Abstract
Gestational diabetes mellitus (GDM) has historically been perceived as a medical complication of pregnancy that also serves as a harbinger of maternal risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent decades, a growing body of evidence has detailed additional lifelong implications that extend beyond T2DM, including an elevated risk of ultimately developing cardiovascular disease. Furthermore, the risk factors that mediate this lifetime cardiovascular risk are evident not only after delivery but are present even before the pregnancy in which GDM is first diagnosed. The concept thus emerging from these data is that the diagnosis of GDM enables the identification of women who are already on an enhanced track of cardiometabolic risk that starts early in life. Studies of the offspring of pregnancies complicated by diabetes now suggest that the earliest underpinnings of this cardiometabolic risk profile may be determined in utero and may first manifest clinically in childhood. Accordingly, from this perspective, GDM is now seen as a chronic metabolic disorder that holds implications across the life span of both mother and child.
Keywords: Diabetes, gestational; Cardiovascular diseases; Child; Life change events
Key figure
INTRODUCTION
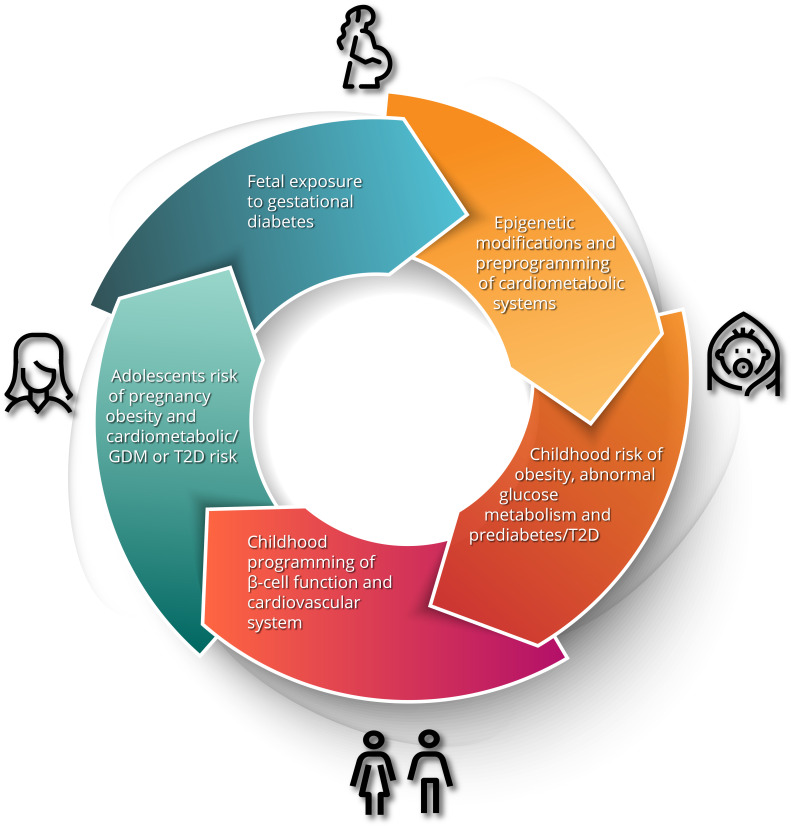
Gestational diabetes mellitus (GDM) is defined as glucose intolerance of varying severity with onset or first recognition during pregnancy. It is considered a medical complication of pregnancy that carries an increased risk of adverse obstetrical outcomes, including macrosomia, shoulder dystocia, birth injury, prematurity, perinatal mortality, and need for Caesarian section [1]. Accordingly, the screening of pregnant women for GDM and the antenatal treatment thereof has become a standard component of obstetrical care. However, the implications of GDM extend well beyond the pregnancy [2]. Most notably, it has long been known that GDM identifies women who are at risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent years, there has been growing recognition that GDM also predicts higher risks of other chronic conditions, including cardiovascular disease (CVD) [2]. Moreover, the risk factors and determinants of these conditions can be detected even before the pregnancy in which GDM is diagnosed. Indeed, the early underpinnings of these risk factors are already evident in the children of GDM pregnancies. As we shall explore in this review, the concept emerging from these data is that, although it is identified and treated in pregnancy, GDM carries implications across the life span for mother and child (Fig. 1).
Fig. 1.
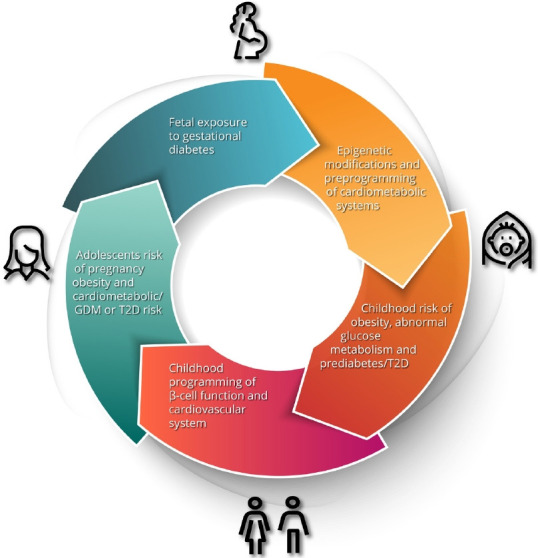
The intergenerational impact of gestational diabetes. Maternal hyperglycemia and resultant fetal hyperinsulinism results in developmental and epigenetic preprogramming of cardiometabolic risk in the offspring including obesity, dysglycemia, dyslipidemia, hypertension and early renal dysfunction. These risks progress to evident cardiometabolic abnormalities in adolescents and predispose young women to the development of gestational diabetes mellitus (GDM) in pregnancy, thereby restarting the cycle. T2D, type 2 diabetes mellitus.
MATERNAL HEALTH AFTER GDM PREGNANCY
Type 2 diabetes mellitus
Since the time of its initial description, GDM has been linked to T2DM. Indeed, in 1964, O’Sullivan and Mahan [3] reported that specific glycemic thresholds on the 3-hour 100 g oral glucose tolerance test (OGTT) in pregnancy could predict a woman’s risk of developing T2DM in the years thereafter, thereby establishing the initial diagnostic criteria for GDM. In the ensuing decades since then, studies have repeatedly demonstrated the truism that maternal glycemia in pregnancy is a predictor of future T2DM [2,4]. Of note, this relationship is evident irrespective of the diagnostic criteria that are applied for identifying GDM. When one considers that the varying sets of criteria (and their respective diagnostic thresholds) label different degrees of gestational glycemia as GDM, it becomes apparent that the relationship with future risk of T2DM must extend to the non-GDM range of glycemia (i.e., as identified by all but the least stringent GDM diagnostic criteria). For example, when compared to their normoglycemic peers, women with an abnormal OGTT that doesn’t meet the diagnosis of GDM by the stringent National Diabetes Database Criteria have an elevated risk of T2DM (albeit lesser than that of those with GDM) [5-7]. In fact, it is now recognized that the any degree of dysglycemia in pregnancy predicts a proportionate risk of progressing to T2DM in the future, with GDM representing the most severe element along both of these continua (i.e., the highest gestational glycemia and the highest risk of T2DM) [6-8]. The physiologic basis for this relationship can found in the underlying pathophysiology that links GDM and T2DM.
The pathophysiologic link in question is pancreatic β-cell dysfunction. Specifically, the progressive insulin resistance that characterizes normal human pregnancy from mid-gestation onwards poses a physiologic challenge (or stress test) for the β-cells, which must increase their secretion of insulin accordingly to maintain normoglycemia. Any insufficiency of β-cell compensation in response to this challenge will lead to dysglycemia in pregnancy, with GDM representing the most severe element thereof. Notably, women who develop GDM have a chronic β-cell defect that first becomes clinically apparent through the antenatal hyperglycemia that arises due to the insufficiency of their compensatory response [9]. Importantly, in addition to underlying the presentation of GDM in pregnancy, this β-cell defect is ultimately responsible for their development of T2DM in the future [10]. Specifically, women with GDM exhibit deterioration of β-cell function in the years after their pregnancy that is apparent even within the first year postpartum while their glucose tolerance may remain in the normal range [7,11]. Over time, however, this deterioration of β-cell function drives their progression from normal glucose tolerance to pre-diabetes and ultimately to T2DM [7,12,13]. Thus, the epidemiologic and clinical link between GDM and T2DM is rooted in a shared underlying pathophysiology of β-cell dysfunction.
Studies estimating the magnitude of risk of T2DM in women with a history of previous GDM have reported considerable variability, partly reflecting the impact of several methodologic factors. These factors include (1) between-study differences in GDM screening protocols and diagnostic criteria (thereby yielding differences in the severity of β-cell dysfunction and hence risk of T2DM); (2) variability in postpartum surveillance protocols and adherence thereto between-study populations; and (3) differences in the non-GDM control population against which women with GDM were compared. When considered collectively (albeit in the context of these limitations), this literature reveals that women with a history of GDM have a 7- to 10-fold higher risk of T2DM than that of their peers [14,15], reflecting an enormous risk increment for a major chronic disease in a population of young women of reproductive age.
Advanced complications associated with T2DM
The relative youth of this high-risk patient population raises the possibility of developing T2DM at a comparatively early age, thereby resulting in longer exposure to the metabolic dysregulation of diabetes over the course of a lifetime. Since cumulative glycemic exposure is a determinant of risk for the vascular complications of T2DM, it is reasonable to anticipate that the incidence of such outcomes may be increased in women with previous GDM [16,17]. Indeed, consistent with this hypothesis, women with a history of GDM have an elevated risk of advanced retinopathy outcomes that is dependent upon the intercurrent development of diabetes [18]. Similarly, this patient population has higher incidence rates of advanced nephropathy (including initiation of dialysis) and hospitalization for foot infection (foot ulcer, lower extremity cellulitis, or lower extremity osteomyelitis, suggestive of neuropathy and/or peripheral vascular disease), with both risks dependent upon intercurrent T2DM [18]. While women with previous GDM do not appear to be at risk of these advanced complications in the absence of T2DM, increased estimated glomerular filtration rate has been reported in this patient population, potentially indicative of early glomerular hyperfiltration and associated renal dysfunction [19]. Similarly, increased microalbuminuria has been demonstrated in women with previous GDM upon progression to pre-diabetes [20], suggesting that renal changes and worsening glucose tolerance may be concomitant processes early in their respective natural histories.
A similar longitudinal relationship over time is evident between GDM and liver disease. Specifically, the presence of liver fat on abdominal ultrasound in early pregnancy has been shown to predict subsequent dysglycemia in 2nd trimester [21]. When compared to their peers in the years after delivery, women with previous GDM show higher rates of fatty liver, the greater severity of which is associated with glucose intolerance [22-25]. Ultimately, women with a history of GDM have an elevated long-term risk of advanced liver disease (hospitalization for cirrhosis, liver failure, or transplantation) that emerges only in those who progress to T2DM in the intervening years [26]. Thus, taken together, the risks of advanced ophthalmologic, nephropathic and hepatic complications in women with a history of GDM appear to be dependent upon the development of T2DM (Table 1), with both renal and liver dysfunction showing evidence of long-term progression of disease processes over time.
Table 1.
Role of intercurrent T2DM in determining the risks of advanced complications in women with a history of gestational diabetes mellitus
| Body site or system | Advanced complications | Is risk fully dependent upon progression to T2DM? | Reference |
|---|---|---|---|
| Eyes | Requiring photocoagulation or vitrectomy | Yes | [18] |
| Kidneys | Requiring renal dialysis | Yes | [18] |
| Liver | Requiring hospitalization for cirrhosis, liver failure, or transplantation | Yes | [26] |
| Feet | Requiring hospitalization for foot ulcer, lower extremity cellulitis, or lower extremity osteomyelitis | Yes | [18] |
| Heart and vasculature | Major adverse cardiovascular events including fatal and non-fatal ischemic heart disease and cerebrovascular events | No | [27] |
T2DM, type 2 diabetes mellitus.
CVD with or without T2DM
A similar long-term relationship exists between GDM and CVD but with one key difference: the elevated risk of CVD outcomes is not fully attributable to T2DM (Table 1) [18,27]. Indeed, over the past decade, a series of studies has shown that women with a history of GDM have a higher incidence of CVD events (fatal and non-fatal ischemic heart disease and cerebrovascular events) than that of their peers [18,27-34]. A meta-analysis of these studies (involving >5 million women and >100,000 events) has revealed that, compared to their peers, women with GDM have a 2-fold higher risk of future CVD events (relative risk [RR], 1.98; 95% confidence interval [CI], 1.57 to 2.50) [27]. Of note, on meta-regression analysis, the rates of incident T2DM across studies did not affect this risk. Furthermore, even when restricting the meta-analysis to only women who did not develop T2DM, GDM predicts a 56% higher risk of future CVD events (RR, 1.56; 95% CI, 1.04 to 2.32) [27]. In addition to major adverse cardiovascular events, GDM has also been associated with higher rates of heart failure [34].
The recognition that incident CVD in women with previous GDM is not fully dependent upon intercurrent T2DM raises the question of the relevant determinants of cardiovascular risk in this patient population. In this context, it is notable that studies have consistently found that, compared to their peers, women with a history of GDM have a higher prevalence of cardiometabolic risk factors, including dyslipidemia, hypertension, overweight/obesity, and metabolic syndrome [35-39]. Moreover, this adverse cardiovascular risk factor profile is evident by 3-month postpartum [37,38]. Its presence so soon after delivery raises the question of whether the adverse risk factor profile is a consequence of the GDM pregnancy or alternatively if it might actually precede the diagnosis of GDM. To address this question, we must consider what is known about the health of women who go on to develop GDM.
Maternal health prior to GDM pregnancy
Various lines of evidence have suggested that, although GDM is typically diagnosed in late 2nd or early 3rd trimester, there are indeed findings that may precede the diagnosis. First, women who go on to develop GDM already exhibit metabolic changes in their amniotic fluid in 1st trimester, including altered levels of glucose, insulin and insulin-like growth factor-binding protein-1 [40]. Second, it has been shown that fetal overgrowth may precede the diagnosis of GDM [41]. Third, an increased likelihood of the subsequent development of GDM can be predicted by 1st trimester measurements of circulating biomarkers and analytes, including glycemic measures, fasting insulin, adiponectin, high density lipoprotein (HDL) cholesterol, triglycerides, C-reactive protein, tissue plasminogen activator antigen, and insulin-like growth factor-binding protein-2 [42]. It thus emerges that there exists a potentially detectable phenotype in early pregnancy that may predict higher risk of subsequent GDM, thereby leading to the question of whether such a presentation could be evident even before conception.
Prior to pregnancy, there are indeed differences between women who go on to develop GDM and those who do not. These differences include higher A1c, fasting glucose, low density lipoprotein (LDL) cholesterol and triglycerides, coupled with lower HDL cholesterol [43-46]. Moreover, in the 5 years prior to the index pregnancy, these cardiovascular risk factors exhibit divergent trajectories between women who go on to develop GDM and their peers, resulting in an amplification of the differences over time [46]. These divergent tracks may also be differentially affected by the pregnancy in women with and without GDM, resulting in further amplification of differences in the postpartum [47]. Ultimately, in enabling the diagnosis of GDM, pregnancy can be seen as a life event that facilitates the identification of a population of young women who are gradually developing a high-risk cardiometabolic phenotype over time [48]. Furthermore, the progressive worsening of their cardiometabolic risk factor burden in the years before pregnancy raises the question of how early in life this pathologic process might actually begin.
HEALTH OF THE OFFSPRING OF GDM PREGNANCY
Evidence of the existence of an unfavorable cardiometabolic profile prior to pregnancy suggests the increased risk for the development of pre-gestational T2DM, GDM, and post-gestational T2DM has early life origins. Following the landmark publication of Barker [49] introducing the concept of developmental origins of health and disease, there has been significant interest in understanding the intergenerational basis for cardiometabolic risk. The impact of in utero exposure to maternal diabetes to the offspring is 2-fold; the immediate perinatal complications and the longer-term risks of cardiometabolic disease. Although the focus of this review is maternal GDM, it is important to note that many of the original studies looking at offspring outcomes of maternal hyperglycemia have combined populations of maternal hyperglycemia, pre-gestational, and maternal GDM exposures.
Postnatal complications of exposure to gestational diabetes in utero
Maternal obesity, elevated gestational weight gain and hyperglycemia are known risk factors for fetal overnutrition, hyperinsulinism, production of insulin-like growth factor-1, and resultant overgrowth. Fetal hyperinsulinism itself has been associated with a disproportionate increase in fetal fat mass, altered fetal lung surfactant [50] resulting in neonatal respiratory distress syndrome, and neonatal hypoglycemia [51]. In addition, maternal hyperglycemia is associated with a relative fetal hypoxia and, devastatingly, fetal asphyxia, and stillbirth [52]. Other developmental impacts of maternal GDM include congenital anomalies of the heart, genitourinary tract, face (cleft lip +/– palate) and central nervous system. A recent large epidemiological study [53] reported an increased overall congenital anomaly risk associated with GDM exposure (odds ratio [OR], 1.28; 95% CI, 1.24 to 1.31), accounting for maternal age, ethnicity, education, smoking, parity, pre-pregnancy body mass index (BMI), hypertension, and infant sex. The perinatal and postnatal morbidity in offspring exposed to maternal hyperglycemia is evident across the spectrum of dysglycemia and is a harbinger of longer-term complications to follow.
Childhood cardiometabolic complications
The impact of fetal overnutrition and overgrowth appears to extend into infancy and childhood with increases in neonatal and infant fat mass and childhood risk of overweight and obesity. The early proposal by Pedersen [54] implicating intrauterine hyperglycemia in pregnancy in offspring obesity and T2DM risk has gained support in recent decades through epidemiological and prospective birth studies. Human observational studies have shown an increased birthweight and neonatal fat mass [55,56], abdominal adiposity [57], and overweight and obesity in those offspring exposed to maternal hyperglycemia and GDM [58-63]. A seminal study of sibships in the Akimal O’odam (Pima) population comparing offspring born before and after a diagnosis of maternal diabetes revealed an increased risk of childhood obesity with maternal diabetes exposure independent of shared genetics and environment [64]. Although reports of the impact of GDM exposure on BMI have been conflicting, more recent data exploring offspring body composition measures beyond weight and BMI from the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS) and Exploring Perinatal Outcomes in Children (EPOCH) study support the independent association between maternal diabetes exposure and elevated offspring adiposity. The HAPO FUS reported that exposure to GDM in pregnancy was associated with an increased odds of childhood obesity by 54%, elevated body fat by 35%, and elevated waist circumference by 34% at age 10 to 14 years [65]. The EPOCH study of multiethnic cohort of youth from Colorado reported a positive correlation of GDM exposure with offspring BMI, waist circumference, and visceral and subcutaneous adiposity; this relationship tracked from childhood (mean age 10.5 years) into adolescence (mean age 16.7 years) and was not significantly impacted by the postnatal environment [66]. Both studies showed attenuated but persistent effects when accounting for maternal pre-pregnancy BMI. Many, but not all [67] studies which have adjusted or accounted for maternal weight (pre-pregnancy BMI and/or gestational weight gain) have shown persistence of a significant childhood obesity risk with maternal diabetes exposure. The degree and timing of excessive childhood adiposity vary depending on the study methodology, suggesting variation in risk based on population, degree and timing of exposure to maternal dysglycemia, treatment of maternal dysglycemia, and other associated environmental exposures. Interestingly not all offspring exposed to diabetes in utero are large-for-gestational-age, have increased childhood adiposity or develop T2DM, suggesting that additional studies focusing on the mechanisms of intergenerational inheritance of risk and the interaction of these mechanisms with postnatal modifiers are urgently needed.
Childhood risk of developing insulin resistance and dysglycemia
In addition to the direct risk associated with obesity in the offspring, elevated maternal glycemia in pregnancy is a predictor of disorders of glucose metabolism including insulin resistance, reduced acute insulin response, impaired glucose tolerance [58,68,69] and T2DM [70] in the offspring. Studies in the Akimal O’odam population provided early evidence that maternal hyperglycemia increases both childhood obesity risk and insulin resistance and T2DM in the offspring [71]. More recent results from the HAPO FUS [69] comparing offspring born to mothers with and without GDM revealed higher plasma glucose levels at 30, 60, and 120 minutes during a 75 g OGTT in offspring born to mothers with GDM. This relationship remained after controlling for measures of current maternal and child obesity. In addition, compared to unexposed offspring, exposure to maternal GDM was associated with decreased insulin sensitivity and β-cell compensation, and an increased odds of developing impaired glucose tolerance (OR, 1.96; 95% CI, 1.41 to 2.73) at follow-up ages 10 to 14 years.
The SEARCH for Diabetes in Youth (SEARCH) study supports these observations as maternal diabetes and obesity exposure in utero accounted for 47% of the risk for developing childhood T2DM in offspring [72]. A large Canadian population-based prospective cohort study demonstrated both GDM and T2DM exposure were important independent risk factors for the development of T2DM and were associated with an accelerated time to diagnosis of T2DM in offspring [70]. Thus, exposure to maternal diabetes (T2DM and GDM) is associated with the development of pre-diabetes and diabetes in the offspring independent of the risk of childhood obesity and excess adiposity, suggesting a potential role of altered β-cell development and ultimately function in early life.
Although the underlying pathophysiology linking maternal diabetes exposure to offspring obesity and metabolic dysregulation remains unknown, accumulating evidence suggests that changes in DNA methylation resulting in altered gene expression link the prenatal environment to later-in-life disease development [73-75]. Altered DNA methylation of genes following exposure to maternal diabetes has been associated with childhood adiposity and T2DM [75-77]. Cord blood analysis in 14 Akimal O’odam offspring exposed to diabetes in pregnancy compared to 14 unexposed offspring revealed differentially methylated regions in promoters controlling insulin signaling and metabolic pathways [75,76,78]. Importantly, prenatal epigenetic marks can persist into adolescence and be associated with changes in glucose and lipid metabolism [76].
Long-term health consequences in offspring exposed to gestational diabetes
The independent impact of GDM exposure on offspring risk of CVD is difficult to determine owing to the frequent co-existence of obesity, central adiposity and dysglycemia of the offspring exposed. Indeed, the long-term cardiovascular risk to exposed offspring is likely multifactorial with increased adiposity, insulin resistance and hyperinsulinism, and T2DM playing important roles. However, studies have attempted to identify whether increased risk of specific cardiovascular risk factors such as hypertension and dyslipidemia can be linked to maternal diabetes exposure in utero. Early clinical studies in the Akimal O’odam population revealed an increase in systolic blood pressure by 11 mm Hg in offspring exposed to maternal diabetes independent of offspring sex, current measures of adiposity, and family history of diabetes [79]. A more recent large population prospective cohort study in Portugal [80] reported elevated systolic and diastolic blood pressures at age 10 in offspring born to mothers with GDM compared to unexposed peers. The relationship was mediated by childhood BMI and was sex-specific, with boys having higher risk than girls.
The risk of hypertension in offspring exposed to maternal diabetes may, in part, result from early renal dysfunction. Reports suggest in some populations up to approximately 50% of youth living with T2DM will develop micro- and macrovascular complications, including end-stage renal disease requiring dialysis, within 15 years of diagnosis (mean age 29 years old) [81,82]. In a cohort of predominantly First Nations adolescents with T2DM, approximately 30% had albuminuria 2-year postdiagnosis, suggesting that kidney disease occurs early and may be independent of persistent hyperglycemia [83]. Supporting evidence from a national surveillance study revealed that 5.4% of Canadian youth with T2DM had albuminuria in the first year of diagnosis and, compared to unexposed youth, were more likely to have been exposed to T2DM in utero [84]. Mechanistically, nephrogenesis is not complete until 36-week of gestation and intrauterine exposure to T2DM may be a critical determinant of nephron endowment [85], and later-in-life function. In mice, exposure to maternal diabetes in utero alters renal development by decreasing branching morphogenesis, impairing nephrogenesis and decreasing nephron endowment at birth [86], which has been shown to cause glomerular hypertrophy, hyperfiltration and subsequently, albuminuria [87].
Studies examining a more comprehensive panel of cardiometabolic risk factors include follow-up data from the EPOCH cohort [88,89] and a large Danish population-based cohort [90]. Short-term follow-up of children in EPOCH revealed associations of maternal GDM with elevated waist circumference, and an early biomarker of endothelial dysfunction, while longer-term follow-up in adolescence reported additional associations with unfavorable lipid profiles in girls (elevated total cholesterol and LDL cholesterol) and elevated systolic blood pressure in boys. Of interest, the relationship with lipid profiles in girls was not significant after adjustment for maternal GDM treatment, suggesting that hyperglycemia is the main culprit to fetal underpinnings of dyslipidemia. The Danish based cohort [90] showed that, after accounting for childhood BMI, exposure to GDM in utero was associated with insulin resistance, elevated fasting blood sugar, insulin and C-peptide levels, and central adiposity in offspring.
Fewer studies have examined hard outcomes such as cardiovascular events in offspring exposed to GDM. Two large population-based cohort studies have shown an increased odds of CVD in offspring at 35 to 40 years. The population-based administrative birth cohort study from Manitoba Canada revealed that intrauterine exposure to GDM is associated with 1.9-fold increased hazard for cardiovascular risk factors (hypertension, dyslipidemia, T2DM) and 1.42-fold increased risk of CVD (incident myocardial infarction, heart failure, cerebral vascular infarction) within 35 years of life compared to unexposed offspring. The authors also noted that increased CVD morbidity was driven by early-onset hypertension, T2DM, and dyslipidemia [91]. The other population-based study from Denmark [33,92] reported GDM exposure to be associated with increased rates of CVD (heart failure, hypertension, deep vein thrombosis, pulmonary embolism) in offspring at 40 years of follow-up (OR, 1.19; 95% CI, 1.07 to 1.32) which persisted when adjusted for maternal and paternal history of CVD. However, the strongest associations were found among the offspring of mothers with a history of diabetes complications or a history of CVD, suggesting a central role of chronic hyperglycemia in the mother in determining the elevated offspring risk.
A causal role for intrauterine exposures in increasing CVD risk is complicated by combined exposures to maternal diabetes and obesity and by postnatal exposures and resultant obesity in offspring. The causal link has been examined in a recent review of animal studies in which maternal GDM or hyperglycemia led to offspring cardiovascular complications including hypertrophy, increased left ventricle wall thickness, systolic and diastolic dysfunction, and high blood pressure in mice and/or rats which is driven in part by cardiac mitochondrial dysfunction [93].
Taken together, the current evidence is compelling for an intergenerational cycle of cardiometabolic dysfunction starting in utero and impacting health throughout the lifespan. The increased risk to offspring predisposes them to metabolic dysfunction and GDM in pregnancy which leads to poor cardiometabolic health in the next generation (Fig. 1). Evidence suggests that a lifecycle approach to prevention strategies is warranted, and reduction of risk will require intervention at several life stages.
CONCLUSIONS
The concept emerging from this literature is that, although diagnosed in pregnancy, GDM identifies women who have a burgeoning cardiometabolic risk factor profile that appears to first diverge from that of their peers early in life. The resultant lifelong exposure to an enhanced risk factor burden ultimately contributes to elevated risks of disease outcomes, including T2DM and CVD. Moreover, intrauterine exposure to this chronic metabolic dysfunction may set in place the replication of this cycle in the offspring of GDM pregnancies. Accordingly, the implications of GDM extend not only across the life span of the affected woman but also that of her child. Conversely, the effect of GDM on intergenerational risk transmission may also offer a unique opportunity for disease modification and possibly prevention. Specifically, it follows that preconception intervention to prevent the clinical manifestation of GDM in pregnancy may provide an opportunity to modify future cardiometabolic risk in both mother and child. As part of the Healthy Life Trajectories Initiative (HeLTI), we are currently conducting such trials of preconception lifestyle intervention to reduce maternal risk of GDM. Ultimately, this approach may enable interruption of the intergenerational transmission of cardiometabolic risk for the benefit of both mother and child.
Acknowledgments
Ravi Retnakaran holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital and his research program is supported by the Sun Life Financial Program to Prevent Diabetes in Women.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None
REFERENCES
- 1.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 2.Fu J, Retnakaran R. The life course perspective of gestational diabetes: an opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine. 2022;45:101294. doi: 10.1016/j.eclinm.2022.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–85. [PubMed] [Google Scholar]
- 4.Saravanan P, Diabetes in Pregnancy Working Group. Maternal Medicine Clinical Study Group. Royal College of Obstetricians and Gynaecologists, UK Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8:793–800. doi: 10.1016/S2213-8587(20)30161-3. [DOI] [PubMed] [Google Scholar]
- 5.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care. 2008;31:1275–81. doi: 10.2337/dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. Risk of early progression to prediabetes or diabetes in women with recent gestational dysglycaemia but normal glucose tolerance at 3-month postpartum. Clin Endocrinol (Oxf) 2010;73:476–83. doi: 10.1111/j.1365-2265.2010.03834.x. [DOI] [PubMed] [Google Scholar]
- 7.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of β-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37:3262–9. doi: 10.2337/dc14-1529. [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Shah BR. Abnormal screening glucose challenge test in pregnancy and future risk of diabetes in young women. Diabet Med. 2009;26:474–7. doi: 10.1111/j.1464-5491.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–91. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–93. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. 2010;33:1798–804. doi: 10.2337/dc10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59:2625–30. doi: 10.2337/db10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care. 2010;33:396–401. doi: 10.2337/dc09-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 15.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and metaanalysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beharier O, Sergienko R, Kessous R, Szaingurten-Solodkin I, Walfisch A, Shusterman E, et al. Gestational diabetes mellitus is a significant risk factor for long-term ophthalmic morbidity. Arch Gynecol Obstet. 2017;295:1477–82. doi: 10.1007/s00404-017-4362-4. [DOI] [PubMed] [Google Scholar]
- 17.Beharier O, Shoham-Vardi I, Pariente G, Sergienko R, Kessous R, Baumfeld Y, et al. Gestational diabetes mellitus is a significant risk factor for long-term maternal renal disease. J Clin Endocrinol Metab. 2015;100:1412–6. doi: 10.1210/jc.2014-4474. [DOI] [PubMed] [Google Scholar]
- 18.Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care. 2017;40:101–8. doi: 10.2337/dc16-1400. [DOI] [PubMed] [Google Scholar]
- 19.Rawal S, Olsen SF, Grunnet LG, Ma RC, Hinkle SN, Granstrom C, et al. Gestational diabetes mellitus and renal function: a prospective study with 9- to 16-year follow-up after pregnancy. Diabetes Care. 2018;41:1378–84. doi: 10.2337/dc17-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kew S, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Postpartum microalbuminuria after gestational diabetes: the impact of current glucose tolerance status. J Clin Endocrinol Metab. 2015;100:1130–6. doi: 10.1210/jc.2014-3814. [DOI] [PubMed] [Google Scholar]
- 21.De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, et al. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: prospective study. Am J Gastroenterol. 2016;111:665–70. doi: 10.1038/ajg.2016.43. [DOI] [PubMed] [Google Scholar]
- 22.Forbes S, Taylor-Robinson SD, Patel N, Allan P, Walker BR, Johnston DG. Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia. 2011;54:641–7. doi: 10.1007/s00125-010-2009-0. [DOI] [PubMed] [Google Scholar]
- 23.Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational diabetes mellitus is strongly associated with non-alcoholic fatty liver disease. Am J Gastroenterol. 2016;111:658–64. doi: 10.1038/ajg.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foghsgaard S, Andreasen C, Vedtofte L, Andersen ES, Bahne E, Strandberg C, et al. Nonalcoholic fatty liver disease is prevalent in women with prior gestational diabetes mellitus and independently associated with insulin resistance and waist circumference. Diabetes Care. 2017;40:109–16. doi: 10.2337/dc16-1017. [DOI] [PubMed] [Google Scholar]
- 25.Mehmood S, Margolis M, Ye C, Maple-Brown L, Hanley AJ, Connelly PW, et al. Hepatic fat and glucose tolerance in women with recent gestational diabetes. BMJ Open Diabetes Res Care. 2018;6:e000549. doi: 10.1136/bmjdrc-2018-000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retnakaran R, Luo J, Shah BR. Gestational diabetes in young women predicts future risk of serious liver disease. Diabetologia. 2019;62:306–10. doi: 10.1007/s00125-018-4775-z. [DOI] [PubMed] [Google Scholar]
- 27.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–14. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 28.Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG. 2014;121:1530–6. doi: 10.1111/1471-0528.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180:41–4. doi: 10.1093/aje/kwu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with longterm cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177:1735–42. doi: 10.1001/jamainternmed.2017.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie-Sampson S, Paradis G, Healy-Profitos J, St-Pierre F, Auger N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol. 2018;55:315–22. doi: 10.1007/s00592-017-1099-2. [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Shah BR. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2019;7:378–84. doi: 10.1016/S2213-8587(19)30077-4. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Soohoo M, Sorensen HT, Li J, Arah OA. Gestational diabetes mellitus and the risks of overall and type-specific cardiovascular diseases: a population- and sibling-matched cohort study. Diabetes Care. 2022;45:151–9. doi: 10.2337/dc21-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Echouffo-Tcheugui JB, Guan J, Retnakaran R, Shah BR. Gestational diabetes and incident heart failure: a cohort study. Diabetes Care. 2021;44:2346–52. doi: 10.2337/dc21-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma A, Boney CM, Tucker R, Vohr BR. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2002;87:3227–35. doi: 10.1210/jcem.87.7.8684. [DOI] [PubMed] [Google Scholar]
- 36.Lauenborg J, Mathiesen E, Hansen T, Glumer C, Jorgensen T, Borch-Johnsen K, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–10. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–7. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. 2010;95:4345–53. doi: 10.1210/jc.2010-0361. [DOI] [PubMed] [Google Scholar]
- 39.Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos A, et al. Risk of developing metabolic syndrome after gestational diabetes mellitus: a systematic review and meta-analysis. J Endocrinol Invest. 2021;44:1139–49. doi: 10.1007/s40618-020-01464-6. [DOI] [PubMed] [Google Scholar]
- 40.Tisi DK, Burns DH, Luskey GW, Koski KG. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care. 2011;34:139–44. doi: 10.2337/dc10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39:982–7. doi: 10.2337/dc16-0160. [DOI] [PubMed] [Google Scholar]
- 42.Retnakaran R. The insulin-like growth factor axis: a new player in gestational diabetes mellitus? Diabetes. 2016;65:3246–8. doi: 10.2337/dbi16-0048. [DOI] [PubMed] [Google Scholar]
- 43.Gunderson EP, Quesenberry CP, Jr, Jacobs DR, Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am J Epidemiol. 2010;172:1131–43. doi: 10.1093/aje/kwq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedderson MM, Darbinian JA, Quesenberry CP, Ferrara A. Pregravid cardiometabolic risk profile and risk for gestational diabetes mellitus. Am J Obstet Gynecol. 2011;205:55. doi: 10.1016/j.ajog.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 45.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22:724–30. doi: 10.1097/EDE.0b013e318225c960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Retnakaran R, Shah BR. Divergent trajectories of cardiovascular risk factors in the years before pregnancy in women with and without gestational diabetes mellitus: a population-based study. Diabetes Care. 2020;43:2500–8. doi: 10.2337/dc20-1037. [DOI] [PubMed] [Google Scholar]
- 47.Retnakaran R, Shah BR. Impact of pregnancy on the trajectories of cardiovascular risk factors in women with and without gestational diabetes. Diabetes Obes Metab. 2021;23:2364–73. doi: 10.1111/dom.14479. [DOI] [PubMed] [Google Scholar]
- 48.Retnakaran R. Diabetes in pregnancy 100 years after the discovery of insulin: hot topics and open questions to be addressed in the coming years. Metabolism. 2021;119:154772. doi: 10.1016/j.metabol.2021.154772. [DOI] [PubMed] [Google Scholar]
- 49.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore TR. A comparison of amniotic fluid fetal pulmonary phospholipids in normal and diabetic pregnancy. Am J Obstet Gynecol. 2002;186:641–50. doi: 10.1067/mob.2002.122851. [DOI] [PubMed] [Google Scholar]
- 51.Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126:e1545–52. doi: 10.1542/peds.2009-2257. [DOI] [PubMed] [Google Scholar]
- 52.Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Clin Perinatol. 2007;34:611–26. doi: 10.1016/j.clp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43:2983–90. doi: 10.2337/dc20-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedersen J. Diabetes mellitus and pregnancy: present status of the hyperglycaemia: hyperinsulinism theory and the weight of the newborn baby. Postgrad Med J. 1971;Suppl:66–7. [PubMed] [Google Scholar]
- 55.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 56.Crume TL, Shapiro AL, Brinton JT, Glueck DH, Martinez M, Kohn M, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015;100:1672–80. doi: 10.1210/jc.2014-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Jarvelin MR, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–21. doi: 10.2337/dc09-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe WL, Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42:372–80. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe WL, Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320:1005–16. doi: 10.1001/jama.2018.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–6. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 61.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10:76–80. doi: 10.2337/diacare.10.1.76. [DOI] [PubMed] [Google Scholar]
- 62.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40 Suppl 2:121–5. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 63.Gomes D, von Kries R, Delius M, Mansmann U, Nast M, Stubert M, et al. Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: an interim analysis from a longitudinal mother-child cohort study. PLoS Med. 2018;15:e1002681. doi: 10.1371/journal.pmed.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–11. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 65.Josefson JL, Catalano PM, Lowe WL, Scholtens DM, Kuang A, Dyer AR, et al. The joint associations of maternal BMI and glycemia with childhood adiposity. J Clin Endocrinol Metab. 2020;105:2177–88. doi: 10.1210/clinem/dgaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hockett CW, Harrall KK, Moore BF, Starling AP, Bellatorre A, Sauder KA, et al. Persistent effects of in utero overnutrition on offspring adiposity: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia. 2019;62:2017–24. doi: 10.1007/s00125-019-04981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patro Golab B, Santos S, Voerman E, Lawlor DA, Jaddoe VW, Gaillard R, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health. 2018;2:812–21. doi: 10.1016/S2352-4642(18)30273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sauder KA, Hockett CW, Ringham BM, Glueck DH, Dabelea D. Fetal overnutrition and offspring insulin resistance and β-cell function: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabet Med. 2017;34:1392–9. doi: 10.1111/dme.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42:381–92. doi: 10.2337/dc18-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wicklow BA, Sellers EA, Sharma AK, Kroeker K, Nickel NC, Philips-Beck W, et al. Association of gestational diabetes and type 2 diabetes exposure in utero with the development of type 2 diabetes in first nations and non-first nations offspring. JAMA Pediatr. 2018;172:724–31. doi: 10.1001/jamapediatrics.2018.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM: role of intrauterine environment. Diabetes. 1988;37:622–8. doi: 10.2337/diab.37.5.622. [DOI] [PubMed] [Google Scholar]
- 72.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Jr, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–6. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr Res. 2007;61(5 Pt 2):38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- 74.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8:935–43. doi: 10.4161/epi.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen P, Piaggi P, Traurig M, Bogardus C, Knowler WC, Baier LJ, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017;60:645–55. doi: 10.1007/s00125-016-4203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fradin D, Boelle PY, Belot MP, Lachaux F, Tost J, Besse C, et al. Genome-wide methylation analysis identifies specific epigenetic marks in severely obese children. Sci Rep. 2017;7:46311. doi: 10.1038/srep46311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–34. doi: 10.2337/db11-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–9. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miranda JO, Cerqueira RJ, Barros H, Areias JC. Maternal diabetes mellitus as a risk factor for high blood pressure in late childhood. Hypertension. 2019;73:e1–7. doi: 10.1161/HYPERTENSIONAHA.118.11761. [DOI] [PubMed] [Google Scholar]
- 81.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37:436–43. doi: 10.2337/dc13-0954. [DOI] [PubMed] [Google Scholar]
- 82.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35:1265–71. doi: 10.2337/dc11-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sellers EA, Dart AB, McGavock J, Wicklow BA. Cardiovascular comorbidity associated with albuminuria in youth-onset type 2 diabetes: analyses from the iCARE study. Can J Diabetes. 2021;45:458–65. doi: 10.1016/j.jcjd.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Sellers EA, Hadjiyannakis S, Amed S, Dart AB, Dyck RF, Hamilton J, et al. Persistent albuminuria in children with type 2 diabetes: a canadian paediatric surveillance program study. J Pediatr. 2016;168:112–7. doi: 10.1016/j.jpeds.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 85.Hoy WE, Ingelfinger JR, Hallan S, Hughson MD, Mott SA, Bertram JF. The early development of the kidney and implications for future health. J Dev Orig Health Dis. 2010;1:216–33. doi: 10.1017/S204017441000022X. [DOI] [PubMed] [Google Scholar]
- 86.Hokke SN, Armitage JA, Puelles VG, Short KM, Jones L, Smyth IM, et al. Altered ureteric branching morphogenesis and nephron endowment in offspring of diabetic and insulin-treated pregnancy. PLoS One. 2013;8:e58243. doi: 10.1371/journal.pone.0058243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–7. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 88.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–7. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perng W, Hockett CW, Sauder KA, Dabelea D. In utero exposure to gestational diabetes mellitus and cardiovascular risk factors in youth: a longitudinal analysis in the EPOCH cohort. Pediatr Obes. 2020;15:e12611. doi: 10.1111/ijpo.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen AC, et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish national birth cohort. Diabetes Care. 2017;40:1746–55. doi: 10.2337/dc17-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guillemette L, Wicklow B, Sellers EA, Dart A, Shen GX, Dolinsky VW, et al. Intrauterine exposure to diabetes and risk of cardiovascular disease in adolescence and early adulthood: a population-based birth cohort study. CMAJ. 2020;192:E1104–13. doi: 10.1503/cmaj.190797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sorensen HT, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. doi: 10.1136/bmj.l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kereliuk SM, Dolinsky VW. Recent experimental studies of maternal obesity, diabetes during pregnancy and the developmental origins of cardiovascular disease. Int J Mol Sci. 2022;23:4467. doi: 10.3390/ijms23084467. [DOI] [PMC free article] [PubMed] [Google Scholar]