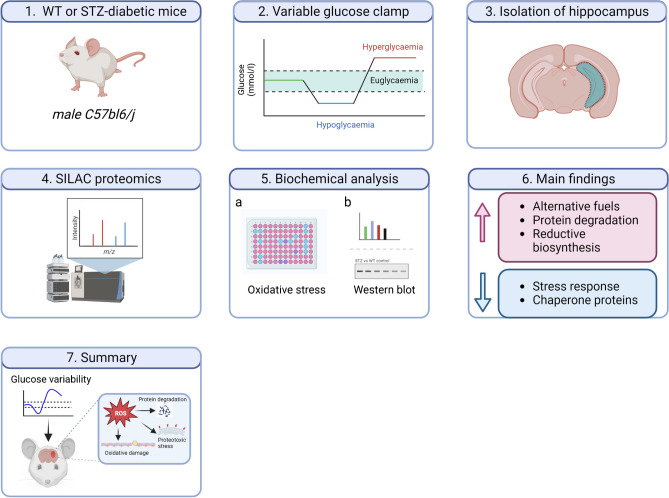
Abstract
Aims/hypothesis
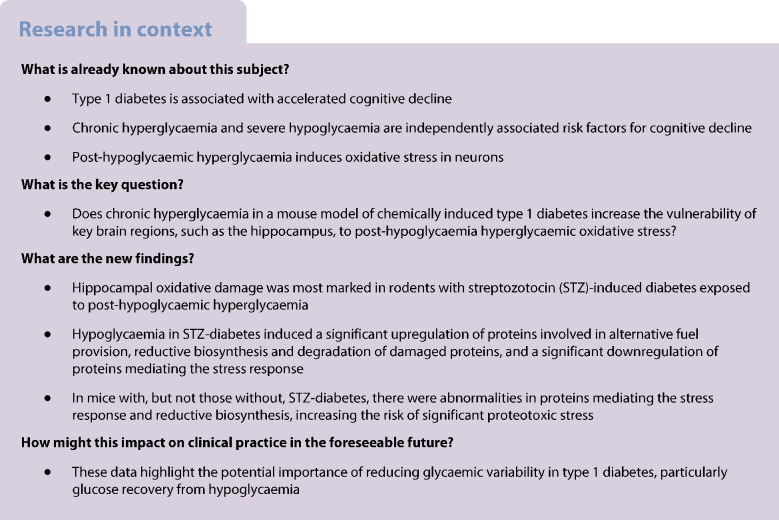
Chronic hyperglycaemia and recurrent hypoglycaemia are independently associated with accelerated cognitive decline in type 1 diabetes. Recurrent hypoglycaemia in rodent models of chemically induced (streptozotocin [STZ]) diabetes leads to cognitive impairment in memory-related tasks associated with hippocampal oxidative damage. This study examined the hypothesis that post-hypoglycaemic hyperglycaemia in STZ-diabetes exacerbates hippocampal oxidative stress and explored potential contributory mechanisms.
Methods
The hyperinsulinaemic glucose clamp technique was used to induce equivalent hypoglycaemia and to control post-hypoglycaemic glucose levels in mice with and without STZ-diabetes and Nrf2−/− mice (lacking Nrf2 [also known as Nfe2l2]). Subsequently, quantitative proteomics based on stable isotope labelling by amino acids in cell culture and biochemical approaches were used to assess oxidative damage and explore contributory pathways.
Results
Evidence of hippocampal oxidative damage was most marked in mice with STZ-diabetes exposed to post-hypoglycaemic hyperglycaemia; these mice also showed induction of Nrf2 and the Nrf2 transcriptional targets Sod2 and Hmox-1. In this group, hypoglycaemia induced a significant upregulation of proteins involved in alternative fuel provision, reductive biosynthesis and degradation of damaged proteins, and a significant downregulation of proteins mediating the stress response. Key differences emerged between mice with and without STZ-diabetes following recovery from hypoglycaemia in proteins mediating the stress response and reductive biosynthesis.
Conclusions/interpretation
There is a disruption of the cellular response to a hypoglycaemic challenge in mice with STZ-induced diabetes that is not seen in wild-type non-diabetic animals. The chronic hyperglycaemia of diabetes and post-hypoglycaemic hyperglycaemia act synergistically to induce oxidative stress and damage in the hippocampus, possibly leading to irreversible damage/modification to proteins or synapses between cells. In conclusion, recurrent hypoglycaemia in sub-optimally controlled diabetes may contribute, at least in part, to accelerated cognitive decline through amplifying oxidative damage in key brain regions, such as the hippocampus.
Data availability
The datasets generated during and/or analysed during the current study are available in ProteomeXchange, accession no. 1-20220824-173727 (www.proteomexchange.org). Additional datasets generated during and/or analysed during the present study are available from the corresponding author upon reasonable request.
Graphical abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-023-05907-6.
Keywords: Glycaemic variability, Hippocampus, Hyperinsulinaemic glucose clamp, Hypoglycaemia, Mouse, Nfe2l2, Nrf2, Oxidative stress, Proteotoxic stress, Type 1 diabetes
Introduction
Short-duration longitudinal studies in young adults with type 1 diabetes compared with matched adults without diabetes have reported small but significant increases in the rate of cognitive decline associated with proliferative retinopathy and systolic hypertension [1, 2]. More recently, the 32-year follow-up of participants enrolled in the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) study reported that higher HbA1c levels over time and elevated systolic BP were associated with a greater rate of cognitive decline collectively equivalent to 9.4 years accelerated brain ageing [3]. Within the EDIC cohort, an fMRI substudy of middle-aged and older adults found brain volume loss and increased vascular injury compared with control individuals without diabetes [4]. Severe hypoglycaemia was also reported to be independently associated with cognitive decline in the EDIC cohort [3], a finding consistent with observational [5–7] and short-duration longitudinal [8] studies.
A reliance on glucose as a fuel, and limited capacity to store fuel, makes the brain especially vulnerable to hypoglycaemia [9]. Certainly, profound hypoglycaemia (to a degree that induces an isoelectric EEG) results in neuronal death in areas of the brain such as the hippocampus [10]. The hippocampus has been extensively researched for its role in memory function, processing speed, and intelligence [11]. Cognitive ageing is associated with loss of hippocampal volume [12]. In a recent report from our laboratory, we showed that rodents with chemically induced type 1 diabetes who had been exposed to recurrent hypoglycaemia demonstrated greater defects in memory function than rodents with type 1 diabetes who had not experienced recurrent hypoglycaemia. This was associated with evidence of lipid peroxidation and protein carbonylation in the hippocampus, markers of oxidative damage [13].
Although reactive oxygen species (ROS) play an integral part in the normal signalling response within many cell types, including neurons, large and frequent disturbances in glucose homeostasis cause excessive ROS production resulting in oxidative stress [14, 15]. Chronic hyperglycaemia [16], severe hypoglycaemia [17] and post-hypoglycaemic glucose recovery all stimulate ROS production. Notably, ROS production post-hypoglycaemia correlates directly with the degree of glucose increase during recovery from hypoglycaemia [18]. Chronic hyperglycaemia also impairs antioxidant defence mechanisms [19, 20]. This led us to hypothesise that marked glycaemic variability may lead to excessive ROS production and irreversible oxidative damage to cells within the brain [13]. What is not clear from these studies is the relative contribution of each to oxidative damage and the key pathways that may underlie this. In the present study, we address this question directly using the hyperinsulinaemic glucose clamp technique combined with the measurement of ROS-induced protein modifications (protein carbonylation and lipid peroxidation) and stable isotope labelling by amino acids in cell culture (SILAC) proteomic analysis of the hippocampus in a variety of mouse models.
Methods
Experimental animals
Male C576BL/6J mice (20–25 g; Charles River, UK) were used. The generation (mice were backcrossed over six generations onto a C57BL/6J background) and genotyping of Nrf2−/− mice lacking Nrf2 (also known as Nfe2l2), kindly provided by K. Itoh and M. Yamamoto (Centre for Tsukuba Advanced Research Alliance and Institute of Basic Medical Sciences, University of Tsukuba, Tsukuba, Japan), were performed as described previously [21]. Mice were housed four per cage with food and water available ad libitum, on a 12 h light–dark schedule. All animal procedures were approved by the University of Dundee Ethical Review Process and performed according to UK Home Office regulations and the ARRIVE 2.0 guidelines (under the auspices of Project License PIL PE82c1898).
Induction of diabetes
C576BL/6J mice were randomly assigned to receive streptozotocin (STZ; 150 mg/kg i.p.) to chemically induce STZ-diabetes or control (Hanks’ Buffered Salt Solution buffer; Gibco, UK; i.p.). At 72 h and 7 days post-injection, blood glucose was measured from tail-vein samples using a hand-held glucose monitor (Accuread, Roche, UK); blood glucose ≥16.0 mmol/l was regarded as diabetic. Any mouse that failed to reach this criterion was given a second injection of STZ, and blood glucose was re-tested. To maintain body weight and health, Linbit insulin implants (LinShin, Canada; at half of the recommended dose [~0.05 U/kg per day]) were inserted subcutaneously under isoflurane anaesthetic as described [13]. Control mice were also anaesthetised.
Vascular surgery and glycaemic clamping
After 4 weeks of stable hyperglycaemia (STZ-diabetes) or euglycaemia (wild-type [WT] control and Nrf2−/−mice), the mice underwent surgery for the insertion of vascular catheters as described previously [22]. Mice were allowed to recover for 5 days (or until they reached pre-surgery weight).
Infusion protocol
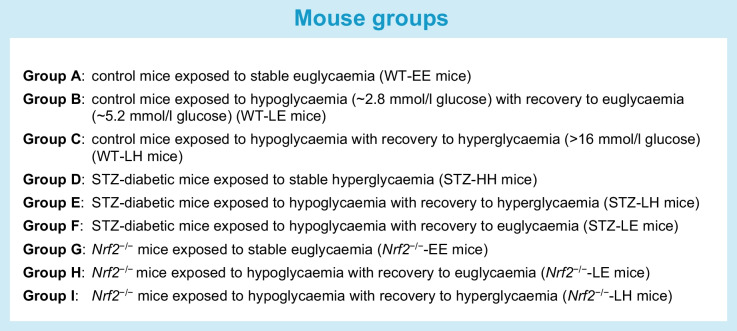
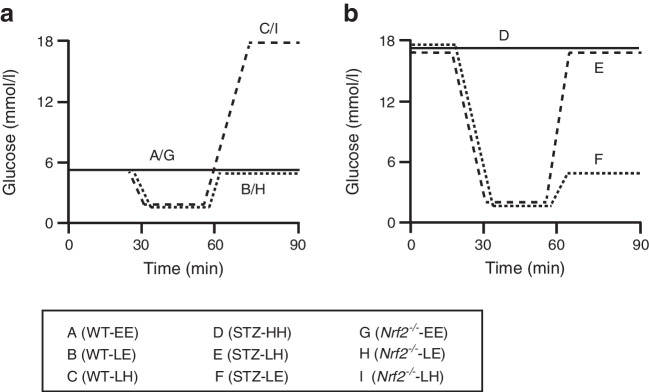
As previously described, a 2 h 4 mU kg−1 min−1 infusion of human short-acting insulin (Actrapid, Novo Nordisk, UK) was initiated in mice fasted for 5 h [23]. Mice were then allocated into groups (see Fig. 1 and the Text box detailing mouse groups). Target glucose levels (5.2 mmol/l [euglycaemia], 2.8 mmol/l [hypoglycaemia] and >16 mmol/l [hyperglycaemia]) were achieved and maintained for at least 30 min using a variable 50% glucose infusion based on frequent plasma glucose determinations. Additional blood samples to measure counterregulatory hormones were taken at the end of the second step of the clamp. At the end of the clamp, the mice were given food and water ad libitum and allowed to recover to their endogenous glucose levels (i.e., hyperglycaemia for STZ-diabetes mice and euglycaemia for WT control and Nrf2−/− mice).
Fig. 1.
Experimental design of mouse glycaemic clamps. (a) Control (A, B, C) and Nrf2−/− (G, H, I) mice were exposed to stable euglycaemia (WT- or Nrf2−/−-EE), hypoglycaemia (~ 2.8 mmol/l) with recovery to euglycaemia (~5.2 mmol/l) (WT- or Nrf2−/−-LE), or hypoglycaemia with recovery to hyperglycaemia (>16 mmol/l) (WT- or Nrf2−/−-LH). (b) STZ-diabetic (D, E, F) mice were exposed to stable hyperglycaemia (STZ-HH), hypoglycaemia with recovery to hyperglycaemia (STZ-LH), or hypoglycaemia with recovery to euglycaemia (STZ-LE)
Hormone analysis
Plasma glucagon and adrenaline levels were measured using commercially available ELISA kits (adrenaline, DEE5100R, Demeditec, Germany; glucagon, 10-1281-01, Mercodia, Sweden).
Biochemical analysis
Sixteen hours after the clamp, mice were killed humanely, and the brain (hippocampus) was dissected and flash-frozen in liquid nitrogen for subsequent biochemical analysis.
Sample preparation and SILAC
Hippocampal samples (single lobe from each mouse) from groups A (WT-EE) and E (STZ-LH) were analysed using SILAC-based proteomic analysis [24]. Samples were randomised, and the analysts were blinded during data acquisition. Samples were homogenised, and the protein extracted in sodium dodecyl sulfate (SDS, 4% wt/vol.) dithiothreitol (DTT, 0.1% wt/vol.), 100 mmol/l Tris-HCl (pH 7.6). After centrifugation, protein lysates from each experimental sample were spiked with an equivalent amount of SILAC protein lysate. After heating to 60°C for 30 min, the samples were alkylated by adding an equal volume of 150 mmol/l iodoacetamide (in 100 mmol/l Tris-HCl buffer [pH 7.6]). The protein was precipitated using the MeOH–chloroform method [25], and the protein concentration was measured using Protein 660 nm reagent (Pierce, UK). Samples were reduced/alkylated, digested with LysC (Pierce, 1:100), and then fractionated using a strong anion exchanger [26].
LC-MS/MS and data processing
The top 6 ms/ms programs (collision-induced dissociation [CID] or pulse-Q dissociation [PQD]) on LTQ-Orbitrap (Thermo Scientific, Germany) peptide identification and protein quantification were assessed using Maxquant (Ver 1.5.0.30; www.maxquant.org) or PEAKS 7.0 (Bioinformatics Solution Inc, Canada; database =Uniprot mouse 2017-02-29). Quantification is based on the methods described [24]. Missing data points were replaced (with 0) only for principal component analysis (PCA). A false detection rate (FDR) was set to 1% at the identified peptide spectrum match level. N-terminal acetylation, cysteine carbamidomethylation, and phosphorylation at S/T/Y were the only permitted post-translational modifications. Normalisation was performed using z-score normalisation in Perseus (Version 1.5.4.0, www.maxquant.org).
Western blot analysis
The second hippocampal lobe from all groups was powdered on liquid nitrogen using a pestle and mortar. A portion of the powder was homogenised in lysis buffer containing protease inhibitors and prepared for western blotting or proteomics analysis as described [27]. The remaining powder was frozen at −80°C for subsequent biochemical analysis. Membranes were probed for the following target proteins identified from SILAC (all from Cell Signalling Technology, UK): heat shock protein 90-β (HSP90B); proteasome subunit α type-2 (PSMA2); proteasome subunit α type-3 (PSMA3); proteasome subunit β type-7 (PSMB7); 6-phosphogluconate dehydrogenase (6PGD); long-chain acyl Co-A dehydrogenase (ACADL); and HSP90 co-chaperone (CDC37). All blots were normalised to the housekeeping protein GAPDH.
Lipid peroxidation
Malondialdehyde concentration was determined in all hippocampal samples (A–I) by the thiobarbituric acid-reactive substances assay [28] using a 96-well plate format. The amount of malondialdehyde was determined spectrophotometrically at 532 nm, and concentrations were determined by standard curve. All samples were assayed in duplicate.
Protein carbonylation
The level of carbonylated protein within the hippocampus of all groups (A–I) was measured by ELISA (Caymen Chemicals, US). Protein carbonyl concentration was calculated using the following equation: protein carbonyl (nmol/ml) = CA/(× 0.011 [mmol/l]−1) (500 ml/200 ml), where CA is the corrected absorbance (mean absorbance of controls – mean absorbance of samples).
RNA extraction and PCR
Total RNA was extracted from hippocampal tissue from all groups (A–I) using TRIzol reagent (Invitrogen, UK). Reverse transcription was performed with 1 ng RNA using SuperScript III First-Strand Synthesis System for RT (Invitrogen). Real-time PCR was performed using TaqMan gene expression assays for the following genes: Nrf2 (encoding for nuclear factor erythroid 2-related factor 2 [NRF2]); Nqo1 (encoding for NAD(P)H: quinone oxidoreductase 1); Hmox-1 (encoding for haem oxygenase 1); and Sod2 (encoding for superoxide dismutase 2). All samples were performed in triplicate and normalised to the housekeeping genes Actb and Ppia. Values are expressed as a fold-change relative to group A (WT-EE) for STZ-diabetic mice and group G (Nrf2−/−-EE) for Nrf2−/− mice.
Statistical analysis
Data were analysed using SPSS version 18 (IBM, UK). One-way ANOVA was used to compare clamp groups within each genotype (groups A–F for control [WT] and STZ-diabetes mice; groups G–I for Nrf2−/− mice). Post hoc analysis was performed using Tukey’s multiple comparisons test. For data that were not normally distributed, Kruskal–Wallis, followed by Dunn’s multiple comparisons test, was performed. Data are expressed as mean values ± SEM. Statistical significance was set at p<0.05.
Results
Hyperinsulinaemic clamp studies on control and STZ-diabetic mice
Stable hypoglycaemic (groups B, C, E and F) and hyperglycaemic plateaus (groups C, D, E and F) were achieved during the clamp procedures (Table 1; p<0.05 for each group vs WT control [group A]). In groups B and C, hypoglycaemia from a euglycaemic baseline resulted in significantly elevated glucagon and adrenaline plasma levels compared with group A. In contrast, in the STZ-diabetic mice (groups E and F), consistent with human type 1 diabetes, the glucagon response to a hypoglycaemic challenge was impaired (Table 1) [29] and the adrenaline response was severely blunted. The hormonal counterregulatory response to hypoglycaemia in Nrf2−/− mice was comparable with that in C57Bl6/J control mice (Table 1).
Table 1.
Mean plasma glucose levels during each phase of the hyperinsulinaemic glucose clamps along with counterregulatory hormone levels (glucagon and adrenaline) measured at the end of the eu/hypoglycaemia period
| Group | Mean glucose (mmol/l) | Glucagon (ng/l) | Adrenaline (pg/ml) | ||
|---|---|---|---|---|---|
| WT | |||||
| A | WT | Ea | E | Ea | Ea |
| 5.6±0.4 | 5.4±0.2 | 5.7±0.3 | 35±3 | 175±43.7 | |
| B | WT | La | E | La | La |
| 5.4±0.3 | 2.7±0.3* | 5.6±0.4 | 143±10** | 802±81.9** | |
| C | WT | La | H | La | La |
| 5.8±0.5 | 2.6±0.1* | 20.8±0.4** | 135±8** | 770±65.5** | |
| STZ-diabetes | |||||
| D | STZ | Ha | H | Ha | Ha |
| 19.3±1.0** | 18.3±2.2** | 18.8±2.3** | 22±6¶¶ | 267±49.1¶¶ | |
| E | STZ | La | H | La | La |
| 22.1±2.9** | 3.3±0.6* | 21.3±1.9** | 48±12¶¶ | 333±92.8¶¶ | |
| F | STZ | La | E | La | La |
| 20.9±1.8** | 2.9±0.7* | 6.1±2.4 | 43±13¶¶ | 355±81.9¶¶ | |
| Nrf2−/− | |||||
| G | Nrf2 | Ea | E | Ea | Ea |
| 6.3±0.3 | 6.3±0.4 | 6.3±0.3 | 42±6 | 251±38.2 | |
| H | Nrf2 | La | E | La | La |
| 6.2±0.2 | 2.5±0.1† | 6.4±0.2 | 116±12†† | 704±54.6†† | |
| I | Nrf2 | La | H | La | La |
| 6.2±0.3 | 2.6±0.1† | 17.1±0.5†† | 125±8†† | 753±81.9†† | |
Results represent mean values ± SEM, n=10–12 per group
aGlucose level during which the hyperinsulinaemic clamp was maintained and glucagon and adrenaline measurements were made
*p<0.05, **p<0.01 vs group A; ¶¶p<0.01 vs both groups B and C; †p<0.05, ††p<0.01 vs group G (one-way ANOVA followed by Tukey’s multiple comparisons test)
E, euglycaemia ~5.2 mmol/l; H, high, hyperglycaemia >16.0 mmol/l; L, low, hypoglycaemia ~2.8 mmol/l, Nrf2, Nrf2−/−
Chronic hyperglycaemia acts synergistically with acute hypoglycaemia to induce NRF2 target genes
To examine the impact of hypoglycaemia on Nrf2 and NRF2 target genes Nqo1, Sod2 and Hmox-1, their expression levels were measured in the hippocampus of all control and STZ-diabetes groups (electronic supplementary material [ESM] Table 1). Transcript levels of Nqo1 and Sod2 were significantly elevated in STZ-diabetic mice following acute hypoglycaemia (STZ-LH vs WT-EE; p<0.05 for both genes), and the levels of Sod2 were further increased (>fivefold) in chronic hyperglycaemia. In WT non-diabetic mice, Sod2 and Hmox-1 transcript levels were significantly elevated by hypoglycaemia (WT-LE vs WT-EE; p<0.05). As anticipated, RNA levels of these NRF2 target genes were unaltered in Nrf2-knockout mice (ESM Table 2), demonstrating NRF2 dependence.
Acute hypoglycaemia in STZ-diabetic mice but not in non-diabetic WT mice induces oxidative damage in the hippocampus
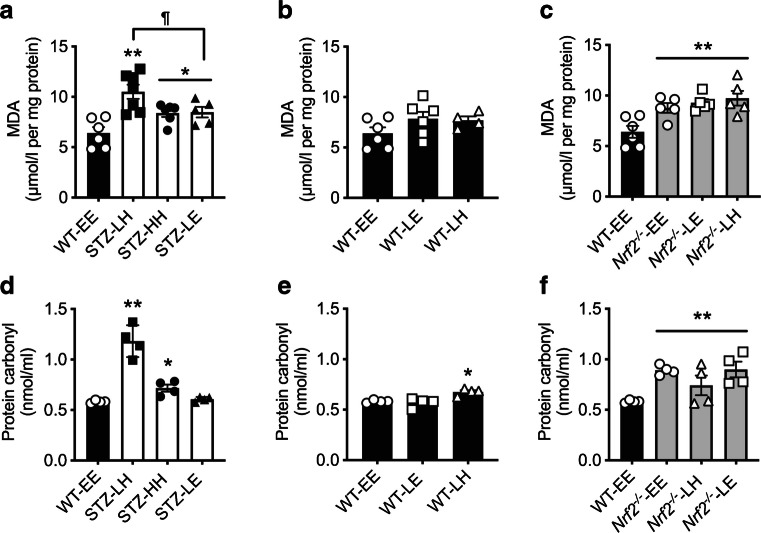
In non-diabetic WT control mice, acute hypoglycaemia did not significantly increase lipid peroxidation irrespective of the glucose level at which the clamp finished (Fig. 2b; WT-EE vs WT-LE, p>0.05; WT-EE vs WT-LH, p>0.05). In contrast, hippocampal lipid peroxidation was significantly increased in all STZ-diabetic models, with the most significant effect seen where there was post-hypoglycaemic hyperglycaemia (Fig. 2a; STZ-LH vs WT-EE, p<0.01). In STZ-diabetes, maintaining post-hypoglycaemic euglycaemia ameliorated this effect (STZ-LE vs STZ-LH, p<0.05). The levels of lipid peroxidation in Nrf2−/− mice were elevated in all conditions when compared with control (WT-EE) mice (Fig. 2c; main effect of genotype, p<0.01).
Fig. 2.
Chronic hyperglycaemia is associated with hippocampal oxidative damage. (a) Levels of hippocampal lipid peroxidation were increased in STZ-diabetic mice (white bars) compared with control (WT) mice (black bars) maintained at euglycaemia. (b) Euglycaemic control mice exposed to an acute episode of hypoglycaemia exhibited no change in hippocampal lipid peroxidation. (c) Euglycaemic Nrf2−/− mice (grey bars) displayed increased levels of hippocampal lipid peroxidation irrespective of hypoglycaemic challenge. (d) Protein carbonylation levels were elevated in STZ-diabetic mice exposed to hyperglycaemia compared with control mice at euglycaemia. (e) Control WT mice exposed to an acute hypoglycaemic episode showed a rise in protein carbonylation only when recovered to a hyperglycaemic state. (f) Nrf2−/− mice displayed increased levels of protein carbonylation irrespective of glycaemic variability. n=4–7/group. Results represent mean values ± SEM. *p<0.05, **p<0.01 vs WT-EE; ¶p<0.05 vs STZ-diabetes (one-way ANOVA followed by Tukey post hoc test). E, euglycaemia; H, high, hyperglycaemia; L, low, hypoglycaemia; MDA, malondialdehyde
Protein carbonylation is commonly used as a biomarker of oxidative damage for many proteins. Levels increase with age, and this increase has been linked to changes in specific enzymes, such as members of the tyrosine kinase family [30], GLUT4 [31] and the 19s and 20s proteasomal subunits [32], and to diseases such as diabetes [33–35]. In non-diabetic WT mice, there was no impact of a single acute hypoglycaemic challenge on levels of carbonylated proteins when returned to euglycaemic levels (Fig. 2e; WT-EE vs WT-LE, p>0.05). In contrast, in STZ-diabetic mice, hypoglycaemia followed by recovery to hyperglycaemia resulted in a marked increase in protein carbonylation (Fig. 2d; WT-EE vs STZ-LH, p<0.01). There were also small but significant increases in carbonylated protein levels in STZ-diabetic mice that had not been exposed to hypoglycaemia (Fig. 2d; WT-EE vs STZ-HH, p<0.05), as well as non-diabetic mice who were exposed to post-hypoglycaemic hyperglycaemia (Fig. 2e; WT-LH vs WT-EE, p<0.05). Interestingly, recovery of STZ-diabetic mice to euglycaemia largely reversed the increase in protein carbonylation (Fig. 2d; WT-EE vs STZ-LE, p>0.05). Notably, levels of carbonylated proteins were significantly elevated in the hippocampus of all Nrf2−/− mice compared with non-diabetic WT mice (Fig. 2f; main effect of genotype, p<0.01).
SILAC quantitative proteomics reveals changes in markers of cellular stress responses to hypoglycaemia
SILAC is a method of accurately quantifying changes in protein expression [24]. In vivo SILAC with label-free proteomics was used to assess changes in hippocampal protein expression in STZ-diabetic mice exposed to post-hypoglycaemic hyperglycaemia (STZ-LH, group E) compared with control mice (WT-EE, group A). This procedure identified 71 proteins that were differentially expressed between groups (ESM Table 3 [upregulated proteins] and ESM Table 4 [downregulated proteins]). Pathway analysis identified significant upregulation of proteins involved in long-chain fatty acid metabolism (predominantly β-oxidation) and components of the proteasome, suggesting an enhanced capacity for long-chain fatty acid oxidation and the degradation of damaged proteins (ESM Table 3). Conversely, significant downregulation of proteins involved in mediating the stress response, including several heat shock proteins, was observed (ESM Table 4).
Dysfunction of markers of protein chaperone function following hypoglycaemia in diabetes
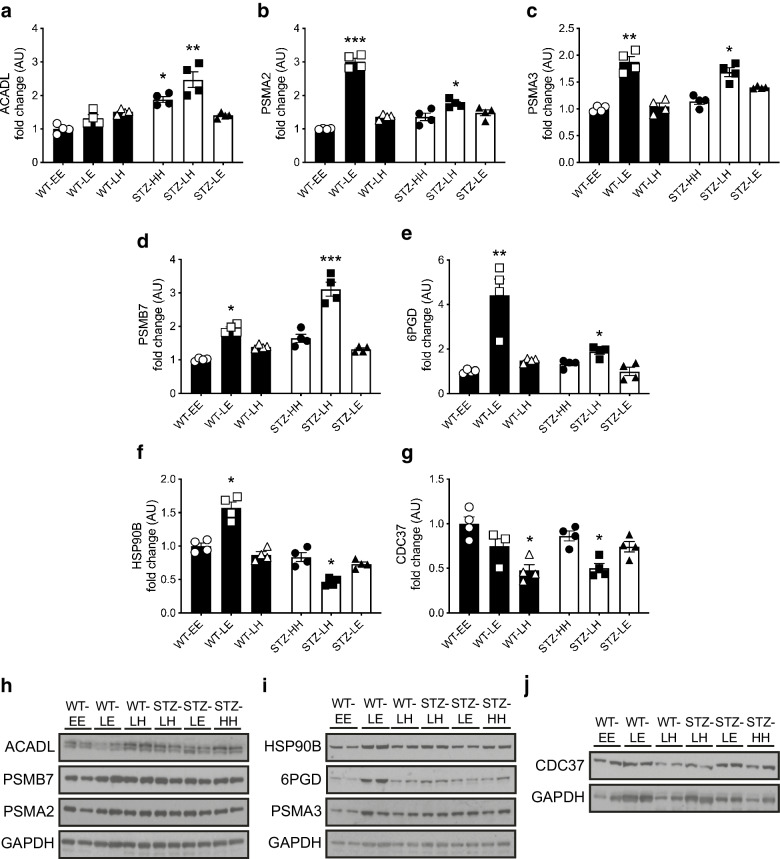
We then examined candidate proteins from the key pathways identified in the SILAC analysis (fatty acid metabolism, proteasomal degradation and chaperone/stress response) across all study groups. ACADL, a mitochondrial protein involved in the initial step of fatty acid β-oxidation, was increased following hypoglycaemia in STZ-diabetic mice, an effect that was not seen when glucose was recovered to euglycaemia (Fig. 3a). In addition, we considered upregulation of 6PGD of interest in relation to the oxidative damage associated with the post-hypoglycaemic hyperglycaemic phase (ESM Table 3). 6PGD is a key enzyme of the oxidative arm of the pentose phosphate pathway (PPP) and the largest contributor to cytosolic NADPH, an important component of cellular antioxidant defences. 6PGD was enhanced in control and STZ-diabetic mice exposed to an acute hypoglycaemic challenge compared with control mice, although the impact of hypoglycaemia was less pronounced in STZ-diabetic mice (Fig. 3e, i; WT-EE vs STZ-LH, p<0.05; WT-EE vs WT-LE, p<0.01).
Fig. 3.
Effect of glycaemic variability on SILAC-outcome selected protein levels in the hippocampus of WT control and STZ-diabetic mice. (a–g) Hippocampal protein levels (ratio of signal intensities to control euglycaemia [WT-EE] data) in mice exposed to an acute hypoglycaemic episode from a euglycaemic (WT control mice, black bars) or hyperglycaemic (STZ-diabetic mice, white bars) baseline and returned to euglycaemia or hyperglycaemia: ACADL (a); PSMA2 (b); PSMA3 (c); PSMB7 (d); 6PGD (e); HSP90B (f); and CDC37 (g). (h–j) Representative immunoblots of ACADL, PSMB7 and PSMA2 (h), HSP90B, 6PGD and PSMA3 (i), and CDC37 (j) and their respective GAPDH loading controls. Results represent mean values ± SEM. *p<0.05, **p<0.01, ***p<0.001 (Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparisons test). E, euglycaemia; H, high, hyperglycaemia; L, low, hypoglycaemia
PSMA2 (Fig. 3b, h), PSMA3 (Fig. 3c, i) and PSMB7 (Fig. 3d, h), which form part of the 20S core structure, were all significantly increased following exposure to hypoglycaemia in both non-diabetic and STZ-diabetic mice (all p<0.05). HSP90, a chaperone protein that assists in correct protein folding and aids degradation of damaged proteins [36], was reduced following hypoglycaemia in STZ-diabetic mice (Fig. 3f, i; WT-EE vs STZ-LH, p<0.05). This contrasts with non-diabetic mice where acute hypoglycaemic challenge induced an increase in expression of HSP90B (Fig. 3f, i; WT-EE vs WT-LE, p<0.05). Similarly, hypoglycaemia in STZ-diabetic but not non-diabetic mice downregulated CDC37, an HSP90B co-chaperone protein (Fig. 3g). Interestingly, this effect was lost when STZ-diabetic mice were recovered to euglycaemia; however, CDC37 was also suppressed in non-diabetic mice recovered from hypoglycaemia to hyperglycaemia, suggesting that post-hypoglycaemic hyperglycaemia suppresses CDC37.
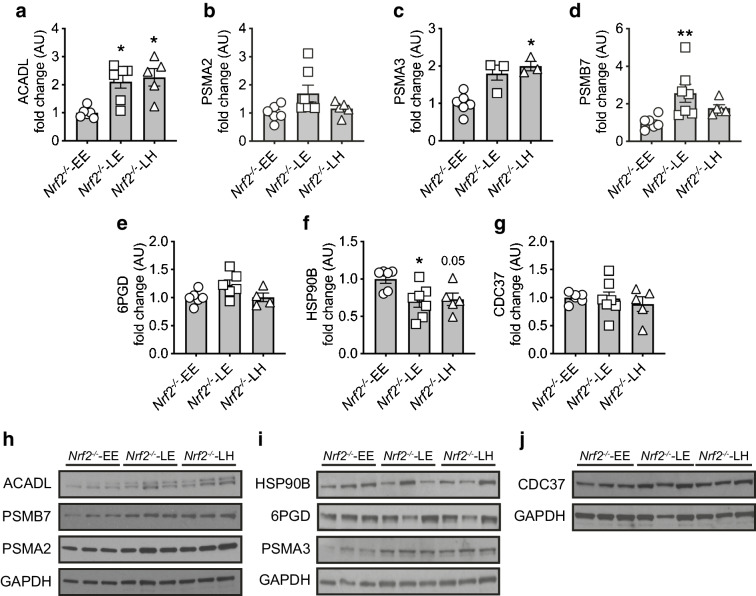
To further examine the role of NRF2 in mediating protection against the oxidative stress associated with both hyper- and hypoglycaemia, we also assessed the impact of acute changes in glycaemia on hippocampal levels of these proteins. Protein abundance of the mitochondrial protein ACADL was significantly elevated, whereas 6PGD did not increase in Nrf2−/− mice (Fig. 4a, e). The increase in 6PGD was also seen in STZ-diabetic mice that had been exposed to acute hypoglycaemia euglycaemia (Fig. 3e). Exposure to hypoglycaemia increased the expression of PSMA3 and PSMB7 (Fig. 4c, i; p<0.05 vs WT-EE; and Fig. 4d, h; p<0.01 vs WT-EE) in Nrf2−/− mouse hippocampus, with a non-statistically significant increase in PSMA2 (Fig. 4b, h; p=0.07). Similarly, the pattern of change in HSP90B after hypoglycaemia in Nrf2−/− mice was also seen in STZ-diabetic mice but not non-diabetic WT mice who experienced post-hypoglycaemia (Fig. 4f). This suggests roles for NRF2 particularly in mediating the increase in reductive biosynthesis and chaperone/stress responses, which appear key pathways in the cellular response to hypoglycaemia.
Fig. 4.
Effect of glycaemic variability on SILAC-outcome selected protein levels in the hippocampus of Nrf2− /− mice. (a–g). Hippocampal protein levels (ratio of signal intensities to Nrf2-/- mice at euglycaemia [Nrf2-/--EE] data) in Nrf2−/− mice exposed to an acute hypoglycaemic episode from a euglycaemic baseline and returned to euglycaemia or hyperglycaemia: ACADL (a); PSMA2 (b); PSMA3 (c); PSMB7 (d); 6PGD (e); HSP90B (f); and CDC37 (g). (h–j) Representative immunoblots of ACADL, PSMB7 and PSMA2 (h), HSP90B, 6PGD and PSMA3 (i), and CDC37 (j), and their respective GAPDH loading controls. Results represent mean values ± SEM. *p<0.05, **p<0.01 (Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparisons test). E, euglycaemia; H, high, hyperglycaemia; L, low, hypoglycaemia
Discussion
In the present study, the hyperinsulinaemic clamp technique was combined with tissue analysis using SILAC proteomics and measures of oxidative stress to reveal a profound disruption in the cellular response to a hypoglycaemic challenge in a mouse model of chemically induced type 1 diabetes that increases the vulnerability of the hippocampus to oxidative damage. Of note, post-hypoglycaemic hyperglycaemia in STZ-diabetes was associated with a downregulation of proteins mediating the stress response and reductive biosynthesis. This is likely to result in proteotoxic stress through a reduced ability of cells to maintain the correct folding of proteins damaged by the stress challenge. This may, in turn, lead to irreversible damage/modification to proteins or synapses between cells within crucial brain regions such as the hippocampus.
In the current study, a single episode of hypoglycaemia in non-diabetic WT mice resulted in significant upregulation of Nrf2 and NRF2 target genes Hmox-1 and Sod2 in WT control mice but with no oxidative damage. This supports long-term studies showing that recurrent non-severe hypoglycaemia in rodents without diabetes has no cognitive sequelae and may even be neuroprotective [13, 36]. NRF2 controls cellular adaptation to oxidative stress and increases during redox perturbation, inflammation and nutrient/energy fluxes, thereby enabling the factor to orchestrate adaptive responses to diverse forms of stress (for review, see [37]). The present study suggests that with a normally functioning NRF2-mediated response to cellular stress, there are no long-term cognitive sequelae to acute hypoglycaemia.
In contrast, when glucose levels were recovered from hypoglycaemia to hyperglycaemia, there was evidence of a small but significant increase in oxidative damage. This is consistent with prior work in neuronal cultures and in vivo models showing that hyperglycaemia after hypoglycaemia results in increased superoxide production and neuronal death [18]. It is of interest that the percentage increases in hippocampal lipid peroxidation and protein carbonylation in the present study is similar to those reported in transgenic mouse models [38, 39] and human post-mortem studies of Alzheimer’s disease [40, 41]. However, it is important to recognise that these represent chronic rather than acute disease models. Another notable finding in the present study is that the increase in hippocampal protein carbonylation was accompanied by a much smaller (1.67-fold vs 4.83-fold) increase in Sod2 expression and the absence of Hmox-1 upregulation in STZ-diabetes compared with non-diabetic WT mice exposed to post-hypoglycaemic hyperglycaemia. This indicates that chronic hyperglycaemia in diabetes may impair the ability to mount a robust antioxidant response.
SILAC labelling and quantitative proteomics of hippocampal tissue revealed that post-hypoglycaemic hyperglycaemia in STZ-diabetic mice resulted in an increased expression of several mitochondrial proteins involved in long-chain lipid-oxidation (hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit α and β [HADHA and HADHB]), lipid transfer (sterol carrier protein 2 [SCP2]) and β-oxidation (ACADL). Previous research has shown a shift towards alternative fuel use following hypoglycaemia [42, 43]. Consistent with this, hypoglycaemia increased levels of ACADL in almost all groups in the current study, including Nrf2−/− mice. The higher levels of ACADL seen in STZ-diabetic mice per se likely reflect increased lipid transport and β-oxidation because of chronic uncontrolled diabetes.
In contrast, clearer differences emerged between groups in the expression of a key enzyme, 6PGD, which sits within the oxidative arm of the PPP. The increase in 6PGD expression, while significant in STZ-diabetic mice exposed to hypoglycaemia, was much smaller than that induced in the non-diabetic WT control mice. Increased flux through this pathway increases the production of the reducing equivalent NADPH required for the reactive biosynthesis of fatty acids and cholesterol and the production of intermediates used in synthesising nucleotides. Increased levels of NADPH are also essential for ameliorating oxidative stress by reducing oxidised glutathione (GSH). Notably, there was no change in 6PGD expression in Nrf2 null mice following hypoglycaemia. This finding is in keeping with a recent report demonstrating that NRF2 regulates the transcription of 6PGD through direct binding to the antioxidant response element within its promoter region [44]. Interruption of glucose supply with reduced PPP and NADPH generation, such as during a hypoglycaemic event in type 1 diabetes (where induction of Sod2 and Hmox-1 is impaired), will further hamper detoxification of ROS and the induction of antioxidant defence proteins. Indeed, previous work has shown that glucose withdrawal abrogates the induction of Hmox-1 by the classical NRF2 activator sulforaphane [45]. This suggests that in STZ-diabetes, there is an impairment in reductive biosynthesis that may increase cellular vulnerability to oxidative stress.
Hypoglycaemia also increased the expression of proteasomal subunits (PSMA2, PSMA3 and PSMB7) in both control and STZ-diabetic mice. The proteasome is an integral part of the ubiquitin–proteasome system (UPS) and corresponding cellular protein quality control (PQC) [46]. If proteasome complex assembly and function are impaired, this can lead to reduced proteolytic activities and the accumulation of damaged or misfolded protein species [47]. In the present study, hypoglycaemia increased levels of proteasomal proteins in all groups, suggesting this response to an oxidative insult is intact, although the rise was less pronounced in Nrf2−/− mice. NRF2 activation has been demonstrated to increase the expression of proteasomal genes and enhance the removal of oxidised proteins following oxidative insult, so this may contribute at least in part to the cellular response to hypoglycaemia [48, 49].
In contrast to the broadly similar impact of hypoglycaemia on the proteasome in all study groups, we found divergent effects of hypoglycaemia on the stress response protein HSP90B when comparing mice with and without STZ-diabetes. Other stress response proteins (heat shock protein 90, α [cytosolic], class A member 1 [HSP-90AA1], heat shock protein family H [HSP110] member 1 [HSPH1] and stress-induced phosphoprotein 1 [STIP1]) were also shown by SILAC to be downregulated in STZ-diabetic mice exposed to hypoglycaemia and recovered to hyperglycaemia. In addition, the HSP90B co-chaperone protein CDC37 was downregulated following acute hypoglycaemia in the STZ-diabetic mice. Interestingly, hypoglycaemia also decreased HSP90B in Nrf2 null mice, independently from CDC37, indicating a possible involvement of NRF2 in this cell protective mechanism. Indeed, STIP1 plays an essential role in the ability of HSP90 to stabilise the NRF2–kelch-like ECH-associated protein 1 (KEAP1) complex [50], supporting functional connectivity between these important cellular stress response pathways. These data suggest that activation of stress response proteins is impaired in STZ-diabetic mice exposed to hypoglycaemia, leading to proteotoxic stress. Furthermore, NRF2 may be required for this aspect of the cellular response to hypoglycaemia.
Limitations of this study include the use of a chemically induced mouse model of type 1 diabetes that does not entirely replicate the human condition, the inclusion of only male mice, and the analysis being performed on the whole hippocampus rather than on isolated neurons or astrocytes. Additionally, lipid peroxidation and protein carbonylation measures provide a global oxidative damage index. Still, they do not allow the identification of specific proteins or pathways that may be directly impacted in this context. It would have been interesting to determine whether there was a correlation between the amount of oxidative damage, depth of hypoglycaemia and degree of post-hypoglycaemic hyperglycaemia, as demonstrated in neuronal cell cultures [18]. However, this requires multiple groups and is best studied ex vivo or in vitro. In addition, it would have been interesting to examine whether normalising glucose levels in the rodent type 1 diabetes model reversed the changes seen. Future studies are planned to address this question.
In conclusion, results from the present study suggest that a functioning NRF2-mediated response to cellular stress in non-diabetic rodents protects the hippocampus from any consequences due to acute non-severe hypoglycaemia. In contrast, in a mouse model of chemically induced type 1 diabetes, the chronic exposure to hyperglycaemia that characterises diabetes (especially when sub-optimally controlled) and post-hypoglycaemic hyperglycaemia result in sufficient oxidative stress to induce oxidative damage in the hippocampus and may then contribute to longer-term cognitive sequelae. Proteomic analysis of hippocampal tissue revealed evidence of disruption in proteins mediating the stress response and reductive biosynthesis in STZ-diabetes mice exposed to a single episode of non-severe hypoglycaemia. This is likely to result in proteotoxic stress through a reduced ability of cells to maintain the correct folding of proteins damaged by the stress challenge and may lead to irreversible damage modification to proteins or synapses between cells within crucial brain regions such as the hippocampus. Future research that more specifically examines underlying mechanisms in neurons, astrocytes and microglia may enable more targeted therapies, such as enhancing NRF2 activity. It is also important to consider the impact of reducing glycaemic variability prior to and/or following hypoglycaemia on oxidative stress in different brain regions.
Supplementary information
(PDF 358 kb)
Acknowledgments
Authors’ relationships and activities
BEG, UPB and RJM are members of the Editorial Board of Diabetologia. RJM has received lecture fees from Sanofi-Aventis and Novo Nordisk. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
ADM, RJM, MLJA and JRG contributed to the design of the work, data collection, analysis and interpretation, and drafting and critical revision of the article. MLE, BEG, UPB, BT, ATDK and JTH contributed to data analysis and interpretation, and drafting and critical revision of the article. All authors approved the final approval of the version to be published. RJM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- ACADL
Long-chain acyl Co-A dehydrogenase
- CDC37
HSP90 co-chaperone
- EDIC
Epidemiology of Diabetes Interventions and Complications
- HSP90B
Heat shock protein 90-β
- NRF2
Nuclear factor erythroid 2-related factor 2
- 6PGD
6-Phosphogluconate dehydrogenase
- PPP
Pentose phosphate pathway
- PSMA2
Proteasome subunit α type-2
- PSMA3
Proteasome subunit α type-3
- PSMB7
Proteasome subunit β type-7
- ROS
Reactive oxygen species
- SILAC
Stable isotope labelling by amino acids in cell culture
- STZ
Streptozotocin
- WT
Wild-type
Funding
This work was supported by the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement no. 777460. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and Type 1 Diabetes Exchange, JDRF, International Diabetes Federation (IDF) and The Leona M. and Harry B. Helmsley Charitable Trust. The industry partners supporting the JU include Abbott Diabetes Care, Eli Lilly, Medtronic, Novo Nordisk and Sanofi-Aventis. This paper reflects the authors’ views and the JU is not responsible for any use that may be made of the information it contains. The University of Cambridge has received salary support for MLE through the National Health Service in the East of England through the Clinical Academic Reserve.
Data availability
The datasets generated during and/or analysed during the current study are available in ProteomeXchange, accession no. 1-20220824-173727 (www.proteomexchange.org). Additional datasets generated during and/or analysed during the present study are available from the corresponding author upon reasonable request.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/12/2024
A Correction to this paper has been published: 10.1007/s00125-024-06188-3
References
- 1.Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia. 2003;46(7):940–948. doi: 10.1007/s00125-003-1128-2. [DOI] [PubMed] [Google Scholar]
- 2.van Duinkerken E, Steenwijk MD, Klein M, et al. Accelerated executive functions decline and grey matter structural changes in middle-aged type 1 diabetes mellitus patients with proliferative retinopathy. J Diabetes. 2018;10(11):835–846. doi: 10.1111/1753-0407.12773. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson AM, Ryan CM, Braffett BH, et al. Cognitive performance declines in older adults with type 1 diabetes: results from 32 years of follow-up in the DCCT and EDIC study. Lancet Diabetes Endocrinol. 2021;9(7):436–445. doi: 10.1016/S2213-8587(21)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson AM, Braffett BH, Erus G et al (2022) Brain structure among middle-aged and older adults with long-standing type 1 diabetes in the DCCT/EDIC study. Diabetes Care. 10.2337/dc21-2438 [DOI] [PMC free article] [PubMed]
- 5.Chaytor NS, Barbosa-Leiker C, Ryan CM, Germine LT, Hirsch IB, Weinstock RS. Clinically significant cognitive impairment in older adults with type 1 diabetes. J Diabetes Complications. 2019;33(1):91–97. doi: 10.1016/j.jdiacomp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA. Severe hypoglycemia and cognitive function in older adults with type 1 diabetes: the study of longevity in diabetes (SOLID) Diabetes Care. 2020;43(3):541–548. doi: 10.2337/dc19-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CM, Klein BEK, Lee KE, Cruickshanks KJ, Klein R. Associations between recent severe hypoglycemia, retinal vessel diameters, and cognition in adults with type 1 diabetes. J Diabetes Complications. 2016;30(8):1513–1518. doi: 10.1016/j.jdiacomp.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duinkerken E, Brands AM, van den Berg E, et al. Cognition in older patients with type 1 diabetes mellitus: a longitudinal study. J Am Geriatr Soc. 2011;59(3):563–565. doi: 10.1111/j.1532-5415.2010.03292.x. [DOI] [PubMed] [Google Scholar]
- 9.Beland-Millar A, Larcher J, Courtemanche J, Yuan T, Messier C. Effects of systemic metabolic fuels on glucose and lactate levels in the brain extracellular compartment of the mouse. Front Neurosci. 2017;11:7. doi: 10.3389/fnins.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004;19(3–4):169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- 11.O'Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 13.McNeilly AD, Gallagher JR, Dinkova-Kostova AT, et al. Nrf2-mediated neuroprotection against recurrent hypoglycemia is insufficient to prevent cognitive impairment in a rodent model of type 1 diabetes. Diabetes. 2016;65(10):3151–3160. doi: 10.2337/db15-1653. [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61(11):2993–2997. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem. 2004;260(1–2):153–159. doi: 10.1023/B:MCBI.0000026067.08356.13. [DOI] [PubMed] [Google Scholar]
- 16.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Alexanian A, Ying R, et al. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol. 2012;32(3):712–720. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117(4):910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marra G, Cotroneo P, Pitocco D, et al. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: a case for gender difference. Diabetes Care. 2002;25(2):370–375. doi: 10.2337/diacare.25.2.370. [DOI] [PubMed] [Google Scholar]
- 20.Santini SA, Marra G, Giardina B, et al. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46(11):1853–1858. doi: 10.2337/diab.46.11.1853. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhry S, Nazmy MH, Meakin PJ, et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48(2):357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 23.McCrimmon RJ, Song Z, Cheng H, et al. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest. 2006;116(6):1723–1730. doi: 10.1172/JCI27775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLeod AK, Fallon PG, Sharp S, Henderson CJ, Wolf CR, Huang JT. An enhanced in vivo stable isotope labeling by amino acids in cell culture (SILAC) model for quantification of drug metabolism enzymes. Mol Cell Proteomics. 2015;14(3):750–760. doi: 10.1074/mcp.M114.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 26.Wisniewski JR, Zougman A, Mann M. Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res. 2009;8(12):5674–5678. doi: 10.1021/pr900748n. [DOI] [PubMed] [Google Scholar]
- 27.McNeilly AD, Gallagher JR, Huang JT, Ashford MLJ, McCrimmon RJ. High-intensity exercise as a dishabituating stimulus restores counterregulatory responses in recurrently hypoglycemic rodents. Diabetes. 2017;66(6):1696–1702. doi: 10.2337/db16-1533. [DOI] [PubMed] [Google Scholar]
- 28.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 29.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32(2):134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- 30.Soreghan BA, Yang F, Thomas SN, Hsu J, Yang AJ. High-throughput proteomic-based identification of oxidatively induced protein carbonylation in mouse brain. Pharm Res. 2003;20(11):1713–1720. doi: 10.1023/b:pham.0000003366.25263.78. [DOI] [PubMed] [Google Scholar]
- 31.Boden G, Homko C, Barrero CA, et al. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;7(304):304re307. doi: 10.1126/scitranslmed.aac4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiken CT, Kaake RM, Wang X, Huang L. Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. 2011;10(5):R110 006924. doi: 10.1074/mcp.M110.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J Proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Sultana R, Perluigi M, Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8(11–12):2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 35.Tanase M, Urbanska AM, Zolla V, et al. Role of carbonyl modifications on aging-associated protein aggregation. Sci Rep. 2016;6:19311. doi: 10.1038/srep19311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53(2):418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- 37.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Loffler T, Flunkert S, Havas D, et al. Neuroinflammation and related neuropathologies in APPSL mice: further value of this in vivo model of Alzheimer's disease. J Neuroinflammation. 2014;11:84. doi: 10.1186/1742-2094-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen L, Chen C, Yang A, Chen Y, Liu Q, Ni J. Redox proteomics identification of specifically carbonylated proteins in the hippocampi of triple transgenic Alzheimer’s disease mice at its earliest pathological stage. J Proteomics. 2015;123:101–113. doi: 10.1016/j.jprot.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45(8):1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 41.Marcus DL, Thomas C, Rodriguez C, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998;150(1):40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 42.Page KA, Williamson A, Yu N, et al. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. 2009;58(5):1237–1244. doi: 10.2337/db08-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weightman Potter PG, Vlachaki Walker JM, Robb JL, et al. Basal fatty acid oxidation increases after recurrent low glucose in human primary astrocytes. Diabetologia. 2019;62(1):187–198. doi: 10.1007/s00125-018-4744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong AJ, Saedi S, Chi NHK, et al. The positive feedback loop between Nrf2 and phosphogluconate dehydrogenase stimulates proliferation and clonogenicity of human hepatoma cells. Free Radic Res. 2020;54(11-12):906–917. doi: 10.1080/10715762.2020.1761547. [DOI] [PubMed] [Google Scholar]
- 45.Heiss EH, Schachner D, Zimmermann K, Dirsch VM. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 2013;1:359–365. doi: 10.1016/j.redox.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleiger G, Mayor T. Perilous journey: a tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014;24(6):352–359. doi: 10.1016/j.tcb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulistio YA, Heese K. The ubiquitin-proteasome system and molecular chaperone deregulation in Alzheimer’s disease. Mol Neurobiol. 2016;53(2):905–931. doi: 10.1007/s12035-014-9063-4. [DOI] [PubMed] [Google Scholar]
- 48.Dreger H, Westphal K, Weller A, et al. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83(2):354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- 49.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23(23):8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Z (2020) The role of Heat Shock Protein 90 in the Keap1/Nrf2 Mediated Oxidative-Stress Response. Electronic Thesis and Dissertation Repository 6761. Available from https://ir.lib.uwo.ca/etd/6761. Accessed 29 March 2023
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 358 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in ProteomeXchange, accession no. 1-20220824-173727 (www.proteomexchange.org). Additional datasets generated during and/or analysed during the present study are available from the corresponding author upon reasonable request.
The datasets generated during and/or analysed during the current study are available in ProteomeXchange, accession no. 1-20220824-173727 (www.proteomexchange.org). Additional datasets generated during and/or analysed during the present study are available from the corresponding author upon reasonable request.