Abstract
Alkylnitriles play important roles in many fields because of their unique electronic properties and structural characteristics. Incorporating cyanoalkyl with characteristic spectroscopy and reactivity properties into amino acids and peptides is of special interest for potential imaging and therapeutic purposes. Here, we report a copper-catalyzed asymmetric cyanoalkylation of C(sp3)-H. In the reactions, glycine derivatives can effectively couple with various cycloalkanone oxime esters with high enantioselectivities, and the reaction can be applied to the late-stage modification of peptides with good yields and excellent stereoselectivities, which is useful for modern peptide synthesis and drug discovery. The mechanistic studies show that the in situ formed copper complex by the coordination of glycine derivatives and chiral phosphine Cu catalyst can not only mediate the single electronic reduction of cycloalkanone oxime ester but also control the stereoselectivity of the cyanoalkylation reaction.
Subject terms: Synthetic chemistry methodology, Stereochemistry, Homogeneous catalysis
Incorporating cyanoalkyl moieties into amino acids and peptides is of interest for potential imaging and therapeutic purposes. Here, the authors developed a Cu-catalyzed asymmetric cyanoalkylation of glycine derivatives for the synthesis of α-cyanoalkylate amino acids and peptide modification.
Introduction
In recent years, the combination of transition-metal catalysis and radical chemistry has emerged as a powerful synthetic method for the construction of a variety of C-C and C-heteroatom bonds under mild conditions1. In particular, the transition metal-catalyzed radical cross-coupling allows the stereoselective construction of C(sp3)-C(sp3) bonds, which is often difficult to realize through the traditional ionic-based cross-coupling reactions2. Although significant progress has been achieved, the stereoselective alkylation via asymmetric radical cross-coupling of non-acidic C(sp3)-H bonds is still challenging and highly desired. This could attribute to the unavailability of p-orbitals of C(sp3)-H bonds to interact with the transition metal catalyst3.
Alkylnitriles play important roles in many application fields due to their unique electronic and structural properties. A large number of pharmaceuticals, bioactive natural products and functional materials contain such structural motifs4–9. Meanwhile, the nitrile group is a versatile synthon as it can be readily transformed into a range of useful functional groups, such as carboxylic acids, amides, amines, aldehydes, ketones, etc.10–14. Ever since the pioneering study of Boivin et al. in the 1990s, the iminyl radical-triggered C-C bond cleavage of cycloalkanone oximes has been successfully applied to synthesize a series of functionalized alkylnitriles under cyanide-free conditions (Fig. 1, eq. 1)15–24. Despite significant progress, the catalytic enantioselective variants of these reactions remain largely unexplored, and the reported reactions were restricted to the Giese additions of alkenes or the couplings with alkynes25–28. In contrast, the direct stereoselective cross-couplings of the key alkylnitrile radicals with other alkyl radicals to form chiral C(sp3)-C(sp3) bonds were still underdeveloped, especially with the C(sp3)-H as alkyl radical precursors.
Fig. 1. Radical functionalization of glycine derivatives and peptides.
(1) The generation of alkylnitrile radicals. (2) Photo-induced Cu-catalyzed asymmetric C(sp3)-H alkylation. (3) Asymmetric C(sp3)-H cyanoalkylation of glycine derivatives and peptides. PC photocatalyst, TM transition metals.
Unnatural amino acids have encountered widespread applications for the preparation of biologically active molecules and peptidomimetic drugs29,30. Introducing functional groups with characteristic spectroscopy and reactivity properties (e.g., -N3, -CN, and etc.) into amino acids and peptides is of special interest for potential imaging and therapeutic purposes31–34. Given the importance of cyano-containing unnatural amino acids in drug discovery and functional material development, the practical and stereoselective synthetic method for constructing this type of compounds is highly desired.
In recent years, copper-catalyzed asymmetric C(sp3)-H functionalization through radical processes has attracted extensive attention35–39. We recently developed a photo-induced and Cu-catalyzed asymmetric C(sp3)-H alkylation of glycine derivatives for the synthesis of unnatural amino acids40. The mechanistic studies revealed that the photo irradiation was essentially required to generate a high-valent organometallic copper (CuIII) intermediate, which was crucial to converting the glycine C(sp3)-H to the corresponding alkyl radical via single electron transfer (SET) reduction and deprotonation sequence (Fig. 1, eq. 2). Interestingly, Gong recently developed a Fe promoted glycine C(sp3)-H cyanoalkylation, in which FeIII served as the SET oxidant to generate the glycine N-radical41. Meanwhile, Zhou et al.42, Zuo et al.28 and Zhang et al.43 independently disclosed that the redox-active cyclobutanone oxime could forge the formation of CuIII species. Inspired by these reports and on the basis of our long-standing interest in Cu-catalyzed radical couplings40,44–50, we hypothesized that trapping of the alkylnitrile radical following ring-opening of cyclobutanone oxime by a Cu complex would in situ form a CuIII complex, which would possibly enable glycine C(sp3)-H to form an alkyl radical under basic conditions. Moreover, with the assistance of a chiral ligand, the asymmetric C(sp3)-H cyanoalkylation might be achieved as a practical complementary approach to the current photo-irradiation strategy40,50. Importantly, the achievement of this reaction would provide a useful tool for preparing cyanoalkylated unnatural α-amino acids and late-stage modification of peptides (Fig. 1, eq. 3).
Results
Investigation of reaction conditions
In this program, we first examined the capability of the chiral Cu complex for catalyzing the redox process and its ability to control the stereoselectivity of radical cyanoalkylation using ethyl (4-methoxyphenyl)glycinate 1a and cyclobutanone oxime ester 2a as model substrates. Asymmetric cyanoalkylation was tested employing different chiral ligands (L1–L7 in Table 1) using DABCO as the base and acetone as the solvent, whereas no stereoselectivities were observed in all cases (Fig. 2, eq. 1). We assume that the intermolecular coordination between the chiral catalyst and alkyl radical is quite weak and random, and it is difficult to control the stereochemistry of competitive racemic background reaction. Thus, enhancing the interaction between the chiral catalyst and substrate is an effective strategy to realize the stereoselective cyanoalkylation of C(sp3)-H40.
Table 1.
Optimization of the reaction conditions
 | |||
|---|---|---|---|
| Entryd | Change from the “standard conditions” | Yield (%)b | Ee (%)c |
| 1 | None | 80 (75) | 93 |
| 2 | Without Cu(MeCN)4PF6 | 0 | -- |
| 3 | Without DABCO | Trace | -- |
| 4 | Without L2 | 28 | 0 |
| 5 | With white LED | 80 | 93 |
| 6 | Under air | 36 | 91 |
aCondition: 1b (0.05 mmol), 2a (1.5 equiv), Cu(MeCN)4PF6 (10 mol%), L2 (11 mol%), DABCO (2.0 equiv), acetone (1.0 mL), room temperature, 12 h, and under argon atmosphere.
bYield was determined by 1H NMR using 4-bromobenzaldehyde as an internal standard and isolated yield in parentheses.
cEe (enantiomeric excess) was determined by HPLC on a Chiralpak IA-H column.
dStandard conditions: 1b (0.1 mmol), 2a (2.0 equiv), Cu(MeCN)4PF6 (10 mol%), L2 (15 mol%), DABCO (2.0 equiv), acetone (0.5 mL), 0 °C, 18 h, and at argon atmosphere.
Fig. 2. Copper-catalyzed asymmetric C(sp3)-H cyanoalkylation of glycine derivatives and peptides.
(1) Asymmetric C(sp3)-H cyanoalkylation of ethyl (4-methoxyphenyl)glycinate. (2) Asymmetric C(sp3)-H cyanoalkylation of tert-butyl (5-methoxyquinolin-8-yl)glycinate. PMP 4 -methoxyphenyl.
Therefore, we embed the easily removed N donor unit into the substrate, by which the chiral catalyst and substrate would jointly constitute a new chiral catalytic system. The results show that electron transfer (or charge transfer) and stereocontrol can be defined within one molecule, and the asymmetric cyanoalkylation of glycine derivatives was achieved through the stereoselective reductive elimination of CuIII species (Fig. 2, eq. 2). Using tert-butyl (5-methoxyquinolin-8-yl)glycinate 1b and cyclobutanone oxime ester 2a as model substrates, to our delight when Cu(MeCN)4PF6 (10 mol%) and (S)-Phanephos (L1, 11 mol%) were employed, the ee of C(sp3)-H cyanoalkylation product was dramatically increased to 80%.
After exploring various chiral ligands, basic additives, Cu catalysts, solvents, and other parameters (see Supplementary Information for details), the optimized reaction conditions were determined (Table 1). The desired product 3ba was obtained in 80% yield and 93% ee at 0 °C for 18 h in the presence of Cu(MeCN)4PF6 (10 mol%), (S)-An-Phanephos (15 mol%), DABCO (2.0 equiv), and acetone (0.2 M) (Table 1, entry 1). Control experiments indicated that copper salt and basic additive are indispensable for the reaction (entries 2–3). Furthermore, Cu(MeCN)4PF6 also can independently catalyze the C(sp3)-H cyanoalkylation of glycine derivative in the absence of (S)-An-Phanephos, leading to the formation of the racemic product in 28% yield (entry 4). Notably, the efficiency of cyanoalkylation was not improved under LED irradiation (entry 5). The coupling reaction was sensitive to oxygen, and the yield dropped sharply to 36% under air conditions (entry 6).
Substrate scopes
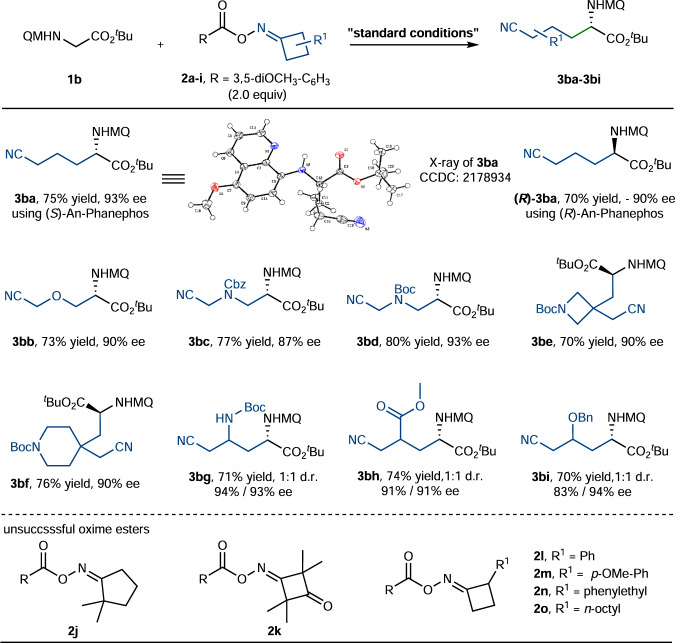
With the optimized reaction conditions in hand, we explored the substrate scope of oxime esters. As summarized in Fig. 3, the catalytic system exhibited a broad substrate scope and good functional group tolerance. It could accommodate cyclobutanone, 3-oxetanone, Cbz and Boc protected azetidinone derived oxime esters (2a-d), delivering products 3ba-bd in satisfactory yields and enantiomeric excesses. X-ray crystallographic analysis of product 3ba confirmed the (S)-configuration of the newly formed stereochemistry. Meanwhile, (R)-3ba was obtained in 70% yield and −90% ee when (R)-An-Phanephos was used instead of (S)-An-Phanephos. The reaction of disubstituted oxime esters 2e and 2 f also proceeded smoothly to afford the desired products 3be and 3bf in good yields and ees, respectively. In addition, the monosubstituted oxime esters 2g-i with -NHBoc, ester and ether groups at the 3-position were also well tolerated, with the corresponding products 3bg-bi isolated in 70–74% yields and 83–94% ees. As a limitation of this protocol, the secondary and tertiary cyanoalkyl radicals derived from 2l-2o were less efficiently captured by the copper catalysts compared to primary cyanoalkyl radicals and gave negative results.
Fig. 3. Substrate scope with respect to oxime esters.
Standard conditions: 1b (0.1 mmol), 2 (2.0 equiv), Cu(MeCN)4PF6 (10 mol%), L2 (15 mol%), DABCO (2.0 equiv), acetone (0.5 mL), 0 °C, 18 h, argon atmosphere. Isolated yields based on 1b after chromatographic purification. Ee was determined by chiral HPLC. D.r. (diastereo ratio) was determined by 1H NMR analysis.
Further expansion of the substrate scope was focused on glycine derivatives (Fig. 4). Glycine amide (1c), glycine ester (1d) and glycine derivative bearing different N-aryl groups (1e) were well tolerated (3ca-ea, 65–77% yields). Furthermore, a variety of dipeptides (Gly-Val, Gly-Pro, Gly-Glu, Gly-Phe, Gly-Met, Gly-Thr and Gly-Lys) substrates were prepared to test the functional group tolerance of this reaction with various amino acid residues incorporated. Gratifyingly, the corresponding cyanoalkylated peptides 3fa-la afforded good yields and excellent stereoselectivities with other amino acid residues untouched (Fig. 4A).
Fig. 4. Substrate scope with respect to glycine derivatives and peptides.
Standard conditions: 1 (0.1 mmol), 2 (2.0 equiv), Cu(MeCN)4PF6 (10 mol%), L2 (15 mol%), DABCO (2.0 equiv), acetone (0.5 mL), 0 °C, 18 h, under Ar. Isolated yields based on 1 after chromatographic purification. Ee was determined by chiral HPLC. D.r. was determined by 1H NMR analysis. aDMF (0.5 mL) as solvent, 36 h. A Substrate scope with respect to glycine derivatives and dipeptides. B Substrate scope with respect to peptides.
Having established proof-of-concept with the above results, we became interested if our reactions could be applied in C(sp3)-H cyanoalkylation of polypeptides (Fig. 4B). Gratifyingly, the late-stage C(sp3)-H cyanoalkylation of pentapeptide (Gly-Leu-Phe-Ser-Lys) and hexapeptide (Gly-Leu-Tyr-Ser-Phe-Ala) derived substrates reacted smoothly and gave the corresponding cyanoalkylation products in good yields (3ma, 63%; 3na, 60%) and high diastereoselectivities (>20:1). It was worth noting that the reactions between hexapeptide (Gly-Leu-Tyr-Ser-Phe-Ala and Gly-Leu-Phe-Gly-D-Thr-Tyr) substrate and different oxime esters (2a, 2b, 2e, and 2f) were achieved in uniformly good yields and high diastereoselectivities, which further highlighted the generality of this method in modification of complex molecules.
Synthetic applications
To further illustrate the application potential of the product, several transformations were conducted (Fig. 5). Firstly, the deprotection of the cyanoalkylation product 3ba proceeded smoothly under simple procedures in high yield and did not erode the ee (Fig. 5, eq. 1). Secondly, treatment of the 3ba with Raney-Ni and H2 in pyridine/EtOH/H2O afforded the corresponding amine 5 in 70% yield and 90% ee (Fig. 5, eq. 2). Furthermore, the cyano group of 3ba was easily converted to amide 6 in the presence of Pd catalyst (Fig. 5, eq. 3).
Fig. 5. Synthetic applications.
(1) Deprotection of 3ba. (2) Conversion of cyanide to amine. (3) Conversion of cyanide to amide. CAN Ce(NH4)2(NO3)6, Py pyridine.
Mechanistic studies
In order to gain some insight into the mechanism, several radical trapping experiments were carried out (Fig. 6). The radical trapping experiments with TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl, completely suppressed) or BHT (2,6-ditert-butyl-4-methylphenol, partially suppressed) indicated that a radical pathway was involved in this transformation. Meanwhile, radical trapping product 7 was isolated in 30% yield in the presence of TEMPO, which suggested the formation of cyanoalkyl radical in the reaction system (see Supplementary Information for details). Furthermore, the HRMS analysis of the original reaction mixture detected the formation of glycinate homo-coupling product 8 when the dosage of 2a was 1.0 equiv (Fig. 6, eq. 1). Moreover, when the reactions of 1b and 2a were carried out under air, oxidized glycine derivative 9 was obtained in 40% yield (Fig. 6, eq. 2). These results indicated that glycinate radical was generated under standard reaction conditions. N-CH3-substituted glycinate 1p did not give any cyanoalkylation product with quantitive recycling of the starting material, indicating that a single free hydrogen atom on N atom is crucial for this reaction (Fig. 6, eq. 3). Notably, imine 1q failed to give the cyanoalkylation product 3da, revealing the Cu-mediated SET pathway likely involved rather than the two-electron oxidation process (Fig. 6, eq. 4).
Fig. 6. Mechanistic studies.
(1) The formation of glycinate homo-coupling product. (2) The α-oxidation of glycinate. (3) The reaction with N-CH3-substituted glycinate. (4) The reaction with imine. (5) The reaction with ethyl-(4-methoxyphenyl) glycinate. (6) The reaction with copper complex A. (7) The reaction with copper complex B.
We prepared ((S)-An-Phanephos)Cu(CH3CN)4PF6 complex A and copper complex B (combined complex A and 1b) according to the literature procedure51. The ground state redox potentials of 2a (Eredox = −2.11 V vs. SCE in CH3CN) and copper complex B (Eredox = −2.38 V vs. SCE in CH3CN) demonstrated that the SET oxidation of 2a by copper complex B was feasible (see Supplementary Information for details). Some control experiments were also carried out, and the results are shown in Fig. 6 (eqs. 5–7). Ethyl (4-methoxyphenyl) glycinate 1a could provide a racemic product under standard conditions (3aa, 30% yield, 0% ee, Fig. 6, eq. 5). The result revealed the importance of the coordination with 5-methoxyquinoline group for the chirality induction. When the isolated Cu complex A was used as the catalyst, the corresponding cyanoalkylation product was obtained in 78% yield and 93% ee (Fig. 6, eq. 6). Furthermore, the isolated Cu complex B (1.0 equiv) was directly used as the substrate without any other catalyst in the system, the coupling product was obtained in 68% yield and 91% ee (Fig. 6, eq. 7). These results indicated that complex B likely formed in the reaction and act as the active catalytic species.
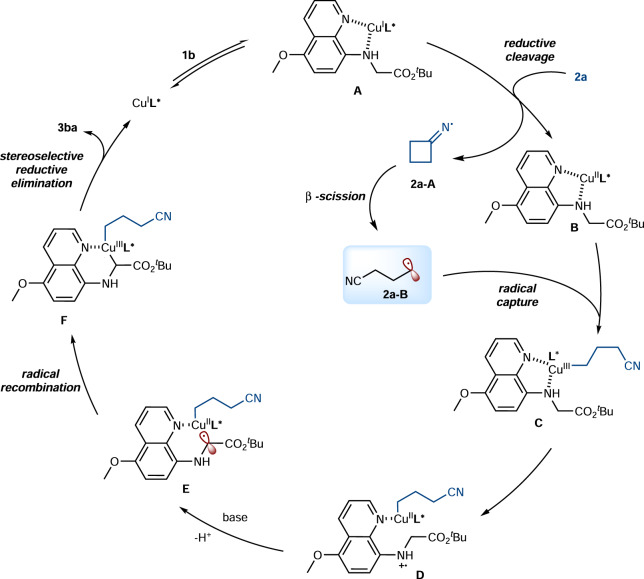
Proposed mechanism
Based on the above mechanistic studies and previous reports52–55, a plausible mechanism was proposed in Fig. 7. The 5-methoxyquinolinyl-8-glycinate ester 1b coordinates to the CuIL* and in situ forms a chiral Cu(I) intermediate A. Then, a SET reduction of oxime ester 2a by intermediate A occurs, followed by fragmentation to afford cyclic iminyl radical 2a-A and oxidized Cu(II) intermediate B. Next, cyclic iminyl radical 2a-A undergoes regioselective ring-opening C-C bond cleavage to form cyanoalkyl radical 2a-B. At this point, cyanoalkyl radical 2a-B was captured by Cu(II) intermediate B to produce the high-valent Cu(III) intermediate C. Then, the copper-mediated intramolecular oxidation of the N atom produces Cu(II) intermediate D, and DABCO-promoted deprotonation gives radical intermediate E, which subsequently attacks the copper center to form a chiral Cu(III) intermediate F. Finally, the stereoselectively reductive elimination delivered the desired product 3ba.
Fig. 7. Proposed mechanism.
Copper-catalyzed asymmetric C(sp3)-H cyanoalkylation of glycine derivatives.
Discussion
In conclusion, we report a copper-catalyzed asymmetric C(sp3)-H cyanoalkylation of glycine derivatives and peptides. The reactions feature mild conditions, excellent enantioselectivity and broad substrate scope. Given the significance of introducing cyanoalkylation into amino acids and peptides for potential imaging and therapeutic purposes, we predict that our asymmetric C(sp3)-H cyanoalkylation would provide new approaches to the synthesis of unnatural α-amino acids and late-stage functionalization of bioactive compounds, and would be useful for modern peptide synthesis and drug discovery.
Methods
General procedure A (3ba-bi, 3ca-la)
To an oven-dried 10-mL quartz test tube with a stirring bar, derivatives of glycine (0.1 mmol) were added, followed by the addition of Cu(MeCN)4PF6 (0.01 mmol, 3.7 mg) and (S)-An-Phanephos or (R)-An-Phanephos (0.015 mmol, 10.5 mg). Then, the air was withdrawn and backfilled with Ar (three times). Acetone (0.25 mL) was added, and the mixture was stirred at room temperature for 40 min. Subsequently, oxime esters (0.2 mmol) and DABCO (0.2 mmol, 22.4 mg) dissolved in acetone (0.25 mL) were added to the abovementioned mixed solution by syringe. Thereafter, the test tube was transferred to a low-temperature device, where it was reacted for 18 h at 0 °C. Then, the reaction was quenched with water (1 mL), extracted with ethyl acetate (3 × 1.5 ml), dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (hexane/ethyl acetate) to give the product.
General procedure B (3ma-oa, 3ob, 3oe, and 3of)
To an oven-dried 10-mL quartz test tube with a stirring bar, peptide substrates (0.1 mmol) were added, followed by the addition of Cu(MeCN)4PF6 (0.01 mmol, 3.7 mg) and (S)-An-Phanephos (0.015 mmol, 10.5 mg). Then, the air was withdrawn and backfilled with Ar (three times). DMF (0.25 mL) was added, and the mixture was stirred at room temperature for 40 min. Subsequently, oxime esters (0.2 mmol) and DABCO (0.2 mmol, 22.4 mg) dissolved in DMF (0.25 mL) were added to the abovementioned mixed solution by syringe. Thereafter, the test tube was transferred to a low-temperature device, where it was reacted for 36 h at 0 °C. Then, the reaction was quenched with water (1 mL), extracted with ethyl acetate (3 × 1.5 ml), dried over anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (hexane/ethyl acetate or dichloromethane/methanol) to give the product.
Supplementary information
Acknowledgements
Supported by the National Natural Science Foundation of China (21971098 and 22271126 for Z.X., 22201112 for C.W.), Innovation Project of Medicine and Health Science and Technology of Chinese Academy of Medical Sciences (2019-I2M-5-074 for Z.X.), Key R&D Project of Gansu Province (22YF7WA010 for Z.X.), and Baiyin Science and Technology Planning Project (2022-2-28G for C.W.).
Author contributions
Z.X. and C.W. conceived the idea, directed the project and designed the experiments; R.Q., Q.C., L.L., Z.M., D.P., H.W. and Z.L. performed the experiments; R.Q. and Q.C. analyzed the data; C.W. and Z.X. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks Baihua Ye and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its supplementary information file and all other data are available from the respective authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2178934. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rupeng Qi, Qiao Chen, Liangyu Liu.
Contributor Information
Chao Wang, Email: wangchao@lzu.edu.cn.
Zhaoqing Xu, Email: zqxu@lzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-38871-1.
References
- 1.Yan M, Lo JC, Edwards JT, Baran PS. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 2016;138:12692–12714. doi: 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi J, Fu GC. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science. 2017;356:eaaf7230. doi: 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garai S, Ghosh KG, Biswas A, Chowdhury S, Sureshkumar D. Diastereoselective palladium-catalyzed C(sp3)-H cyanomethylation of amino acid and carboxylic acid derivatives. Chem. Commun. 2022;58:7793–7796. doi: 10.1039/D2CC03106J. [DOI] [PubMed] [Google Scholar]
- 4.Fleming FF. Nitrile-containing natural products. Nat. Prod. Rep. 1999;16:597–606. doi: 10.1039/a804370a. [DOI] [Google Scholar]
- 5.Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshima J, et al. Photophysical properties of 3-[2-cyano-4-(dimethylamino)phenyl]alanine: a highly fluorescent and environment-sensitive amino acid with small molecular size. Chem. Lett. 2006;35:620–621. doi: 10.1246/cl.2006.620. [DOI] [Google Scholar]
- 7.Ressler C, Nigam SN, Giza Y-H, Nelson J. Isolation and identification from common vetch of γ-L-glutamyl-β-cyano-L-alanine, a bound form of the neurotoxin β-cyano-L-alanine. J. Am. Chem. Soc. 1963;85:3311–3312. doi: 10.1021/ja00903a069. [DOI] [Google Scholar]
- 8.Naruse N, et al. β-Cyanoglutamic acid, a new antifungal amino acid from a streptomycete. J. Antibiot. 1993;46:685–686. doi: 10.7164/antibiotics.46.685. [DOI] [PubMed] [Google Scholar]
- 9.Gonzàlez R, et al. C3’-cis-substituted carboxycyclopropyl glycines as metabotropic glutamate 2/3 receptor agonists: synthesis and SAR studies. Bioorg. Med. Chem. 2005;13:6556–6570. doi: 10.1016/j.bmc.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Fleming FF, Wang Q. Unsaturated nitriles: conjugate additions of carbon nucleophiles to a recalcitrant class of acceptors. Chem. Rev. 2003;103:2035–2077. doi: 10.1021/cr020045d. [DOI] [PubMed] [Google Scholar]
- 11.Fleming FF, Zhang Z. Cyclic nitriles: tactical advantages in synthesis. Tetrahedron. 2005;61:747–789. doi: 10.1016/j.tet.2004.11.012. [DOI] [Google Scholar]
- 12.Wang M-X. Enantioselective biotransformations of nitriles in organic synthesis. Acc. Chem. Res. 2015;48:602–611. doi: 10.1021/ar500406s. [DOI] [PubMed] [Google Scholar]
- 13.Bagal DB, Bhanage BM. Recent advances in transition metal-catalyzed hydrogenation of nitriles. Adv. Synth. Catal. 2015;357:883–900. doi: 10.1002/adsc.201400940. [DOI] [Google Scholar]
- 14.Yang X, Fleming FF. C- and N-metalated nitriles: the relationship between structure and selectivity. Acc. Chem. Res. 2017;50:2556–2568. doi: 10.1021/acs.accounts.7b00329. [DOI] [PubMed] [Google Scholar]
- 15.Boivin J, Fouquet E, Zard SZ. Ring opening induced by iminyl radicals derived from cyclobutanones: new aspects of tin hydride cleavage of S-phenyl sulfenylimines. J. Am. Chem. Soc. 1991;113:1055–1057. doi: 10.1021/ja00003a057. [DOI] [Google Scholar]
- 16.Boivin J, Fouquet E, Zard SZ. Iminyl radicals: Part II. Ring opening of cyclobutyl- and cyclopentyliminyl radicals. Tetrahedron. 1994;50:1757–1768. doi: 10.1016/S0040-4020(01)80850-4. [DOI] [Google Scholar]
- 17.Xiao F, Guo Y, Zeng Y-F. Recent developments in radical cross-coupling of redox-active cycloketone oximes. Adv. Synth. Catal. 2021;363:120–143. doi: 10.1002/adsc.202001093. [DOI] [Google Scholar]
- 18.Xiao W, Wu J. Recent advances for the photoinduced C-C bond cleavage of cycloketone oximes. Chin. Chem. Lett. 2020;31:3083–3094. doi: 10.1016/j.cclet.2020.07.035. [DOI] [Google Scholar]
- 19.Yu X-Y, Chen J-R, Xiao W-J. Visible light-driven radical-mediated C-C bond cleavage/functionalization in organic synthesis. Chem. Rev. 2021;121:506–561. doi: 10.1021/acs.chemrev.0c00030. [DOI] [PubMed] [Google Scholar]
- 20.Kwon K, Simons RT, Nandakumar M, Roizen JL. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis. Chem. Rev. 2022;122:2353–2428. doi: 10.1021/acs.chemrev.1c00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivaguru P, Wang Z, Zanoni G, Bi X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 2019;48:2615–2656. doi: 10.1039/C8CS00386F. [DOI] [PubMed] [Google Scholar]
- 22.Yu X-Y, Zhao Q-Q, Chen J, Xiao W-J, Chen J-R. When light meets nitrogen-centered radicals: from reagents to catalysts. Acc. Chem. Res. 2020;53:1066–1083. doi: 10.1021/acs.accounts.0c00090. [DOI] [PubMed] [Google Scholar]
- 23.Lou J, Ma J, Xu B-H, Zhou Y-G, Yu Z. Photoinduced, copper-catalyzed three-component annulation of gem-gialkylthio enynes. Org. Lett. 2020;22:5202–5206. doi: 10.1021/acs.orglett.0c01645. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S-Y, et al. Visible-light-driven photoredox-catalyzed C(sp3)-C(sp3) cross-coupling of N-arylamines with cycloketone oxime esters. Org. Chem. Front. 2022;9:2534–2540. doi: 10.1039/D2QO00128D. [DOI] [Google Scholar]
- 25.Wang P-Z, et al. Asymmetric three-component olefin dicarbofunctionalization enabled by photoredox and copper dual catalysis. Nat. Commun. 2021;12:1815–1824. doi: 10.1038/s41467-021-22127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P-Z, et al. Photoinduced copper-catalyzed asymmetric three-component coupling of 1,3-dienes: an alternative to Kharasch-Sosnovsky reaction. Angew. Chem. Int. Ed. Engl. 2021;60:22956–22962. doi: 10.1002/anie.202110084. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, et al. Photoinduced copper-catalyzed asymmetric C-O cross-coupling. J. Am. Chem. Soc. 2021;143:13382–13392. doi: 10.1021/jacs.1c06535. [DOI] [PubMed] [Google Scholar]
- 28.Zuo H-D, et al. Copper-catalyzed asymmetric deconstructive alkynylation of cyclic oximes. ACS Catal. 2021;11:6010–6019. doi: 10.1021/acscatal.1c00842. [DOI] [Google Scholar]
- 29.Lang K, Chin JW. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 2014;114:4764–4806. doi: 10.1021/cr400355w. [DOI] [PubMed] [Google Scholar]
- 30.Blaskovich MAT, et al. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016;59:10807–10836. doi: 10.1021/acs.jmedchem.6b00319. [DOI] [PubMed] [Google Scholar]
- 31.VanBrunt MP, et al. Genetically encoded azide containing amino acid in mammalian cells enables site-specific antibody-drug conjugates using click cycloaddition chemistry. Bioconjug. Chem. 2015;26:2249–2260. doi: 10.1021/acs.bioconjchem.5b00359. [DOI] [PubMed] [Google Scholar]
- 32.Jo H, Culik RM, Korendovych IV, DeGrado WF, Gai F. Selective incorporation of nitrile-based infrared probes into proteins via cysteine alkylation. Biochemistry. 2010;49:10354–10356. doi: 10.1021/bi101711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J, Pazos IM, Zhang W, Culik RM, Gai F. Site-specific infrared probes of proteins. Annu. Rev. Phys. Chem. 2015;66:357–377. doi: 10.1146/annurev-physchem-040214-121802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getahun Z, et al. Using nitrile-derivatized amino acids as infrared probes of local environment. J. Am. Chem. Soc. 2003;125:405–411. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Chen P, Liu G. Copper-catalyzed radical relay in C(sp3)-H functionalization. Chem. Soc. Rev. 2022;51:1640–1658. doi: 10.1039/D1CS00727K. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, et al. Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay. Science. 2016;353:1014–1018. doi: 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Lei M, Gong L. Photocatalytic regio- and stereoselective C(sp3)-H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2019;2:1016–1026. doi: 10.1038/s41929-019-0357-9. [DOI] [Google Scholar]
- 38.Li J, et al. Site-specific allylic C-H bond functionalization with a copper-bound N-centred radical. Nature. 2019;574:516–521. doi: 10.1038/s41586-019-1655-8. [DOI] [PubMed] [Google Scholar]
- 39.Cai C-Y, et al. Photoelectrochemical asymmetric catalysis enables site- and enantioselective cyanation of benzylic C-H bonds. Nat. Catal. 2022;5:943–951. doi: 10.1038/s41929-022-00855-7. [DOI] [Google Scholar]
- 40.Qi R, et al. Visible light induced Cu-catalyzed asymmetric C(sp3)-H alkylation. J. Am. Chem. Soc. 2021;143:12777–12783. doi: 10.1021/jacs.1c05890. [DOI] [PubMed] [Google Scholar]
- 41.Lu D, Cui J, Yang S, Gong Y. Iron-catalyzed cyanoalkylation of glycine derivatives promoted by pyridine-oxazoline ligands. ACS Catal. 2021;11:4288–4293. doi: 10.1021/acscatal.1c00557. [DOI] [Google Scholar]
- 42.Zhou X-S, et al. Copper-catalyzed radical cross-coupling of oxime esters and sulfinates for synthesis of cyanoalkylated sulfones. Chem. Cat. Chem. 2019;11:1–7. [Google Scholar]
- 43.Zhang H, et al. β‑Lactam synthesis via copper-catalyzed directed aminoalkylation of unactivated alkenes with cyclobutanone o‑benzoyloximes. Org. Lett. 2021;23:3620–3625. doi: 10.1021/acs.orglett.1c01007. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, et al. Photoinduced, copper-promoted regio- and stereoselective decarboxylative alkylation of α,β-unsaturated acids with alkyl iodides. Org. Lett. 2017;19:6412–6415. doi: 10.1021/acs.orglett.7b03289. [DOI] [PubMed] [Google Scholar]
- 45.Guo Q, Wang M, Wang Y, Xu Z, Wang R. Photoinduced, copper-catalyzed three components cyanofluoroalkylation of alkenes with fluoroalkyl iodides as fluoroalkylation reagents. Chem. Commun. 2017;53:12317–12320. doi: 10.1039/C7CC07128K. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Guo Q, Chen C, Wang M, Xu Z. Photo-induced, Cu-catalyzed three component azidofluoroalkylation of alkenes with CF3I and RfI as fluoroalkylation reagents. Org. Chem. Front. 2018;5:1522–1526. doi: 10.1039/C8QO00120K. [DOI] [Google Scholar]
- 47.Wang C, et al. Visible-light-driven, copper-catalyzed decarboxylative C(sp3)-H alkylation of glycine and peptides. Angew. Chem. Int. Ed. Engl. 2018;57:15841–15846. doi: 10.1002/anie.201809400. [DOI] [PubMed] [Google Scholar]
- 48.Guo Q, et al. Dual-functional chiral Cu-catalyst-induced photoredox asymmetric cyanofluoroalkylation of alkenes. ACS Catal. 2019;9:4470–4476. doi: 10.1021/acscatal.9b00209. [DOI] [Google Scholar]
- 49.Wang C, et al. Cu-catalyzed cyanoalkylation of electron-deficient alkenes with unactivated alkyl bromides. Chem. Commun. 2019;55:9991–9994. doi: 10.1039/C9CC04432A. [DOI] [PubMed] [Google Scholar]
- 50.Qi R, et al. Visible-light-promoted stereoselective C(sp3)-H glycosylation for the synthesis of C-glycoamino acids and C-glycopeptides. Angew. Chem. Int. Ed. Engl. 2022;61:e202200822. doi: 10.1002/anie.202200822. [DOI] [PubMed] [Google Scholar]
- 51.Caprioli F, Madduri AVR, Minnaard AJ, Harutyunyan SR. Asymmetric amplification in the catalytic enantioselective 1,2- addition of Grignard reagents to enones. Chem. Commun. 2013;49:5450–5452. doi: 10.1039/c3cc41892h. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, et al. Enantioselective radical ring-opening cyanation of oxime esters by dual photoredox and copper catalysis. Org. Lett. 2019;21:9763–9768. doi: 10.1021/acs.orglett.9b03970. [DOI] [PubMed] [Google Scholar]
- 53.Wang T, et al. Enantioselective cyanation via radical-mediated C-C single bond cleavage for synthesis of chiral dinitriles. Nat. Commun. 2019;10:5373–5382. doi: 10.1038/s41467-019-13369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F, Chen P, Liu G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 2018;51:2036–2046. doi: 10.1021/acs.accounts.8b00265. [DOI] [PubMed] [Google Scholar]
- 55.Zhou H, Li Z-L, Gu Q-S, Liu X-Y. Ligand-enabled copper(I)-catalyzed asymmetric radical C(sp3)-C cross-coupling reactions. ACS Catal. 2021;11:7978–7986. doi: 10.1021/acscatal.1c01970. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its supplementary information file and all other data are available from the respective authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2178934. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/.