs was weighed, and the concentration was adjusted to 200 μg/mL. An accurately weighed 0.05 g of SA was thoroughly mixed with 3 mL of AMPs solution to make a solution of SA-AMPs. An appropriate amount of CaCO3 was added to 1 mL of AMPs solution and mixed to produce a suspension of CaCO3-AMPs. Similarly, an appropriate amount of GDL was added to another 1 mL of AMPs solution separately and thoroughly mixed to produce a solution of GDL-AMPs. All solutions were then thoroughly mixed for 30 s with a vortex mixer, and allowed to form a gel under ambient conditions, where SA hydrogel and AMPs encapsulation are concurrently completed. During the procedure, a fixed concentration of AMPs (200 μg/mL) and SA (1 wt.%) were used to prepare the AMPs hydrogel.
Fig. 1. Schematic representation of preparation and application of antibacterial hydrogel. SA, CaCO3, and GDL were mixed in antimicrobial peptides solution to prepare antibacterial hydrogel, which was then applied to the wound infected with bacteria. Both skin tissue and serum were collected after 10 days of hydrogel treatment. Anti-inflammatory analysis refers to the determination of expression levels of IL-1β and tumor necrosis factor-α in serum by enzyme-linked immunosorbent assay.
SA, sodium alginate; CaCO3, calcium carbonate; GDL, glucono-δ-lactone.
Morphology of hydrogel
The internal morphology of developed hydrogels was examined using scanning electron microscopy (SEM; Hitachi Regulus8100, Japan) with a voltage of 3 kV and a resolution of 1.1 nm [20]. Hydrogels were placed in deionized water at room temperature until swelling equilibrium was reached, followed by freeze-drying. The freeze-dried samples were then sliced, affixed to a specimen holder, and sputter-coated with gold. The prepared samples were then viewed with SEM for cross-sectional morphology and photographed.
Analysis of basic properties of hydrogels
Swelling index
The swelling index of hydrogels varies with the change in quality of the hydrogel over time [21]. The initial weight (W0) of developed hydrogels was determined and recorded at room temperature followed by immersion hydrogels in deionized water for 2 h, and the final weight (Wt) was each hydrogel sample was recorded at a time when the weight no longer changes, showing that the swelling equilibrium has been reached. The surface moisture was gently removed by tapping with an absorbent paper, and the final weight (Wt) of the hydrogel was then recorded. The swelling ratio of the hydrogel is computed using the following formula:
Water content
The percent water contents were determined by employing same method as described for swelling index, with only difference in the equation used for its calculation. The water content was calculated as follows [22]:
Water retention ratio
For calculation of water retention ratio, the hydrogels were placed in deionized water to reach the swelling equilibrium and removed. After the adhered moisture from hydrogels were removed by tapping with an absorbent paper, the initial weight W0 of the hydrogels was recorded. The hydrogels were then placed at room temperature under 35% relative humidity, and the final weight (Wt) was measured every 2 h. The following formula was used to compute the water retention ratio of hydrogel [23]:
In vitro AMPs release profile
AMPs release ratio from hydrogels was calculated using a previously published method with slight modifications [24]. Briefly, drug loaded hydrogels were introduced into a 30 mL deionized water. Sample aliquots of 3 mL were withdrawn at regular time intervals of 2, 4, 6, 8, 12, 14 and 24 h. An equal volume of deionized water was added at each step of sample withdrawn to keep the release medium volume constant throughout the experiment. AMPs concentration released as a function of time was measured using an ultravioletray spectrophotometer (MAPADA UV-1800PC, China) at λmax 257 nm. The concentration of AMPs is calculated by comparing optical density of the samples with a standard curve (Supplementary Fig. 1). AMPs concentration released thus determined was plotted as cumulative drug release vs. time.
Antibacterial activity of hydrogel-AMPs
The in vitro antibacterial activity of developed hydrogels was determined by Kirby-Bauer diffusion [25]. P. aeruginosa GIM1.551, S. aureus ATCC 25923, P. suis PM-29, and S. epidermidis LY8 were cultured for 12 h at 37°C in Tryptic Soy Broth (TSB; Qingdao Hope Bio-Technology Co., Ltd., China) liquid medium, the concentration of the bacterial suspension was further adjusted to 0.5 McFarland turbidity, and then 200 μL of bacterial suspension was uniformly transferred to the TSB solid medium. The antibacterial hydrogel containing 10 µg Chol-37(F34-R) was affixed to the TSB solid medium containing bacterial culture and incubated again at 37°C for 18 h in an incubator. Following incubation, the zones of inhibition were then calculated by measuring the diameter of each clear zone around the hydrogel.
Sustained release properties of antibacterial hydrogel
The drug sustained-release of the antibacterial hydrogel was examined using a previously described method [26]. S. aureus ATCC 25923 was cultured overnight in an oscillating incubator at 37°C and 150 rpm. The concentration of the bacterial suspension was further adjusted to 0.5 McFarland turbidity. The surface of TSB solid medium was uniformly smeared with 200 µL of bacterial suspension, followed by placing the hydrogel containing Chol-37(F34-R) on the surface of TSB solid medium. The hydrogel was placed on a new TSB solid medium every 12 h, and inhibition zone diameters were recorded after 12 h of incubation. The diameter of inhibition zone at a particular time was used to assess the drug sustained release rate of the antibacterial hydrogel.
Antibacterial barrier activity of hydrogel
The bacterial barrier activity of the hydrogel was determined using a previously described method [27]. Briefly, P. aeruginosa GIM1.551 was cultured overnight in a shaking incubator at 37°C operated at 150 rpm. The hydrogel (without AMPs, the same below) was first sterilized using ultraviolet light and then sliced into discs (10 mm in diameter, 1.2 mm thickness) and placed on TSB solid medium. Following diluting the bacterial suspension to 1 × 109 CFU/mL, 50 µL of the bacterial suspension was placed in the middle of the hydrogel sample carefully and uniformly distributed across the surface of the hydrogel. The blank control group consisted of the bare surface of TSB solid medium without the hydrogel. After incubating at 37°C for 3, 6, and 12 h, the hydrogel discs in plates were removed, and the plates were cultured for another 12 h under the same conditions. Finally, the bacteria colony formation was detected.
In vivo evaluation of infected wound healing
An in vivo infected wound mice model was developed using P. aeruginosa GIM1.551 [9]. Specific pathogen-free BALB/c mice (4–6 weeks, 20 ± 3 g, with equal numbers of males and females) were housed in College of Animal Science and Technology, Henan University of Science and Technology. Animal management and experimental procedures were approved by the Institutional Animal Care and Use Committee (20211015002). All animals were humanely handled. Mice were randomly divided into 6 groups: healthy, blank control, hydrogel, Chol-37(F34-R), hydrogel comprising Chol-37(F34-R), and CPBO. Mice in the healthy group did not create wounds nor were treated. The dorsal areas of mice in the remaining groups were shaved with a blade and cleaned with 75% ethanol. A 1.5 cm diameter circular wound was created on the upper back of the mice by knife cutting, and the wound was infected with 6.5 × 1010 CFU/mL P. aeruginosa GIM1.551. The route of bacterial inoculation was wound daubing inoculation. The wounds of the blank control group mice were only infected with bacteria but were not subjected to drug treatment, and the wounds were wrapped using sterile medical gauze as a control. The initial therapy was administered at 2 h after infection, and the wounds are covered with hydrogel once a day for 10 days. The hydrogels were mounted on the injury site using an elastic adhesive bandage. The wound size was photographed each day, and the condition was monitored until the wound healed. The mice were euthanized with 120 mg/kg intraperitoneal sodium pentobarbitalmice after 10 days of treatment, and the wound healing sites and skin tissues of the mice were collected for histopathological analysis. Histopathological scoring of mouse tissue was carried out as previously described [28]. Furthermore, 1 mL of blood was withdrawn from each mouse and then allowed to stand for 2 h. After centrifuge for 15 min at 1,000 rpm, the expression levels of interleukin (IL)-1β and tumor necrosis factor (TNF)-α were analyzed using enzyme-linked immunosorbent assay (ELISA) Kit (Wuhan Myhalic Biotechnological Co., Ltd., China).
Statistical analysis
All statistical analyses were performed by using GraphPad Prism 8 software (GraphPad Software, USA). All results were given as mean ± standard deviation values. Statistical analyses were performed using one-way ANOVA F-statistics or unpaired t-tests, and differences were considered significant at p < 0.05 or p < 0.01.
RESULTS
Hydrogel formation and loading of AMPs
All formulations successfully formed gel like structure as shown in Fig. 2A. However, the hydrogels A and B failed to form a uniform gel like structure, having partial transparent liquid above the test tube owing to reduced cross linking activity. In contrast, the physical appearance of hydrogel C was stable, with a smooth and elastic surface. The microstructural properties of hydrogels are vital to their performance. Uniform hydrogel pore structures provide optimal air permeability and aid in releasing loading drugs in hydrogel. As depicted in Fig. 2B, hydrogel A had a flaky connection structure with no pores, while there are no pores in hydrogel B as well showing a cotton-like inner texture. On the other hand, hydrogels C has a uniform three-dimensional pore size with linked pore structures, excellent physical form, and the most uniform internal pores.
Fig. 2. The physical appearance (A) and SEM images (B) of hydrogels. Sodium alginate solution without Ca2+ is clear and does not form (a). There was a small amount of clarified liquid above hydrogel A (b) and hydrogel B (c), which did not react completely. The hydrogel C (d) reacted completely. SEM results showed the internal structure of the three kinds of hydrogels. Among them, hydrogel C showed a homogeneously porous internal structure. Scale bar: (B) 1 µm.
Analysis of basic properties of hydrogels
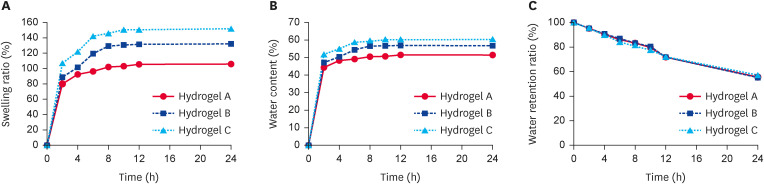
The results of basic properties analysis of the hydrogels are shown in Fig. 3. The hydrogel C was found to exhibit the highest swelling index compared to other formulations, where reached to 106.99% in the first 2 h, where the swelling equilibrium established within 10 h with final swelling index recorded to be 151.53% (Fig. 3A). As the hydrogel swell, the water content also steadily rises, which were found to be 60.24% after 24 h in case of hydrogel C formulation (Fig. 3B). Since ordinary gauze bandages cannot prevent wound getting dried out by the uncontrolled and excessive loss of transepidermal water, which further delays the wound healing process. As displayed in Fig. 3C, the water retention performance of all hydrogel formulations was similarly maintained at about 60% after 24 h.
Fig. 3. Basic properties of hydrogels. (A) Swelling ratio of hydrogels. (B) Water content of hydrogels. (C) Water retention ratio of hydrogels.
In vitro AMPs release profile
The in vitro drug release profile of AMPs from three different hydrogels is shown in Fig. 4. All formulations showed a multistage release pattern, where the hydrogel B failed to sustain the drug release with only 2 h accounting for roughly 60% of the overall release. The release rate was too fast to create a better-controlled release impact. Although the release ratio of AMPs in hydrogel A was slow, the final release ratio was relatively low, and AMPs release were more diminutive. AMPs in hydrogel C were continually released at an almost constant rate between 4 and 8 h. After 8 h, the release rate slowed down. The cumulative drug release for hydrogel C was found to be 71.15% in 24 h. The antibacterial hydrogel fabricated from hydrogel C not only exhibited sustained release of AMPs, but the final release amount was also higher.
Fig. 4. In vitro antimicrobial peptides release profile. The curve of Chol-37(F34-R) released from hydrogels over time.
By comparing the physical form, internal morphology, swelling ratio, water content, water retention ratio, and release ratio of the three hydrogels, all properties of hydrogel C were superior. Therefore, hydrogel C was selected for subsequent analysis.
Antibacterial activity of hydrogel-AMPs
Table 1 showed the experimental results, and Supplementary Fig. 2 displayed the inhibition zones. The results showed that the blank hydrogel failed to produce the inhibition zone, indicating that there is no bacteriostatic effect. Compared with the blank hydrogel group, the Chol-37(F34-R) hydrogel group exhibited broad-spectrum antibacterial effects against P. suis PM-29, S. aureus ATCC 25923, S. epidermidis LY8, and P. aeruginosa GIM1.551. The antibacterial hydrogel group containing Chol-37(F34-R) demonstrated a more substantial antibacterial effect when compared to the blank hydrogel group. Furthermore, there was no significant difference in antimicrobial activity between the antibacterial hydrogel group containing Chol-37(F34-R) and the Chol-37(F34-R) alone group. This demonstrates that Chol-37(F34-R) antibacterial activity was not hindered by the hydrogel structure and was continuously released. In addition, the shelf life of the antibacterial hydrogel was determined by using Kirby-Bauer diffusion method employing S. aureus ATCC 25923 as the test bacteria. The antibacterial activity of developed hydrogel can be maintained for approximately 40 days under storage conditions of 4°C (Supplementary Fig. 3), but only for approximately 8 days under storage conditions of room temperature (Supplementary Fig. 4), indicating that the prepared hydrogels are best suited to be stored at low temperatures to keep the activity for extended period.
Table 1. Antibacterial activity of Chol-37(F34-R) and Chol-37(F34-R) hydrogel against pathogenic bacteria evaluated by agar diffusion method.
| Bacteria strain | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| A | B | C | D | |
| P. aeruginosa GIM1.551 | 28 ± 0.9** | 24 ± 1.2NS | 19 ± 0.4 | - |
| S. aureus ATCC 25923 | 39 ± 1.1** | 36 ± 0.8NS | 25 ± 0.6 | - |
| P. suis PM-29 | 45 ± 0.7* | 42 ± 0.9NS | 40 ± 1.3 | - |
| S. epidermidis LY8 | 12 ± 0.8** | 10 ± 0.6NS | 20 ± 0.5 | - |
Four different bacteria were collected to detect antibacterial activity of antibacterial hydrogel. The experimental groups are as follows: A indicates Chol-37(F34-R); B indicates antibacterial hydrogel containing Chol-37(F34-R); C indicates ceftiofur sodium (CS); D indicates blank hydrogel; - indicates no antibacterial activity; *p < 0.05 or **p < 0.01 indicates significant difference in A compared to C; NS indicates nonsignificant difference in B compared to A.
Drug sustained release properties of hydrogel-AMPs
The consistent release of drugs is vital in clinical applications. It can improve drug absorption and bioavailability in the host and drug stability and efficacy through sustained drug release [20]. In this study, S. aureus ATCC 25923 was employed as a test bacterium in Kirby-Bauer diffusion experiments to examine the hydrogel's sustained release function. As depicted in Fig. 5, the inhibition zone of the developed hydrogel against S. aureus ATCC 25923 remained wide after 24 h, indicating that the hydrogel has a sustained antibacterial effect. Since hydrogels release the drug over time, therefore, with passage of time, the zone of inhibition also started to reduce in diameter with no visible zone of inhibition observed until 48 h, indicating that Chol-37(F34-R) from hydrogel has been completely release and the release process has also ended. These results affirmed the conclusion that the antimicrobial hydrogel had a drug sustained-release effect by hindering the rapid release of the drug. Sustained drug release helps prolong the drug action thereby reducing the dosage frequency in clinical applications, which not only ensures maintaining higher drug levels at wound site but also improve patient compliance.
Fig. 5. Drug sustained release properties of antibacterial hydrogel. The drug sustained release properties of antibacterial hydrogel were investigated by Kirby-Bauer diffusion experiments. Until 48 h, the inhibition zone no longer appeared, indicating that Chol-37(F34-R) in the hydrogel was no longer released.
Antibacterial barrier activity of hydrogel
The results of barrier formation by the hydrogels are demonstrated in Fig. 6. The positive control group had a large amount of colony formation of P. aeruginosa GIM1.551. In contrast, when culture plates were covered with antimicrobial hydrogel, no bacterial colonies formation were observed until 3 and 6 h of incubation, but few colonies start to emerge after 12 h of incubation, depicting that SA hydrogels were able to form a protective barrier against bacterial thus can help prevent infiltration of microbes into the wound bed.
Fig. 6. Antibacterial barrier activity of Hydrogel. P. aeruginosa was coated on the hydrogel film (The diameter of the hydrogel film was 10 mm and the thickness was 1.2 mm), and compared with the control group, the colony on TSB solid medium surface covered by hydrogel film decreased significantly. Blank indicates TSB medium without any treatment.
In vivo evaluation of infected wound healing
The photographs of the animals taken during the course of treatment at day 0, day 6 and day 10 are depicted in Fig. 7. It has been observed that as the number of treatments applied increases, the wounded area of mice in each group gradually decreased. The animal group treated with Chol-37(F34-R) hydrogel showed complete healing of the wound which was more efficient in comparison to other animal groups. The skin tissues were collected and stained with H&E. The results revealed that collagen fiber fiber was formated in each treatment group and a good healing effect (Fig. 8). There were more evident with clear hierarchical structure between the epidermis and dermis in case of antimicrobial hydrogel treatment. In contrast, the samples taken from control animal group still presented varying degree of inflammation with visible inflammatory cells in the dermis. Similarly, the control group possessed fewer hair follicles and a low degree of differentiation after 10 days, while the hair follicles and degree of differentiation was improved in each treatment group. The hydrogel group contained the most hair follicles, most of which had turned spindle-shaped and were on the verge of breaking through the outer skin. Both tissue and adipose tissue were significantly thickened and healed ideally.
Fig. 7. The healing of infected wounds after treatment. Photographs of wounds treated with hydrogel, Chol-37(F34-R), antimicrobial hydrogel, and CPBO over 10 days. The control group did not receive treatment. Gel indicated that SA hydrogel and Chol-37(F34-R)-Gel indicated SA hydrogel containing Chol-37(F34-R). CPBO indicates compound polymyxin B ointment, d indicates the number of days mice were treated.
Fig. 8. H&E staining of wound sections and their histopathological scoring. A indicated recovery of skin tissues in each group after 10 days of treatment. The images are presented at a magnification of 100× or 200×. Histopathological scoring of wounded skin was shown in B. Gel indicated that SA hydrogel and Chol-37(F34-R)-Gel indicated SA hydrogel containing Chol-37(F34-R). CPBO indicates compound polymyxin B ointment.
**p < 0.01, compared with control; ##p < 0.01, compared with Chol-37 (F34-R); NS, nonsignificant difference.
The expression levels of IL-1β and TNF-α in serum are shown in Fig. 9. In the antibacterial hydrogel group, IL-1β and TNF-α values were significantly lower than those in the control group, hydrogel and AMPs groups, depicting exertion of a potent anti-inflammatory effect. In comparison to antibiotics, the value of IL-1β in mice was slightly higher, while TNF-α value was much lower than that of CPBO group.
Fig. 9. Expression level of IL-1β and TNF-α. After 10 days of treatment, the expression levels of IL-1β (A) and TNF-α (B) in serum of each group. The results are presented as mean ± standard deviation and * indicates significant difference compared with control (p < 0.05); ** indicates extremely significant difference compared with control (p < 0.01); ## indicates significant extremely difference in the control compared to the healthy (p < 0.01); n = 5.
IL, interleukin; TNF, tumor necrosis factor.
DISCUSSION
In our study, we employed an in-situ release method for preparation of hydrogel by employing CaCO3 as Ca2+ donating entity. Three hydrogels were designed based on the molar ratio (f) of Ca2+ in CaCO3 to -COOH in SA and the molar ratio (n) of GDL and Ca2+. From all formulated hydrogels following preliminary testing, the one was selected to be used as a matrix platform for formulation of antimicrobial hydrogel.
The swelling ratio of SA hydrogel is determined by the uniform porous structure and the degree of cross-linking with Ca2+ [29]. When Ca2+ levels are high, SA reacts quickly with it, causing the gel to become non-uniform, resulting in tight polymer cross-linking and smaller pores, which are unfavorable for water absorption. When there is insufficient Ca2+, the polymer cross-linking density is low, and the formed hydrogel is softer and friable. The porous structure, high swelling, and water content of the hydrogel help to absorb wound exudates without hindering the delivery of loaded drugs, thus helping to minimize the secondary damage caused by wound secretion when removing dressings, and maintaining the wet environment of the wound bed to promote healing [30]. Besides, the swelling ratio of the hydrogel is proportional to the drug release ratio [31]. For example, Das et al. [32] prepared α-tricalcium phosphate (α-TCP) with different concentrations (10:1, 2:1, and 1:10 W/W) in a 6% SA solution by ultrasonic method, forming three kinds of composite hydrogels. The release rates of bovine serum albumin (BSA), tetracycline (TCN), and dimethyloxallyl glycine (DMOG) of three hydrogels were measured. The results revealed that the release rates of BSA, TCN, and DMOG were higher in the 10:1 mixed gel. α-TCP in the 10:1 mixed gel has the lowest content, larger pore space and pore size, while the swelling ratio was maximum [32]. Findings in the present analysis are similar to those of the previous results reported by Das et al. [32]. The hydrogel C proved superior in terms of swelling ratio, water retention, and release ratio, hence hydrogel C was then selected for fabrication of antimicrobial hydrogel as novel wound healing platform.
The hydrogel had to stay on the wound for a longer duration before healing, the antibacterial ability assessment of wound dressings is significant. Wound dressings with antibacterial activity can help promote wound healing by preventing bacterial contamination [33]. Therefore, whether the hydrogels have antibacterial activity is required as wound dressings in clinical applications. The hydrogel wound dressing designed in this study used Chol-37(F34-R) as an antibacterial drug, which had a substantial antibacterial effect. This may be due to the fact that Chol-37(F34-R) could not be completely released from the hydrogel, similar to the hydrogel release ratio results in the preceding trials. By comparing the antibacterial activity of hydrogel groups and Chol-37(F34-R) group, no significant difference was observed. This depicts that despite of cross linked hydrogel network, the hydrogel itself did not significantly alter the effect of AMPs and the release of Chol-37(F34-R) from hydrogels did lose the broad-spectrum antibacterial activity. A similar antibacterial experiment was reported by Yang et al., who used chitosan hydrogel to encapsulate LL-37 [34]. The results of antibacterial experiments revealed that LL-37 and LL-37/CS hydrogel could reduce S. aureus growth by approximately 40% and 25%, respectively, similar to the results of our study [34]. Moreover, the antibacterial hydrogel demonstrated a sustained release effect on the nutrient agar plate. After 24 h, the transfer of antibacterial hydrogel onto the new agar plate still had a strong antibacterial activity. It enables sustained delivery of drugs which is envisaged to reduce the frequency of administration in practical applications. This also help maintain the higher local levels of drug at a wound site, which has great advantages as a wound infection dressing [35].
Bacterial barrier activity is an excellent method of preventing wound infection. Bacterial infection will not only exacerbate the development of wound exudate but also delay the wound healing during the recovery period. Therefore, an appropriate wound dressing should protect the wound against bacterial infection. Many hydrogel wound dressings have been reported to the antibacterial activity [36,37]. Poly (vinyl alcohol) (PVA), human-like collagen (HLC), and SA were used to prepare a multifunctional PVA/HLC/SA composite hydrogel [37]. The gelatin sponge was used as a control to test the bacterial barrier activity of PVA/HLC/SA hydrogel on S. aureus and Escherichia coli. The results indicated that PVA/HLC/SA hydrogel exhibited significantly lower bacterial penetration than the gelatin sponge. The least amount of bacterial penetration indicates a good bacterial barrier activity [37]. In our study, we prepared a SA hydrogel loaded with active moieties and assessed the bacterial barrier effect by culturing P. aeruginosa GIM1.551 on a hydrogel on nutrient agar to evaluate the wound-protective function. The results demonstrated that, compared to the control group, the colony number of the nutrient agar surface covered with hydrogel film was greatly reduced, thus exerting an excellent barrier protective effect. In addition, when the hydrogel film was removed from the nutrient agar surface, the nutrient agar was not damaged, indicating that SA hydrogel could avoid the repeated injury caused by dressing removal from the wound. Generally, bacterial colony growth was also not found on nutritional agar when the hydrogel possessed antibacterial action, while previous susceptibility assays revealed that SA hydrogel lacked antibacterial activity. In summary, the bacterial barrier activity of SA hydrogel can prevent further bacterial infection and accelerate wound healing, which has the potential to be a good wound dressing.
In this study, the antimicrobial hydrogel was applied to the infected skin wound in mice to observe the healing effect in vivo. Compared with the control group, the antimicrobial hydrogel exerted a significant wound healing effect in mice with more hair growth on the regenerated skin. The histopathological analysis of skin from the healing site also demonstrated that the skin tissue of antibacterial hydrogel group was better in appearance than other groups. Inflammation is a natural human defense mechanism mediated by releasing various inflammatory mediators and pro-inflammatory cytokines which play a pivotal role in wound healing, but excessive and prolonged inflammation is not beneficial with perspective of skin regeneration. Hence, it is envisaged that by reducing the expression levels of various pro-inflammatory cytokines may create a favorable micro-environment for wound repair [38]. In this study, pro-inflammatory cytokines IL-1β and TNF-α in the serum of mice were studied using ELISA. In the antibacterial hydrogel group, the levels of IL-1β and TNF-α were significantly different and lower than those of control group, hydrogel, and AMPs group. The continuous release of Chol-37(F34-R) from the hydrogel not only increased the bioavailability of AMPs, but also exerts bactericidal effect against P. aeruginosa GIM1.551 and hence promoted wound healing. The same effect was also reported by Wu et al. who prepared a wound dressing by immobilizing polyhexamethylene guanidine phosphate on the surface of a P(M-Arg/NIPAAm) hybrid hydrogel [26]. Compared with the control group, the wounds of mice treated with P(M-Arg/NIPAAm) hydrogel had significantly lower levels of IL-1β and TNF-α in serum. These results demonstrate that the antibacterial hydrogel containing Chol-37(F34-R) prepared in this study has great application potential in wound dressings.
In this study, an antibacterial hydrogel wound dressing was developed by employing Chol-37(F34-R) as an antibacterial agent and SA hydrogel as the matrix. The antibacterial hydrogel showed excellent swelling index, higher water content, sustained the drug release, and exerted significant antibacterial activity. The hydrogel also ensured excellent barrier forming ability on the wound bed. The antibacterial hydrogel efficiently promoted wound healing by lowering the levels of pro-inflammatory cytokines throughout the wound healing process. SA hydrogel-loaded with Chol-37(F34-R) presents a novel platform for wound healing applications with an ability to compensate for the disadvantage of instability and easy degradation of AMPs as an antibacterial agents for clinical application. Using antibacterial peptide hydrogels as wound healing platforms may open new horizons in treatment of infectious wounds.
ACKNOWLEDGEMENTS
We thank Ningbo freescience Information Technology Co., Ltd. for editing the manuscript.
Footnotes
Funding: This research was supported by the National Natural Science Foundation of China (U2004151 and 31802159) and the youth backbone teachers training program of Henan University of Science and Technology (13450009).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Liao C.
- Data curation: Shi S, Dong H, Chen X, Xu S, Song Y, Wang X, Liao C.
- Formal analysis: Dong H.
- Funding acquisition: Liao C.
- Investigation: Shi S, Dong H, Song Y, Niu M.
- Methodology: Shi S, Dong H, Chen X, Xu S, Li M, Yan Z, Wang X, Niu M.
- Project administration: Zhang M, Liao C.
- Resources: Liao C.
- Supervision: Liao C.
- Validation: Zhang M.
- Writing - original draft: Dong H, Shi SB.
- Writing - review & editing: Yan Z, Wang X, Niu M, Zhang M, Liao C.
SUPPLEMENTARY MATERIALS
A standard curve was drawn with OD values as ordinate and the standard substance concentration as abscissa. The equation for the standard curve was y = 0.00216*x + 0.00128, R2= 0.99914. The OD of the samples was measured spectrophotometrically at a 257 nm wavelength, and then the OD value of the samples was substituted into the standard curve equation to calculate the sample concentration.
Inhibition zone of bacteria by antibacterial hydrogel. Gel indicated that SA hydrogel and Chol-37(F34-R)-Gel indicated SA hydrogel containing Chol-37(F34-R). CS indicates ceftiofur sodium. The antibacterial activity of the antibacterial hydrogel was measured by agar diffusion method. Antibacterial activity was represented by the appearance of an inhibition zone, and the antibacterial hydrogel still showed high antibacterial activity. The SA hydrogel without peptide had no antibacterial activity.
The maintenance time of antibacterial activity of antibacterial hydrogel under 4°C preservation condition. S. aureus ATCC 25923 was used as test bacteria, d represents days of hydrogel preservation. The antibacterial activity maintenance time of the antibacterial hydrogel was determined by agar diffusion experiments. At intervals, the antibacterial hydrogel kept at 4°C was taken out and its antibacterial activity was determined. The results showed that its antibacterial activity could be maintained for approximately 40 days.
The maintenance time of antibacterial activity of antibacterial hydrogel under 25°C preservation condition. S. aureus ATCC 25923 was used as test bacteria, d represents days of hydrogel preservation. The antibacterial activity maintenance time of the antibacterial hydrogel was determined by agar diffusion experiments. At intervals, the antibacterial hydrogel kept at 25°C was taken out and its antibacterial activity was determined. The results showed that its antibacterial activity could be maintained for approximately 8 days.
References
- 1.Rezaei N, Hamidabadi HG, Khosravimelal S, Zahiri M, Ahovan ZA, Bojnordi MN, et al. Antimicrobial peptides-loaded smart chitosan hydrogel: release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int J Biol Macromol. 2020;164:855–862. doi: 10.1016/j.ijbiomac.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Al-Wrafy F, Brzozowska E, Górska S, Gamian A. Pathogenic factors of Pseudomonas aeruginosa - the role of biofilm in pathogenicity and as a target for phage therapy. Postepy Hig Med Dosw (Online) 2017;71:78–91. doi: 10.5604/01.3001.0010.3792. [DOI] [PubMed] [Google Scholar]
- 3.Abushaheen MA, Muzaheed, Fatani AJ, Alosaimi M, Mansy W, George M, et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6):100971. doi: 10.1016/j.disamonth.2020.100971. [DOI] [PubMed] [Google Scholar]
- 4.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat. 2015;21-22:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Rima M, Rima M, Fajloun Z, Sabatier JM, Bechinger B, Naas T. Antimicrobial peptides: a potent alternative to antibiotics. Antibiotics (Basel) 2021;10(9):1095. doi: 10.3390/antibiotics10091095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Shen T, Liu Y, Chen L, Wang C, Liao C. Porcine myeloid antimicrobial peptides: a review of the activity and latest advances. Front Vet Sci. 2021;8:664139. doi: 10.3389/fvets.2021.664139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Shen T, Liu Y, Zhou J, Shi S, Wang Y, et al. Enhancing the antibacterial activity of antimicrobial peptide PMAP-37(F34-R) by cholesterol modification. BMC Vet Res. 2020;16(1):419. doi: 10.1186/s12917-020-02630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapa RK, Diep DB, Tønnesen HH. Topical antimicrobial peptide formulations for wound healing: current developments and future prospects. Acta Biomater. 2020;103:52–67. doi: 10.1016/j.actbio.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Hlaing SP, Cao J, Hasan N, Ahn HJ, Song KW, et al. In situ hydrogel-forming/nitric oxide-releasing wound dressing for enhanced antibacterial activity and healing in mice with infected wounds. Pharmaceutics. 2019;11(10):496. doi: 10.3390/pharmaceutics11100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forero-Doria O, Polo E, Marican A, Guzmán L, Venegas B, Vijayakumar S, et al. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr Polym. 2020;242:116383. doi: 10.1016/j.carbpol.2020.116383. [DOI] [PubMed] [Google Scholar]
- 13.Yan T, Kong S, Ouyang Q, Li C, Hou T, Chen Y, et al. Chitosan-gentamicin conjugate hydrogel promoting skin scald repair. Mar Drugs. 2020;18(5):233. doi: 10.3390/md18050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza F, Zafar H, Zhu Y, Ren Y, -Ullah A, Khan AU, et al. A review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics. 2018;10(1):16. doi: 10.3390/pharmaceutics10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinstraesser L, Koehler T, Jacobsen F, Daigeler A, Goertz O, Langer S, et al. Host defense peptides in wound healing. Mol Med. 2008;14(7-8):528–537. doi: 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Guan S, Zhang K, Li J. Benlysta-loaded sodium alginate hydrogel and its selective functions in promoting skin cell growth and inhibiting inflammation. ACS Omega. 2020;5(18):10395–10400. doi: 10.1021/acsomega.0c00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salesa B, Llorens-Gámez M, Serrano-Aroca Á. Study of 1D and 2D carbon nanomaterial in alginate films. Nanomaterials (Basel) 2020;10(2):206. doi: 10.3390/nano10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Liu H, Gao Q, Liu X, Tong Z. Alginate-calcium carbonate porous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr Polym. 2008;71(3):476–480. [Google Scholar]
- 19.Babavalian H, Latifi AM, Shokrgozar MA, Bonakdar S, Mohammadi S, Moosazadeh Moghaddam M. Analysis of healing effect of alginate sulfate hydrogel dressing containing antimicrobial peptide on wound infection caused by methicillin-resistant Staphylococcus aureus. Jundishapur J Microbiol. 2015;8(9):e28320. doi: 10.5812/jjm.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong L, Liu Q, Gao Y, Jia H, Dai W, Guo L, et al. The effect of collagen hydrogels on chondrocyte behaviors through restricting the contraction of cell/hydrogel constructs. Regen Biomater. 2021;8(4):rbab030. doi: 10.1093/rb/rbab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Zhang H, Yang X, Zhang W, Jiang M, Wen T, et al. Preparation and application of quaternized chitosan-and AgNPs-base synergistic antibacterial hydrogel for burn wound healing. Molecules. 2021;26(13):4037. doi: 10.3390/molecules26134037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Xue Y, Wang J, Zhu Y, Zhu Y, Zhang X, et al. A composite hydrogel with high mechanical strength, fluorescence, and degradable behavior for bone tissue engineering. Polymers (Basel) 2019;11(7):1112. doi: 10.3390/polym11071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Călina I, Demeter M, Scărișoreanu A, Sătulu V, Mitu B. One step e-beam radiation cross-linking of quaternary hydrogels dressings based on chitosan-poly(Vinyl-pyrrolidone)-poly(ethylene glycol)-poly(acrylic acid) Int J Mol Sci. 2020;21(23):9236. doi: 10.3390/ijms21239236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin M, Wang X, Yu Z, Wang Y, Wang X, Deng M, et al. γ-PGA hydrogel loaded with cell-free fat extract promotes the healing of diabetic wounds. J Mater Chem B Mater Biol Med. 2020;8(36):8395–8404. doi: 10.1039/d0tb01190h. [DOI] [PubMed] [Google Scholar]
- 25.Pramono A, Fauzantoro A, Hidayati IR, Hygea A, Puspita OS, Muktamiroh H, et al. In vitro assay of ethanolic heat reflux extract of Nicotiana tabacum L. var virginia against nosocomial bacteria pathogen. J Phys Conf Ser. 2018;970:012021 [Google Scholar]
- 26.Wu DQ, Zhu J, Han H, Zhang JZ, Wu FF, Qin XH, et al. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: in vitro and in vivo study. Acta Biomater. 2018;65:305–316. doi: 10.1016/j.actbio.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Li L, Peng H, Zheng H, Cao S, Lv G, et al. A spray-filming self-healing hydrogel fabricated from modified sodium alginate and gelatin as a bacterial barrier. Macromol Biosci. 2020;20(2):e1900303. doi: 10.1002/mabi.201900303. [DOI] [PubMed] [Google Scholar]
- 28.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171(2):484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. doi: 10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan WS, Arulselvan P, Ng SF, Taib CN, Sarian MN, Fakurazi S. Healing effect of Vicenin-2 (VCN-2) on human dermal fibroblast (HDF) and development VCN-2 hydrocolloid film based on alginate as potential wound dressing. BioMed Res Int. 2020;2020:4730858. doi: 10.1155/2020/4730858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patra P, Rameshbabu AP, Das D, Dhara S, Panda AB, Pal S. Stimuli-responsive, biocompatible hydrogel derived from glycogen and poly(N -isopropylacrylamide) for colon targeted delivery of ornidazole and 5-amino salicylic acid. Polym Chem. 2016;7(34):5426–5435. [Google Scholar]
- 32.Das D, Bang S, Zhang S, Noh I. Bioactive molecules release and cellular responses of alginate-tricalcium phosphate particles hybrid gel. Nanomaterials (Basel) 2017;7(11):389. doi: 10.3390/nano7110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YH, Rao ZF, Liu YJ, Liu XS, Liu YF, Xu LJ, et al. Multifunctional injectable hydrogel loaded with cerium-containing bioactive glass nanoparticles for diabetic wound healing. Biomolecules. 2021;11(5):702. doi: 10.3390/biom11050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Guo JL, Han J, Si RJ, Liu PP, Zhang ZR, et al. Chitosan hydrogel encapsulated with LL-37 peptide promotes deep tissue injury healing in a mouse model. Mil Med Res. 2020;7(1):20. doi: 10.1186/s40779-020-00249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng J, Fan R, Wu H, Yao H, Yan Y, Liu J, et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2019;10(1):1604. doi: 10.1038/s41467-019-09601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Guo B, Wu H, Liang Y, Ma PX. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat Commun. 2018;9(1):2784. doi: 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H, Fan D, Duan Z, Zhu C, Fu R, Li X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater Sci Eng C. 2019;105:110118. doi: 10.1016/j.msec.2019.110118. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Xu P, Yao Z, Fang Q, Feng L, Guo R, et al. Preparation of antimicrobial hyaluronic acid/quaternized chitosan hydrogels for the promotion of seawater-immersion wound healing. Front Bioeng Biotechnol. 2019;7:360. doi: 10.3389/fbioe.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A standard curve was drawn with OD values as ordinate and the standard substance concentration as abscissa. The equation for the standard curve was y = 0.00216*x + 0.00128, R2= 0.99914. The OD of the samples was measured spectrophotometrically at a 257 nm wavelength, and then the OD value of the samples was substituted into the standard curve equation to calculate the sample concentration.
Inhibition zone of bacteria by antibacterial hydrogel. Gel indicated that SA hydrogel and Chol-37(F34-R)-Gel indicated SA hydrogel containing Chol-37(F34-R). CS indicates ceftiofur sodium. The antibacterial activity of the antibacterial hydrogel was measured by agar diffusion method. Antibacterial activity was represented by the appearance of an inhibition zone, and the antibacterial hydrogel still showed high antibacterial activity. The SA hydrogel without peptide had no antibacterial activity.
The maintenance time of antibacterial activity of antibacterial hydrogel under 4°C preservation condition. S. aureus ATCC 25923 was used as test bacteria, d represents days of hydrogel preservation. The antibacterial activity maintenance time of the antibacterial hydrogel was determined by agar diffusion experiments. At intervals, the antibacterial hydrogel kept at 4°C was taken out and its antibacterial activity was determined. The results showed that its antibacterial activity could be maintained for approximately 40 days.
The maintenance time of antibacterial activity of antibacterial hydrogel under 25°C preservation condition. S. aureus ATCC 25923 was used as test bacteria, d represents days of hydrogel preservation. The antibacterial activity maintenance time of the antibacterial hydrogel was determined by agar diffusion experiments. At intervals, the antibacterial hydrogel kept at 25°C was taken out and its antibacterial activity was determined. The results showed that its antibacterial activity could be maintained for approximately 8 days.