Abstract
Upon understanding the boosting role of carotenoids on the endogenous anti-inflammatory system, it is vital to explore their role in reducing the use of high doses of non-steroidal anti-inflammatory drug (NSAIDs), and their mediated secondary toxicity during the treatment of chronic diseases. The current study investigates the carotenoids potential on inhibition of secondary complications induced by NSAIDs, aspirin (ASA) against lipopolysaccharide (LPS) stimulated inflammation. Initially, this study evaluated a minimal cytotoxic dose of ASA and carotenoids (β-carotene, BC/lutein, LUT/astaxanthin, AST/fucoxanthin FUCO) in Raw 264.7, U937, and peripheral blood mononuclear cells (PBMCs). In all three cells, carotenoids + ASA treatment reduced the LDH release, NO, and PGE2 efficiently than an equivalent dose of carotenoid or ASA treated alone. Based on cytotoxicity and sensitivity results, RAW 264.7 cells were selected for further cell-based assay. Among carotenoids, FUCO + ASA exhibited an efficient reduction of LDH release, NO, and PGE2 than the other carotenoids (BC + ASA, LUT + ASA, and AST + ASA) treatment. FUCO + ASA combination decreased LPS/ASA induced oxidative stress, pro-inflammatory mediators (iNOS, COX-2, and NF-κB), and cytokines (IL-6, TNF-α, and IL-1β) efficiently. Further, apoptosis was inhibited by 69.2% in FUCO + ASA, and 46.7% in ASA than LPS treated cells. A drastic decrease in intracellular ROS generation with the increase in GSH was observed in FUCO + ASA compared to LPS/ASA groups. The results documented on the low dose of ASA with a relative physiological concentration of FUCO suggested greater importance for alleviating secondary complications and optimize prolonged chronic disease treatments with NSAID’s associated side effects.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03632-w.
Keywords: Carotenoids, Aspirin, Secondary toxicity, Reactive oxygen species, Macrophages
Introduction
Global incidences of chronic diseases drastically escalated due to systemic inflammation mediated by oxidative stress under many circumstances (Reuter et al. 2010; GBD 2017). Currently, upon understanding the boosting role of carotenoids on the endogenous anti-inflammatory system, it is important to elucidate the carotenoid's influence on higher doses of NSAIDs and mediated toxicity during chronic disease conditions. Natural compounds such as terpenoids, flavonoids, polyphenols, and vitamins are recognized as pivotal bioactive dietary supplements and they found to be associated with a decreased risk of chronic diseases. Recently, bio-functionality of natural compounds has been concomitant with the intracellular signalling cascades, thereby influencing the gene expression related to progression or control of specific health problems, including cancers and other chronic diseases (Knekt et al.2002; Fletcher and Fairfield 2002; Han et al. 2005; Fiedor and Burd 2014; Cory et al. 2018; Raju et al.2021). Among natural compounds, carotenoids were extensively screened from the past 3 decades due to their predominant accumulation in human tissues and their circulation in the blood via dietary consumption of fruits, vegetables, and microbial sources (Khachik 1997; Sommerburg et al. 1998; Scarmo et al. 2010; Kotake-Nara and Nagao 2011; Eggersdorfer and Wyss 2018). Epidemiological and clinical studies demonstrate that regular dietary intake of carotenoids-rich foods reduce the risk of atherosclerosis, cancers, and age-related degenerative diseases. In this context, many notable studies have correlated the plasma carotenoid level and decreased risk of oxidative stress and inflammatory responses (Haegele et al. 2000; Hozawa et al. 2007; Thomson et al. 2007; Buttala et al. 2012; Kaulmann and Bohn 2014; Cocate et al. 2015; Park et al. 2022). Though, the influence of carotenoids on biochemical and molecular levels of anti-inflammatory activity is exploring previously. However, the efficiency of individual or mixture of carotenoids at relative physiological concentrations against secondary toxicity/complications induced by potent anti-inflammatory drugs is not detailed. Generally, the progression of chronic diseases occurs through the production of pro-inflammatory cytokines, interleukin, tumor necrosis factor (TNF-α), and mediators of inflammation, for instance, reactive oxygen species (ROS), reactive nitrogen species (RNS), and prostaglandin E2 (PGE2) in cells and tissues (Kaulmann and Bohn 2014). In general, macrophages are preferred to evaluate in vitro, since they perform three vital functions such as antigen presentation, phagocytosis, and immunomodulation through the production of cytokines and growth factors. Further, macrophages play a role in the initiation, maintenance, and anti-inflammation process, as they take part in the auto regulatory loop to produce a wide range of signals, which involves both beneficial and detrimental effects in inflammation. Therefore, therapeutic intervention studies targeted macrophages to explore the various bioactive molecules, including carotenoids to manage inflammatory diseases. It has been known that the generation of above optimal levels of oxidative stress elicits a wide range of responses in the macrophages, such as activation of P13K, PTEN, NIK, and MAPKs signaling mediators linked to NF-κB regulation (Han et al. 2005; Kaulmann and Bohn 2014; Finkel and Holbrook 2000; Li and Engelhardt 2006). Currently, administration of many potent anti-inflammatory drugs and nutrients is practiced for treating chronic diseases, such as cardiovascular diseases and cancer; however, they might possess adverse events, especially when exposed to higher doses with prolonged treatment (Rayburn et al. 2009; Hossain et al. 2012; Ittaman et al. 2014; McNeil et al. 2018). Currently, clinicians recommend and practice synthetic drugs to treat chronic inflammatory disease. However, chronic usage of potent non-steroidal anti-inflammatory drugs (NSAIDs) results in gastrointestinal ulcerations, bleeding, hepato-renal dysfunction, organ failure, and skin reactions (Rainsford 1999; Sostres et al. 2010; Morris et al. 2009; Lavie et al. 2017; Rothwell et al. 2018). Optimization of drug doses for effective control of inflammation with lesser/nil side effects is of utmost importance and needs to be evaluated precisely. The benefit of such targeted treatments or therapy may depend on a delicate balance between conquer inflammation and inferring normal cellular function. In this context, selectively targeting specific NF-κB subunits, IκB proteins, or kinases through natural compounds may improve therapeutic efficacy and minimize systemic toxicity. Previously, we demonstrated that secondary toxicity inhibition in normal cells when treated with anticancer drug plus carotenoids without compromising cytotoxicity and selective killing of cancer cells. Further, we hypothesized that the mechanistic difference of anticancer drug doses on the mitigation of secondary toxicity and improved therapeutic efficiency of a low dose of the anticancer drug with carotenoids on cancer therapy (Vijay et al. 2018). Although carotenoids and their products are identified in blood and tissues, no studies were attempted to correlate their role in chronic inflammation-mediated or secondary complications of high dose NSAID drug treatments. Nevertheless, studies have reported that carotenoids inhibit pro-inflammatory mediators and exert anti-inflammatory effects by inhibiting MAPKs, including JNK, ERK, p38, and NF-κB regulation in lipopolysaccharide -induced pro-inflammation (Kaulmann and Bohn 2014; Choi et al. 2016; Zhao et al. 2017). Aspirin (acetylsalicylic acid, ASA) is a widely known anti-inflammatory drug used to treat chronic inflammatory conditions by inhibiting pro-inflammatory mediators in humans and animals (Thun et al. 2002; Dannenberg and DuBois 2003; Bosetti et al 2009; Cuzick et al. 2009; Elwood et al. 2009). In contrast to these aspects, several reports have demonstrated secondary complications of prolonged usage of the moderately high dose of NSAID, particularly aspirin (Chan et al. 2005; Rothwell et al. 2018). The mechanism of these drugs is possibly mediated through the hindrance of prostaglandin-endoperoxide synthase or cyclooxygenase (COX) enzymes (Rainsford 1999). The cytotoxic damage of ROS/RNS depends on their concentration and balance between endogenous antioxidant molecules or enzymes. When this equilibrium is imbalanced, cells are exposed to the above threshold levels of reactive oxygen species thereby inducing oxidative stress. Under oxidative stress or an imbalanced situation, cells required external antioxidants to mitigate the oxidative stress, thus avoiding oxidative-mediated insults. Oxidative stress is an important factor in the biomedical realm since it is associated with various chronic diseases. Carotenoids are well-known antioxidants that can block oxidative stress by interacting with the nuclear factor erythroid 2–related factor 2 pathway, enhancing its translocation into the nucleus, and activating antioxidant enzymes. In this context, upon understood the potential anti-inflammatory function of carotenoids, we hypothesized to test the role of carotenoids against higher dose of NSAIDs and its associated secondary toxicity in chronic diseases treatments. Further, we used macrophages cells as model system to explore carotenoids role in minimization of high dose of aspirin mediated secondary toxicity in macrophage cells pre-treated with LPS. We intend to test and evaluate the influence of carotenoids which are recognized as vital molecules circulated in blood and tissue with a minimal dose of aspirin as a potent drug molecule with no or less secondary toxicity in Raw 264.7, U937, and peripheral blood mononuclear cells (PBMCs). Also, the current study also covered the therapeutic efficiency of different carotenoids of unique structural properties combined with aspirin against impeding secondary complications.
Materials and methods
Chemicals
Standard lutein (99%), β-carotene (98%), astaxanthin (99%), fucoxanthin (98%) bovine serum albumin, propidium iodide (PI), Poly-d-lysine, 4,6-diamidino-2-phenylindole dilactate (DAPI), propidium iodide (PI), poly-d-lysine, acridine orange (AO), ethidium bromide (EtBr), aspirin (ASA), and cell culture grade dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF) (stabilized with 0.25% BHT) were obtained from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals and solvents used were of analytical and HPLC grade purchased from Sisco Research Laboratories (Mumbai, India). Dulbecco's modified eagle medium (DMEM), Roswell park memorial institute medium (RPMI), fetal bovine serum, antibiotic–antimycotic solution, calcium, and magnesium-free phosphate buffer saline, 3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), trypan blue, cell culture consumables and neutral aluminum oxide (particle size: 70–230 mesh) were obtained from Hi-Media chemical laboratories (Mumbai, India). PE Annexin-V Apoptosis detection (Batch: 6,179,925) assay kit was purchased from BD pharmingen (BD Bioscience, San Diego, CA), ELISA kit (R and D Systems Inc., MN, USA). BCA assay reagent procured from Thermoscientific (USA). β-actin, iNOS, TNF-α, COX-2, IL-1β, IL-6, and NF-κB primary antibodies purchased from Cell Signaling Technology, Inc (USA). Goat anti-rabbit/mouse IgG-HRP secondary antibodies and western blotting luminol reagent purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). CLX-posure™ film (8 × 10 inches) procured from Thermo Fisher Scientific (USA).
LC–MS (APCI) + analysis
The isolated carotenoids from spinach [β-carotene (BC) and lutein (LUT)], shrimp [astaxanthin (AST)], and seaweed [fucoxanthin (FUCO)] by open column chromatography were quantified (n = 3) from the HPLC peak area of respective reference standards before cell culture treatments (Sangeetha et al. 2010; Sowmya et al. 2017). The peak identity and λmax of individual isolated carotenoids were confirmed with their retention time, UV–Vis, and LC–MS (APCI)+ spectra of standards recorded under similar conditions (Lakshminarayana et al. 2008). The details of conditions adopted for purification and quantification were similar to the previously described (Vijay et al. 2018). The purified carotenoids fractions obtained by column chromatography showed high purity (%) of BC (97 ± 2), LUT (96 ± 2.3), AST (95 ± 3), and FUCO (96 ± 2.1) under standard condition.
Culture and treatments of PBMCs, RAW 264.7 and U937 cells
Peripheral blood mononuclear cells (PBMCs) were isolated according to the method of Yoshiaki et al. (1999) with slight modifications. In brief, 10 mL of blood was drawn from a healthy volunteer and added into a tube containing 0.5 M EDTA. Diluted 10 mL blood (1:1 dilution with PBS) was carefully layered over 10 mL of Ficoll-Paque gradient and centrifuged (1600 rpm for 30 min at 4 °C). The centrifuged sample consists of different layers from top to bottom as in the following order; Plasma–Platelets–PBMC–Ficoll–Red blood cells (with granulocytes). The upper plasma and platelets layer discarded, and the buffy coat of PBMCs is carefully aspirated and transferred to a fresh tube. Then, PBS added to PBMCs to make up the volume to 50 ml and centrifuged (1200 rpm for 10 min at 18 °C). The supernatant fraction discarded, and the pellet was re-suspended in PBS and centrifuged again (3000 rpm for 10 min at 18 °C) to obtain a cell pellet, and this process repeated three times. Then pellet was mixed in RPMI-1640 medium, then PBMCs cells counted and seeded (density of 4 × 105) in a tissue culture grade 96-well plate for experiments detailed elsewhere. Macrophage cells (RAW 264.7 and U937) were procured from NCCS (Pune, India). PBMCs (RPMI) and RAW 264.7 (DMEM) cells were cultured as a monolayer, and U937 cells (RPMI) were cultured as a suspension in media supplemented with 10% heat-inactivated fetal bovine serum, 100 U penicillin, and 100 μg/mL streptomycin. Cells maintained in 95% of humidified atmospheric oxygen and 5% CO2 at 37 ºC in a carbon dioxide incubator. Macrophages wee regularly passaged (twice a week), and passages between 4 and 20 were considered for experiments. Exponentially growing cells (70–90%) were seeded (5 × 103 cells/ well) in a 96-well plate supplemented with 200 μL culture medium and incubated for 24 h. Then, cultures were pre-stimulated with 1 μg/mL of lipopolysaccharide (LPS), subsequently replenish with 200 μL serum-free media containing 20 μM carotenoids (BC or LUT or AST or FUCO), and parallel control was maintained and incubated for another 24 h. In this study, we chose 20 μM carotenoids as a saturated concentration for screening to establish the anti-inflammatory activity of selected cell lines (Sowmya et al. 2015). Based on the cell survival and sensitivity to carotenoid treatment on PBMCs and macrophages (reduced % cell viability), we selected RAW 264.7 cells as a better model for further drug interaction study (Supplementary Fig. 1). For the elucidation of anti-inflammatory drug interaction with carotenoids, we selected aspirin (ASA) as a chemical model drug (a common drug widely used to treat chronic inflammation) to understand the secondary complications in the prospect of prolonged usage Vs chronic inflammation (Cuzick et al. 2009; Elwood et al. 2009; Graham 2006; Carlson et al. 2013). Before the drug interaction study, we screened different ASA concentrations ranging from 0 to 3 µM and selected 0.8 µM as a minimal cytotoxic dose (85–90% cell viability) to test all other biochemical and molecular levels in combined treatment with carotenoids. Briefly, cells were treated with 20 µM purified carotenoids (BC or LUT or AST or FUCO) with a minimal anti-inflammatory drug of ASA as established. The combination of 20 µM carotenoid (Sugawara et al. 2001) with 0.8 µM ASA resulted in a higher percentage of cell death within 6 h of treatment. Therefore, we used lower carotenoid concentration (2–10 µM) for 12 h treatment. Unfortunately, the selected carotenoids concentrations were relatively equivalent to attainable physiological concentrations of 0.4–5.0 µM as reported earlier (Kaplan et al. 1990; Prince and Frisoli 1993; Liu et al. 2000; Hadad 2012). Each carotenoid is delivered separately with a suitable solvent system (Sowmya et al. 2015). BC and LUT were delivered in THF, whereas AST and FUCO were delivered in DMSO. For combination treatment of ASA and carotenoid prepared in a respective solvent system and delivered one after the other within a permissible limit not to exceed 0.5%. Vehicles used for the delivery of specific carotenoids treated alone were considered as control groups. Carotenoid combination with ASA was solubilised with DMSO and diluted with DMEM before cell treatment. After combination treatment, cell viability was measured by MTT assay (Lakshminarayana et al. 2013). The viability of control cells is represented as 100% (Vijay et al. 2018). The percentage of viable and dead cells was analyzed using a trypan blue dye exclusion method. The cells were trypsinized, washed with PBS, and counted before and after each experiment using a hemocytometer (Sowmya et al. 2015).
Cellular uptake of carotenoids
Macrophages cells (106 /mL) treated (12 h) with aspirin plus carotenoids were harvested, washed with DMEM, and extracted carotenoid (s) with suitable solvent systems. Carotenoids content of all the treated cells was quantified by HPLC and expressed after subtracting the carotenoids content of adherent to the cell surface determined at 4 °C (Sowmya et al. 2017).
Lactate dehydrogenase assay
The cytotoxicity induced by carotenoids plus ASA or only ASA or without them (control) was quantitated by measuring lactate dehydrogenase (LDH) release using the LDH assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific, USA). Cytotoxicity of experimental samples was measured as %LDH release against control cells without LPS treatment. Cells treated with 1% Triton X-100 are referred to as positive control. A value of > 5% LDH release into the culture media was considered cytotoxic.
Nitric oxide scavenging assay
Nitric oxide (NO) scavenging activity of carotenoids plus ASA was measured as per the established procedure with slight modifications (Islam et al. 2013). In brief, PBMCs and macrophages cells (106 cells/mL) were seeded separately on 24-well tissue culture plates and pre-incubated for 12 h at 37 °C to achieve stable culture adherence. Further, LPS-stimulated cells treated either with or without carotenoids plus ASA or incubated only ASA for another 12 h to monitor NO production by measuring nitrite levels in the culture media using Griess reagent.
Determination of PGE2 levels
PBMCs and macrophages (106 cells/ mL) pre-incubated with LPS were treated with carotenoids plus ASA or only ASA or without them (control) for 12 h. After incubation, a PGE2 level in the macrophage cell lysate was determined using an enzyme immunoassay kit (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. Celecoxib (3 μM) was used as a positive control (Soontornchaiboon et al. 2012). Based on the efficiency of cytoprotection and toxicity levels, further, we selected RAW264.7 cells as a better model system and FUCO (epoxy-carotenoid) as an efficient carotenoid molecule to elucidate other cell-based assays unless otherwise mentioned.
Measurement of pro-inflammatory cytokines production
The inhibitory effect of carotenoid combination with ASA on the pro-inflammatory cytokines (IL-1β and TNF-α) and COX2 production in LPS (1 μg/ml) treated macrophage was assessed according to the manufacturer protocol using mouse ELISA kit (R and D Systems Inc., MN, USA). The supernatant of cell lysate was used for measuring pro-inflammatory cytokines.
Apoptosis detection by Annexin FITC and PI staining
Apoptosis detection was performed with FITC Annexin V apoptosis detection kit (BD pharmingen, BD Bioscience, San Diego, CA) according to the manufacturer’s instructions. Briefly, after 12 h (LPS stimulated) of incubation of macrophages either with or without carotenoids plus ASA or only ASA treatments were harvested, washed with ice-cold PBS, and then centrifuged at 3000 rpm for 5 min at 4 °C. The cell pellet was re-suspended in ice-cold binding buffer, then added FITC Annexin V (1 µL/mL) and PI (10 µL/mL) solutions were in a sample tube and incubated at room temperature for 15 min in dark condition before flow cytometry analyses (Sowmya et al. 2015). The typical apoptotic morphological changes and nuclear condensation of cells were observed under a fluorescence microscope using appropriate staining similar to a previously reported our group (Sowmya et al. 2017).
Analysis of cell cycle distribution, reactive oxygen species, and glutathione levels.
Cells (106 cells) treated (12 h) with carotenoids plus ASA or only ASA were harvested, washed, and re-suspended in PBS. Then cells were stained with DCF-DA (10 μM) for 15 min in 5% CO2 at 37 °C and immediately subjected to FACS analysis. The intercellular ROS levels were measured after calibration of flow cytometry with negative (unstained) and positive control (50 μM H2O2) cells. The results expressed as fluorescence intensity of dichlorofluorescein in control, treatments, and positive control. For the analysis of GSH, cell lysate was prepared and used as per the previous method with slight modifications (Sowmya et al. 2017). The cell cycle progression of treated cells was analyzed using flow cytometry (Vijay et al. 2018). Briefly, after incubation either with control or LPS or ASA* or ASA + FUCO were harvested and washed with the ice-cold PBS and centrifuged at 3000 rpm for 5 min at 4 ºC. The cells grown as a monolayer included both harvested by trypsinization and floating in the medium. The cell pellet was re-suspended in an ice cold binding buffer, followed by FITC Annexin V (1 μL/mL) and PI (10 μL/mL) solutions were added. Sample tubes incubated for 15 min in a dark at room temperature before flow cytometry analyses (Sowmya et al. 2017). The processing of cell pellet, cell lysate, protein estimation, and reagent preparation is done as per the standardized procedures by our group Sowmya et al. (2015).
Western blot analysis
Cells (10 × 106 cells) treated with ASA and its combination with FUCO were washed twice with ice-cold PBS and lysed using ice-cold lysis buffer, and then centrifuged at 10,000 rpm for 10 min at 4 °C to obtain a supernatant. The total protein content in the supernatant of cell lysate was estimated using a BCA assay. Cell lysate (30 μg protein) was separated on 10% SDS-PAGE and electro-blotted onto nitrocellulose membranes. Then, membranes were blocked for 1 h using a blocking buffer and incubated overnight at 4 °C with respective antibodies such as β-actin (loading control), iNOS, TNF-α, COX-2, IL-1β, IL-6, and NF-κB. The primary antibody was detected using HRP labeled goat anti-rabbit/mouse immunoglobulin G secondary antibodies. A chemiluminescence kit (Santa Cruz Biotechnology, Santa Cruz, CA) was used to visualize the protein bands. Three X-ray film exposures (10–15 min) of the same blots were scanned and calculated protein expression levels using densitometry analysis in each membrane.
Statistical analysis
The data were analyzed for normality using Shapiro–wilk test. It was rejected that means the data was non-normal. Since the objective of the present work was to compare the effect of different groups and different levels of carotenoids concentration on % cell viability. Two-way ART ANOVA used whenever the effect was found significant. Post hoc test (pairwise comparisons using BH method) was carried out to find the source of significance. The data and results were represented graphically using boxplots and interaction plots.
Results
Influence of a combination of different carotenoids with ASA on macrophages and PBMCs cell viability.
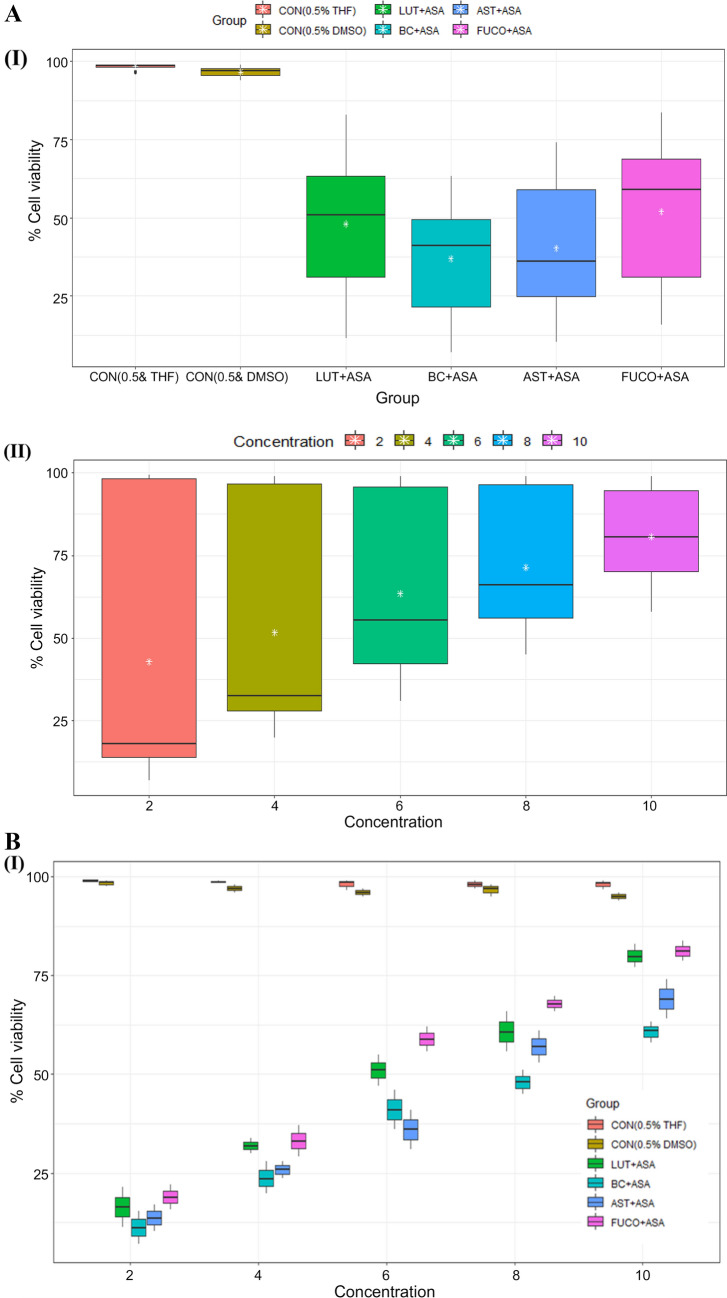
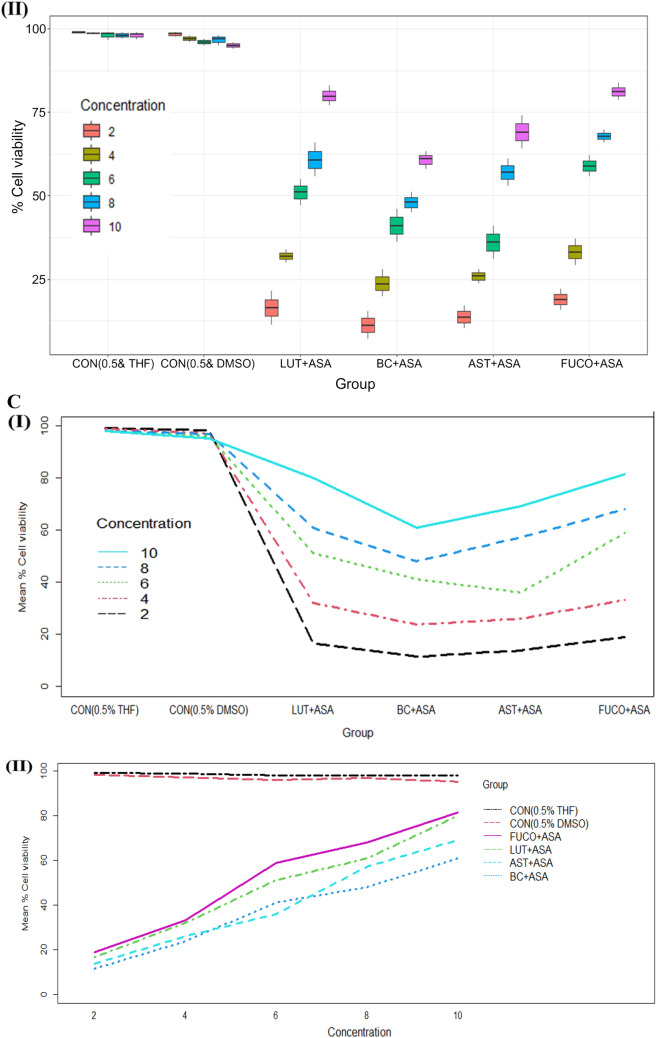
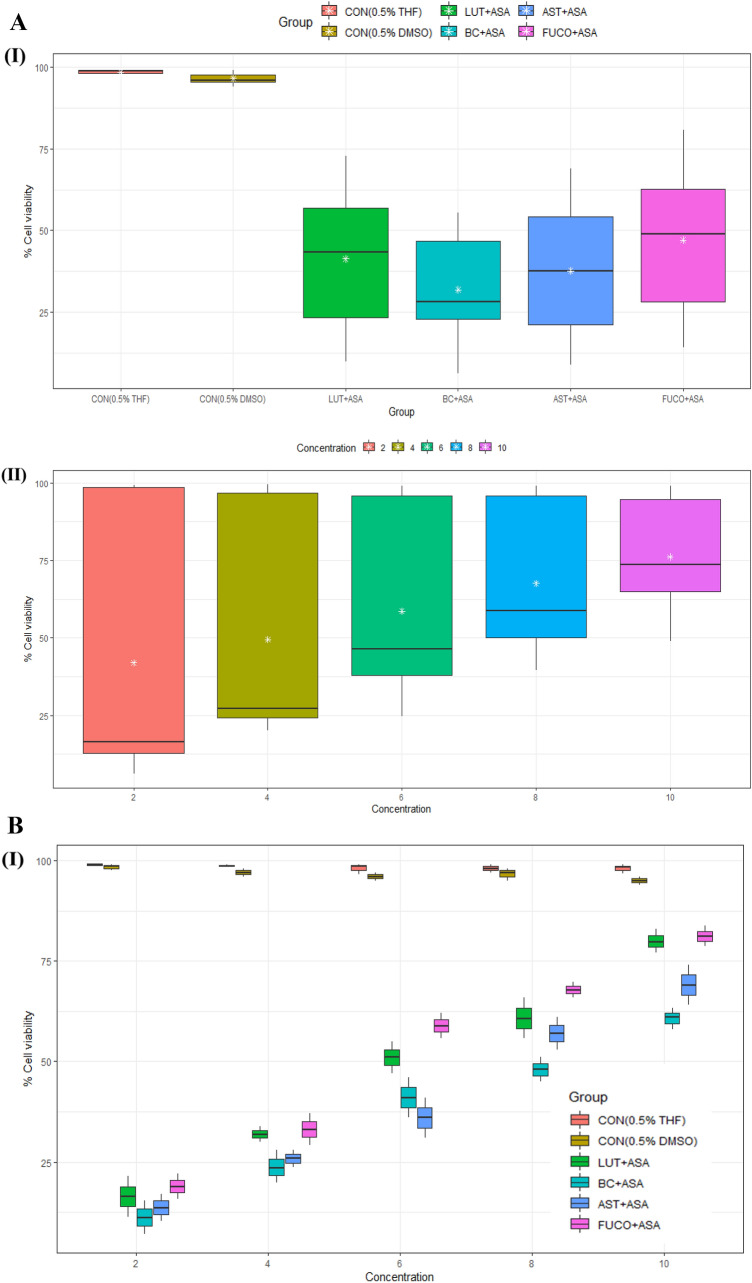
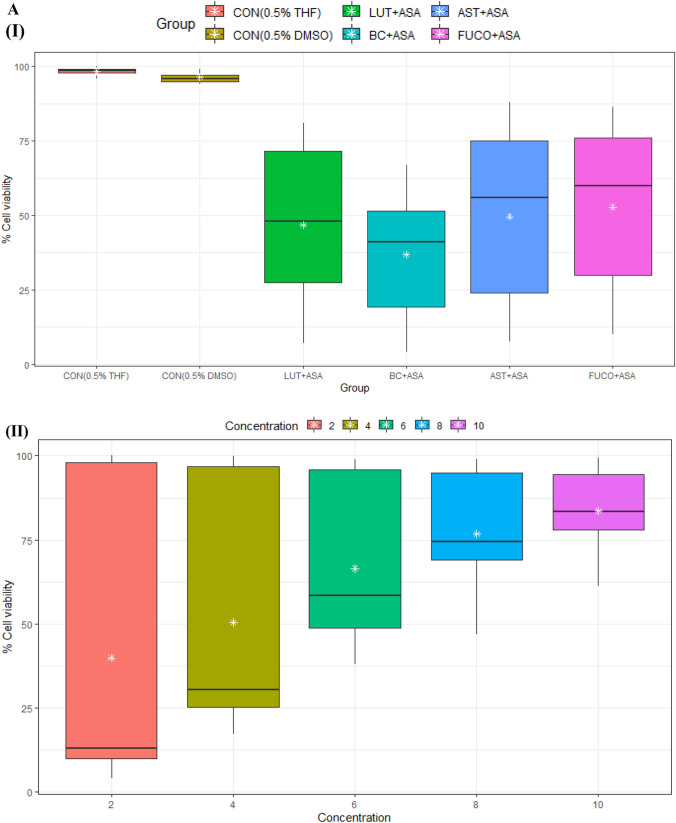
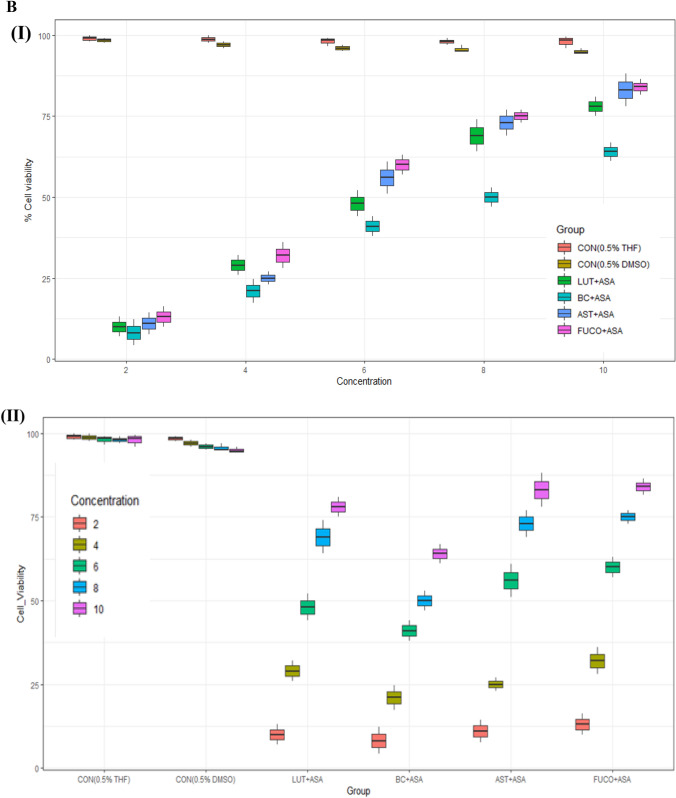
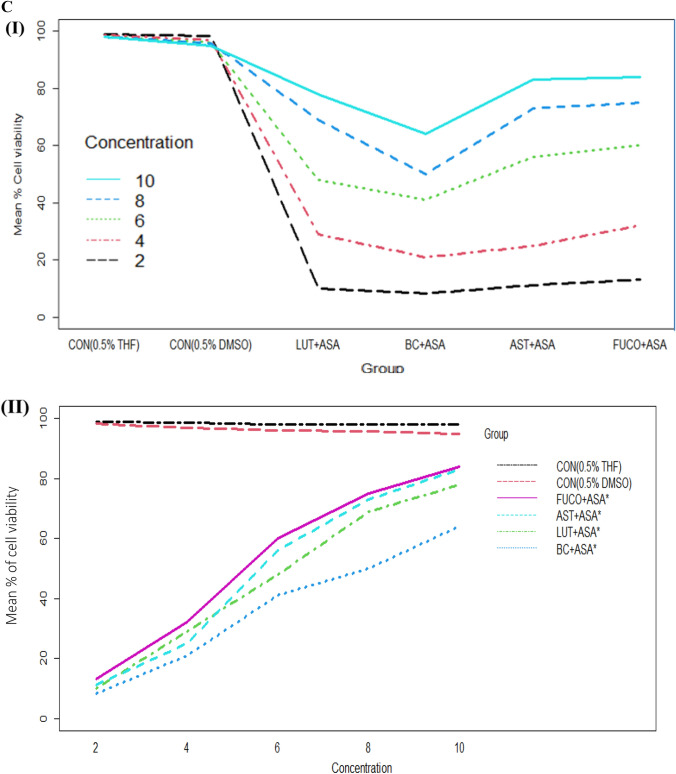
The cell viability/toxicity levels of purified carotenoids were comparable to standard carotenoids results with no significant difference. The macrophages (RAW 264.7 and U937) and PBMCs treated (24 h) with an increasing concentration of each carotenoid significantly increased the cell viability in a dose-dependent manner (Figs. 1, 2, 3). The cell viability percentage confirmed the cytoprotective activity of carotenoids in all the treated cells (Figs. 1, 2, 3). Further, the IC50 concentration of each carotenoid on cell viability of macrophages/ PBMCs found in the following order: FUCO > LUT > AST > BC (Table 1). The cells were pre-incubated with LPS was treated with a minimal cytotoxic dose (85–90% cell viability) of ASA plus physiological equivalent concentration/dose of carotenoids significantly increased cell viability by 66% (BC), 78% (LUT), 70% (AST), and 77% (FUCO) in RAW 264.7 cells as compared to only LPS treated cells, respectively. Likewise, in U937 cells, the cell viability was found to be 58% (BC), 63% (LUT), 68% (AST), and 69% (FUCO), while in PBMCs, it found to be 51% (BC), 62% (LUT), 63% (AST) and 67% (FUCO). These results demonstrated that the carotenoid combination with ASA significantly increased cell viability compared to an equivalent dose of individual carotenoid or ASA treatment in LPS- treated cells (Figs. 1, 2, 3 and Table 1). Furthermore, ASA treated with FUCO or LUT increased cell viability more significantly than the combination with AST and BC in pre-treated LPS. The IC50 value of ASA* was found to be 1.5 ± 0.3 μM in RAW 264.7, 2.2 ± 0.3 μM in U937 cells, and 1.4 ± 0.2 μM in PBMCs treated cells. There is no significant difference found in cell numbers between control (untreated) and vehicle control, demonstrating that up to 0.5% of DMSO or THF does not affect cell growth (Fig. 1, 2, 3). Overall, these results attributed that treatment with a physiological concentration of carotenoids (~ 5 µM) with low-dose ASA (~ 0.3 µM) has shown efficiency in macrophages and PBMCs growth compared to carotenoid or ASA alone treatments in the LPS pre-incubated cells. The synergistic influence of carotenoids combination with ASA on cell growth/proliferation in all three cell types was evident and found to be in the following order FUCO > LUT > AST > BC (Table 1). Moreover, cellular uptake of carotenoids in RAW 264.7 cells was higher in individual carotenoid-treated cells than in the carotenoid combination with the ASA group. The results clearly demonstrated that there is a significant difference in the % cell viability between treated different groups and concentration of carotenoids (except under the two control groups) in all the three cell lines (Figs. 1, 2, 3 and Table 2.). Furthermore, interaction between concentration and group observed (Figs. 1C, 2C, 3C and Table 2.).
Fig. 1.
A Boxplot of percent cell viability of Raw 264.7 cells treated different groups (I) and concentrations (II). B Percentcell viability of Raw 264.7cells treated different concentration (I) of carotenoids and groups (II). C Interaction between treated group and concentration (I) and concentration and group (II) of mean of cell viability in Raw 264.7 cells. CON control; LPS lipopolysaccharide; ASA aspirin; BC β-carotene; LUT lutein; AST astaxanthin; FUCO fucoxanthin. Control (CON) represents a treatment of THF or DMSO vehicle alone. Refer to Table 1 for abbreviations and treatment doses
Fig. 2.
A Boxplot of percent cell viability of U397 cells treated different group (I) and concentration (II). B Percent cell viability of U397 cells treated different concentration (I) of carotenoids and groups (II). C Interaction between treated group and concentration (I) and concentration and group (II) of mean of cell viability in U937 cells. CON control; LPS lipopolysaccharide; ASA aspirin; BC β-carotene; LUT lutein; AST astaxanthin; FUCO fucoxanthin. Control (CON) represents a treatment of THF or DMSO vehicle alone. Refer to Table 1 for abbreviations and treatment doses
Fig. 3.
A Boxplot of percent cell viability of PBMCs treated different groups (I) and concentrations (II). B Percent cell viability of PBMCs treated different concentration (I) of carotenoids and groups (II). C Interaction between treated group and concentration (I) and concentration and group (II) on mean of cell viability in PBMCs. CON control; LPS lipopolysaccharide; ASA aspirin; BC β-carotene; LUT lutein; AST astaxanthin; FUCO fucoxanthin. Control (CON) represents a treatment of THF or DMSO vehicle alone. Refer to Table 1 for abbreviations and treatment doses
Table 1.
Influence of carotenoids combination with ASA on cytotoxicity and cellular uptake of carotenoids in macrophages and PBMCs
| Experimental groups | IC50 values (µM) | Cellular uptake (p moles/106cells) |
||||
|---|---|---|---|---|---|---|
| RAW 264.7 | U937 | PBMCs | RAW 264.7 | U937 | PBMCs | |
| Individual treatments | ||||||
| ASA* | 1.5 | 2.2 | 1.4 | – | – | – |
| BC | 20.6 | 22.3 | 22.3 | 310 ± 4.2c | 255 ± 3.3 d | 327 ± 4.3d |
| LUT | 21.1 | 22.3 | 22.3 | 411 ± 4.8b | 342 ± 5.6 b | 449 ± 4.1b |
| AST | 23.5 | 24.2 | 24.2 | 467 ± 7.3a | 446 ± 3.9a | 498 ± 3.9a |
| FUCO | 22.6 | 23.4 | 23.4 | 382 ± 5.5b | 311 ± 5.1 | 413 ± 2.9c |
| Combination treatments | ||||||
| BC + ASA | 6.1 | 6.9 | 6.5 | 79 ± 2.3d | 78 ± 3.4d | 89 ± 2.9d |
| LUT + ASA | 8.2 | 9.4 | 8.8 | 131 ± 3.1c | 135 ± 2.8c | 149 ± 3.3c |
| AST + ASA | 7.3 | 7.8 | 7.3 | 259 ± 4.5a | 334 ± 3.9a | 397 ± 5.7a |
| FUCO + ASA | 5.1 | 6.1 | 5.3 | 163 ± 5.2b | 199 ± 4.7b | 204 ± 3.1b |
The data represent mean ± SD (n = 5). Values not sharing a common superscript letter within a column under individual or combination treatments are significantly different (p < 0.05) as determined by one-way ANOVA followed by Tukey’s test. ASA*-IC50 concentration; ASA, minimal dose of ASA (10–13% cell survival) in RAW 264.7 (0.1 µM), U937 (0.21 µM) and PBMCs (0.17 µM), respectively
CON control; ASA aspirin; BC β-carotene; LUT lutein; AST Astaxanthin; FUCO fucoxanthin
Table 2.
Two-way ART ANOVA for analysis of carotenoid concentration and its combination treatment of ASA in three different cells
| Source | df | df of error | F value | p value |
|---|---|---|---|---|
| Raw 264.7 cells | ||||
| Group | 5 | 60 | 137.848 | < 2.22e-16*** |
| Concentration | 4 | 60 | 311.383 | < 2.22e-16*** |
| Group: Concentration | 20 | 60 | 40.199 | < 2.22e-16*** |
| U937 cells | ||||
| Group | 5 | 60 | 144.737 | < 2.22e-16*** |
| Concentration | 4 | 60 | 332.778 | < 2.22e-16*** |
| Group: concentration | 20 | 60 | 35.223 | < 2.22e-16*** |
| PBMCs | ||||
| Group | 5 | 60 | 109.386 | < 2.22e-16*** |
| Concentration | 4 | 60 | 326.054 | < 2.22e-16*** |
| Group: concentration | 10 | 60 | 57.108 | < 2.22e-16*** |
Inference: The main effects of group and concentration of carotenoids are significant. That is, the % cell viability differs significantly between different groups. Similarly, it also differs significantly between different concentrations. The p value for corresponding to all the three sources; group, concentration and the interaction is almost zero, which is a clear indication that the different sources are highly significant. Post hoc test results: Since the main effects and the interactions were found significant, post hoc test (pairwise comparisons using BH method) was carried out to test out the source of significance. Groups: The results say that, there exists significant difference between any two groups. Concentration: The same was true, with respect to the concentration between any two levels of the concentration there exists significant difference. Interaction effect: Most of the pairwise combinations were found to be significant
However, cellular uptake of carotenoids from the unique carotenoid treatments or in combination with ASA resulted in the following order: AST < LUT < FUCO < BC (Table 1). The treatment of ASA with a carotenoid combination did not affect the cellular uptake of carotenoids (Table 1).
Cytotoxicity influence of carotenoids plus ASA treatments in LPS-stimulated macrophages and PBMCs
The cytotoxicity influence of carotenoids plus ASA treatments in LPS-stimulated macrophages and PBMCs is shown in Fig. 4. The LDH assay demonstrated an apparent increase of % LDH in the culture medium of the LPS-treated group. Whereas the percentage of LDH release was lower by 75 and 62% in FUCO + ASA, followed by 62.5 and 56% in LUT + ASA (%), 56.2 and 52% in AST + ASA, 37.5 and 38% in BC + ASA, and 33.3 and 30% in ASA treated groups of in RAW 264.7 and U937 cells compared to LPS alone treated cells, respectively. Similarly, LDH levels in PBMCs was lowered by63.4% in FUCO + ASA, 51.2% in LUT + ASA, 36.5% in AST + ASA, 19.5% in BC + ASA, and 26.8% in ASA treated cells compared to LPS treated group. This observation indicates that FUCO + ASA treatment increased cell viability significantly compared to the other carotenoids plus ASA-treated groups against LPS-stimulated inflammation.
Fig. 4.
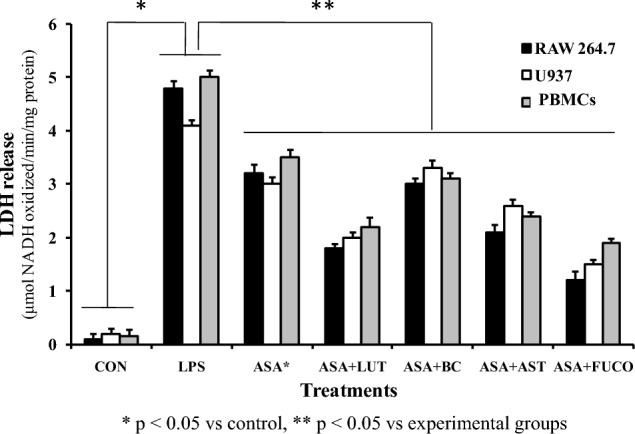
Effect of combined treatment of carotenoids with ASA on LPS induced LDH to release in macrophages and PBMCs incubated for 12 h. The data represent mean ± SD (n = 5). a,b,c,d,e,fValues not sharing common superscript letters between treatment groups are significantly different (p < 0.05) from their respective control as analyzed by one-way ANOVA followed by Tukey's test. Refer to Table 1 and Fig. 1 for abbreviations and doses for treatment groups
Combined treatment of carotenoids with ASA on nitric oxide scavenging and PGE2 levels in macrophages and PBMCs.
The combination of carotenoids with ASA treatments significantly decreased the LPS-stimulated NO production in RAW 264.7 and U937 cells treated with FUCO + ASA (58.8% and 53.4%), LUT + ASA (44.4% and 32.8%), AST + ASA (39.8% and 30.2%), BC + ASA (30.2% and 22.6%), and ASA (28.9% and 20.1%) as compared to only LPS treated cells, respectively. Likewise, NO levels in PBMCs significantly decreased after the treatment of FUCO + ASA (51.1%), LUT + ASA (46.5%), AST + ASA (40.1%), BC + ASA (31.3%), and ASA (30.2%) than the LPS treated cells. Correspondingly, PGE2 levels decreased in RAW 264.7 and U937 cells treated with FUCO + ASA (62.5% and 52%), LUT + ASA (45% and 39.8%), AST + ASA (42% and 34.4%), BC + ASA (31.3% and 28.7%), and ASA (30.2% and 23.4%) compared to LPS alone -treated cells, respectively. Likewise, PGE2 levels decreased in PBMCs cells treated with FUCO + ASA (51%), LUT + ASA (40.8%), AST + ASA (31.4%), BC + ASA (23.3%), and ASA (22.2%) compared to LPS treated cells (Fig. 5A and B). These results strongly suggest that the FUCO + ASA combination has shown higher protection against LPS-induced inflammation than other carotenoid and ASA combination groups. Based on the cytoprotection and toxicity levels, we chose RAW264.7 cells and FUCO (epoxy-carotenoid) as efficient carotenoid molecules to elucidate other cell-based assays.
Fig. 5.
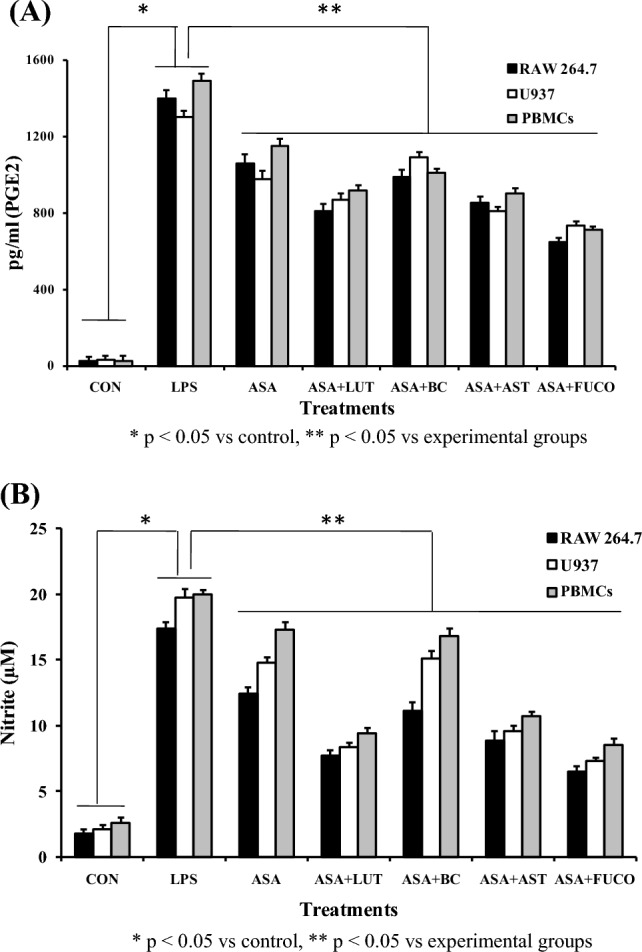
Effect of combined treatment of carotenoids with ASA on PGE2 (A) and nitrite (B) levels in LPS induced macrophages and PBMCs incubated for 12 h. The data represent mean ± SD (n = 5). a,b,c,d,e,fValues not sharing common superscript letters between treatment groups are significantly different (p < 0.05) from their respective control as analyzed by one-way ANOVA followed by Tukey's test. Refer to Table 1 and Fig. 1 for abbreviations and treatment doses
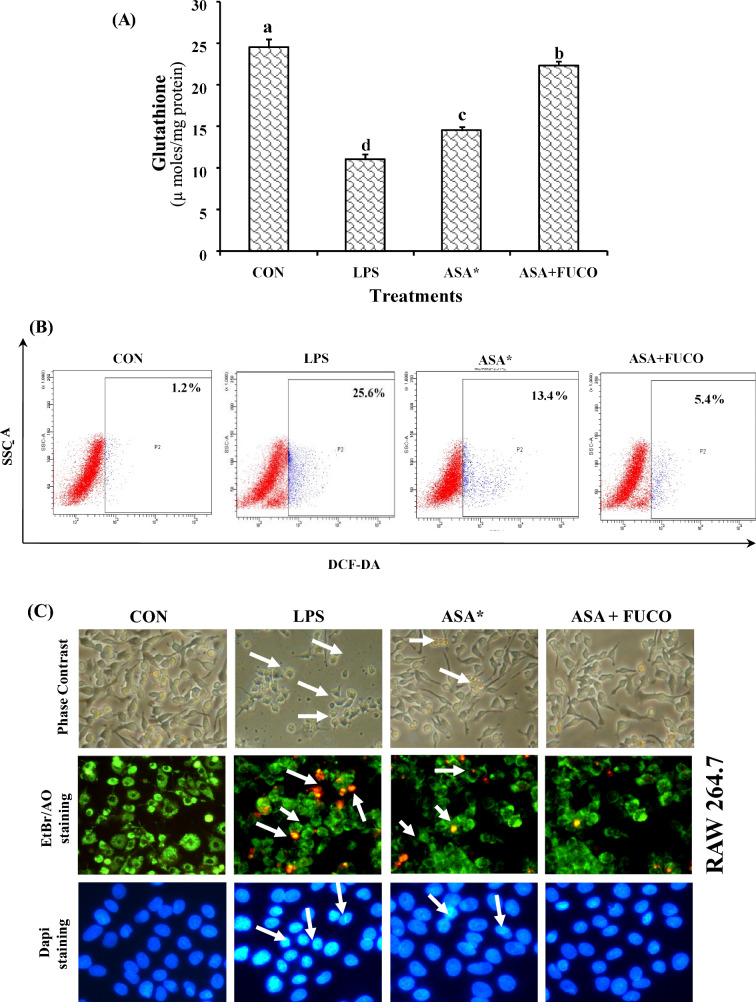
Effect of carotenoids plus ASA combination treatment on oxidative status, generation of intracellular ROS, and cell cycle progression in RAW 264.7 cells
The effect of a relative physiological dose of carotenoids with a low-dose of the anti-inflammatory drug on the oxidative status of RAW 264.7 cells is shown in Fig. 6A and B. The cells treated with FUCO + ASA and ASA have significantly reduced GSH levels by 48.8% and 37.4% in LPS pre-incubated cells compared to LPS alone treated cells, respectively. Likewise, treatment of FUCO with ASA decreased intracellular ROS generation by 33% and 15% (Fig. 6B). Whereas the percentage (%) of ROS in the positive control (H2O2) cells was found to be 65%. The FUCO combination with ASA treatment (12 h) on cell cycle distribution analyzed using flow cytometry is shown in Table 3. The relative percentage of apoptotic cells decreased by 69.2% in FUCO + ASA, and 46.7% in ASA treated RAW 264.7 cells as compared to LPS treated cells. A significant change in morphology and nuclear condensation was observed in cells treated with carotenoid plus ASA than any other treated groups (Fig. 6C). This confirms the influence of FUCO on the protection against the LPS-induced damage.
Fig. 6.
Effect of combined treatment of carotenoid (FUCO) with ASA on glutathione (GSH) levels (A) and Intracellular ROS generation in RAW 264.7 cells pre-treated with LPS (B) (Cells treated with H2O2 (ROS, 65%) was considered a positive control). Morphological observations of carotenoid (FUCO) and their combination with ASA in RAW 264.7 cells with ethidium bromide and acridine orange staining (C) (arrows indicate early and late apoptotic cells (200 ×). DAPI was used as a nuclear marker). The microscopic field for ethidium bromide and acridine orange staining and DAPI are different with the same cell lineages. Refer to Table 1 and Fig. 1 for abbreviations and treatment doses. The data represent mean ± SD (n = 5). a,b,c,dValues not sharing common superscript letters between treatments are significantly different (p < 0.05) as analyzed by one-way ANOVA followed by Tukey's test. Refer to Table 1 and Fig. 1 for abbreviations and treatment doses
Table 3.
Effect of ASA and ASA + FUCO on cell cycle distribution and induction of apoptosis in RAW-264.7 cells
| RAW 264.7 | ||||
|---|---|---|---|---|
| Experimental groups | Cell cycle distribution (%) | Apoptosis (%) | ||
| G0/G1 | S | G2/M | ||
| CON | 57.8 ± 0.6b | 23.1 ± 1.6b | 18.5 ± 1.8b | 0.6 ± 0.2a |
| LPS | 75.1 ± 0.2d | 13.2 ± 0.8a | 11.7 ± 0.1a | 78.4 ± 1.2d |
| ASA* | 59.9 ± 1.3c | 23.2 ± 1.5b | 18.9 ± 1.5b | 33.1 ± 1.3c |
| ASA + FUCO | 40.3 ± 1.7a | 38.5 ± 1.7c | 21.2 ± 0.8c | 21.3 ± 2.1b |
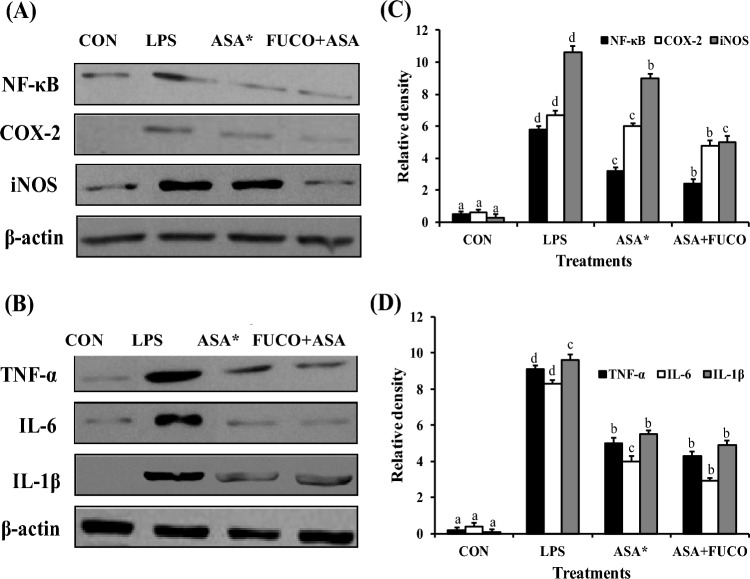
Role of FUCO with ASA on LPS-induced IL-1β, COX-2, and TNF-α expression
The effect of FUCO combination with ASA on the production of pro-inflammatory cytokines (IL-1β and TNF-α) and COX-2 in cells incubated (12 h) with LPS is shown in Fig. 7A–C. The combined treatment of FUCO and ASA significantly reduced IL-1β, COX-2, and TNF-α production by 68.4%, 59.7%, and 35.3% compared to LPS-treated cells, respectively.
Fig. 7.
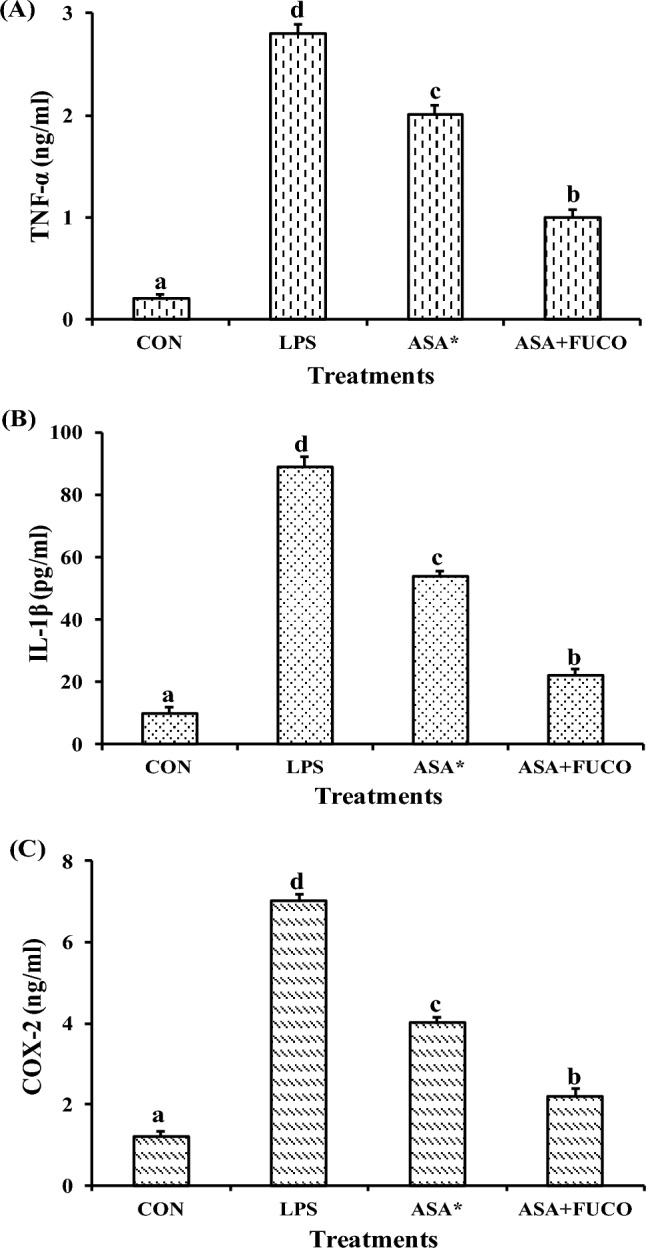
Influence of carotenoid plus ASA treatment on TNF-α (A), IL-1β (B) and COX-2 (C) protein concentration against pre-treated with LPS in RAW 264.7 cells. The data represent mean ± SD (n = 5). a,b,c,d Values not sharing a common superscript letters between treatments are significantly different (p < 0.05) as analyzed by one-way ANOVA followed by Tukey's test. Refer to Table 1 and Fig. 1 for abbreviations and treatment doses
Effect of FUCO combination with ASA on the regulation of NF-κB-regulated gene expression in RAW 264.7 cells
The influence of FUCO or its combination treatment with ASA on the suppression of NF-κB regulation is shown in Fig. 8. To determine the efficient reduction of NO and PGE2 production by FUCO + ASA treatment in LPS-induced cells, we further analyzed other LPS-induced intermediate pro-inflammatory markers (iNOS, COX-2, IL-1β, TNF-α, and NF-κB protein expression) in RAW 264.7 cells (Fig. 8). The expression of iNOS, COX-2, IL-1β, TNF-α, and NF-κB significantly increased in macrophage cells treated with LPS compared to ASA or FUCO + ASA groups. However, FUCO combination with ASA significantly down-regulated the expression of iNOS (1.3 and 2.1 fold), COX-2 (1.0 and 2.2 fold), IL-1β (1.1 and 2.3 fold), TNF-α (1.4 and 2.8 fold), and NF-κB (1.0 and 2.7 fold) as compared to LPS treated cells, respectively.
Fig. 8.
Effect of carotenoids on NF-κB, COX-2, iNOS, TNF-α, IL-6 and IL-1β expression in RAW 264.7 cells treated for 12 h. Panels A and B representative of western blot analyses; panels C and D represent the densitometric analysis of three different determinations in RAW 264.7 cells. The data represent mean ± SD (n = 5). a,b,c,dValues not sharing common superscript letters between treatments are significantly different (p < 0.05) as analyzed by one-way ANOVA followed by Tukey's test. Refer to Table 1 and Fig. 1 for abbreviations and treatment doses
Discussion
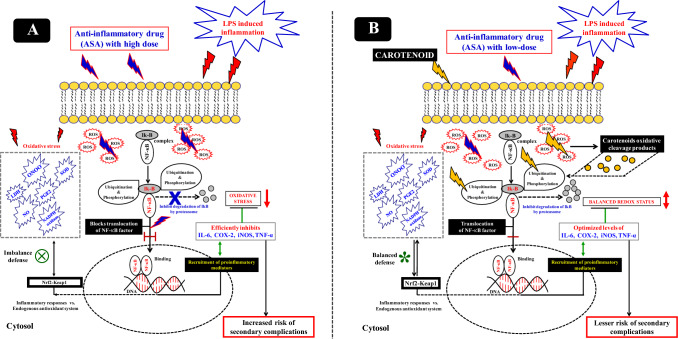
This study demonstrated the influence of carotenoid efficiency on minimizing the secondary toxicity associated with NSAIDs on the selective inhibition of inflammatory mediators in macrophages and PBMCs (Fig. 9). Earlier, studies have reported that inhibition of pro-inflammatory mediators by high doses of carotenoids (lycopene, β-carotene, and lutein) (Bai et al. 2005; Feng et al. 2010), and polyphenols (carnosic acid, resveratrol, or gallic acid) (Ciz et al. 2008; Hadad 2012) in LPS-stimulated macrophages. Currently, we reveal the inhibitory effect of pro-inflammatory mediators by combination treatment of carotenoids plus ASA at a lower concentration/dose, which is relatively equivalent in the range of human plasma levels. LPS pre-treated (1 h) macrophages were incubated (12 h) with carotenoid plus ASA resulting in significant inhibition of NO and PGE2 production (Fig. 5). Specifically, combined FUCO and ASA inhibited efficiency compared to other carotenoids combined with ASA. Previous studies have demonstrated a remarkable synergistic effect of carotenoids with polyphenols (Fuhrman et al. 2000; Lo et al. 2002; Linnewiel-Hermoni et al. 2015) and vitamins (Liu et al. 2008). Our findings indicated a low-dose of carotenoid plus ASA combination mechanistically linked to the down-regulation of iNOS, TNF-α, and COX-2 by regulating NF-κB expression (Figs. 7 and 8). However, their expression level is more prominent in cells treated with ASA or LPS. Often, the reactive oxygen species (superoxide and nitric oxide levels) play a role in host defense (Baran et al. 1996) at optimum levels; however, their intensity when crossing the threshold levels (oxidative stress) may increase inflammatory responses, ultimately damaging the cells (Kaulmann and Bohn 2014). Furthermore, the reaction between reactive oxygen species/free radicals resulted in peroxynitrite (ONOO) production, the most toxic derivative of NO, which can cause immense cellular damage and induce chronic inflammation (Grisham et al. 1999). The generation of superoxide radicals by NADPH oxidase and coupled oxidative stress occurs due to xenometabolism, stimulated by lifestyle-associated factors, and microbial infections. These situations resulted in the activation of diverse cell signalling by optimizing MAP kinases regulation and NF-κB gene expressions, eventually up-regulating the gene expressions encoding pro-inflammatory markers (Kaulmann and Bohn 2014). Carotenoids are linked to their action against oxidative stress mechanisms and allied cell signalling. Exploring vital nutraceuticals to prevent secondary complications and mediated oxidative stress due to xenobiotics or synthetic chemotherapeutic drugs requires an elaborative study. Consequently, interference with their actions is of significant relevance to repairing the inflammatory process, which is one of the primary aims to reduce chronic inflammation and our current hypothesis. Despite the adverse cardiovascular and gastrointestinal effects of non-steroidal anti-inflammatory COX-2-inhibitory drug (Graham 2006) is widely used to treat inflammatory diseases (Baron and Sandler 2000). By and large, LPS is known to mediate oxidative stress and up-regulate superoxides, NO, PGE2, and expression of COX-2, iNOS, and TNF-α through NF-κB regulation in macrophages. In the present study, elevated levels of NO and PGE2 by LPS were optimized by combined treatment of carotenoids plus ASA (Fig. 4). Alternatively, few studies explored TNF-α antagonists for treating chronic inflammatory diseases; however, this approach impels a greater risk, particularly gastric ulcers (Sostres et al. 2010). As revealed persuasively, this study opinion that combinations of a permissible dose of the potent anti-inflammatory drug like ASA with a vital natural compound like carotenoid FUCO could be more appropriate for treating chronic inflammation. Further, this approach may help prevent secondary toxicity concomitant with the optimization of drugs with carotenoids contrary to the high dose of NSAIDs for the effective control of inflammation associated with secondary complications. Previously, Hossainet al. (2012) suggested the benefit of a combined anti-inflammatory and anti-cancer drug approach substantiating the relevance of treatment for preventing inflammation that can control iNOS and COX-2 expression. In our study, the combined treatment of FUCO + ASA has shown significant inhibition of iNOS and COX-2 expression compared to ASA or LPS alone treated cells. Nonetheless, optimization of COX-2 expression to control the inflammatory process by the combinational treatment of FUCO + ASA is a promising approach to persistent over-expression of COX-2 complications. This observation gives a greater advantage for various therapies. This indicates that NSAIDs like ASA control the pro-inflammatory markers activation at low doses; however, up-lifting its action by FUCO as compared to a high dose of drug-mediated secondary toxicity documented with LPS alone treatment. So far, no detailed studies are available with this concept, which may be due to a lack of efficiency in natural compounds vs. chemically derived drugs at low doses. However, certain studies have revealed the synergistic influence of carotenoids with other phytocompounds on oxidative stress and allied chronic problems (Fuhrman et al 2000; Hadad 2012; Linnewiel-Hermoni et al. 2015). Stahl et al. (1998) have shown that a combination of lycopene with β-carotene was more protective against oxidative damage than the treatment of lycopene with lutein, emphasizing the opinion that the differences related to physicochemical properties of carotenoids and their location in the membrane. It has been attributed that xanthophylls span the membrane through a polar end group attached at the membrane polar sites, whereas hydrocarbon carotenoid lycopene and β-carotene lack hydrophilic substituents and remain placed within the membrane affecting fluidity. Furthermore, they can influence defense mechanisms against reactive oxygen species and free radicals by improving the defense system (Agamey et al. 2004).
Fig. 9.
A scheme of possible influence of low doses of marine carotenoids plus ASA on anti-inflammation (B), vs. high dose of ASA with secondary complications (A)
The efficiency of the combination treatment of carotenoids with ASA is attributed to involving a few potential ways, such as counteracting aspirin derivatives as antioxidants may reduce the burden of carotenoid reaction with the ROS or free radicals and their reactant products such as peroxynitrite in the cytosol. Alternatively, reduced oxidative stress by aspirin may activate carotenoids ability to scavenge or deactivate ROS and mediate stress more porously. Since the efficiency of FUCO as an antioxidant is decided based on oxidative stress or oxygen tension levels at the cellular levels, certain carotenoids behave as pro-oxidants at higher oxygen tension (Agamey et al. 2004; Burton and Ingold 1984). Further, the synergistic action of FUCO with ASA may be due to the electrophilicity and polarity of the molecules, which is more for activated translocation of NF-κB transcription factor from the cytosol to the nucleus. Hence, FUCO was found to be more actively involved in the inhibition of NF-κB expression as compared to other carotenoids. β-Carotene is highly recognized as non-polar and spans its membrane within the lipophilic region, whereas cytosol mobilizes those molecules that are hydrophobic. Several studies have documented that the regulation of NF-κB gene expression is one of the key signalling pathways to induce apoptosis. In contrast, under induced inflammatory conditions (LPS/H2O2), enhanced levels of ROS and their reactants stimulate phosphorylation of NF-κB-IκB complex and execute translocation of NF-κB transcription factor into the nucleus for further regulation of NF-κB gene expression. Since carotenoids/FUCO as antioxidants could down-regulate oxidative stress-mediated NF-κB translocation into the nucleus. In this context, carotenoids may act as superior antioxidants at cellular levels with a concomitant reduction in peroxy-nitrite and ROS radicals (Fig. 9B). The reaction mechanism between carotenoids and ROS may produce oxidative cleavage products. Previously our research group (Lakshminarayana et al. 2008), we speculated that enzymatic and oxidative cleavage products might also affect the regulation of antioxidant and anti-inflammatory transcription systems (Fig. 9B); however, the mechanism is not much detailed (Arathi et al. 2015, 2018). Recently, Takatani al. (2021) identified and elucidated the pivotal roles of fucoxanthin‐derived apo-carotenoids against LPS-induced macrophages and adipocytes. The mechanistic differences of inflammation found in the treatment of either low-dose of ASA or a combined physiological dose of carotenoid with ASA may be due to elevated oxidative stress through multifactor ROS controlled MAPKs pathway (Feng et al. 2010). Earlier studies have correlated the carotenoids-mediated are down-regulation of NF-κB through the MAPKs pathway (Feng et al. 2010; Kaulmann and Bohn 2014). This study unrevealed the potential anti-inflammatory property of marine carotenoid FUCO as it alleviates/reduces secondary complications of ASA and is also more significant than other carotenoids. The differences between carotenoids may occur due to the chemical and structural properties of carotenoids. These results provide greater insight into marine carotenois, particularly to combat anti-inflammatory drugs mediated secondary drug toxicity in cardiovascular diseases and cancer treatments. Prevention of cancer at the primary level is considered an important public health issue, and using selective drugs for chemoprevention is equally vital for effective treatment (Wild et al. 2015). Also, many studies attempted to elucidate the possible anti-cancer effect of aspirin with extensive research work. However, few studies address its effectively on breast, gastric, colorectal, lung, and prostate cancer control (Patrignani and Patrono 2015; Cuzick 2017; Bosetti et al. 2020). It has attributed that about 50% of cancers can be prevented by evading risk factors and implementing evidence-based treatment strategies (Colditz et al. 2006). In contrast, studies have reported an inverse relationship linking the incidence of various cancers and cardiovascular disease mortality with using high doses of aspirin. In this context, the preliminary evidence of the present study suggested that the application of a potent drug like ASA with carotenoid shown with lesser or no secondary toxicity is considered vital for further exploitation under in vivo models.
In the present study, the synergistic anti-inflammatory action of ASA with FUCO observe at a low dose than a higher dose of ASA treated alone (Fig. 9A and B). However, exploration of the proper dose of ASA for the potential anti-inflammatory effect in animals should be determined through clinical trials. Although, NSAIDs provide long established commonly used medications, their use as a combination of treatment with specifically recognized nutraceutical agents is a new and promising approach to treat chronic diseases. Additionally, bioactive efficiency, proper dosage, and long-term side effects of the ASA and FUCO combination in vivo conditions need to be detailed and most warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors acknowledge the DST-SERB (NO.EEQ/2019/000282.Dtd 04.11.2019) and Bangalore University (Uni.order No.DEV.D2a: BU-RP: 2020-21. Dtd.07.01.2022) for project grants and support.
Author contributions
KV, PRS, RA, RL involved in design experiment and writing the original draft; ARR, RG, MH, MBM, RM involved in revising and editing the manuscript, and SR involved in statistical analysis.
Data availability
Not applicable.
Declarations
Conflict of interest
Authors declare that there is no conflict of interest.
References
- Agamey AE, Lowe GM, McGarveya DJ, Mortensenc A, Phillip DM, Truscott TG, Young AJ. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Arathi BP, Sowmya PR, Vijay K, Baskaran V, Lakshminarayana R. Metabolomics of carotenoids: the challenges and prospects e a review. Trends Food Sci Technol. 2015;45:10–117. doi: 10.1016/j.tifs.2015.06.003. [DOI] [Google Scholar]
- Arathi BP, Sowmya PR, Kuriakose GC, Shilpa S, Shwetha HJ, Kumar S, Raju M, Baskaran V, Lakshminarayana R. Fractionation and characterization of lycopene-oxidation products by LC-MS/MS (ESI)+: elucidation of the chemopreventative potency of oxidized lycopene in breast-cancer cell lines. J Agric Food Chem. 2018;66:11362–11371. doi: 10.1021/acs.jafc.8b04850. [DOI] [PubMed] [Google Scholar]
- Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kwon YG, Chung CK, Kim YM. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide stimulated macrophages by suppressing redox-based NF-κB activation. Exp Mol Med. 2005;37:323–334. doi: 10.1038/emm.2005.42. [DOI] [PubMed] [Google Scholar]
- Baran J, Guzik K, Hryniewicz W, Ernst M, Flad HD, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;64:4242–4248. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Sandler RS. Non-steroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res. 2009;181:231–251. doi: 10.1007/978-3-540-69297-3_22. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31:558–568. doi: 10.1016/j.annonc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Burton GW, Ingold KU. β-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Butalla AC, Crane TE, Patil B, Wertheim BC, Thompson P, Thomson CA. Effects of a carrot juice intervention on plasma carotenoids, oxidative stress, and inflammation in overweight breast cancer survivors. Nutr Cancer. 2012;64:331–341. doi: 10.1080/01635581.2012.650779. [DOI] [PubMed] [Google Scholar]
- Carlson LM, Rasmuson A, Idborg H, Segerström L, Jakobsson PJ, Sveinbjörnsson B, Kogner P. Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis. 2013;34:1081–1088. doi: 10.1093/carcin/bgt009. [DOI] [PubMed] [Google Scholar]
- Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and non-steroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Kim NH, Kim SJ, Lee HJ, Kim S. Fucoxanthin inhibits the inflammation response in paw edema model through suppressing MAPKs, Akt, and NFkB. J Biochem Mol Toxicol. 2016;30:111–119. doi: 10.1002/jbt.21769. [DOI] [PubMed] [Google Scholar]
- Ciz M, Pavelkova M, Gallova L, Kralova J, Kubala L, Lojek A. The influence of wine polyphenols on reactive oxygen and nitrogen species production by murine macrophages RAW 264.7. Physiol Res. 2008;57:393–402. doi: 10.33549/physiolres.931088. [DOI] [PubMed] [Google Scholar]
- Cocate PG, Natali AJ, Alfenas RCG, de Oliveira A, Dos Santos EC, Hermsdorff HHM. Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br J Nutr. 2015;114:257–264. doi: 10.1017/S0007114515001622. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Sellers TA, Trapido E. Epidemiology—identifying the causes and preventability of cancer? Nat Rev Cancer. 2006;6:75–83. doi: 10.1038/nrc1784. [DOI] [PubMed] [Google Scholar]
- Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J. Preventive therapy for cancer. Lancet Oncol. 2017;18:e472–e482. doi: 10.1016/S1470-2045(17)30536-3. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, DuBois RN. COX-2: a new target for cancer prevention and treatment (Progress in experimental tumor research), Basel, Switzerland. Karger. 2003;2003:37. [Google Scholar]
- Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;15:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Gallagher AM, Duthie GG, Mur LAJ, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- Feng D, Ling W, Duan R. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kB in macrophages. Inflamm Res. 2010;59:115–121. doi: 10.1007/s00011-009-0077-8. [DOI] [PubMed] [Google Scholar]
- Fiedor J, Burd K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults. JAMA. 2002;2002(287):3127–3129. doi: 10.1001/jama.287.23.3127. [DOI] [PubMed] [Google Scholar]
- Fuhrman B, Volkova N, Rosenblat M, Aviram M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, orgarlic. Antioxid Redox Signal. 2000;2:491–506. doi: 10.1089/15230860050192279. [DOI] [PubMed] [Google Scholar]
- GBD (Global Burden of Disease Study) Risk factors collaborators, global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DJ. COX-2 inhibitors, other NSAIDs, and cardiovascular risk: the seduction of common sense. JAMA. 2006;296:653–1656. doi: 10.1001/jama.296.13.jed60058. [DOI] [PubMed] [Google Scholar]
- Grisham M, JourdHeuil D, Wink D. Nitric oxide physiological chemistry of nitric oxide and its metabolites implications in inflammation. Am J Physiol. 1999;276:G315–321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- Hadad N. Levy R (2012) The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic Biol Med. 2012;53:1381–1391. doi: 10.1016/j.freeradbiomed.2012.07.078. [DOI] [PubMed] [Google Scholar]
- Haegele AD, Gillete C, O’Neil C, Wolfe P, Heimendinger J, Sedlacek S, Thompson HJ. Plasma xanthophyll carotenoids correlate inversely with indices of oxidative DNA damage and lipid peroxidation. Cancer Epidemiol Biomark Prev. 2000;9:421–425. [PubMed] [Google Scholar]
- Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-κB activation: role of H2O2 and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide. 2005;5:504–513. doi: 10.1006/niox.2001.0367. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, Kim ND. Aspirin enhances doxorubicin-induced apoptosis and reduces tumor growth in human hepatocellular carcinoma cells in vitro and in vivo. Int J Oncol. 2012;40:1636–1642. doi: 10.3892/ijo.2011.1304. [DOI] [PubMed] [Google Scholar]
- Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee DH. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: The coronary artery risk development in young adults (CARDIA)/young adult longitudinal trends in antioxidants (YALTA) study. Clin Chem. 2007;53:447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MD, Ishita IJ, Jin SE, Choi RJ, Lee CM, Kim YS, Jung HA, Choi SJ. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem Toxicol. 2013;55:541–548. doi: 10.1016/j.fct.2013.01.054. [DOI] [PubMed] [Google Scholar]
- Ittaman SV, VanWormer JJ, Rezkalla SH. The role of aspirin in the prevention of cardiovascular disease. Clin Med Res. 2014;12:147–154. doi: 10.3121/cmr.2013.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34:907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Khachik F. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthamol vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- Kotake-Nara E, Nagao A. Absorption and metabolism of xanthophylls. Mar Drugs. 2011;9:1024–1037. doi: 10.3390/md9061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayana R, Aruna G, Sangeetha RK, Bhaskar N, Divakar S, Baskaran V. Possible degradation/biotransformation of lutein in vitro and in vivo: isolation and structural elucidation of lutein metabolites by HPLC and LC–MS (atmospheric pressure chemical ionization) Free Radic Biol Med. 2008;45:982–993. doi: 10.1016/j.freeradbiomed.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Lakshminarayana R, Aruna G, Sathisha UV, Dharmesh SM, Baskaran V. Structural elucidation of possible lutein oxidation products mediated through peroxyl radical inducer 2, 2′-Azobis (2-methylpropionamidine) dihydrochloride: Antioxidant and cytotoxic influence of oxidized lutein in HeLa cells. Chem Biol Interact. 2013;203:448–455. doi: 10.1016/j.cbi.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Howden CW, Scheiman J, Tursi J. Upper gastrointestinal toxicity associated with long-term aspirin therapy: consequences and prevention. Curr Probl Cardiol. 2017;42:146–164. doi: 10.1016/j.cpcardiol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Li Q, Engelhardt JF. Interleukin-1β induction of NF-κB is partially regulated by H2O2-mediated activation of NF-κB-inducing kinase. J Biol Chem. 2006;281:11495–14505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- Linnewiel-Hermoni K, Khanin M, Danilenko M, Zango G, Amosi Y, Levy J, Sharoni Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch Biochim Biophys. 2015;572:28–35. doi: 10.1016/j.abb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological β-carotene supplementation on cell proliferation and histopathalogical changes in lungs of cigarette smoke-exposed ferrets. Carcinogensis. 2000;21:2245–2253. doi: 10.1093/carcin/21.12.2245. [DOI] [PubMed] [Google Scholar]
- Liu D, Shi J, Colina Ibarra A, Kakuda Y, Xue SJ. The scavenging capacity and synergistic effects of lycopene, vitamin-E, vitamin-C, and β-carotene mixtures on the DPPH free radical. Food Sci Technol. 2008;41:1344–1349. [Google Scholar]
- Lo A, Liang Y, Lin-Shiau S, Ho C, Lin J. Carnosol, an antioxidant in rosemary, suppresses inducible nitricoxide synthase through down-regulating nuclear factor-kappa-B in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Murray AM. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunology. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- Park YMM, Lilyquist J, Erve TJV, O'Brien KM, Nichols HB, Milne GL, Weinberg CR, Sandler DP. Association of dietary and plasma carotenoids with urinary F2-isoprostanes. Eur J Nutr. 2022;61:2711–2723. doi: 10.1007/s00394-022-02837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani P, Patrono C. Cyclooxygenase inhibitors: from pharmacology to clinical read-outs. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851:422–432. doi: 10.1016/j.bbalip.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Prince MR, Frisoli JK. β-carotene accumulation in serum and skin. Am J Clin Nutr. 1993;57:175–181. doi: 10.1093/ajcn/57.2.175. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. Profile and mechanisms of gastrointestinal and other side effects of non-steroidal anti-inflammatory drugs (NSAIDs) Am J Med. 1999;107:27S–35S. doi: 10.1016/S0002-9343(99)00365-4. [DOI] [PubMed] [Google Scholar]
- Raju M, Sowmya PR, Ambedkar R, Arathi BP, Lakshminarayana R. Carotenoids metabolic pathways and their functional role in health and diseases. In: Ravishankar G, Ambati R, editors. Global perspectives on astaxanthin: from industrial production to food, health, and pharmaceutical applications. Academic Press; 2021. pp. 671–691. [Google Scholar]
- Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol. 2009;1:29–43. doi: 10.4255/mcpharmacol.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwa BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, Morimoto T, Mehta Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomized trials. Lancet. 2018;392:387–399. doi: 10.1016/S0140-6736(18)31133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangeetha RK, Bhaskar N, Divakar S, Baskaran V. Bioavailability and metabolism of fucoxanthin in rats: structural characterization of metabolites by LC-MS (APCI) Mol Cell Biochem. 2010;333:299–310. doi: 10.1007/s11010-009-0231-1. [DOI] [PubMed] [Google Scholar]
- Scarmo S, Cartmel B, Lin H, Leffell DJ, Welch E, Bhosale P, Bernstein PS, Mayne ST. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch Biochem Biophys. 2010;504:34–39. doi: 10.1016/j.abb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerburg O, Keunen JEE, Bird AC, van Kuijk FJGM. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornchaiboon W, Joo SS, Kim SM. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull. 2012;35:1137–1144. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:121–132. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Sowmya PR, Arathi BP, Vijay K, Baskaran V, Lakshminarayana R. Role of different vehicles in carotenoids delivery and their influence on cell viability, cell cycle progression, and induction of apoptosis in HeLa cells. Mol Cell Biochem. 2015;406:245–253. doi: 10.1007/s11010-015-2442-y. [DOI] [PubMed] [Google Scholar]
- Sowmya PR, Arathi BP, Vijay K, Baskaran V, Lakshminarayana R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem Toxicol. 2017;106:58–69. doi: 10.1016/j.fct.2017.05.024. [DOI] [PubMed] [Google Scholar]
- Stahl W, Junghans A, de Boer B, Dromina ES, Briviba K, Sies H. Carotenoid mixtures protect multi-lamellar liposomes against oxidative damage: synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305–308. doi: 10.1016/S0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131:2921–2927. doi: 10.1093/jn/131.11.2921. [DOI] [PubMed] [Google Scholar]
- Takatani N, Taya D, Katsuki A, Beppu F, Yamano Y, Wada A, Miyashita K, Hosokawa M. Identification of paracentrone in fucoxanthin-fed mice and anti-inflammatory effect against lipopolysaccharide-stimulated macrophages and adipocytes. Mol Nutr Food Res. 2021;65:2000405. doi: 10.1002/mnfr.202000405. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Stendell-Hollis NR, Rock CL, Cussler EC, Flatt SW, Pierce JP. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2008–2015. doi: 10.1158/1055-9965.EPI-07-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Non-steroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Vijay K, Sowmya PR, Arathi BP, Shilpa S, Shwetha HJ, Raju M, Baskaran V, Lakshminarayana R. Low-dose doxorubicin with carotenoids selectively alters redox status and up-regulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem Toxicol. 2018;118:675–690. doi: 10.1016/j.fct.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Wild CP, Bucher JR, de Jong BW, Dillner J, von Gertten C, Groopman JD, et al. Translational cancer research: balancing prevention and treatment to combat cancer globally. J Natl Cancer Inst. 2015;107:353. doi: 10.1093/jnci/dju353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiaki A, Shu H, Takashi H. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharma Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Zhao D, Kwon SH, Chun YS, Gu MY, Yang HO. Anti-neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial cells. Neurochem Res. 2017;42:667–677. doi: 10.1007/s11064-016-2123-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.