Abstract
Tumor necrosis factor α (TNF-α) is a pro-inflammatory cytokine capable of inducing extrinsic apoptosis and necroptosis. Tumor necrosis factor receptor-associated factor 6 (TRAF6), an E3 ligase, is a member of the TRAF family of proteins, which mediates inflammatory signals by activating nuclear factor kappa B (NFкB) and mitogen-activated protein kinase (MAPK). Although the functions of TRAF6 have been identified, its role in TNF-α-induced cell death remains poorly understood. Here, we report that TRAF6 is a negative modulator of TNF-α-induced cell death but does not affect TNF-α-induced NFκB activation. TRAF6 deficiency accelerates both TNF-α-induced apoptosis and necroptosis; however, the acceleration can be reversed by reconstituting TRAF6 or TRAF6C70A, suggesting that E3 ligase activity is not required for this activity. Mechanistically, TRAF6 directly interacts with RIPK1 during TNF-α-induced cell death signaling, which prevents RIPK1 from interacting with components of the cell death complex such as itself, FADD or RIPK3. These processes suppress the assembly of the death complex. Notably, IKK was required for TRAF6 to interact with RIPK1. In vivo, Traf6-/- embryos exhibited higher levels of cell death in the liver but could be rescued by the simultaneous knockout of Tnf. Finally, TRAF6 knockdown xenografts were highly sensitive to necroptotic stimuli. We concluded that TRAF6 suppresses TNF-α-induced cell death in coordination with IKK complexes in vivo and in vitro by suppressing the assembly of cell death complex.
Subject terms: Cell biology, Molecular biology
Introduction
Tumor necrosis factor α (TNF-α) is a multifunctional cytokine that induces a pro-inflammatory response and regulates cell death cascades in recipient cells. The ligation of TNF-α to the TNF receptor (TNFR) induces NFкB activation, leading to the expression of pro-inflammatory and pro-survival genes [1, 2]. However, inhibition of the NFκB activation cascade through TNF-α signaling pathways results in the assembly of the complex II or necrosome, which respectively induce extrinsic apoptosis and necroptosis [3, 4]. In TNF-α-dependent apoptosis, complex II is mainly composed of RIPK1, FADD, and caspase-8, which lead to a caspase activation cascade. As a result, the cells undergo apoptosis via plasma membrane blebbing, DNA degradation, and chromatin condensation. The apoptotic bodies are engulfed by macrophages to prevent adverse inflammatory responses [3, 4]. Blocking the activation of both NFкB and caspases leads to the recruitment of RIPK3 and MLKL by RIPK1 to form the necrosome. However, inhibition of NFкB alone has been reported to potentially lead to necroptosis [5]. These processes are accompanied by the subsequent phosphorylation of RIPK1, RIPK3, and MLKL. Phosphorylated MLKL oligomerizes and reduces membrane integrity, which causes necrosis-like cell death characterized by cell swelling, membrane rupture, and cytolysis. Exposure to damage-associated and pathogen-associated molecular patterns in the microenvironment exacerbates inflammatory responses [3, 4, 6]. Because it can determine cellular responses and fates, TNF-α signaling has a wide range of physiological effects on development, immune responses, and tissue homeostasis [1, 2, 7].
Tumor necrosis factor receptor-associated factor 6 (TRAF6) is a member of the TRAF protein family. TRAF6 mediates signals from diverse receptors, including CD40, IL-1R, RANK, TGFR, and TLRs, to activate NFкB and MAPK; however, it is not known to regulate TNFR complex signaling [8–10]. In addition to receptor complex signaling, TRAF6 also regulates various substrates such as p53, STAT6, Beclin1, p62, mTOR, Rac1, AKT, PIK3CA, Notch, hnRNPA1, MAVS, IRF7, HIF1α, MST1, YOD1, DNA2, and cGAS [11–27]. Thus, TRAF6 has diverse physiological functions in immune responses and tissue homeostasis [9, 28–31].
TRAF6 is reportedly both a promotive and suppressive regulator of apoptosis. TRAF6 mediates p75NTR receptor signaling to activate c-Jun, which induces pro-apoptotic gene expression [32]. TRAF6 also activates caspase-8 during death receptor signaling. As a result, cells with higher TRAF6 expression undergo spontaneous apoptosis and are more sensitive to FasL-, TRAIL-, and MG132-induced apoptosis [33, 34]. In contrast, TRAF6 protects cancer cells from apoptosis [35]. For example, TRAF6 protects cancer cells from doxorubicin and gemcitabine [36, 37]. Other reports have demonstrated that TRAF6 represses ROS production in TNF-α-treated cells, preventing cell death [38, 39]. However, the detailed molecular mechanisms of how TRAF6 regulates TNF-α-induced extrinsic cell death have not been fully elucidated.
Here, we describe the novel role of TRAF6 as an anti-apoptotic and anti-necroptotic suppressor of RIPK1-mediated cell death in TNF-α- and smac-mimetic treated cells. TRAF6-depleted cell lines were highly sensitized to TNF-α/birinapant-induced apoptosis and TNF-α/birinapant/zVAD-fmk-induced necroptosis, whereas TNF-α-induced NFκB and MAPK activation remained unaffected [10]. Mechanistically, TRAF6 blocks the protein-protein interaction of RIPK1 with itself, FADD, and RIPK3, independent of its E3 ligase activity. Depletion or inhibition of IKKα/β blocks the interaction between TRAF6 and RIPK1 and accelerates cell death, indicating that IKK is a prerequisite for TRAF6 and RIPK1 interaction. In Traf6-/- mice embryos, cell death was activated in liver tissue. Double knockout of Tnf with Traf6 reduced deregulated cell death in embryos, highlighting the importance of TRAF6 in preventing TNF-α-induced cell death in vivo. In addition, results from a tumor xenograft model revealed that depletion of TRAF6 sensitizes tumors to necroptotic stimuli.
Results
TRAF6 deficiency accelerates TNF-α-induced cell death
To investigate the role of TRAF6 in TNF-α-induced cell death, we generated stable knockdown (KD) and knockout (KO) cell lines using shRNAs and gRNAs targeting TRAF6 (Figs. 1A, C, F). Treating TRAF6-depleted HeLa cell lines with TNF-α and birinapant (T/Bi) resulted in an increase in apoptotic cell death compared to that in the control (Fig. 1B, S1A). Consistently, in HT-29 cells, apoptosis induced by treatment with T/Bi or necroptosis induced by treatment with TNF-α, birinapant, and zVAD-fmk (T/Bi/zVAD) was enhanced in TRAF6 KO cells (Figs. 1D, E, S1B). Traf6 KO L929 cells were also sensitized to apoptotic and necroptotic stimuli (Figs. 1G, H, S1C). To investigate the role of TRAF6 E3 ligase activity in suppressing cell death, we induced the expression of TRAF6WT or TRAF6C70A which is defective in E3 ligase activity (Fig. S2A, B) [40–42]. The overexpression of either TRAF6WT or TRAF6C70A in HeLa and HT-29 cells suppressed apoptosis and necroptosis, respectively (Fig. S2C, D, S3A). Moreover, reconstitution of either TRAF6WT or TRAF6C70A in TRAF6 KD HT-29 cells also repressed necroptosis, suggesting that the E3 ligase activity of TRAF6 might not be required for protection against apoptosis or necroptosis (Fig. S3B). Overall, these results indicate that TRAF6 negatively regulates TNF-α-mediated apoptosis and necroptosis without affecting the expression levels of proteins involved in cell death pathways. Moreover, the E3 ligase activity of TRAF6 is not required to suppress cell death.
Fig. 1. TRAF6 deficiency accelerates TNF-α-induced cell death.
A HeLa cells were lentivirally transfected to generate stable TRAF6-knockdown (KD) cell lines. The expression of RIPK1, FADD, caspase-8, and TRAF6 in each cell line was analyzed using western blotting. B Traf6 KD and control HeLa cell lines were treated with 10 ng/ml TNF-α and 1 μM birinapant to induce apoptosis. ZVAD-fmk (10 μM) was used as the control to inhibit caspase-mediated cell death. The cells were harvested at the indicated time points and stained with annexin V-FITC. The proportion of apoptotic cells in each cell line was measured using flow cytometry (n = 3). C gRNAs targeting TRAF6 were lentivirally transfected into HT-29 cells. The expression of RIPK1, RIPK3, MLKL, and TRAF6 in TRAF6 knockout (KO) cell lines were analyzed using western blotting. D TRAF6 KO and control HT-29 cells were treated with 30 ng/ml TNF-α and 2 μM birinapant. Apoptosis rates were measured using annexin V- FITC staining and flow cytometry (n = 3). E TRAF6 KO and control HT-29 cells were treated with 30 ng/ml TNF-α, 2 μM birinapant, and 30 μM zVAD-fmk to induce necroptosis at the indicated time points. GSK’963 (100 nM) was used to inhibit necroptosis. Cells were stained with annexin V-FITC and necroptosis was measured using flow cytometry (n = 3). F gRNAs targeting the mouse Traf6 gene were lentivirally transfected into the L929 cell line. Expression of PARP, caspase-3, RIPK3, MLKL, and TRAF6 in Traf6 KO cell lines were analyzed using western blotting. G Traf6 KO and control L929 cells were treated with mouse TNF-α (10 ng/ml) to induce apoptosis at the indicated time points. Cells were stained with annexin V-FITC, and apoptosis was measured using flow cytometry (n = 3). H Traf6 KO and control L929 cells were treated with mouse TNF-α (10 ng/mL) and zVAD-fmk (10 μM) to induce necroptosis at the indicated time points. GSK’963 (100 nM) was used to inhibit necroptosis. Cells were stained with annexin V-FITC, and necroptosis was measured using flow cytometry (n = 3). Data are presented as means ± standard deviations (S.D.s), n = 3. Significance between groups was determined using two-sided Students’ test. (*p < 0.05, **p < 0.005, ***p < 0.0005) (Figs. 1B, D, E, G, H).
TRAF6 is not involved in TNF-α-induced NFκB activation
Because TRAF6 suppresses apoptosis and necroptosis induced by T/Bi and T/Bi/zVAD, we also investigated the possible roles of TRAF6 in promoting cell survival by enhancing NFκB activation. In TRAF6-deficient HeLa and HT-29 cell lines treated with TNF-α, the phosphorylation and degradation of IκBα and the phosphorylation of p65 were unaffected compared with the controls (Fig. S4A, B). In addition, the phosphorylation of JNK and ERK, which has been reported to be regulated by TRAF6 [43, 44], was not affected by TRAF6 KO in TNF-α-treated cells (Fig. S4A, B). Immunoprecipitation of TNFR1 revealed that the ubiquitination and phosphorylation of RIPK1 in the TNFR1 complex were not affected by TRAF6 depletion (Fig. S4C). These data are consistent with those of a previous study reporting that TRAF6 does not regulate NFκB activation induced by TNF-α [10]. Based on these results, the possibility of TRAF6 affecting TNF-α-mediated survival pathways can be excluded.
TRAF6 suppresses cell death by blocking complex II and necrosome assembly
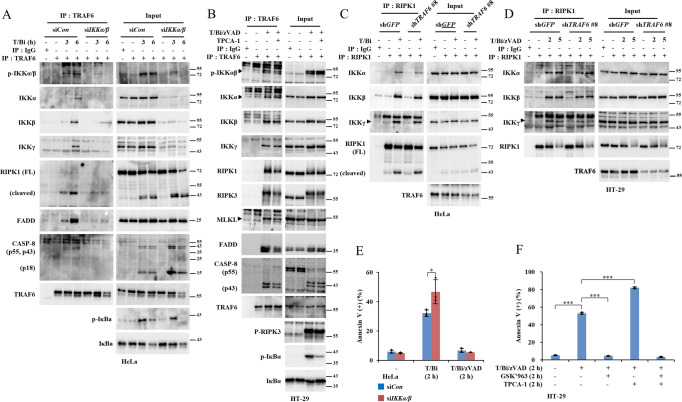
Subsequently, we compared the molecular signaling cascades involved in TNF-α-induced cell death in TRAF6-deficient and control cells. Concordantly, the cleavage of PARP and caspase-8 was augmented in TRAF6-depleted HeLa cells (Fig. 2A). Furthermore, immunoprecipitation of caspase-8 in T/Bi-treated cell lysates exhibited more than twofold increase in complex II assembly in TRAF6-depleted cells compared to that in control cells (Fig. 2B). The induction of necroptosis by T/Bi/zVAD in HT-29 cells also increased the phosphorylation of RIPK1, RIPK3, and MLKL after TRAF6 depletion (Fig. 2C). Immunoprecipitation of RIPK3 in T/Bi/zVAD-treated cell lines indicated that the depletion of TRAF6 increased the assembly of necrosomes twofold higher compared to that in the control cells (Fig. 2D). An accelerated increase in cell death markers in TRAF6-depleted cells was reversed after the induction of TRAF6WT and TRAF6C70A expression, indicating that E3 ligase activity was not required to suppress TNF-α-induced necroptosis (Fig. S3C). Overall, TRAF6 did not have a discernable effect on complex I. In contrast, a significant increase in complex II and necrosome formation was observed after TRAF6 depletion.
Fig. 2. TRAF6 deficiency promotes the assembly of the cell death complex.
A TRAF6 KD and control HeLa cells were treated with 10 ng/ml TNF-α and 1 μM birinapant (T/Bi) to induce apoptosis. ZVAD-fmk (10 μM) was used as the negative control for apoptosis. The cells were harvested and lysed at the indicated time points. Cleavage of PARP and caspase-8 and the expression of TRAF6 were analyzed using western blotting. B T/Bi treated TRAF6 KD and control HeLa cells were lysed at the indicated time points. Anti-caspase-8 antibody and protein-G agarose beads were used to pull down the complex II. The protein-bead conjugates were washed, and the proteins were separated by SDS-PAGE. The immunoprecipitated proteins were analyzed using western blotting. C TRAF6 KO and control HT-29 cells were treated with 30 ng/ml TNF-α, 2 μM birinapant, and 30 μM zVAD-fmk (T/Bi/zVAD) to induce necroptosis. GSK’963 (100 nM) was used to inhibit RIPK1-mediated necroptosis. Cells were harvested at the indicated time points and analyzed using western blotting. D T/Bi/zVAD-treated TRAF6 KD and control HT-29 cells were lysed at the indicated time points. The necrosome was pulled down using an anti-RIPK3 antibody and protein-G agarose beads. The protein-bead conjugate was washed and sampled. The assembly of the RIPK1-RIPK3-MLKL complex was analyzed using western blotting.
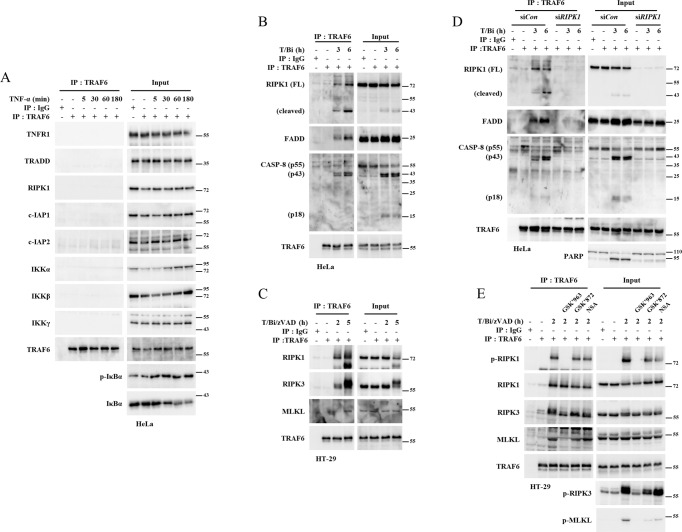
TRAF6 interacts with components of TNF-α-induced cell death upon cell death signals
Although TRAF6 did not have any effect on the NFκB-mediated survival pathway, its depletion led to an increase in complex II and necrosome formation. These observations led us to investigate whether TRAF6 interacts with the components of these complexes. TRAF6 did not immunoprecipitate with complex I components, including RIPK1 and IKK, in TNF-α-treated HeLa cell lysates (Fig. 3A). However, treating HeLa cells with T/Bi induced the interaction of TRAF6 with complex II components, including RIPK1, FADD, and caspase-8, while TRAF6 did not interact with TNFR1 complex components such as TNFR1, TRADD, cIAP1, or cIAP2 (Fig. 3B, S5A, B). Similarly, the interaction of TRAF6 with RIPK1, RIPK3, and MLKL was induced upon the treatment of HT-29 cells with T/Bi/zVAD (Fig. 3C, S5C). These interactions were progressively reduced at the later time points, which highly correlate with the progression of cell death complex formation kinetics (Fig S5D, E). Therefore, TRAF6 seems to bind to components of cell death complex while they are highly activated. Because RIPK1, a crucial regulator of both apoptosis and necroptosis, is expressed in both HeLa and HT-29 cell lines, we investigated whether RIPK1 could be the major regulatory target of TRAF6. RIPK1 KD by siRNA prevented the interaction of TRAF6 with caspase-8 and FADD in HeLa cells (Fig. 3D). In the T/Bi/zVAD-stimulated HT-29 cell line, inhibition of RIPK1 kinase activity (by GSK’963) significantly suppressed the association between TRAF6 to RIPK3 or MLKL. Of importance, the binding of TRAF6 to RIPK1 was not affected. In contrast, inhibition of RIPK3 kinase activity (by GSK’872), or MLKL oligomerization (by NSA) did not affect the interaction of TRAF6 with RIPK1, RIPK3, and MLKL (Fig. 3E). These results indicated that RIPK1 kinase activity is required for the interaction of TRAF6 with RIPK3 and MLKL. Since TRAF6 does not interact with TNFR1, while RIPK1 does under cell death stress, we analyzed whether TRAF6-RIPK1 interaction is induced in the cytosol. In T/Bi/zVAD or T/Bi/zVAD/GSK’963-treated cells, caspase-8 was immunoprecipitated with RIPK1, and FADD. This presents the cytosolic cell death complex formation even under treatment of GSK’963 (Fig S6A). In the same cell lysate, cytosolic interaction of TRAF6 with RIPK1 was further confirmed by depleting TNFR1 by anti-TNFR1 antibody (Fig S6B). Since HeLa and L929 are known to secrete autonomous TNF-α [45, 46], we tested both cell lines without TNF-α. While there were no significant effects of long-term treatment of birinapant or zVAD on L929 (Fig. S7A), HeLa cells underwent apoptotic cell death by birinapant treatment. Furthermore, TRAF6 KD HeLa cells were more sensitive to long-term treatment of birinapant (Fig. S7B). This phenomena was partially suppressed by anti-TNF-α antibody treatment. Knockdown of RIPK1 by siRNA completely blocks the birinapant-induced cell death. Particulary, RIPK1/FADD/CASP-8 complex assembly was increased in TRAF6-depleted cell line (Fig. S7C). Collectively, these data suggest that RIPK1 might be the primary target of TRAF6 in regulating TNF-α-induced cell death. Apoptotic and necroptotic TNF-α stimuli induced the cytosolic interaction between TRAF6 and RIPK1. The interactions between TRAF6 and other factors in the complex II or necrosome depend on the presence or activation of RIPK1. These results suggest that TRAF6 mainly targets RIPK1 to alleviate TNF-α-induced cell death.
Fig. 3. TRAF6 interacts with the components of cytosolic cell death complex in an RIPK1-dependent manner.
A HeLa cells were treated with 10 ng/ml TNF-α and lysed at the indicated time points. The lysates were incubated with anti-TRAF6 antibodies and protein-G agarose beads. The beads were then washed and subjected to SDS-PAGE. The recruitment of complex I components to TRAF6 was analyzed using western blotting. B HeLa cells were treated with 10 ng/ml TNF-α and 1 μM birinapant (T/Bi). Cells were lysed at the indicated time points and incubated with anti-TRAF6 antibodies and protein-G agarose beads to pull down TRAF6. The beads were then washed and subjected to SDS-PAGE. The recruitment of RIPK1, FADD, and caspase-8 to TRAF6 was analyzed using western blotting. C HT-29 cells were treated with 30 ng/ml TNF-α, 2 μM birinapant, and 30 μM zVAD-fmk (T/Bi/zVAD). The cells were lysed at the indicated time points and incubated with anti-TRAF6 antibodies and protein-G agarose beads. The beads were then washed and subjected to SDS-PAGE. The recruitment of RIPK1, RIPK3, and MLKL to TRAF6 was analyzed using western blotting. D HeLa cells were transfected with siRNA against RIPK1 or control siRNA. RIPK1 knockdown and control cells were treated with T/Bi. The cells were lysed at the indicated time points and incubated with anti-TRAF6 antibodies and protein-G agarose beads to pull down TRAF6. The beads were then washed and subjected to SDS-PAGE. The recruitment of RIPK1, FADD, and caspase-8 to TRAF6 was analyzed using western blotting. E HT-29 cells were treated with T/Bi/zVAD. GSK’ 963 (100 nM) to inhibit RIPK1 kinase activity. GSK’872 (3 μM) was used to inhibit RIPK3 kinase activity. NSA (2 μM) was used to block MLKL oligomerization. The cells were lysed at the indicated time points and incubated with anti-TRAF6 antibodies and protein-G agarose beads. The beads were then washed and subjected to SDS-PAGE. The recruitment of RIPK1, RIPK3, and MLKL to TRAF6 was analyzed using western blotting.
TRAF6 suppresses TNF-α–mediated cell death by forming complexes with RIPK1
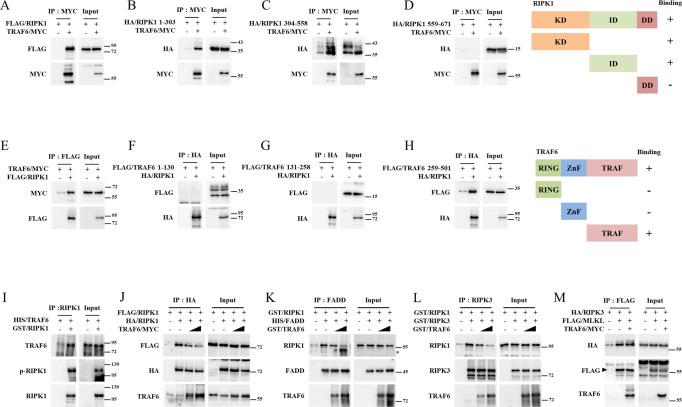
Because previous data suggested that RIPK1 is the primary binding substrate for TRAF6 in cell death-stimulated cells, we focused on its ability to interact with RIPK1. We confirmed that overexpressed TRAF6 and RIPK1 interacted in HEK-293FT cells (Figs. 4A, E). We also observed that TRAF6 bound to the kinase (RIPK1 1-303) (Fig. 4B) and intermediate domains (RIPK1 304-558) (Fig. 4C) but did not bind to the death domain (RIPK1 558-671) (Fig. 4D). In contrast, full-length TRAF6 and the TRAF domain (TRAF6 259-522) bound to RIPK1 (Fig. 4E–H). Recombinant TRAF6 and RIPK1 purified from E. coli were also co-immunoprecipitated when an anti-RIPK1 antibody was used in the procedure (Fig. 4I). Overall, the ability of the C-terminal TRAF domain of TRAF6 to bind to the kinase and intermediate domains of RIPK1 may be necessary for it to suppress cell death. Because TRAF6C70A suppresses TNF-α-mediated cell death (Fig. S2, S3), we hypothesized that the protein-protein interaction of TRAF6 and RIPK1, rather than ubiquitination, might suppress complex II or necrosome assembly. As expected, TRAF6 suppressed RIPK1 homo-interactions in a dose-dependent manner (Fig. 4J). Furthermore, RIPK1/FADD and RIPK1/RIPK3 interactions were kinetically blocked by TRAF6 (Figs. 4K, L). Notably, these competitive interactions in vitro without E1, E2, ubiquitin, or ATP support the idea that E3-ligase activity is not required for this function. However, TRAF6 could not suppress the interaction between RIPK3 and MLKL, suggesting that direct interaction between TRAF6 and RIPK1 is critical for preventing cell death (Fig. 4M). In summary, these data suggest that the primary suppressive target of TRAF6 is RIPK1 and that it prevents cell death by inhibiting the interaction of RIPK1 with itself, FADD, or RIPK3, independently of E3 ligase activity.
Fig. 4. TRAF6 suppresses interaction between RIPK1 and components of the cell death complex.
A HEK 293-FT cells were transfected with pCMV/TRAF6-MYC and pcDNA3-FLAG/RIPK1 by polyethylenimine (PEI). The cells were lysed and incubated with anti-MYC antibodies and protein-G agarose beads to pull down TRAF6/MYC. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 was analyzed using western blotting. B–D pcDNA3-HA/RIPK1 1-303 (kinase domain), 304-558 (intermediate domain), or 559-671 (death domain), and pCMV/TRAF6-MYC were overexpressed in HEK-293 FT cells by PEI-transfection. The cells were lysed and incubated with anti-MYC antibodies and protein-G agarose beads to pull down TRAF6/MYC. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 domain was analyzed using western blotting. E HEK 293-FT cells were transfected with pcDNA3-FLAG/RIPK1 and pCMV/TRAF6-MYC by PEI. The cells were lysed and incubated with anti-FLAG antibodies and protein-G agarose beads to pull down FLAG/RIPK1. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 was analyzed using western blotting. F–H pcDNA3-FLAG/TRAF6 1-130 (RING domain), 131-258 (Zinc finger domain), or 259-522 (TRAF domain) and pcDNA3-HA/RIPK1 were overexpressed in HEK-293 FT cells by PEI-transfection. The cells were lysed and incubated with anti-HA antibody and protein-G agarose beads to pull down HA/RIPK1. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 was analyzed using western blotting. I Recombinant HIS/TRAF6 and GST/RIPK1 were purified from E.coli. Recombinant proteins were mixed in lysis buffer and incubated with anti-RIPK1 antibodies and protein-G agarose beads to pull down GST/RIPK1. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 was analyzed using western blotting. J HEK-293 FT cells were transfected with pcDNA3-HA/RIPK1, pcDNA3-FLAG/RIPK1, and pCMV/TRAF6-MYC by PEI. Cell lysates were incubated with anti-FLAG antibodies and protein-G agarose beads to pull down FLAG/RIPK1. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitation of TRAF6 and RIPK1 was analyzed using western blotting. K Recombinant GST/RIPK1, GST/TRAF6, and HIS/FADD were purified from E. coli. The proteins were mixed and incubated with anti-FADD antibodies. Protein-G agarose beads were used to pull down the protein-antibody conjugate. The protein-bead conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitated proteins were analyzed using western blotting. (*: TRAF6 band). L Recombinant GST/RIPK1, GST/TRAF6, and GST/RIPK3 were purified from E. coli. The proteins were mixed and incubated with anti-RIPK1 antibodies. Protein-G agarose beads were used to pull down protein-antibody conjugates. The protein-antibody conjugate was washed and sampled for SDS-PAGE analysis. The immunoprecipitated proteins were analyzed using western blotting. M. HEK-293FT cells were transfected with pcDNA3-HA/RIPK3, pcDNA3-FLAG/MLKL, and/or pCMV/TRAF6-MYC. The cells were lysed and immunoprecipitated using anti-FLAG antibodies and Protein-G agarose beads to pull down FLAG/MLKL. Protein-protein interactions were analyzed using western blotting.
IKK is required for induction of RIPK1/TRAF6 interaction
TRAF6 interacts with RIPK1 and other cell death-associated factors only at the onset of apoptosis or necroptosis (Fig. 3). This phenomenon implies that putative upstream factors trigger the interaction between TRAF6 and RIPK1. IKK has been reported to be involved in regulating RIPK1-mediated cell death in TNF-α-treated cells [47, 48] and TRAF6 signal transduction [28]. Therefore, we investigated whether IKK is an upstream regulator of the TRAF6/RIPK1 regulatory axis. Knockdown of both IKKα and IKKβ significantly reduced the interactions between TRAF6 and RIPK1, FADD, and caspase-8 (Fig. 5A). The presence of either IKKα or IKKβ was found to be sufficient for the interaction between TRAF6 and RIPK1 (Fig. S9A). Similarly, exposing T/Bi/zVAD-treated HT-29 cells to TPCA-1, an inhibitor of IKKα/β kinase activity, reduced the interaction of TRAF6 with RIPK1, RIPK3, MLKL, FADD, and caspase-8 (Fig. 5B). Furthermore, IKK co-immunoprecipitated with TRAF6 and RIPK1 in response to cell death stimuli (Fig. 5A–D). TRAF6 is reportedly a critical upstream mediator of IKK in other types of receptor signaling [49]. However, TRAF6 depletion did not block the interaction between RIPK1 and IKK (Figs. 5C, D). Consistent with these observations, IKKα/β KD or treatment with TPCA-1 increased apoptosis and necroptosis in HeLa and HT-29 cells, respectively (Figs. 5E, F). Inhibiting the kinase activity of RIPK1 by GSK’963 did not affect the interaction between TRAF6 and IKK (Fig. S9B). These data support the hypothesis that IKK is an upstream inducer of TRAF6 interactions with RIPK1. IKK plays a pro-survival role by phosphorylating RIPK1 Ser25 in complex I after TNF-α or TNF-α/cycloheximide treatment [47, 48]. In contrast, in T/Bi-or T/Bi/zVAD-treated cells, we demonstrated that IKK complexes with cytosolic cell death complexes (Fig. S9C, D). This finding correlates with data suggesting that TRAF6 interacts with RIPK1 in the cytosol after cell death stimuli, further implicating the IKK/TRAF6/RIPK1 regulatory axis (Fig. 3). However, the interaction of TRAF6 with RIPK1 was not inhibited by alanine substitution of serine residues on RIPK1 reported to be phosphorylated by IKK in TNFR complexes. This finding suggests that IKK might have different target sites or regulatory mechanisms than hypothesized (Fig. S10A-G) [48]. In summary, apoptotic and necroptotic stimuli induced IKK to mediate the recruitment of TRAF6 to RIPK1, which suppressed RIPK1-mediated cell death.
Fig. 5. IKK is required for TRAF6-RIPK1 interactions.
A HeLa cells were transfected with siRNAs targeting IKKα and IKKβ. After 48 h, the cells were treated with 10 ng/ml TNF-α and 1 μM birinapant (T/Bi) to induce apoptosis. The cells were harvested and lysed at the indicated time points. The cell lysates were incubated with anti-TRAF6 antibodies and protein-G agarose beads to pull down TRAF6-interacting proteins. The beads were washed and sampled for SDS-PAGE analysis. B HT-29 cells were treated with 30 ng/ml TNF-α, 2 μM birinapant, 30 μM zVAD-fmk (T/Bi/zVAD), and 5 μM TPCA-1 for the indicated durations. The cells were harvested and lysed at the indicated time points. The cell lysates were incubated with anti-TRAF6 antibodies and protein-G agarose beads to pull down TRAF6-interacting proteins. The beads were washed and sampled for SDS-PAGE analysis. C T/Bi treated TRAF6 knockdown and control HeLa cells were lysed at the indicated time points. Anti-RIPK1 antibody and protein-G agarose beads were used to pull down the complex II. The protein-bead conjugates were washed, and the proteins were separated by SDS-PAGE. The immunoprecipitated proteins were analyzed using western blotting. D shGFP and shTRAF6 transfected HT-29 cells were treated with T/Bi/zVAD. The cells were lysed and incubated with anti-RIPK1. Protein-G agarose beads were used to precipitate the proteins. Protein interactions were analyzed using western blotting. E HeLa cells were transfected with siRNAs targeting IKKα and IKKβ. After 48 h, the cells were treated with T/Bi for 2 h to induce apoptosis. Cells were treated with 10 μM zVAD-fmk to inhibit apoptosis. Cell death was measured using annexin-V staining and flow cytometry (n = 3). F HT-29 cells were treated with T/Bi/zVAD and 5 μM TPCA-1 for 2 h. The cells were treated with 100 nM GSK’963 to inhibit necroptosis. Cell death was measured using annexin-V staining and flow cytometry (n = 3). Data are presented as means ± standard deviations (S.D.s), n = 3. Significance was determined using the two-sided Students’ test. (*p < 0.05, **p < 0.005, ***p < 0.0005).
Traf6 depletion deregulated cell death in the embryonic liver and tumor xenograft model
Subsequently, we assessed the in vivo suppressive roles of TRAF6 in extrinsic cell death. Traf6–/– mice reportedly exhibit perinatal and postnatal mortality [10]. Therefore, we investigated whether cell death was deregulated in Traf6–/– KO embryos. On embryonic day 17.5, Traf6–/– embryos exhibited increased TUNEL in their liver tissues (Fig. S11A, B). In addition, Traf6–/–/Tnf–/– embryos exhibited reduced cell death signals compared to Traf6–/– embryos (Fig. S11A, B). TRAF6 is an oncogenic protein that protects cancer cells from death induced by DNA-damaging anticancer drugs [29, 36, 37]. Thus, we investigated whether TRAF6 could protect cancer cells from extrinsic cell death. First, we established a TRAF6-deficient Molm-13 leukemia cell line (Fig. S12A). There was no difference in cell growth rates between wild type and TRAF6 KD cell lines under normal conditions (Fig. S12B). Since this cell line was known to secrete TNF-α autonomously [50–52], we treated the cells with Bi/zVAD without TNF-α treatment. The data show that TRAF6 deficient cell line is more susceptible to TNF-α-induced cell death, which are completely blocked by the treatment of GSK’963 (Fig. S12C, D). While TRAF6 was reported to suppress apoptosis induced by DNA-damaging agents [29, 36, 37], TRAF6 KD Molm-13 was not sensitized to etoposide and cisplatin (Fig. S13A, B). This finding indicates that suppression of the DNA-damage-induced apoptosis by TRAF6 depends on cell types. Tumor xenografts of the Molm-13 cell lines were established in nude mice to measure necroptosis in autocrine-TNF-α secretion dependent manner [50–52]. The xenografts established with TRAF6 KD Molm-13 were highly sensitive to necroptotic stimuli and exhibited reduced growth (Fig. 6A–D). Overall, TRAF6 appears to function as an anti-cell death factor in both embryos and cancer xenografts.
Fig. 6. TRAF6 suppresses cell death ex vivo.
A–D Tumor xenografts were established using control or TRAF6 KD Molm-13 cell lines (n = 5). The mice were treated with birinapant (2 mg/kg) and emricasan (1 mg/kg) every two days via peritoneal injection for a total of six times. A Representative images of the xenografts. B The tumors were lysed, and TRAF6 expression was analyzed using western blotting. C Tumor weights were measured after dissection. D Tumor growth was measured every four days during the experiment. Data are presented as means ± standard deviations (S.D.s). Significance was determined using the two-sided Students’ test (n.s: non-significance, *p < 0.05, **p < 0.005, ***p < 0.0005).
Discussion
Here, we report a novel role for TRAF6 as a suppressor of TNF-α/smac-mimetics-induced cell death downstream of IKK. TRAF6 is a critical regulator of NFκB/MAPK activation in receptor signaling [9, 29]. We revealed that the TNFR pathway is not regulated by TRAF6 [10] (Fig. S4). However, TNF-α is upregulated and performs various functions in tumors [53], and TRAF6 is overexpressed in tumors and suppresses intrinsic cell death [29, 36, 37]. This suggests that TNF-α-induced cell death might be suppressed by TRAF6. As shown in this study, TRAF6 suppressed cell death induced by treatment of various cancer cell lines and a xenograft model with the smac-mimetic birinapant and TNF-α (Figs. 1, 6). These findings suggest an additional tumor-promoting role for TRAF6. They also imply that a combination of smac-mimetics and TRAF6 inhibitors could be used to sensitize cancer cells [54]. In addition to cancer tissue, TNF-α is expressed in developing mouse embryos and is a critical regulator of hepatic homeostasis [55–57]. Furthermore, abnormal TNFR signaling sensitizes hepatic cells to apoptosis [58]. Our study revealed deregulated cell death in the Traf6-/- liver tissue, which was reversed by simultaneous knockout of Tnf. In this regard, our research suggests the critical role of TRAF6 in normal liver development, where tight regulation of TNF-α is required. Despite the reversal of cell death in fetal liver by Tnf KO, the lethality of Traf6 KO mice was not reversed (Fig. S11C). While the increased cell death is limited in the liver (Fig. S11A), Traf6 KO mice display defects in various physiological homeostasis in bone, the immune system, stem cells, skin appendices, Schwann cells, etc [9]. Further studies are required to employ liver-specific DKO of Traf6 crossed with other cell death factors such as Tnf, Caspase, and Ripk3 or their doubl KO to identify specific types of cell death, and to provide strict genetic controls. IKK was found to suppress TNF-α-induced cell death by phosphorylating RIPK1 in the complex I independently of NFκB activation [47, 48]. In TNF-α and smac-mimetics-treated cells, we examined the cytosolic regulation of RIPK1 by TRAF6, which requires the presence of IKK. Our observations differed from those reported previously, which indicates that IKK acts downstream of TRAF6 to activate NFκB in other receptor signaling pathways [28]. Because TRAF6 does not participate in complex I signaling, IKK seems to be activated independently of TRAF6 as an upstream suppressor of RIPK1 in TNF-α/smac-mimetic-treated cell death. Future studies should focus on comprehensively investigating the mechanisms of IKK activation in apoptotic and necroptic cells and the detailed mechanism of IKK inducing TRAF6/RIPK1 interaction to suppress cell death. In summary, we propose a molecular mechanism by which TRAF6 regulates RIPK1-mediated cell death and its physiological implications.
Materials and methods
Cell culture, plasmids, and transfection
The HT-29, HeLa, Molm-13, L929, and HEK-293 FT cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). Cell lines were maintained according to the instructions provided by the ATCC (Manassas, VA, United States). Plasmids were prepared as previously described. The TRAF6 plasmid was purchased from Sinobiological (Beijing, China, #HG17419-CM). Plasmids were transfected using polyethylenimine (#408727; Sigma-Aldrich, St. Louis, MO, USA). The adenoviruses were purchased from VectorBuilder (Chicago, IL, USA). The siRNAs used in this study were siRIPK1 (Dharmacon, CO, USA; #L-004445-00), siCHUK (#L-003474-00; Dharmacon), and siIKBKB (#L-003503-00; Dharmacon). Lipofectamine RNAiMAX reagent (#13778150; Thermo Fisher, Waltham, MA, USA) was used to transfect the siRNAs according to the manufacturer’s instructions.
Stable cell line generation
The LentiCRISPRv2 vector was provided by Feng Zhang (Addgene plasmid #52961). The inserted guide RNA sequences were designed using CRISPOR (http://crispor.tefor.net) and cloned into the vector, as described in the GeCKO protocol. To generate stable knockdown (KD) and knockout (KO) cell lines, HEK-293FT cells were transfected with plasmids for virus production. The virus-containing media were harvested by filtration with a 0.45-μm syringe filter. The target cells were treated with the virus-containing medium and selected using puromycin. Depletion efficacy was assessed using western blotting. The sequences used are listed in Supplementary Table 1.
Immunoprecipitation and immunoblotting
For immunoprecipitation, cells were harvested and lysed in lysis buffer (50 mM Tris-HCl, pH = 7.6, 150 mM NaCl, 10 % glycerol, 0.1 % Triton X-100, and protease inhibitors) using 26 G needle syringe. The protein concentration of the lysates was measured using a BCA assay kit (Thermo Fisher; #23225). The lysates were treated with antibodies against bait proteins and incubated at 4 °C. Protein-G agarose beads (Invitrogen, Waltham, MA, United States; #15920-010) were used to pull down bait protein-antibody conjugates and incubated at 4 °C. After incubation, the beads were washed with DISC buffer and samples for western blotting were prepared using Laemmli buffer. In other experiments, cells were collected by scraping or trypsinization, and samples were prepared using Laemmli buffer. Bio-Rad (Hercules, CA, United States) labware was used for SDS-PAGE, and the proteins were transferred onto a nitrocellulose membrane (Cytiva, AmershamTM PotranTM; #10600003). Membranes were blocked with 5 % skim milk in TBS-T (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1 % Tween®20). The membranes were then incubated with antibodies for immunoblotting at 4 °C. The membranes were then washed with TBS-T and incubated with the appropriate secondary antibodies. The membranes were washed with TBS-T, and an enhanced chemiluminescence solution was used to visualize the bands. Luminescence was detected using the FUSION Solo X imaging system (Vilber, 24 rue de Lamirault, 77090 Collégien, France). The antibodies used in this study are listed in Supplementary Table 2.
Purification of recombinant protein
Plasmids were transformed into Escherichia coli BL21 cells. Protein synthesis was induced by treating the E. coli culture with 1 mM IPTG (Biopure) at 4 °C. After 16 h, the cells were harvested and sonicated in lysis buffer (50 mM Tris-HCl, adjusted to pH 8 for 6HIS-proteins and pH 7.6 for GST-proteins. 500 mM NaCl, 1% Triton X-100, and 10 % glycerol, The buffer for 6HIS proteins also contained 10 mM imidazole (Sigma-Aldrich; #I202). The soluble fraction was collected via centrifugation. 6HIS-tagged proteins were incubated with Ni2+-NTA beads (Qiagen, Hilden, Germany; #30210), and GST-tagged proteins were treated with glutathione sepharose 4B (Cytiva, AmershamTM PotranTM; #17-0756-1). After incubation, the beads were washed with lysis buffer. Subsequently, the proteins were eluted using an elution buffer containing 250 mM imidazole for 6HIS-tagged proteins and 20 mM reduced glutathione for GST-tagged proteins (Sigma-Aldrich; #G4251-2). Eluted proteins were dialyzed using dialysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1 % Triton X-100, 10% glycerol).
Chemicals
To induce apoptosis, HeLa cells were treated with 10 ng/ml TNF-α and 1 μM birinapant, HT-29 cells were treated with 30 ng/ml TNF-α and 2 μM birinapant, and L929 cells were treated with 10 ng/ml mouse TNF-α. Molm-13 cells were treated with 20 ng/ml TNF-α and 2 μM birinapant. ZVAD-fmk (10 μM) was used to inhibit the apoptosis of HeLa cells. To induce necroptosis, HT-29 cells were treated with 30 ng/ml TNF-α, 2 μM birinapant, or 30 μM zVAD-fmk. The L929 cells were treated with 10 ng/ml mouse TNF-α and 10 μM zVAD-fmk. The Molm-13 cells were treated with 20 ng/ml TNF-α, 2 μM birinapant and 30 μM zVAD-fmk or 30 μM emricasan. To inhibit RIPK1, RIPK3, and MLKL, 100 nM GSK’963, 3 μM GSK’872, and 2 μM necrosulfonamide were used. IKK activity was inhibited by treatment with 5 μM of TPCA-1. Information on the chemicals used is provided in Supplementary Table 3.
FACS
Biological triplicates of each treatment were harvested by trypsinization before cell death evaluations. Annexin V (BD Biosciences, San Diego, CA, USA; #556419) staining was performed according to the manufacturer’s instructions. Cell fluorescence was measured using an Accuri 6 instrument (BD Biosciences).
Mouse experiments
Traf6-/- mouse was provided by the RIKEN BRC through the National BioResource. Project of MEXT/AMDED, Japan (RBRC04950). Tnf-/- mice were purchased from the JACKSON Laboratory. To analyze embryo tissues, pregnant mice were dissected at embryonic day 17.5, and the embryos were fixed with formalin (Sigma-Aldrich; #HT501128) and paraffin blocks. Sagittal slices of embryos were stained with the TUNNEL assay kit which was performed in blind by T&P BIO (Gwangju-si, Gyeonggi-do, Korea, http://www.tnpbio.co.kr/index.asp). IHC staining was scored with Image J [59]. Xenografts were established as described previously [52]. Briefly, 5×105 cells in 50 μl PBS and 50 μl Matrigel (Corning, NY, USA; #354234) shGFP or shTRAF6 #8 Molm-13 cells were injected into the flanks of 6-week-old female BALB/c nude mice (Seoul, Korea, Narabiotech). Then, 2 mg/kg birinapant and 1 mg/kg emricasan were injected intraperitoneally every two days for a total of six times.
Statistical analysis
Cell death and IHC staining values were compared using means and standard deviations. Statistical significance was assessed using Student’s t-test and analyzed using GraphPad Prism software.
Supplementary information
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (MSIP) (2015R1A3A2066581), in part by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2020R1A5A1019023), and in part by the Brain Korea 21(BK21) FOUR program.
Author contributions
C-SL designed, performed, and interpreted all experiments and prepared the manuscript. GH prepared materials and performed the xenograft model experiment. YWN and CHH designed and interpreted the experimental results. JS directed the experiments and revised the manuscript.
Competing interests
The authors declare no competing interests.
Ethics
The mouse embryo and xenograft model studies were approved by the Yonsei University Institutional Animal Care and Use Committee (IACUC-A-202111-1368-01 and IACUC-A-202205-1465-01).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01161-w.
References
- 1.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott M. Tumour necrosis factor signalling in health and disease. F1000Res. 2019;8:111. doi: 10.12688/f1000research.17023.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumari S, Van TM, Preukschat D, Schuenke H, Basic M, Bleich A, et al. NF-kappaB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation. Life Sci Alliance. 2021;4:e202000956. [DOI] [PMC free article] [PubMed]
- 6.Seo J, Nam YW, Kim S, Oh DB, Song J. Necroptosis molecular mechanisms: recent findings regarding novel necroptosis regulators. Exp Mol Med. 2021;53:1007–17. doi: 10.1038/s12276-021-00634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HH. Structure of TRAF family: current understanding of receptor recognition. Front Immunol. 2018;9:1999. doi: 10.3389/fimmu.2018.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Gohda J, Akiyama T, Inoue JI. TNF receptor-associated factor 6 (TRAF6) plays crucial roles in multiple biological systems through polyubiquitination-mediated NF-kappa B activation. P Jpn Acad B-Phys. 2021;97:145–60. doi: 10.2183/pjab.97.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimmack G, Schorpp K, Kutzner K, Gehring T, Brenke JK, Hadian K, et al. YOD1/TRAF6 association balances p62-dependent IL-1 signaling to NF-kappaB. Elife. 2018;6:e22416. doi: 10.7554/eLife.22416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang J, Bolanos LC, Choi K, Liu X, Christie S, Akunuru S, et al. Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia. Nat Immunol. 2017;18:236–45. doi: 10.1038/ni.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Chen Y. Ubiquitination of cGAS by TRAF6 regulates anti-DNA viral innate immune responses. Biochem Biophys Res Commun. 2019;514:659–64.. doi: 10.1016/j.bbrc.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Qin JJ, Yang X, Ji YX, Guo F, Cheng WL, et al. The ubiquitin E3 ligase TRAF6 exacerbates ischemic stroke by ubiquitinating and activating Rac1. J Neurosci. 2017;37:12123–40. doi: 10.1523/JNEUROSCI.1751-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H, Li XB, Meng Y, Fan L, Li M, Fang J. TRAF6 upregulates expression of HIF-1alpha and promotes tumor angiogenesis. Cancer Res. 2013;73:4950–9. doi: 10.1158/0008-5472.CAN-13-0370. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Li CF, Zhang L, Wu CY, Han L, Jin G, et al. TRAF6 restricts p53 mitochondrial translocation, apoptosis, and tumor suppression. Mol Cell. 2016;64:803–14. doi: 10.1016/j.molcel.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JA, Kuang TT, Pu N, Fang Y, Han X, Zhang L, et al. TRAF6 regulates YAP signaling by promoting the ubiquitination and degradation of MST1 in pancreatic cancer. Clin Exp Med. 2019;19:211–8. doi: 10.1007/s10238-018-00543-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C, Lu C, Pu H, Li D, Zhang L. TRAF6 promotes IL-4-induced M2 macrophage activation by stabilizing STAT6. Mol Immunol. 2020;127:223–9. doi: 10.1016/j.molimm.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Meng Y, Liu C, Shen L, Zhou M, Liu W, Kowolik C, et al. TRAF6 mediates human DNA2 polyubiquitination and nuclear localization to maintain nuclear genome integrity. Nucleic Acids Res. 2019;47:7564–79. doi: 10.1093/nar/gkz537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra AK, Sachan N, Mutsuddi M, Mukherjee A. TRAF6 is a novel regulator of Notch signaling in Drosophila melanogaster. Cell Signal. 2014;26:3016–26. doi: 10.1016/j.cellsig.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Liu Y, Huang S, Fang M. TRAF6 interacts with and ubiquitinates PIK3CA to enhance PI3K activation. FEBS Lett. 2018;592:1882–92. doi: 10.1002/1873-3468.13080. [DOI] [PubMed] [Google Scholar]
- 22.Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3:ra42. doi: 10.1126/scisignal.2000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling T, Weng GX, Li J, Li C, Wang W, Cao L, et al. TARBP2 inhibits IRF7 activation by suppressing TRAF6-mediated K63-linked ubiquitination of IRF7. Mol Immunol. 2019;109:116–25. doi: 10.1016/j.molimm.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Hindi SM, Paul PK, Dahiya S, Mishra V, Bhatnagar S, Kuang S, et al. Reciprocal Interaction between TRAF6 and notch signaling regulates adult myofiber regeneration upon injury. Mol Cell Biol. 2012;32:4833–45. doi: 10.1128/MCB.00717-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Chen J, Cai X, Wu J, Chen X, Wu YT, et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–96. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–8. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dainichi T, Matsumoto R, Mostafa A, Kabashima K. Immune Control by TRAF6-Mediated Pathways of Epithelial Cells in the EIME (Epithelial Immune Microenvironment) Front Immunol. 2019;10:1107. doi: 10.3389/fimmu.2019.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JD, Liu NA, Tang L, Yan B, Chen X, Zhang JL, et al. The relationship between TRAF6 and tumors. Cancer Cell Int. 2020;20:429. doi: 10.1186/s12935-020-01517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191:1395–411. doi: 10.1083/jcb.201006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlantis K, Polykratis A, Welz PS, van Loo G, Pasparakis M, Wullaert A. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut. 2016;65:935–43. doi: 10.1136/gutjnl-2014-308323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisiswa L, Fernandez-Suarez D, Sergaki MC, Ibanez CF. RIP2 gates TRAF6 interaction with death receptor p75(NTR) to regulate cerebellar granule neuron survival. Cell Rep. 2018;24:1013–24. doi: 10.1016/j.celrep.2018.06.098. [DOI] [PubMed] [Google Scholar]
- 33.He L, Wu X, Siegel R, Lipsky PE. TRAF6 regulates cell fate decisions by inducing caspase 8-dependent apoptosis and the activation of NF-kappaB. J Biol Chem. 2006;281:11235–49. doi: 10.1074/jbc.M508779200. [DOI] [PubMed] [Google Scholar]
- 34.Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Curr Biol. 2006;16:1666–71. doi: 10.1016/j.cub.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 35.Fan Z, Wu Z, Yang B. The Effect of miR-361-3p targeting TRAF6 on apoptosis of multiple myeloma cells. J Microbiol Biotechnol. 2021;31:197–206. doi: 10.4014/jmb.2010.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Q, Liang C, Hua J, Zhang B, Liu J, Zhang Y, et al. A miR-146a-5p/TRAF6/NF-kB p65 axis regulates pancreatic cancer chemoresistance: functional validation and clinical significance. Theranostics. 2020;10:3967–79.. doi: 10.7150/thno.40566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie C, Zhang LZ, Chen ZL, Zhong WJ, Fang JH, Zhu Y, et al. A hMTR4-PDIA3P1-miR-125/124-TRAF6 regulatory axis and its function in NF kappa B signaling and chemoresistance. Hepatology. 2020;71:1660–77. doi: 10.1002/hep.30931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon K, Jung EJ, Lee SR, Kim J, Choi Y, Lee SY. TRAF6 deficiency promotes TNF-induced cell death through inactivation of GSK3 beta. Cell Death Differ. 2008;15:730–8. doi: 10.1038/sj.cdd.4402304. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa D, Funakoshi-Tago M, Aizu-Yokota E, Sonoda Y, Inoue J, Kasahara T. TNF-receptor associated factor 6-deficient fibroblast is sensitive to the TNF-alpha-induced cell death: involvement of reactive oxygen species. Biochem Biophys Res Commun. 2006;351:93–8. doi: 10.1016/j.bbrc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Strickson S, Emmerich CH, Goh ETH, Zhang J, Kelsall IR, Macartney T, et al. Roles of the TRAF6 and Pellino E3 ligases in MyD88 and RANKL signaling. Proc Natl Acad Sci USA. 2017;114:E3481–E9. doi: 10.1073/pnas.1702367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. Plos One. 2008;3:e4064. doi: 10.1371/journal.pone.0004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X, et al. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat Commun. 2016;7:11267. doi: 10.1038/ncomms11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFκB and MAPK pathways in response to IL-1 and RANKL. Plos One. 2008;3:e4064. doi: 10.1371/journal.pone.0004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabio G, Davis RJ, editors. TNF and MAP kinase signalling pathways. Seminars in immunology; 2014: Elsevier. [DOI] [PMC free article] [PubMed]
- 45.Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell. 2011;145:92–103. doi: 10.1016/j.cell.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 46.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dondelinger Y, Delanghe T, Priem D, Wynosky-Dolfi MA, Sorobetea D, Rojas-Rivera D, et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat Commun. 2019;10:1729. doi: 10.1038/s41467-019-09690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Molecular cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Wu X, Jiang G, Tai G. Mechanism by which TRAF6 participates in the immune regulation of autoimmune diseases and cancer. BioMed Research International. 2018;2020:4607197. doi: 10.1155/2020/4607197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo J, Seong D, Nam YW, Hwang CH, Lee SR, Lee CS, et al. Beclin 1 functions as a negative modulator of MLKL oligomerisation by integrating into the necrosome complex. Cell Death Differ. 2020;27:3065–81. doi: 10.1038/s41418-020-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brumatti G, Ma C, Lalaoui N, Nguyen NY, Navarro M, Tanzer MC, et al. The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia. Sci Transl Med. 2016;8:339ra69. doi: 10.1126/scitranslmed.aad3099. [DOI] [PubMed] [Google Scholar]
- 52.Seong D, Jeong M, Seo J, Lee JY, Hwang CH, Shin HC, et al. Identification of MYC as an antinecroptotic protein that stifles RIPK1-RIPK3 complex formation. Proc Natl Acad Sci USA. 2020;117:19982–93. doi: 10.1073/pnas.2000979117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275–88. doi: 10.1111/j.1745-7254.2008.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Townsend PA, Kozhevnikova MV, Cexus ONF, Zamyatnin AA, Jr, Soond SM. BH3-mimetics: recent developments in cancer therapy. J Exp Clin Cancer Res. 2021;40:355. doi: 10.1186/s13046-021-02157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chie K, Katsuo N, Yoshiyuki T, Den-Ichi M, Gen-Ichiro S. Constitutive expression of TNF-α and-β genes in mouse embryo: roles of cytokines as regulator and effector on development. Int J Biochem. 1994;26:111–9. doi: 10.1016/0020-711X(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 56.Kamiya A, Gonzalez FJ. TNF-alpha regulates mouse fetal hepatic maturation induced by oncostatin M and extracellular matrices. Hepatology. 2004;40:527–36. doi: 10.1002/hep.20362. [DOI] [PubMed] [Google Scholar]
- 57.Tiegs G, Horst AK. TNF in the liver: targeting a central player in inflammation. Semin Immunopathol. 2022;44:445–59. doi: 10.1007/s00281-022-00910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;156:997–1007. doi: 10.1016/S0002-9440(10)64967-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9:e3465. doi: 10.21769/BioProtoc.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.