Abstract
Polyploidy is widespread and particularly common in angiosperms. The prevalence of polyploidy in the plant suggests it as a crucial driver of diversification and speciation. The paleopolyploid soybean (Glycine max) is one of the most important crops of plant protein and oil for humans and livestock. Soybean experienced two rounds of whole genome duplication around 13 and 59 million years ago. Due to the relatively slow process of post-polyploid diploidization, most genes are present in multiple copies across the soybean genome. Growing evidence suggests that polyploidization and diploidization could cause rapid and dramatic changes in genomic structure and epigenetic modifications, including gene loss, transposon amplification, and reorganization of chromatin architecture. This review is focused on recent progresses about genetic and epigenetic changes during polyploidization and diploidization of soybean and represents the challenges and potentials for application of polyploidy in soybean breeding.
Keywords: Polyploidy, Diploidization, Genetic regulation, Epigenetic changes, Soybean
Introduction
Polyploidy, the presence of two or more sets of chromosomes in an organism or cell, is a prominent feature of species evolution of eukaryotes including plants, animals, and fungi (Chen 2007). Polyploidy provides increased organism complexity and novel genetic combinations and has been suggested as a key driver of speciation and biodiversification (del Pozo and Ramirez-Parra 2015). Polyploidy is widespread in plants and estimates indicate two rounds of ancestral whole genome duplications (WGDs) occurred before the divergence of extant seed plants and angiosperms (Jiao et al. 2011). Neopolyploids are classified into two main categories, autopolyploids and allopolyploids, according to their origin and composition of chromosomes (Chen 2007; Song and Chen 2015). Autopolyploids arise by genome duplication of a single species, whereas allopolyploids result from hybridized species following with genome doubling or spontaneously crossing related species via unreduced gametes. Allopolyploids are considered more common than autopolyploids especially in plants, as allopolyploidy fixes both heterozygosity and genetic redundancy which contribute to immediate acquisition of novel traits and improved adaptation to various natural environments (Van de Peer et al. 2017). Although polyploids have some adaptive advantage and could occupy new habitats, polyploidy was traditionally considered an evolutionary dead end (Arrigo and Barker 2012). Polyploidy entails several drawbacks. For example, WGD could cause meiotic irregularities and results in reduced fertility compared with the diploid progenitors (Parisod et al. 2010). After WGD, polyploids undergo rapid and dynamic changes in genomic structure and gene expression (Conant et al. 2014). Genome downsizing is observed in many polyploids over evolutionary timescales, which may be due to natural selection as part of the diploidization process (Doyle and Coate 2019). The diploidization involves chromosomal rearrangement, pseudogenization and subfunctionalization of genes, transposable element (TE) elimination or amplification, and epigenetic changes (Wolfe 2001; Li et al. 2021). Most land plants, such as maize and soybean, are known to be paleopolyploids (ancient polyploids) which have undergone diploidization (Rieseberg and Willis 2007; Wood et al. 2009; Jiao et al. 2011).
Soybean (Glycine max) is a well-documented paleopolyploid and one of the most important sources of plant protein and oil for humans and livestock. Cytogenetic studies have shown that most papilionoids are diploids with x = 10 or 11, whereas soybean has 2n = 40 chromosomes. Soybean and other papilionoid legumes share an ancient WGD which is estimated to have occurred around 59 million years ago (MYA) (Lavin et al. 2005; Schmutz et al. 2010, 2014). In addition, soybean experienced an independent genus Glycine-specific WGD at ~ 13 MYA (Schmutz et al. 2010). Based on classical and molecular taxonomy, the genus Glycine is composed of two subgenera, Soja and Glycine. The subgenus Soja includes cultivated soybean (Glycine max) and its wild progenitor (Glycine soja), both of which are annual species. The subgenus Glycine is indigenous to Australia and contains around 30 perennial species (Doyle et al. 2003; Sherman-Broyles et al. 2014). The perennial Glycine species could be divided into eight different genome groups (A–F, H, I) within which species are reproductively compatible (Sherman-Broyles et al. 2014). The perennial soybeans include diploid (2n = 38 or 40) and polyploid (2n = 78 or 80) species, the former confined to Australia and neighboring islands and the latter more widespread (Doyle et al. 2003). Of the wild perennial Glycine species, Glycine tomentella is a species complex consisting of four cytotypes (2n = 38, 40, 78, 80). An apparent aneuploidy event leading to formation of the G. tomentella D1 lineage (2n = 38) occurred at some point since its common ancestor with G. tomentella D3 lineage (2n = 40) within the last 1.8 MYA (Sherman-Broyles et al. 2014). Aneuallotetraploid (DDEE, AAEE; 2n = 78) and allotetraploid (AADD; 2n = 80) G. tomentella were produced by somatic chromosome doubling of 2n = 39 and 2n = 40 F1 hybrids (Ratnaparkhe et al. 2011). Due to the relatively slow process of diploidization, most genes are present in multiple copies across the soybean genome (Schmutz et al. 2010). Therefore, soybean offers a good model for studying effects of polyploidization and diploidization on species evolution.
In this review, we describe the recent progresses in polyploidization and diploidization during soybean evolution. We want to provide an overview of the current knowledge about the genetic and epigenetic changes promoted by polyploidization and diploidization in soybean. Furthermore, we want to discuss the challenges and potentials for application of polyploidy in soybean breeding.
The genetic changes in paleopolyploid soybean
Following polyploidization, duplicated genomes experience nonequivalent genomic changes, including chromosomal rearrangements, gene translocations and transpositions, and transposon insertions (Adams and Wendel 2005). Due to the multiple rounds of WGD and genome reorganization by diploidization, duplicated genomic segments can involve multiple chromosomes in soybean genome (Schmutz et al. 2010). About 38.6% of the homoeologous genes are found in blocks that are distributed in more than two chromosomes (Schmutz et al. 2010). Uneven DNA fragmentation levels are observed in chromosomes of soybean genome (Williams 82), of which chromosome 14 seems to be a highly fragmented chromosome with block matches to the highest number (14) of all chromosomes (Schmutz et al. 2010). In both Brassica and maize genomes, the homoeologs in the underfractionated subgenome tend to have higher levels of gene expression and lower rates of TE accumulation than homoeologs in the overfractionated subgenome, which is called subgenome dominance (Zhao et al. 2017; Cheng et al. 2012; Schnable et al. 2011). However, subgenome dominance is not observed in soybean and there is also no evidence for unbalanced fractionation between duplicated syntenic blocks (Garsmeur et al. 2014; Zhao et al. 2017). Subgenome dominance is suggested to be associated with the degree of differences between the subgenomes when polyploidization occurs (Garsmeur et al. 2014). It is proposed that the two subgenomes are far less distinct prior to polyploidization in soybean than those in maize (Zhao et al. 2017). Although high percentage (50%) of duplicate pairs are retained in soybean, at least 27.9% of the duplicated blocks have changed orientations, and 23.3% of the duplicated block pairs have experienced switches from euchromatic to heterochromatic status or vice versa (Zhao et al. 2017).
Analyses of synthetic polyploids show that genome downsizing and gene loss often arise with the onset of polyploidization (Gaeta et al. 2007; Liu et al. 1998; Kashkush et al. 2002). The rate of gene loss following the Glycine-specific WGD is 4.36% of genes per million years, which is higher than that (1.28% of genes per million years) following the early-legume WGD (Schmutz et al. 2010). This differential in gene loss rates suggests gene loss is very rapid shortly after WGD, subsequently slowing down. The duplicated genes could undergo progressive loss, pseudogenization, subfunctionalization, and neofunctionalization after WGD (Lynch and Force 2000). One of the more intriguing aspects of chromosomal rearrangement concerns which duplicated genes are lost and which are retained following polyploidy. The soybean genes can be divided into WGD genes, small-scale duplicated genes (tandem, proximal, and dispersed duplicated genes), and singleton genes (Wang et al. 2012). The ratio of single-copy genes to WGD genes in pericentromeric regions is 7.3 times higher than that in chromosomal arms, indicating biased accumulation of singletons in pericentromeric regions (Du et al. 2012). The single-copy genes are not randomly distributed among gene families in polyploid species (Duarte et al. 2010; De Smet et al. 2013). Genes that are convergently restored to single copy after WGD have been referred to as “duplication-resistant” (DR) genes (Duarte et al. 2010; Paterson et al. 2006). There is strong correlation between gene category and functional feature (Xu et al. 2018). For example, photosynthesis genes are over‐represented in singletons, whereas transcription factors (TFs) are more prone to be enriched in WGD genes (Xu et al. 2018; Moharana and Venancio 2020). The TF expansions are strongly associated with WGD events and the expanded TFs are preferentially transcribed in roots and nodules in soybean and other legume species, implying their role in the evolution of nodulation in the legumes (Moharana and Venancio 2020). The DR genes consistently belong to functional GO categories such as “housekeeping roles” and “DNA repair” (De Smet et al. 2013; Duarte et al. 2010). Although two copies are retained in WGD genes, the two copies might have experienced subfunctionalization (function divergence) or neofunctionalization (gain of new function). The divergence of expression between WGD gene pairs increases with age of WGD event (Xu et al. 2018).
The WGD genes exhibit significantly higher expression levels and are expressed in more organs than the small-scale duplicated genes and the singletons (Fig. 1) (Du et al. 2012; Wang et al. 2021b; Kim et al. 2015). Interestingly, the singletons are more frequently tandemly duplicated than the WGD genes in soybean, which may counteract the genome imbalance caused by gene loss (Zhao et al. 2017). The WGD genes have significantly lower Ka (nonsynonymous nucleotide substitution rate) than the singletons, whereas no significant difference is observed in Ks (synonymous nucleotide substitution rate) between the WGD genes and the singletons (Du et al. 2012). The WGD genes may have undergone a stronger level of functional constraint than the singletons. On the other hand, the Ka/Ks (the ratio of nonsynonymous to synonymous nucleotide substitution rate) is significantly lower in the copies of WGD genes with higher expression levels relative to the homoeologs with lower expression levels, suggesting greater selective constraint on the higher-expression members of WGD genes (Zhao et al. 2017). Accordingly, TEs are significantly farther from the higher expression copies of WGD genes. In addition, the mean Ks of WGD genes in the chromosomal arms is significantly higher than that of their homoeologous copies in the pericentromeric regions, suggesting asymmetric evolution of WGD gene pairs with different chromatin status (Du et al. 2012).
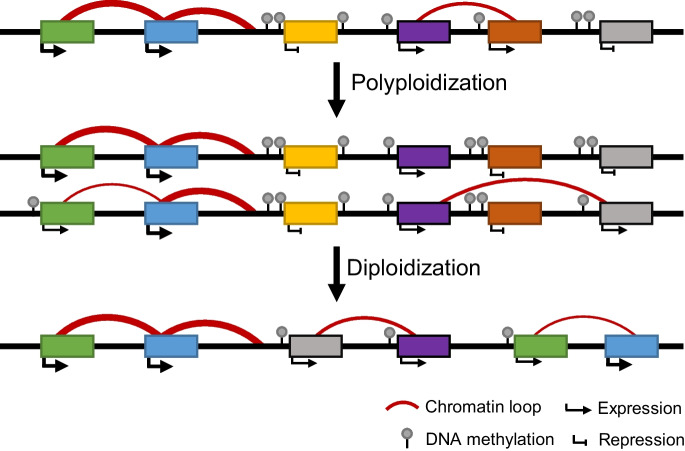
Fig. 1.
Epigenetic regulation of gene expression during polyploidization and diploidization of soybean. The changes of chromatin loops and DNA methylation contribute to expression variations and gene rearrangement during polyploidization and diploidization of soybean. The thickness of arrow and red curve indicates expression level and chromatin interaction intensity, respectively
The epigenetic regulation in paleopolyploid soybean
In addition to genetic changes, various epigenetic changes including DNA methylation and histone modifications occur during polyploidization and diploidization in plant (Song et al. 2017; Liu et al. 2021; Wang et al. 2018). These epigenetic modifications can alter homoeologous gene expression and reprogram gene expression networks, which allows polyploids to establish new habits and promote adaptation in local environments (Song and Chen 2015). Compared with singletons and small-scale duplicated genes, WGD genes display more long-range chromosomal interactions and are coupled with higher levels of active histone marks and chromatin accessibilities but void of DNA methylation around transcription sites in soybean (Fig. 1) (Wang et al. 2021b). However, CG body-methylated genes were abundant in WGD genes compared with singletons (Kim et al. 2015). In contrast to CG methylation, the highest non-CG methylation is observed in gene body of singletons (Kim et al. 2015). In soybean, epigenetic modifications are likely involved in pseudogenization followed by gene loss after WGD (El Baidouri et al. 2018). Within the gene body, highly expressed genes showed higher levels of CG methylation and lower levels of non-CG methylation than the underexpressed homoeologs in WGD genes (Kim et al. 2015). In addition, higher-expressed copies in WGD genes were coupled with more chromatin interactions as well as higher levels of chromatin accessibilities and activated histone modifications (Fig. 1) (Wang et al. 2021b). In contrast, the WGD gene pairs that were not differentially expressed showed similar levels of chromatin contacts, chromatin accessibilities, and epigenetic modifications (Wang et al. 2021b).
Recent studies suggest polyploidization could reshape the higher-order chromatin architecture in polyploid plants (Wang et al. 2018; Jia et al. 2021; Concia et al. 2020; Yuan et al. 2022). Compared with common bean, soybean underwent Glycine-specific polyploidy and diploidization. More orthologous genes are located in topologically associated domains (TADs) in soybean than in common bean, which suggests that polyploidy and subsequent diploidization may facilitate formation and distribution of TAD (Wang et al. 2021b). The soybean genome experienced dramatic chromosomal rearrangements during diploidization (Schmutz et al. 2010). Chromosomal rearrangements are overrepresented in the regions with higher levels of chromatin contacts, DNA accessibility, and active histone modifications (Wang et al. 2021b). In addition, chromosomal rearrangements tend to take place outside of TADs during diploidization of soybean (Wang et al. 2021b), indicating chromatin architecture is involved in chromosomal rearrangements during diploidization.
Not only protein-encoding genes (PEGs), but also the MIRNA genes have experienced dramatic changes following recent WGD of soybean (Zhao et al. 2015). The MIRNA genes show significantly higher ratio of singletons to duplicates than PEGs in soybean genome (Zhao et al. 2015). The overrepresentation of MIRNA singletons is proposed to be caused by a combination of deletion of one of the two copies of duplicated MIRNA pairs, asymmetric tandem duplication of copies of MIRNA gene pairs, and sequence variation within amplified TEs (Zhao et al. 2015). Strikingly, PEG targets of MIRNA duplicates tend to be retained as duplicates compared with PEG targets of MIRNA singletons, suggesting coevolution between MIRNAs and their PEG targets during polyploidization and diploidization of soybean (Zhao et al. 2015).
Impact of polyploidy on stress tolerance and biotic interactions
The origin of many polyploids coincides with major periods of global climatic change and/or periods of mass extinction (Van de Peer et al. 2017). The analyses of a large number plant genomes and transcriptomes suggest that a wave of WGDs occurred close to the Cretaceous-Paleogene (K-P) boundary (Fawcett et al. 2009). The K-P boundary is marked by a number of cataclysmic events, such as a meteor impact near Chicxulub in Mexico. Numerous studies have suggested that polyploids occupy extremely dry and cold habitats that the related diploids cannot in those same clades (Van de Peer et al. 2021; Syngelaki et al. 2020; Gunn et al. 2020). It has long been recognized that the levels of unreduced gametes can increase in response to external stimuli, such as environmental stress (Van de Peer et al. 2021). For example, the frequency of higher-order polyploids increases with latitude in the Arctic (Rice et al. 2019). Many natural and synthetic polyploids show increased abiotic tolerance (Van de Peer et al. 2021). For instance, tetraploid Arabidopsis, tetraploid rice, hexaploid wheat, and allopolyploid Glycine dolichocarpa exhibit increased salt stress tolerance compared to their related diploids or tetraploids (Chao et al. 2013; Wang et al. 2021a; Yang et al. 2014; Luo et al. 2017; Coate et al. 2013). In polyploids, a number of genes show extensive expression changes under salt stress, including genes related to jasmonic acid synthesis and signaling and genes encoding potassium-channel proteins (Wang et al. 2021a; Chao et al. 2013).
Besides the increased abiotic tolerance, polyploid leguminous plants show enhanced interactions with bacteria and fungi. For instance, in synthetic neotetraploid alfalfa (Medicago sativa subsp. caerulea), nodule size and the internal interface are immediately increased relative to the related diploid alfalfa (Forrester and Ashman 2020). The allopolyploid Glycine dolichocarpa shows enhanced nodulation responses relative to the diploid progenitors, accompanied with non-additive expression of genes associated with hormonal signaling and nodulation (Powell and Doyle 2017). Such an effect can increase the efficiency of nitrogen fixation by rhizobial symbionts, expand the range of abiotic tolerances, and provide resistance to root pathogens (Van de Peer et al. 2021; Powell and Doyle 2017, 2016). Ploidy-mediated changes in root exudates are significantly different between diploids and polyploids, which modify the rhizosphere soil conditions in terms of nutrients, microbes, and soil nematodes (Segraves 2017). These modifications may change the entire network of species within community (Segraves 2017). Therefore, the polyploid leguminous plant can increase abiotic and biotic tolerance through gene expression and metabolism changes or indirect effects on mutualisms and rhizosphere soil conditions.
Conclusions and prospects
The increase of soybean production becomes a great challenge for worldwide demand due to extreme climate changes, environmental pollution, and shrinking farmland (Burroughs et al. 2022; da Silva et al. 2021). Over the last few decades, high-density planting, intercropping, and utilization of saline-alkali land have proven to be effective in increasing soybean production (Vogel et al. 2021; Liu et al. 2020). Studies on diverse species have shown polyploid plants often exhibit enhanced tolerance to biotic and abiotic stresses relative to their diploid counterparts (Wang et al. 2021a; Yang et al. 2014; Tossi et al. 2022; Chao et al. 2013). Soybean is a salt-sensitive crop, and its production is severely affected by saline soils (Phang et al. 2008). Development of neotetraploid soybean by artificial polyploidization likely promotes tolerance to abiotic stresses including salt and heat for soybean (Fig. 2), which could accelerate utilization of saline‐alkali land and thus enhance increase of soybean production.
Fig. 2.
Development of autotetraploid and allotetraploid soybeans. Autopolyploidy and allopolyploidy have the potential to optimize multiple traits in soybean, including tolerance to abiotic stresses, pathogen resistance, seeds per pod, podding rate, seed weight, and nitrogen fixation efficiency
Severe genetic bottlenecks have been incurred during soybean domestication and improvement (Zhou et al. 2015). Wild relatives offer a vast gene pool for genetic improvement of cultivated soybean. The subgenus Glycine harbors desirable agronomic traits for soybean improvement, including resistance to cyst nematode and fungal pathogens, tolerance to drought and salt stresses, and large number of seed set per pod (Zhuang et al. 2022; Sherman-Broyles et al. 2014). Hybridization between cultivated soybean and perennial Glycine species causes inviable seed formation (Singh 2019). The immature seed rescue is applied to overcome interspecific hybridization barriers between soybean and wild perennial species and obtain intersubgeneric plants (Singh and Nelson 2015), although the efficiency of immature seed rescue is low and the genetic introgression from perennial species in soybean is successfully achieved only using G. tomentella as the maternal parent. Polyploidization and epimutagenesis could effectively bypass interspecific hybridization barrier in Arabidopsis and Capsella species (Josefsson et al. 2006; Huc et al. 2022). Developing effective methods for overcoming crossability barriers will contribute to generate allotetraploid soybean and introgression lines by crossing soybean with perennial species (Fig. 2), which could transfer beneficial trait of perennial species into cultivated soybean to meet the growing demand for soybean.
Author contribution
J.Y. drafted the manuscript. Q.S. conceived the article and revised the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD1201400).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
On behalf of all authors, the corresponding author provides the consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Soybean Functional Genomics.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams KL, Wendel JF. Polyploidy and genome evolution in plants. Curr Opin Plant Biol. 2005;8(2):135–141. doi: 10.1016/j.pbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Arrigo N, Barker MS. Rarely successful polyploids and their legacy in plant genomes. Curr Opin Plant Biol. 2012;15(2):140–146. doi: 10.1016/j.pbi.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Burroughs CH, Montes CM, Moller CA, Mitchell NG, Michael AM, Peng B, Kimm H, Pederson TL, Lipka AE, Bernacchi CJ, Guan K, Ainsworth EA. Reductions in leaf area index, pod production, seed size and harvest index drive yield loss to high temperatures in soybean. J Exp Bot. 2022 doi: 10.1093/jxb/erac503. [DOI] [PubMed] [Google Scholar]
- Chao DY, Dilkes B, Luo H, Douglas A, Yakubova E, Lahner B, Salt DE. Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science. 2013;341(6146):658–659. doi: 10.1126/science.1240561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol. 2007;58:377–406. doi: 10.1146/annurev.arplant.58.032806.103835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Wu J, Fang L, Sun S, Liu B, Lin K, Bonnema G, Wang X. Biased gene fractionation and dominant gene expression among the subgenomes of Brassica rapa. PLoS One. 2012;7(5):e36442. doi: 10.1371/journal.pone.0036442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate JE, Powell AF, Owens TG, Doyle JJ. Transgressive physiological and transcriptomic responses to light stress in allopolyploid Glycine dolichocarpa (Leguminosae) Heredity (edinb) 2013;110(2):160–170. doi: 10.1038/hdy.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant GC, Birchler JA, Pires JC. Dosage, duplication, and diploidization: clarifying the interplay of multiple models for duplicate gene evolution over time. Curr Opin Plant Biol. 2014;19:91–98. doi: 10.1016/j.pbi.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Concia L, Veluchamy A, Ramirez-Prado JS, Martin-Ramirez A, Huang Y, Perez M, Domenichini S, Rodriguez Granados NY, Kim S, Blein T, Duncan S, Pichot C, Manza-Mianza D, Juery C, Paux E, Moore G, Hirt H, Bergounioux C, Crespi M, Mahfouz MM, Bendahmane A, Liu C, Hall A, Raynaud C, Latrasse D, Benhamed M. Wheat chromatin architecture is organized in genome territories and transcription factories. Genome Biol. 2020;21(1):104. doi: 10.1186/s13059-020-01998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RFB, Vina A, Moran EF, Dou Y, Batistella M, Liu J. Socioeconomic and environmental effects of soybean production in metacoupled systems. Sci Rep. 2021;11(1):18662. doi: 10.1038/s41598-021-98256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet R, Adams KL, Vandepoele K, Van Montagu MC, Maere S, Van de Peer Y. Convergent gene loss following gene and genome duplications creates single-copy families in flowering plants. Proc Natl Acad Sci U S A. 2013;110(8):2898–2903. doi: 10.1073/pnas.1300127110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Ramirez-Parra E. Whole genome duplications in plants: an overview from Arabidopsis. J Exp Bot. 2015;66(22):6991–7003. doi: 10.1093/jxb/erv432. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Coate JE. Polyploidy, the nucleotype, and novelty: the impact of genome doubling on the biology of the cell. Int J Plant Sci. 2019;180(1):1–52. doi: 10.1086/700636. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL, Rauscher JT, Brown AHD. Diploid and polyploid reticulate evolution throughout the history of the perennial soybeans (Glycine subgenus Glycine) New Phytol. 2003;161(1):121–132. doi: 10.1046/j.1469-8137.2003.00949.x. [DOI] [Google Scholar]
- Du J, Tian Z, Sui Y, Zhao M, Song Q, Cannon SB, Cregan P, Ma J. Pericentromeric effects shape the patterns of divergence, retention, and expression of duplicated genes in the paleopolyploid soybean. Plant Cell. 2012;24(1):21–32. doi: 10.1105/tpc.111.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Wall PK, Edger PP, Landherr LL, Ma H, Pires JC, Leebens-Mack J, dePamphilis CW. Identification of shared single copy nuclear genes in Arabidopsis, Populus, Vitis and Oryza and their phylogenetic utility across various taxonomic levels. BMC Evol Biol. 2010;10:61. doi: 10.1186/1471-2148-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Baidouri M, Kim KD, Abernathy B, Li YH, Qiu LJ, Jackson SA. Genic C-methylation in soybean is associated with gene paralogs relocated to transposable element-rich pericentromeres. Mol Plant. 2018;11(3):485–495. doi: 10.1016/j.molp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Fawcett JA, Maere S, Van de Peer Y. Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event. Proc Natl Acad Sci U S A. 2009;106(14):5737–5742. doi: 10.1073/pnas.0900906106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester NJ, Ashman TL. Autopolyploidy alters nodule-level interactions in the legume-rhizobium mutualism. Am J Bot. 2020;107(2):179–185. doi: 10.1002/ajb2.1375. [DOI] [PubMed] [Google Scholar]
- Gaeta RT, Pires JC, Iniguez-Luy F, Leon E, Osborn TC. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell. 2007;19(11):3403–3417. doi: 10.1105/tpc.107.054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsmeur O, Schnable JC, Almeida A, Jourda C, D’Hont A, Freeling M. Two evolutionarily distinct classes of paleopolyploidy. Mol Biol Evol. 2014;31(2):448–454. doi: 10.1093/molbev/mst230. [DOI] [PubMed] [Google Scholar]
- Gunn BF, Murphy DJ, Walsh NG, Conran JG, Pires JC, Macfarlane TD, Birch JL. Evolution of Lomandroideae: multiple origins of polyploidy and biome occupancy in Australia. Mol Phylogenet Evol. 2020;149:106836. doi: 10.1016/j.ympev.2020.106836. [DOI] [PubMed] [Google Scholar]
- Huc J, Dziasek K, Pachamuthu K, Woh T, Kohler C, Borges F. Bypassing reproductive barriers in hybrid seeds using chemically induced epimutagenesis. Plant Cell. 2022;34(3):989–1001. doi: 10.1093/plcell/koab284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Xie Y, Cheng J, Kong C, Wang M, Gao L, Zhao F, Guo J, Wang K, Li G, Cui D, Hu T, Zhao G, Wang D, Ru Z, Zhang Y. Homology-mediated inter-chromosomal interactions in hexaploid wheat lead to specific subgenome territories following polyploidization and introgression. Genome Biol. 2021;22(1):26. doi: 10.1186/s13059-020-02225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473(7345):97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Josefsson C, Dilkes B, Comai L. Parent-dependent loss of gene silencing during interspecies hybridization. Curr Biol. 2006;16(13):1322–1328. doi: 10.1016/j.cub.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Kashkush K, Feldman M, Levy AA. Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics. 2002;160(4):1651–1659. doi: 10.1093/genetics/160.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, El Baidouri M, Abernathy B, Iwata-Otsubo A, Chavarro C, Gonzales M, Libault M, Grimwood J, Jackson SA. A comparative epigenomic analysis of polyploidy-derived genes in soybean and common bean. Plant Physiol. 2015;168(4):1433–1447. doi: 10.1104/pp.15.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin M, Herendeen PS, Wojciechowski MF. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst Biol. 2005;54(4):575–594. doi: 10.1080/10635150590947131. [DOI] [PubMed] [Google Scholar]
- Li Z, McKibben MTW, Finch GS, Blischak PD, Sutherland BL, Barker MS. Patterns and processes of diploidization in land plants. Annu Rev Plant Biol. 2021;72:387–410. doi: 10.1146/annurev-arplant-050718-100344. [DOI] [PubMed] [Google Scholar]
- Liu B, Vega JM, Feldman M. Rapid genomic changes in newly synthesized amphiploids of Triticum and Aegilops. II. Changes in low-copy coding DNA sequences. Genome. 1998;41(4):535–542. doi: 10.1139/g98-052. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhang M, Feng F, Tian Z. Toward a “Green Revolution” for soybean. Mol Plant. 2020;13(5):688–697. doi: 10.1016/j.molp.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yuan J, Jia G, Ye W, Jeffrey Chen Z, Song Q. Histone H3K27 dimethylation landscapes contribute to genome stability and genetic recombination during wheat polyploidization. Plant J Cell Mol Biol. 2021;105(3):678–690. doi: 10.1111/tpj.15063. [DOI] [PubMed] [Google Scholar]
- Luo Q, Peng M, Zhang X, Lei P, Ji X, Chow W, Meng F, Sun G. Comparative mitochondrial proteomic, physiological, biochemical and ultrastructural profiling reveal factors underpinning salt tolerance in tetraploid black locust (Robinia pseudoacacia L.) BMC Genomics. 2017;18(1):648. doi: 10.1186/s12864-017-4038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154(1):459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharana KC, Venancio TM. Polyploidization events shaped the transcription factor repertoires in legumes (Fabaceae) Plant J Cell Mol Biol. 2020;103(2):726–741. doi: 10.1111/tpj.14765. [DOI] [PubMed] [Google Scholar]
- Parisod C, Holderegger R, Brochmann C. Evolutionary consequences of autopolyploidy. New Phytol. 2010;186(1):5–17. doi: 10.1111/j.1469-8137.2009.03142.x. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Chapman BA, Kissinger JC, Bowers JE, Feltus FA, Estill JC. Many gene and domain families have convergent fates following independent whole-genome duplication events in Arabidopsis, Oryza. Saccharomyces Tetraodon Trends Genet. 2006;22(11):597–602. doi: 10.1016/j.tig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Phang TH, Shao G, Lam HM. Salt tolerance in soybean. J Integr Plant Biol. 2008;50(10):1196–1212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Powell AF, Doyle JJ (2017) Non-additive transcriptomic responses to inoculation with rhizobia in a young allopolyploid compared with its diploid progenitors. Genes (Basel) 8(12). 10.3390/genes8120357 [DOI] [PMC free article] [PubMed]
- Powell AF, Doyle JJ. Enhanced rhizobial symbiotic capacity in an allopolyploid species of Glycine (Leguminosae) Am J Bot. 2016;103(10):1771–1782. doi: 10.3732/ajb.1600060. [DOI] [PubMed] [Google Scholar]
- Ratnaparkhe MB, Singh RJ, Doyle JJ. Glycine. In: Kole C, editor. Wild crop relatives: genomic and breeding resources. Berlin, Germany: Springer; 2011. pp. 83–116. [Google Scholar]
- Rice A, Smarda P, Novosolov M, Drori M, Glick L, Sabath N, Meiri S, Belmaker J, Mayrose I. The global biogeography of polyploid plants. Nat Ecol Evol. 2019;3(2):265–273. doi: 10.1038/s41559-018-0787-9. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317(5840):910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Schmutz J, McClean PE, Mamidi S, Wu GA, Cannon SB, Grimwood J, Jenkins J, Shu S, Song Q, Chavarro C, Torres-Torres M, Geffroy V, Moghaddam SM, Gao D, Abernathy B, Barry K, Blair M, Brick MA, Chovatia M, Gepts P, Goodstein DM, Gonzales M, Hellsten U, Hyten DL, Jia G, Kelly JD, Kudrna D, Lee R, Richard MM, Miklas PN, Osorno JM, Rodrigues J, Thareau V, Urrea CA, Wang M, Yu Y, Zhang M, Wing RA, Cregan PB, Rokhsar DS, Jackson SA. A reference genome for common bean and genome-wide analysis of dual domestications. Nat Genet. 2014;46(7):707–713. doi: 10.1038/ng.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable JC, Springer NM, Freeling M. Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proc Natl Acad Sci U S A. 2011;108(10):4069–4074. doi: 10.1073/pnas.1101368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves KA. The effects of genome duplications in a community context. New Phytol. 2017;215(1):57–69. doi: 10.1111/nph.14564. [DOI] [PubMed] [Google Scholar]
- Sherman-Broyles S, Bombarely A, Powell AF, Doyle JL, Egan AN, Coate JE, Doyle JJ. The wild side of a major crop: soybean’s perennial cousins from Down Under. Am J Bot. 2014;101(10):1651–1665. doi: 10.3732/ajb.1400121. [DOI] [PubMed] [Google Scholar]
- Singh RJ. Cytogenetics and genetic introgression from wild relatives in soybean. Nucleus. 2019;62(1):3–14. doi: 10.1007/s13237-019-00263-6. [DOI] [Google Scholar]
- Singh RJ, Nelson RL. Intersubgeneric hybridization between Glycine max and G. tomentella: production of F(1), amphidiploid, BC(1), BC(2), BC(3), and fertile soybean plants. Theor Appl Genet. 2015;128(6):1117–1136. doi: 10.1007/s00122-015-2494-0. [DOI] [PubMed] [Google Scholar]
- Song Q, Chen ZJ. Epigenetic and developmental regulation in plant polyploids. Curr Opin Plant Biol. 2015;24:101–109. doi: 10.1016/j.pbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Zhang T, Stelly DM, Chen ZJ. Epigenomic and functional analyses reveal roles of epialleles in the loss of photoperiod sensitivity during domestication of allotetraploid cottons. Genome Biol. 2017;18(1):99. doi: 10.1186/s13059-017-1229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syngelaki E, Schinkel CCF, Klatt S, Horandl E. Effects of temperature treatments on cytosine-methylation profiles of diploid and autotetraploid plants of the alpine species Ranunculus kuepferi (Ranunculaceae) Front Plant Sci. 2020;11:435. doi: 10.3389/fpls.2020.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossi VE, MartínezTosar LJ, Laino LE, Iannicelli J, Regalado JJ, Escandón AS, Baroli I, Causin HF, Pitta-Álvarez SI. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front Plant Sci. 2022;13:869423. doi: 10.3389/fpls.2022.869423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y, Mizrachi E, Marchal K. The evolutionary significance of polyploidy. Nat Rev Genet. 2017;18(7):411–424. doi: 10.1038/nrg.2017.26. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y, Ashman TL, Soltis PS, Soltis DE. Polyploidy: an evolutionary and ecological force in stressful times. Plant Cell. 2021;33(1):11–26. doi: 10.1093/plcell/koaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JT, Liu W, Olhoft P, Crafts-Brandner SJ, Pennycooke JC, Christiansen N. Soybean yield formation physiology - a foundation for precision breeding based improvement. Front Plant Sci. 2021;12:719706. doi: 10.3389/fpls.2021.719706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang P, Lin M, Ye Z, Li G, Tu L, Shen C, Li J, Yang Q, Zhang X. Evolutionary dynamics of 3D genome architecture following polyploidization in cotton. Nat Plants. 2018;4(2):90–97. doi: 10.1038/s41477-017-0096-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Jia G, Jiang X, Cao S, Chen ZJ, Song Q. Altered chromatin architecture and gene expression during polyploidization and domestication of soybean. Plant Cell. 2021;33(5):1430–1446. doi: 10.1093/plcell/koab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao S, Wang P, Lu K, Song Q, Zhao FJ, Chen ZJ (2021a) DNA hypomethylation in tetraploid rice potentiates stress-responsive gene expression for salt tolerance. Proc Natl Acad Sci U S A 118(13). 10.1073/pnas.2023981118 [DOI] [PMC free article] [PubMed]
- Wolfe KH. Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet. 2001;2(5):333–341. doi: 10.1038/35072009. [DOI] [PubMed] [Google Scholar]
- Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci U S A. 2009;106(33):13875–13879. doi: 10.1073/pnas.0811575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Nadon BD, Kim KD, Jackson SA. Genetic and epigenetic divergence of duplicate genes in two legume species. Plant Cell Environ. 2018;41(9):2033–2044. doi: 10.1111/pce.13127. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhao L, Zhang H, Yang Z, Wang H, Wen S, Zhang C, Rustgi S, von Wettstein D, Liu B. Evolution of physiological responses to salt stress in hexaploid wheat. Proc Natl Acad Sci U S A. 2014;111(32):11882–11887. doi: 10.1073/pnas.1412839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Sun H, Wang Y, Li L, Chen S, Jiao W, Jia G, Wang L, Mao J, Ni Z, Wang X, Song Q. Open chromatin interaction maps reveal functional regulatory elements and chromatin architecture variations during wheat evolution. Genome Biol. 2022;23(1):34. doi: 10.1186/s13059-022-02611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Meyers BC, Cai C, Xu W, Ma J. Evolutionary patterns and coevolutionary consequences of MIRNA genes and microRNA targets triggered by multiple mechanisms of genomic duplications in soybean. Plant Cell. 2015;27(3):546–562. doi: 10.1105/tpc.15.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Zhang B, Lisch D, Ma J. Patterns and consequences of subgenome differentiation provide insights into the nature of paleopolyploidy in plants. Plant Cell. 2017;29(12):2974–2994. doi: 10.1105/tpc.17.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Jiang Y, Wang Z, Gou Z, Lyu J, Li W, Yu Y, Shu L, Zhao Y, Ma Y, Fang C, Shen Y, Liu T, Li C, Li Q, Wu M, Wang M, Wu Y, Dong Y, Wan W, Wang X, Ding Z, Gao Y, Xiang H, Zhu B, Lee SH, Wang W, Tian Z. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat Biotechnol. 2015;33(4):408–414. doi: 10.1038/nbt.3096. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Wang X, Li X, Hu J, Fan L, Landis JB, Cannon SB, Grimwood J, Schmutz J, Jackson SA, Doyle JJ, Zhang XS, Zhang D, Ma J. Phylogenomics of the genus Glycine sheds light on polyploid evolution and life-strategy transition. Nat Plants. 2022;8(3):233–244. doi: 10.1038/s41477-022-01102-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.