Abstract
For a deeper and comprehensive understanding of the composition and function of rhizosphere microbiomes, we need to focus at the scale of individual roots in standardized growth containers. Root exudation patterns are known to vary along distinct parts of the root even in juvenile plants giving rise to spatially distinct microbial niches. To address this, we analyzed the microbial community from two spatially distinct zones of the developing primary root (tip and base) in young Brachypodium distachyon grown in natural soil using standardized fabricated ecosystems known as EcoFABs as well as in more conventional pot and tubes. 16S rRNA based community analysis showed a strong rhizosphere effect resulting in significant enrichment of several OTUs belonging to Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria. However, microbial community composition did not differ between root tips and root base or across different growth containers. Functional analysis of bulk metagenomics revealed significant differences between root tips and bulk soil. The genes associated with different metabolic pathways and root colonization were enriched in root tips. On the other hand, genes associated with nutrient-limitation and environmental stress were prominent in the bulk soil compared to root tips, implying the absence of easily available, labile carbon and nutrients in bulk soil relative to roots. Such insights into the relationships between developing root and microbial communities are critical for judicious understanding of plant-microbe interactions in early developmental stages of plants.
Subject terms: Biogeography, Microbial ecology
Introduction
Plants exude 20–40% of their photosynthetically fixed carbon through intact root cells into the surrounding soil [1]. Besides root phenology, root exudates are a key determinant for development of rhizosphere community. These root exudates contain low-molecular weight organic compounds, and together with mucilage and sloughed off root tissues mainly expelled from root tips, provide a major source of nutrients for the rhizosphere microbiome [2]. These compounds create an exclusive environment in the rhizosphere that is physiochemically distinct from the surrounding bulk soil and play a key role in recruiting and selecting relevant beneficial microbes to develop a unique rhizosphere microbiome [3].
Root exudation patterns have been shown to vary spatially along the root system very early in developing plants, exudates from rapidly dividing root tips differ in composition from exudates released from older sections of the root [4]. While the assembly of the microbial community along different parts of roots (biogeography) is considered an important parameter in rhizosphere dynamics, systematic and standardized studies probing this deeper are lacking. Most rhizosphere microbiome studies, where plants are grown in soil, do not compartmentalize the roots based on their length, but rather based on radial distance from the root axis (rhizosphere, rhizoplane and endosphere). As a result, capturing the effect of spatial differences along the roots is unexplored, causing a gap in understanding how these differences impact microbial assembly in the rhizoplane in juvenile plants.
Furthermore, while few studies have demonstrated influence of plant growth container type on plant morphology [5–9], direct impacts of growth containers on the rhizosphere microbiome is relatively unexplored under highly controlled experimental conditions. Complex biochemical processes and interactions occur at microscale dimensions surrounding the root and the ability to interrogate these processes within highly reproduceable and controlled growth containers will propel our understanding of rhizosphere spatial heterogeneity [10].
In this study, we investigated rhizosphere biogeography from two distinct root zones of young Brachypodium distachyon grown in natural soil, in three different types of growth containers- conventional pots, tubes and specially fabricated EcoFABs [11] to assess (a) microbiome structure and function across root tips, root base and bulk soil; and (b) the suitability of standardized growth containers to study plant-microbe interactions at such finer scales in juvenile plants. We also tested these different containers under open or closed environments (encased within secondary containment). The EcoFABs had demonstrated high value in standardized investigations of plant phenotypic traits and metabolite production [12], but their applicability to study spatially resolved rhizosphere in juvenile plants had not yet been explored. We used long read 16S rRNA amplicon sequencing and shotgun metagenomic sequencing to delineate differences between these diverse containers and distinct root zones (root tips, root base). We observed significant differences in microbial structure and functional potential between root tips and bulk soil even in young developing plant roots.
Materials and methods
Soil and plant growth conditions
Soil for plant growth was collected from the south meadow field site at the Angelo Coast Range Reserve in northern California (39° 44′ 21.4′′ N 123° 37′ 51.0′′ W) in August 2020. The upper layer (0–10 cm) was collected in clean collection bags, immediately transported on ice and stored at 4 °C until further processing. The collected soil was passed through a 2 mm sieve to remove larger particles like dry roots and rocks prior to use.
In this study, we used three types of containers, EcoFAB, test tubes and plastic pots to grow B. distachyon (Bd21-3 plant line). EcoFABs (n = 11) were fabricated as reported earlier [13] with slight modifications. Briefly, the oval-shaped polydimethylsiloxane (PDMS) cast measuring 7.7 cm × 5.7 cm × 0.5 cm (height × width × depth) providing a container volume of 10 mL was held together by metal clamps and screws. Sterile plastic test tubes (n = 14) used to grow plants were 10 cm long with a diameter of 1.5 cm, and had a hole drilled at the bottom to drain excess water. The pots (n = 14) used were 10 cm × 10 cm squares with a depth of 10.5 cm, tapered from top to bottom. The weight of soil in test tube and EcoFAB was kept at 15 g each while the pot contained 600 g. The vertical distance between the sown seed to the bottom of the container was 8 cm for EcoFAB and 9 cm for both pot and test tube. Except for soil, all components were sterilized by UV sterilization or autoclaving. In addition, approximately half of all containers were kept sterile in closed Microbox containers (Sac O2, Belgium) while others were kept open to the environment.
Cold-treated Brachypodium distachyon seeds were de-husked, surface-sterilized in 70% ethanol followed by 50% household bleach for 5 min each and rinsed thoroughly in sterile water. They were germinated on sterile 0.8% noble agar plates under sunlight at room temperature for two days. Germinated seedlings were transferred into the containers taking care to place it 0.5 cm below the soil surface, watered once at 100% capacity with sterile water. Subsequent watering was done at 15% holding capacity, every 2 and 4 days for the open and closed containers respectively. The plants were placed in a greenhouse with a 16-h photoperiod, 87.5% relative humidity, and average day and nighttime temperatures of 19.9 °C and 17.9 °C respectively.
Plant phenotypic measurements
Plants were harvested from all containers 14 days after sowing when the primary root had reached bottom of EcoFAB, and key plant phenotypic characteristics were measured. After excising the roots from the base of plant shoot, dry shoot weight was obtained by oven drying the shoots at 80 °C for 24 h followed by cooling to room temperature and measuring dry weight [14–16]. Shoot length was measured from end of the longest leaf to the point where root starts [17]. Root length was measured from root base to tip of the primary root.
Rhizosphere and bulk soil sample collection
At the time of harvest, most plants had only one fine primary axile root. The roots were excised carefully from soil under aseptic conditions and lightly shaken to remove loosely attached bulk soil. Root tip and root base samples were harvested as 2 cm cuttings, measured from tip of the root, and from base of the plant shoot respectively. Due to complications during sampling resulting in physical damage to the roots, some samples were discarded reducing the number of root samples to n = 8, n = 11, and n = 7 originating from EcoFAB, test tube, and pot respectively. The loosely-bound rhizosphere soil was obtained by vortexing the root in 5 mM sodium pyrophosphate for 15 s, three times. The root was then placed in fresh pyrophosphate buffer and sonicated for 5 min to extract tightly-bound fraction. To ensure the complete representation of the rhizosphere microbiome, both the loosely- and tightly-bound fractions were pooled for subsequent DNA extraction. Bulk soil (0.5 g) was collected from containers at least 1 cm away from the roots and kept frozen before DNA extraction.
DNA extraction and sequencing
Genomic DNA was extracted using DNeasy PowerLyzer Powersoil kit (Qiagen, US) following the manufacturer’s instructions and the eluted genomic DNA was quantified using QubitTM dsDNA High Sensitivity assay kit (Thermofisher, US).
For bacterial full-length 16S rRNA amplification and sequencing, genomic DNA from all the available different root locations and bulk soil were sent to Loop Genomics (US). Briefly, the DNA was amplified with indexed forward (5’ CTGCCTAGAACA [Index, F] AGAGTTTGATCMTGGCTCAG 3’) and reverse primers (5’ TGCCTAGAACAG [Index, R] TACCTTGTTACGACTT 3’) and sequenced using the Illumina sequencing platform via paired end (150 bp X 2) mode followed by the standard Loop Genomics informatics pipeline that uses short reads to construct synthetic long reads [18].
For metagenomic sequencing, replicates of each sample type (root tip, root base or bulk soil from each type of container) was pooled to accommodate the 200 ng DNA concentration requirement, resulting in a total of 9 samples. These samples were sent to QB3-Berkeley Functional Genomics Laboratory (University of California, Berkeley, US) (http://qb3.berkeley.edu/fgl/) for library prep and subsequent sequencing using Illumina 150 bp X 2 paired end reads with a depth of 20 Gb per sample.
16S rRNA community analysis
16S amplicon samples which contained less than 1000 reads after demultiplexing were discarded before analysis. We ensured that there were at least 3 replicate samples for every type of sample under the three variables tested; 1. Container (EcoFAB, pot or test tube), 2. Location (root tip, root base or bulk soil) and 3. Condition (Closed or Open). The demultiplexed data from loop genomics was then clustered into OTUs using usearch (version 11.0.667) for comparative analyses as follows [19]. Briefly, FASTQ files were 1st trimmed (1400 bps) and quality filtered (maximum expected error cutoff 1.0) before initial clustering and chimera filtering using Unoise 3 command. The resulting OTUs were further clustered to 97% identity before generating the OTU table, taxonomic assignments and comparative analyses.
From the OTUs generated through usearch, DECIPHER v2.0 (r studio package) was used to obtain taxonomic information based on the SILVA SSU version 138 [20, 21] following default parameters. The generated OTU samples were subjected to Hellinger transformation using decostand method in vegan R package version 2.5-7 [22] to standardize differences in sequencing depth prior to diversity analysis. Differential abundance of microbial OTUs across different containers and sample locations were determined using the DESeq2 package (version 1.14.1) in R [23]. Pairwise comparison between sample locations coupled to each container was carried out using a full DESeq2 model (design = ~Container_Location + Condition). OTUs showing significant log-fold changes (padj < 0.05) in at least one of these comparisons was further selected and visualized on a phylogenetic tree in iToL [24]. The log fold-change values were tested for correlation using Spearman’s test through custom python script. Afterwards, pairwise comparisons were repeated with a reduced model (design = ~Location + Container + Condition) to study the effect on sample location while controlling container and condition variations. Using the transformed data, homogeneity of multivariate dispersions was analyzed for each sample location in each container using betadisper from vegan R package.
Metagenome assembly, annotation, and binning
Shotgun metagenomic sequence for the 9 samples (3 containers * 3 locations) were individually assembled using IDBA-UD v1.1.3 [25] with the parameters: -pre_correction -mink 20 -maxk 150 -step 10. Following metagenome assembly, all samples were filtered to remove contigs smaller than 1 kb using pullseq (https://github.com/bcthomas/pullseq). Open reading frames were then predicted on all contigs using Prodigal v2.6.3 [26] with the parameters: -m -p meta. KEGG KO annotations were predicted using KofamScan [27] using HMM models from release r02_18_2020 using default options. In cases where multiple HMMs matched a protein above threshold, the HMM with the lowest E-value had its annotation transferred to the protein.
Metagenome assemblies were binned into draft genomes using a combination of 4 automated binning methods. Briefly, reads from all 9 samples were mapped to assembled contigs ≥2.5 kbp using Bowtie2, and a differential coverage profile for each contig across all samples was used as input for the following differential coverage binners: MaxBin2, CONCOCT, vamb, and MetaBAT [28–31]. The algorithm DasTool [32], was then used to select the highest quality bins across the 4 binning outputs for each metagenome assembly. Finally, the full genome set across all samples (n = 146 genomes) was de-replicated at the species level (Average Nucleotide Identity ≥95%) using dRep [33] with the following parameters: -p 16 -comp 10 -ms 10000 -sa 0.95, resulting in a total of 42 species representative genomes. Species representatives were further selected to have ≥60% completeness and ≤10% contamination as estimated by checkM [34], this resulted in a final set of 32 species representative genomes meeting the criteria. 16S rRNA sequences were extracted from genomes with ContEst16S tool available online (https://www.ezbiocloud.net/tools/contest16s, last accessed on August 17, 2022) [35]. These 16S rRNA sequences were compared with the OTUs obtained from amplicon sequencing using BLAST+ [36] to check for taxonomic consistency.
Phylogenetic and abundance analysis of genome bins
Phylum level taxonomic assignments of 32 de-replicated genome bins and 1 genome (P. calidifontis - GCA000015805) included as an outgroup were inferred using GTDB-Tk v1.5.1 [37] with reference data version r202; phylogenetic relationships between de-replicated genome bins were inferred using GToTree v1.5.22 based on a set of 25 marker genes, and a phylogenetic tree was produced using FastTree2 [38].The tree was displayed and rooted in Geneious Prime v2020.2.4. The relative abundance of the 32 genome bins in all samples was assessed by cross mapping reads from each of the 9 samples back to the genome bins using Bowtie2, followed by quantification of coverage of genomes in each sample using coverM (https://github.com/wwood/CoverM). Differential abundance of genomes between rhizosphere spatial locations was assessed using the DESeq2 package in R [23]. Detailed steps can be found in Supplementary material.
Bulk metagenome analysis
Phylum level taxonomic composition of bulk metagenomes was assessed directly from raw sample reads using graftM [39] run with a custom ribosomal protein L6 (rpL6) marker database constructed from the r202 release of the GTDB database. Differentially abundant KO genes across the different sample locations were determined using the DESeq2 package (version 1.14.1) in R [23]. Pairwise comparison between sample locations was carried out using a reduced DESeq2 model (design = ~Location). Heatmap of differentially abundant genes were plotted in R using the variance stabilized abundance values.
Results
Container type has minimal impact on plant phenotypic growth
We measured three major phenotypes of plant growth, i.e., dry shoot weight, shoot length, root length, to determine container impacts on general plant growth. The only significant difference was between plants grown in pots in open vs. closed conditions (Supplementary Fig. S1). The microbox used to maintain sterile condition (closed) was found to trap a visibly higher amount of moisture inside the box and likely created higher water retention promoting plant growth. Regardless, no other significant difference was detected within or among containers despite differences in container architecture.
Microbiomes in root tips and root base are largely similar yet distinct from the bulk soil
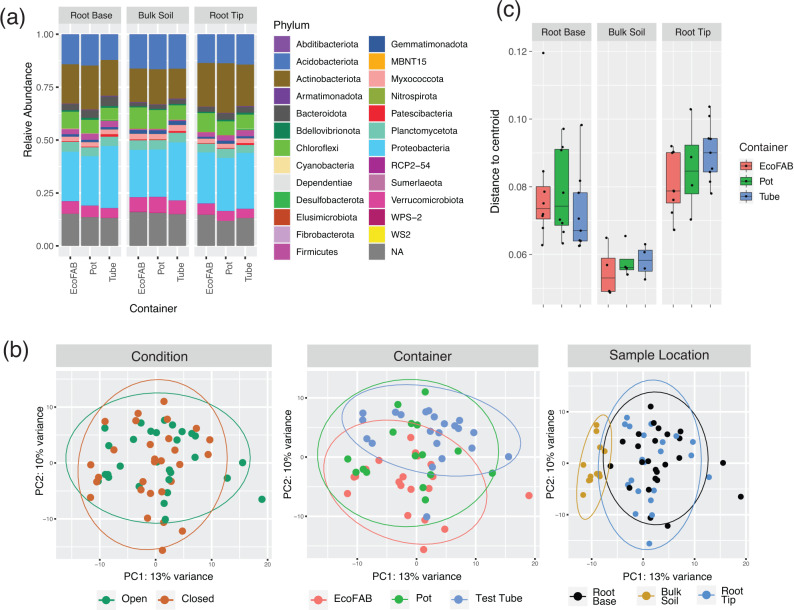
B. distachyon, a model grass species for wheat family, was chosen as it produces only one fine primary axile root from the base of the embryo [40] on which the microbial spatial analysis was performed. We analyzed the rhizosphere microbial community from two different root locations of a 14-day old B. distachyon and the bulk soil using full length 16S rRNA obtained using synthetic long read technology. Among the 3674 OTUs obtained after quality filtering, 25 different phyla were identified which corresponded to ~80–87.5% of all reads among the samples. Microbial relative abundance showed on average a dominance of the bacterial phyla Proteobacteria (22.3–29.3%), Actinobacteriota (14.2–23.5%), Acidobacteriota(12.2–16.5%), Chloroflexi(6.3–10.1%), Planctomycetota (3.7–4.7%), Verrucomicrobiota (4.2–7.4%), Bacteriodota (1.6–4.5%) and Myxococcota (1.9–2.6%) in all samples (Fig. 1a). Interestingly, phyla Firmicutes had lower relative abundance in bulk (average - 0.4%) compared to root tip and root base samples (average - 2.6%). Alpha diversity was lower in root tip compared to bulk soil (p < 0.005, Anova and Tukey) or root base (p < 0.05, Anova and Tukey) in all three diversity metrics analyzed (species number, Shannon and inverse Simpson) (Supplementary Fig. S2). On the other hand, no significant difference in alpha diversity was observed between root base and bulk soil. When root tip samples were compared between the three containers, there was no significant difference in alpha diversity (p > 0.05, Anova and Tukey) indicating negligible container impact.
Fig. 1. Analysis of 16S rRNA amplicon sequencing of rhizosphere (root tip and root base) and bulk soil samples from 14-day old Brachypodium distachyon grown in three different containers: EcoFAB, pot and test tubes.
a Microbial relative abundance, b PCA plot of variance stabilized 16S amplicon data, the samples are then visualized according to the three different variables examined: condition, container or sample location, c Boxplot showing multivariate homogeneity of group dispersions grouped according to sample location and container.
Beta diversity analysis was then carried out to investigate the influence of three parameters tested, i.e., container type, location on root and open or closed condition. Principal Components Analysis (PCA) of the samples showed no clear separation among the two conditions or among the three container types whereas a distinct separation was observed between bulk soil samples compared to root base or root tip (Fig. 1b). However, no distinction was seen when comparing root base and root tip based on ordination analysis. This was supported statistically using MANOVA/adonis which showed the highest dissimilarity contributed by sample location (R2 = 0.10934, p = 9.99e−05) followed by container type (R2 = 0.06336, p = 0.00069) but no significant dissimilarity caused by either open or closed conditions (R2 = 0.02149, p = 0.8119). Next, we examined whether the homogeneity within samples could be influenced by container type. Overall, the EcoFAB samples exhibited a comparable homogeneity among replicates of the same sample location compared to the other two conventional containers (Fig. 1c).
Pairwise comparison between sample locations showed the same differentially abundant OTUs regardless of container type
The OTUs which showed a statistically significant change in any of the pairwise comparisons between sample locations within each container type were selected and visualized using a neighbor-joining tree (Fig. 2a, Supplementary Table S1–S3). Distinct log-fold changes could be observed for comparisons looking at rhizosphere (root base or root tip) vs. bulk soil; the container type has no impact on this trend. Only two OTUs had significant abundance difference between root tip vs. root base, indicating that root tip and root base microbiomes may not be very distinct from one another in young developing plant roots. Further, analysis with Spearman’s correlation coefficient showed that the overall log-fold changes of each OTU were statistically positively correlated in most comparisons regardless of container (Supplementary Table S4), with the only exception being the root tip vs. root base changes observed in pot vs test tube (rho = −0.02, p = 0.78). In all three comparisons, results from EcoFAB samples were consistent with the others. Henceforth, we disregard the container variable to focus on spatial differences.
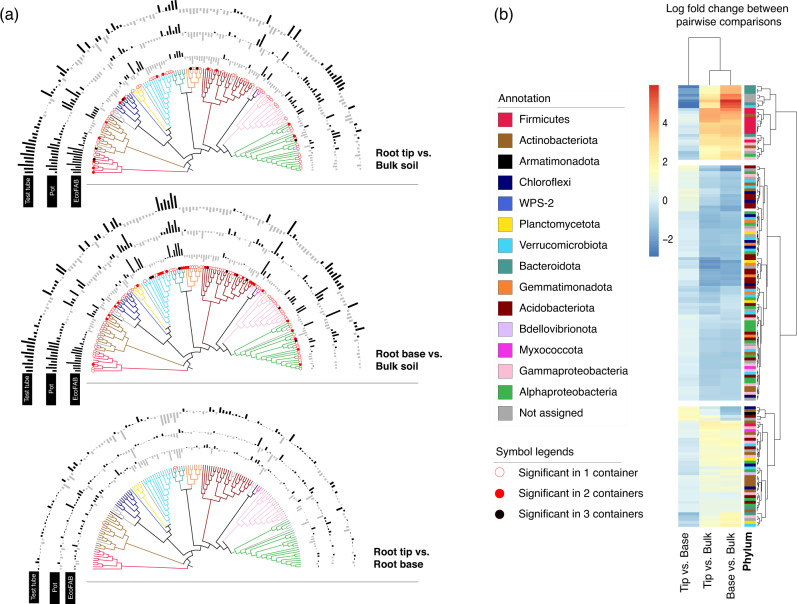
Fig. 2. Differentially abundant OTUs between different sample locations.
a Neighbor joining tree of selected OTUs which showed significant log fold changes during pairwise analysis of sample locations. The top tree depicts a pairwise comparison between root tip and bulk soil, middle tree between root base and bulk soil, and the bottom tree depicts the comparison between root tip and root base. The bar chart around the tree corresponds to log fold changes for each OTU in each of the different containers - test tube, pot or EcoFAB. An outward bar away from the tree represents a positive log fold change in the and an inward bar towards the tree represents a negative fold change in the respective OTU. The significant changes are indicated at the bottom of each node with a symbol. No symbol at the bottom of the node means the fold change is not statistically significant. b Clustering of selected OTUs based on pairwise comparison between sampling locations (ignoring containers) reveals three different clusters. Each OTU is colored by the phylum it belongs to.
Using comparisons solely based on sample location, the OTUs could be grouped into three distinct clusters (Fig. 2b, Supplementary Table S5). The first and smallest cluster showed the OTUs exhibiting significant increase in the rhizosphere (root base or root tip) compared to the bulk soil. Among them are multiple OTUs belonging to Mucilaginibacter (Bacteriodota), Bacillus (Firmicutes), Paenibacillus (Firmicutes), and unclassified Oxalobacteraceae (Gammaproteobacteria). The biggest cluster was for OTUs with a large decrease in the rhizosphere which included the phyla Acidobacteriota, Gemmatimonadota and Chloroflexi. The third cluster contained OTUs with minimal increase or decrease compared within sample locations and contained a mix of phyla.
Taxonomic analysis from metagenomics shows similar community composition to 16S rRNA based amplicon data
Read data from shotgun metagenome samples was directly assessed for bulk taxonomic composition using the ribosomal protein L6 (rpL6) marker gene. The phylum-level relative abundance in all samples showed dominance by the Proteobacteria, Actinobacteriota, Acidobacteriota, Planctomycetota and Verrucomicrobiota (Supplementary Fig. S3a), similar to the 16S rRNA based community composition (Fig. 1a). A PCA plot also illustrated a clustering of the bulk soil samples distinctly from the rhizosphere samples as seen earlier in the corresponding 16S amplicon data (Supplementary Fig. S3b). Overall, the metagenomic taxonomy was in correspondence with the 16S amplicon data and both types of analysis revealed minimal changes contributed by container differences.
Metagenome assembled genomes (MAGs) represent a small fraction of the total reads
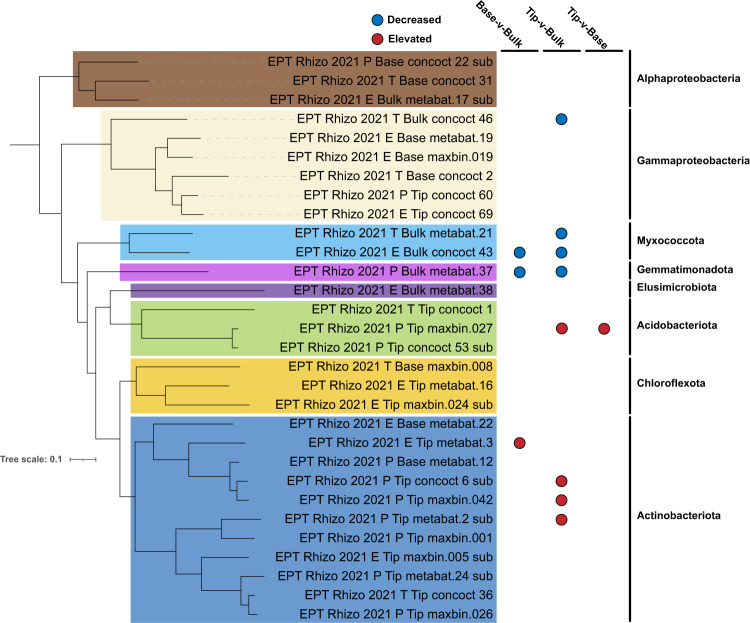
Out of the 32 representative MAGs generated from 9 metagenomes after dereplication and quality filtering (Fig. 3), 11 MAGs belonged to Actinobacteriota; 6 MAGs from Gammaproteobacteria; 4 MAGs from Acidobacteriota and Alphaproteobacteriota; 3 MAGs each from Chloroflexota; 2 MAGs from Myxococcota and 1 MAG each from Gemmatimonadota and Elusimicrobiota (Supplementary Table S6). As expected in systems with higher diversity, the total coverage of these genomes was rather low, representing ~3% of the read data. 10 MAGs were identified to be differentially abundant across sample locations (Fig. 3). It is interesting to note that one Acidobacterial MAG (Edaphobacter sp.) had increased abundance in root tip compared to both bulk and base. Members of Edaphobacter genus are reported to be associated with ectomycorrhizal fungi and are important in their root colonization [41]. Only 6 MAGs had 16S rRNA and all these sequences had a 97–100% match with OTUs obtained from amplicon sequencing and similar phylogenetic classification.
Fig. 3. Phylogenetic tree and differential abundance of MAGs.
Phylogenetic tree of 30 of 32 dereplicated MAGs passing tree building criteria (P. calidifontis - GCA000015805 included as outgroup for rooting; not displayed) along with their differential abundance (significantly elevated or decreased; Wald Test - FDR ≤ 0.05) based on sample location. MAG names are colored based on their phylum-level classification and phyla names displayed on the right. Tree was inferred using a set of 25 phylogenetically informative marker genes conserved between Archaea and Bacteria.
Metagenome analysis reveals metabolic differences between root tip and bulk
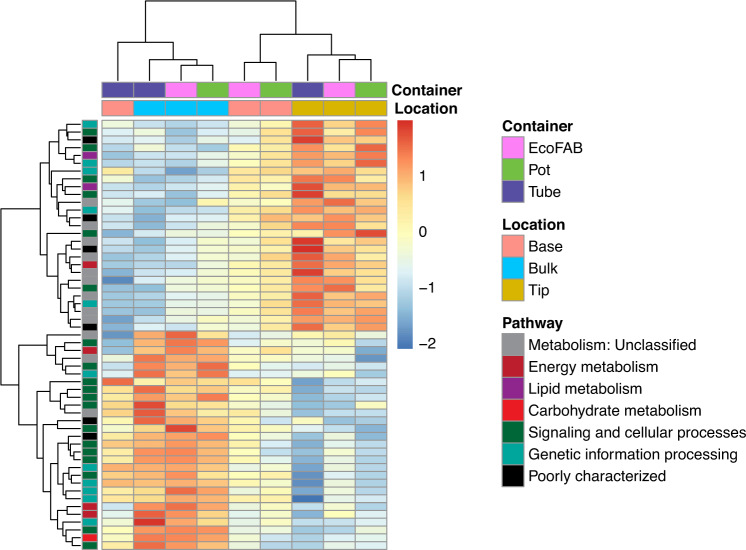
5783 unique KEGG orthology groups (KOs) were annotated in the metagenomes, accounting for ~30% of the total proteins predicted in each metagenome. PCA plot of KEGG Orthology (KO) composition of samples indicated that samples cluster by location irrespective of the container type (Supplementary Fig. S4) and hence container parameter was excluded from further DESeq analysis. There were no differentially abundant KOs when root tip was compared to base, in congruence with observations from PCA analysis of OTUs (Section 3.2). Among the 55 differentially abundant KOs identified (Fig. 4, Supplementary Table S7), 27 were enriched in root tip compared to bulk, while other 27 were decreased in tip vs. bulk and 2 KOs (one KO shared with decreased tip vs. bulk comparison) increased in bulk over base.
Fig. 4. Heatmap of abundance of 55 differentially abundant KEGG Orthology genes across different locations.
Heatmap depicts nine metagenome samples were analyzed using DESeq (corrected p-value < 0.1) and normalized by z-score across all datasets. Each row represents a gene, colored by its KEGG level I classification. 27 KOs were enriched in root tip compared to bulk, 27 KOs were enriched in bulk compared to tip and 2 KOs in bulk over base.
KOs involved in different metabolic pathways were over-represented in tip compared to the bulk suggesting an active microbial population utilizing plant-derived compounds. These KOs, which could be broadly categorized as either enzymes, transcriptional regulators or transporters, play a critical role in substrate utilization as well as root colonization. Enzymes encoded were peptidases (ampS, cwlO), nucleases (nucS), kinases (rsbW, fakA), and other enzymes involved in fatty acid degradation (acd), lipid storage (tgs/wax-dgat), cell wall synthesis (tagTUV), and redox regulation (gshA, fqr). Transcriptional factors/regulator genes enriched in root tips were involved in regulation of purine catabolism (pucR), arabinogalactan biosynthesis (embR), biofilm formation (sigB) [42], sulfur utilization (sutR) and other functions (tetR). The enzyme, peptidoglycan DL-endopeptidase encoded by cwlO, has been shown to regulate biofilm formation and consequently root colonization in plant-beneficial rhizobacterium Bacillus velezensis SQR9 [43]. Interestingly, the anti-sigma factor rsbW and sigma factor sigB were identified as adjacent genes of sigB gene cluster and play important roles in stress resistance, biofilm formation and root colonization in Bacillus cereus 905 [42].Transporters involved in acquisition of copper (ycnJ), amino acid translocation (rhtA), ion transport (nhaA), and other nutrients (MFS (mmr) and ABC transporters (mlaD/linD)) were elevated in root tips. 4 other poorly characterized genes and gene involved in oxidative phosphorylation (qcrC) were also increased in the root tips over bulk metagenomes.
Microbes in the bulk soil do not have ready access to the labile carbon and nitrogen compounds in the exudates and hence may have to invest more in the machinery for nutrient acquisition. Genes involved in heme uptake (exbBD and tonB) [44] and nitrogen assimilation/quorum sensing (rpoN) [45] were increased in bulk soil. In addition, KOs involved in glycogen synthesis (glgA), lipopolysaccharide export (lptF), polysaccharide biosynthesis/export (wza/gfcE), maintenance of cellular integrity under acidic stress (ompA-ompF porin), production of coenzymes (pqqL) involved in free-radical scavenging, regulation of exopolysaccharide production (hprK), periplasmic divalent cation tolerance (cutA) and osmotic stress genes (osmY) may confer resistance to environmental stressors like osmotic stress and desiccation [46, 47] present in bulk soil. KOs corresponding to transfer RNA biogenesis (mnmE/trmE, gidA/mnmG), transcriptional regulation (rho, ada), ribosome biogenesis (rlmI), and sulfur metabolism (dmsBC) were also enriched in the soil.
Discussion
We investigated the utility of EcoFABs as a possible alternative to conventional containers such as pots and tubes for studying the spatial microbial biogeography of the rhizosphere. Although studies have shown that container design parameters such as size, density, depth can affect root growth and basic plant physiological traits during early developmental stages [5–9], our study with young Brachypodium plants showed that containers had no significant impact. While most of these studies looked at container sizes around 50cm3, they were performed using woody tree seedlings such as Pinus sp. (Pine tree species) and Quercus sp. (Oak tree species). Container impacts may not apply to softer plants such as B. distachyon to a discernible extent, emphasizing the importance of using the correct standardized experimental systems and containers to perform accurate study comparisons for the plant under investigation.
Next, we investigated the impact of microbial community assembly on the root impacted by container differences using both 16S amplicon sequencing and metagenomics. Based on 16S amplicon sequencing results, microbial community of each location with respect to root showed relatively similar composition across all containers. Differences were observed mostly in root tip or base locations compared to the bulk soil. At root tips, a decrease of bacterial OTU richness and alpha diversity when compared to bulk soil has been previously reported [3, 48]. This reduction in microbial diversity in the rhizosphere is commonly observed [49] as the root exudates create a selective environment, recruiting selected microbes from bulk soil. We further observed that root tips had lower bacterial diversity (richness and evenness) than root base, which concurs with the other studies conducted on Brachypodium roots [50, 51]. Root tip environment appears to be more stochastic and dynamic compared to the root base as the assembly patterns appear to be more deterministic in older parts of the root [49]. This is partially true in our study as well, there were a higher number of significant OTUs in the comparison of base vs bulk than comparing tip vs bulk (Fig. 2a). Nonetheless, overall correlations show a significantly positive correlation which meant that the rhizosphere effect is already developing at the tip even for 2-week old juvenile plants of Brachypodium. Usually, microbial composition studies tend to occur at later stages of Brachypodium growth [50–52] because the plant often takes 30 – 35 days to reach maturity [40]. Our study, however, shows that a rhizosphere effect may be occurring as early as 14 days into the plant growth.
Only some of the dominant rhizosphere community members such as Gammaproteobacteria and Bacteriodota matched the observations in a previous study with Brachypodium rhizosphere [50]. Phyla such as Betaproteobacteria, which were highly enriched in a previous study with mature plants [50], were neither abundant nor showed enrichment in the rhizosphere. Nonetheless, other rhizosphere enriched groups in this study include Actinobacteria, Acidobacteria and Verrucomicrobia which seems to be more of an effect of the low pH soil characteristic of our field site [53]. Additionally, in that study [50] Brachypodium was grown in sand amended soil which could explain the differences. Actinobacteria, for instance, is associated with rhizosphere in soils with high organic content [54, 55]. In another study where fine scale sampling of 4-week-old Brachypodium roots was performed, Firmicutes were more abundant in root tips compared to root base, whereas opposite trend was observed for Verrucomicrobia [51]. Phyla such as Actinobacteria, Proteobacteria and Bacteriodota were reported to be enriched in wheat rhizosphere [56]. Thus, in line with prior studies, our data also suggests that a combination of root exudates and edaphic factors are working in tandem to enrich a specific rhizosphere community.
Among 150 OTUs which were differentially abundant between different sampling locations, all OTUs belonging to phylum Firmicutes and Bacteriodota were enriched in rhizosphere over bulk soil. These included genera Bacillus and Paenibacillus (Firmicutes) and Mucilaginibacter (Bacteriodota). Members of Paenibacillus have been isolated from rhizosphere of wide variety of plants; several of these are capable of fixing-nitrogen [57–59]. Similarly, several Mucilaginibacter strains have been isolated from rhizosphere, and a comparative analysis of various strains in this genus highlighted the presence of diverse carbohydrate active enzymes including cellulose-degrading enzymes [60]. Impacts of different Bacillus isolates on Brachypodium plants have been characterized previously; Bacillus can accelerate growth, provide drought protection [61], influence root architecture [61] and can modulate plant hormone homeostasis. Some Bacillus, could be classified as r-strategists, and quickly grow in response to nutrient availability in rhizosphere [51].
Majority of differentially abundant OTUs belonging to Gemmatimonodota, Acidobacteria and Verrucomicrobia had reduced abundance in the rhizosphere compared to bulk. These bacterial groups are slow-growing and oligotrophic [62–64], thus more suited to survive in bulk soil away from the nutrient-rich rhizosphere. OTUs belonging to Actinobacteria, Gammaproteobacteria and Alphaproteobacteria showed no clear trends.
We observed congruence between taxonomic results obtained by 16S rRNA gene sequencing and metagenomics (rpL6 marker gene), demonstrating reliability of different sequencing methodologies for bacterial profiling (short read Illumina vs. long-read technology). Comparative analysis of metagenomic functional potential between various sampling locations revealed significant differences between root tips and bulk soil. KO genes involved in different metabolic pathways and root colonization were over-represented in tip compared to the bulk suggesting an active microbial population capable of utilizing plant-derived exudates and occupying the rhizosphere. KO genes associated with machinery for nutrient acquisition and stress-tolerance were prominent in bulk soil where readily available substrates are scarce in comparison to the vicinity of roots. These findings are consistent with other metagenomic studies comparing rhizosphere vs. bulk soil [65] and also in agreement with the results from 16S amplicon sequencing, where rhizosphere is abundant in fast-growing groups and bacterial assembly in root tips is stochastic, while bulk soil is enriched with groups that are more oligotrophic and adapted to survive in nutrient-limited conditions.
We would also like to highlight a few shortcomings and potential improvements in follow up studies. As a result of low DNA yields from juvenile roots, samples were pooled for metagenomics which led to low sample numbers. In addition, genome-resolved metagenomics yielded fewer genomes making statistical analysis of genome relative abundance and metabolic potential analysis challenging. Differences in gene abundance were observed only between root tips and bulk soil, thus differences within rhizosphere compartments (tip vs. base) are unclear which is probably due to sampling of young plants. Size of current EcoFABs limit how long plants can be grown, but can be addressed with bigger devices in future versions.
Thus, in conclusion, we have demonstrated the influence of developing roots in shaping microbial communities in comparison with bulk soil in 14-day old juvenile Brachypodium plants through 16S rRNA amplicon sequencing and metagenomic analyses. While we did not observe distinct differences in microbiomes between the two root zones; based on previous work [51] in older plants, there is a need for high-resolution sampling of rhizosphere to understand biological interactions occurring at finer scales. To further probe into the physiology of root-enriched microbes, we are currently performing high-throughput enrichment of this rhizobiome on known root exudate compounds to create reduced complexity communities and working on engineering materials that can be integrated into EcoFABs to enable localized, sub-millimeter scale sampling at different timepoints.
Supplementary information
Acknowledgements
This research work was funded through the Microbial Community Analysis and Functional Evaluation in Soils (m-CAFEs) Science Focus Area Program at Lawrence Berkeley National Laboratory funded by the U.S. Department of Energy, Office of Science, Office of Biological & Environmental Research Awards DE-AC02-05CH11231.
Data availability
The 16S rRNA amplicon sequences and metagenome-assembled genomes generated during the current study are available in the NCBI SRA repository, under the BioProject ID PRJNA902408. The full assemblies for each metagenome sample are publicly available at our in-house analysis platform, ggKbase (https://ggkbase.berkeley.edu).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shwetha M. Acharya, Mon Oo Yee.
Supplementary information
The online version contains supplementary material available at 10.1038/s43705-023-00265-1.
References
- 1.Pausch J, Kuzyakov Y. Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol. 2018;24:1–12. doi: 10.1111/gcb.13850. [DOI] [PubMed] [Google Scholar]
- 2.Dennis PG, Miller AJ, Hirsch PR. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol. 2010;72:313–27. doi: 10.1111/j.1574-6941.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 3.Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807–38. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 4.Aufrecht J, Khalid M, Walton CL, Tate K, Cahill JF, Retterer ST. Hotspots of root-exuded amino acids are created within a rhizosphere-on-a-chip. Lab Chip. 2022;22:954–63. doi: 10.1039/D1LC00705J. [DOI] [PubMed] [Google Scholar]
- 5.Howell KD, Harrington TB. Nursery practices influence seedling morphology, field performance, and cost efficiency of containerized cherrybark oak. Southern J Appl For. 2004;28:152–62. doi: 10.1093/sjaf/28.3.152. [DOI] [Google Scholar]
- 6.South DB, Harris SW, Barnett JP, Hainds MJ, Gjerstad DH. Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, U.S.A. Forest Ecol Manag. 2005;204:385–98. doi: 10.1016/j.foreco.2004.09.016. [DOI] [Google Scholar]
- 7.Tsakaldimi M, Zagas T, Tsitsoni T, Ganatsas P. Root morphology, stem growth and field performance of seedlings of two mediterranean evergreen oak species raised in different container types. Plant Soil. 2005;278:85–93. doi: 10.1007/s11104-005-2580-1. [DOI] [Google Scholar]
- 8.Kostopoulou P, Radoglou K, Papanastasi OD, Adamidou C. Effect of mini-plug container depth on root and shoot growth of four forest tree species during early developmental stages. Turkish J Agric For. 2011;35:379–90.
- 9.Chu X, Wang X, Zhang D, Wu X, Zhou Z. Effects of fertilization and container-type on nutrient uptake and utilization by four subtropical tree seedlings. J For Res. 2020;31:1201–13. doi: 10.1007/s11676-019-01070-0. [DOI] [Google Scholar]
- 10.Yee MO, Kim P, Li Y, Singh AK, Northen TR, Chakraborty R. Specialized plant growth chamber designs to study complex rhizosphere interactions. Front Microbiol. 2021;12:625752. doi: 10.3389/fmicb.2021.625752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zengler K, Hofmockel K, Baliga NS, Behie SW, Bernstein HC, Brown JB, et al. EcoFABs: advancing microbiome science through standardized fabricated ecosystems. Nat Methods. 2019;16:567–71. doi: 10.1038/s41592-019-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasse J, Kant J, Cole BJ, Klein AP, Arsova B, Schlaepfer P, et al. Multilab EcoFAB study shows highly reproducible physiology and depletion of soil metabolites by a model grass. New Phytol. 2019;222:1149–60. doi: 10.1111/nph.15662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Sasse J, Lewald KM, Zhalnina K, Cornmesser LT, Duncombe TA, et al. Ecosystem fabrication (EcoFAB) protocols for the construction of laboratory ecosystems designed to study plant-microbe interactions. J Vis Exp. 2018;134:57170. [DOI] [PMC free article] [PubMed]
- 14.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 1962;15:413. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 15.Darko E, Végh B, Khalil R, Marček T, Szalai G, Pál M, et al. Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions. PLoS One. 2019;14:e0226151. doi: 10.1371/journal.pone.0226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukami A, Ngetich A, Mweu C, Oduor RO, Muthangya M, Mbinda WM. Differential characterization of physiological and biochemical responses during drought stress in finger millet varieties. Physiol Mol Biol Plants. 2019;25:837–46. doi: 10.1007/s12298-019-00679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bresolin APS, Dos Santos RS, Wolter RCD, de Sousa RO, da Maia LC, Costa de Oliveira A. Iron tolerance in rice: an efficient method for performing quick early genotype screening. BMC Res Notes. 2019;12:361. doi: 10.1186/s13104-019-4362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan BJ, Grinevich D, Thakur S, Balamotis MA, Yehezkel TB. Ultra-accurate microbial amplicon sequencing with synthetic long reads. Microbiome. 2021;9:130. doi: 10.1186/s40168-021-01072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar RC, Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31:3476–82. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- 20.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright ES. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 2016;8:352. doi: 10.32614/RJ-2016-025. [DOI] [Google Scholar]
- 22.Jari O. vegan: Community Ecology Package. R package version 1.8-5. http://www.cran.r-project.org 2007.
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y, Leung HCM, Yiu SM, Chin FYL. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–8. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 26.Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2. doi: 10.1093/bioinformatics/btz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y-W, Simmons BA, Singer SW. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–7. doi: 10.1093/bioinformatics/btv638. [DOI] [PubMed] [Google Scholar]
- 29.Alneberg J, Bjarnason BS, de Bruijn I, Schirmer M, Quick J, Ijaz UZ, et al. Binning metagenomic contigs by coverage and composition. Nat Methods. 2014;11:1144–6. doi: 10.1038/nmeth.3103. [DOI] [PubMed] [Google Scholar]
- 30.Nissen JN, Johansen J, Allesøe RL, Sønderby CK, Armenteros JJA, Grønbech CH, et al. Improved metagenome binning and assembly using deep variational autoencoders. Nat Biotechnol. 2021;39:555–60. doi: 10.1038/s41587-020-00777-4. [DOI] [PubMed] [Google Scholar]
- 31.Kang DD, Froula J, Egan R, Wang Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ. 2015;3:e1165. doi: 10.7717/peerj.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat Microbiol. 2018;3:836–43. doi: 10.1038/s41564-018-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 2017;11:2864–8. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–55. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee I, Chalita M, Ha S-M, Na S-I, Yoon S-H, Chun J. ContEst16S: an algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int J Syst Evol Microbiol. 2017;67:2053–7. doi: 10.1099/ijsem.0.001872. [DOI] [PubMed] [Google Scholar]
- 36.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaumeil P-A, Mussig AJ, Hugenholtz P, Parks DH. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–7. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price MN, Dehal PS, Arkin AP. FastTree 2 — approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd JA, Woodcroft BJ, Tyson GW. GraftM: a tool for scalable, phylogenetically informed classification of genes within metagenomes. Nucleic Acids Res. 2018;46:e59. doi: 10.1093/nar/gky174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watt M, Schneebeli K, Dong P, Wilson IW. The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops. Functional Plant Biol. 2009;36:960. doi: 10.1071/FP09214. [DOI] [PubMed] [Google Scholar]
- 41.Yu W-Y, Peng M-H, Wang J-J, Ye W-Y, Li Y-L, Zhang T, et al. Microbial community associated with ectomycorrhizal Russula symbiosis and dominated nature areas in southern China. FEMS Microbiol Lett. 2021;368:fnab028. doi: 10.1093/femsle/fnab028. [DOI] [PubMed] [Google Scholar]
- 42.Gao T, Li Y, Chai Y, Wang Q, Ding M. SigB regulates stress resistance, glucose starvation, MnSOD production, biofilm formation, and root colonization in Bacillus cereus 905. Appl Microbiol Biotechnol. 2021;105:5943–57. doi: 10.1007/s00253-021-11402-y. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Li Z, Li X, Xia L, Zhou X, Xu Z, et al. FtsEX-CwlO regulates biofilm formation by a plant-beneficial rhizobacterium Bacillus velezensis SQR9. Res Microbiol. 2018;169:166–76. doi: 10.1016/j.resmic.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Nienaber A, Hennecke H, Fischer HM. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol Microbiol. 2001;41:787–800. doi: 10.1046/j.1365-2958.2001.02555.x. [DOI] [PubMed] [Google Scholar]
- 45.Chuckran PF, Hungate BA, Schwartz E, Dijkstra P. Variation in genomic traits of microbial communities among ecosystems. FEMS Microbes 2021;2:xtab020. [DOI] [PMC free article] [PubMed]
- 46.Feng S, Qiu Y, Huang Z, Yin Y, Zhang H, Zhu D, et al. The adaptation mechanisms of Acidithiobacillus caldus CCTCC M 2018054 to extreme acid stress: bioleaching performance, physiology, and transcriptomics. Environ Res. 2021;199:111341. doi: 10.1016/j.envres.2021.111341. [DOI] [PubMed] [Google Scholar]
- 47.Dsouza M, Taylor MW, Turner SJ, Aislabie J. Genomic and phenotypic insights into the ecology of Arthrobacter from Antarctic soils. BMC Genomics. 2015;16:36. doi: 10.1186/s12864-015-1220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK. The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett. 2016;19:926–36. doi: 10.1111/ele.12630. [DOI] [PubMed] [Google Scholar]
- 49.Rüger L, Feng K, Dumack K, Freudenthal J, Chen Y, Sun R, et al. Assembly patterns of the rhizosphere microbiome along the longitudinal root axis of maize (Zea mays L.) Front Microbiol. 2021;12:614501. doi: 10.3389/fmicb.2021.614501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki A, Donn S, Ryan PR, Mathesius U, Devilla R, Jones A, et al. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS One. 2016;11:e0164533. doi: 10.1371/journal.pone.0164533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei S, Jacquiod S, Philippot L, Blouin M, Sørensen SJ. Spatial analysis of the root system coupled to microbial community inoculation shed light on rhizosphere bacterial community assembly. Biol Fertil Soils. 2021;57:973–89. doi: 10.1007/s00374-021-01590-0. [DOI] [Google Scholar]
- 52.Donn S, Kawasaki A, Delroy B, Chochois V, Watt M, Powell JR. Root type is not an important driver of mycorrhizal colonisation in Brachypodium distachyon. Pedobiologia. 2017;65:5–15. doi: 10.1016/j.pedobi.2017.08.001. [DOI] [Google Scholar]
- 53.Diamond S, Andeer PF, Li Z, Crits-Christoph A, Burstein D, Anantharaman K, et al. Mediterranean grassland soil C-N compound turnover is dependent on rainfall and depth, and is mediated by genomically divergent microorganisms. Nat Microbiol. 2019;4:1356–67. doi: 10.1038/s41564-019-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tkacz A, Cheema J, Chandra G, Grant A, Poole PS. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 2015;9:2349–59. doi: 10.1038/ismej.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kopecky J, Kyselkova M, Omelka M, Cermak L, Novotna J, Grundmann GL, et al. Actinobacterial community dominated by a distinct clade in acidic soil of a waterlogged deciduous forest. FEMS Microbiol Ecol. 2011;78:386–94. doi: 10.1111/j.1574-6941.2011.01173.x. [DOI] [PubMed] [Google Scholar]
- 56.Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M. Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol. 2015;17:610–21. doi: 10.1111/1462-2920.12452. [DOI] [PubMed] [Google Scholar]
- 57.Ripa FA, Tong S, Cao W-D, Wang ET, Wang T, Liu HC, et al. Paenibacillus rhizophilus sp. nov., a nitrogen-fixing bacterium isolated from the rhizosphere of wheat (Triticum aestivum L.) Int J Syst Evol Microbiol. 2019;69:3689–95. doi: 10.1099/ijsem.0.003472. [DOI] [PubMed] [Google Scholar]
- 58.Kämpfer P, Busse H-J, McInroy JA, Clermont D, Criscuolo A, Glaeser SP. Paenibacillus allorhizosphaerae sp. nov., from soil of the rhizosphere of Zea mays. Int J Syst Evol Microbiol. 2021;71:005051. doi: 10.1099/ijsem.0.005051. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Li Q, Chen S. Diazotroph Paenibacillus triticisoli BJ-18 drives the variation in bacterial, diazotrophic and fungal communities in the rhizosphere and root/shoot endosphere of maize. Int J Mol Sci. 2021;22:1460. doi: 10.3390/ijms22031460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ZY, Wang RX, Zhou JS, Cheng JF, Li YH. An assessment of the genomics, comparative genomics and cellulose degradation potential of Mucilaginibacter polytrichastristrain RG4-7. Bioresour Technol. 2020;297:122389. doi: 10.1016/j.biortech.2019.122389. [DOI] [PubMed] [Google Scholar]
- 61.Gagné-Bourque F, Mayer BF, Charron J-B, Vali H, Bertrand A, Jabaji S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS One. 2015;10:e0130456. doi: 10.1371/journal.pone.0130456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S, et al. Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems. 2013;16:810–22. doi: 10.1007/s10021-013-9650-7. [DOI] [Google Scholar]
- 63.Barber NA, Chantos-Davidson KM, Amel Peralta R, Sherwood JP, Swingley WD. Soil microbial community composition in tallgrass prairie restorations converge with remnants across a 27-year chronosequence. Environ Microbiol. 2017;19:3118–31. doi: 10.1111/1462-2920.13785. [DOI] [PubMed] [Google Scholar]
- 64.Vieira S, Sikorski J, Dietz S, Herz K, Schrumpf M, Bruelheide H, et al. Drivers of the composition of active rhizosphere bacterial communities in temperate grasslands. ISME J. 2020;14:463–75. doi: 10.1038/s41396-019-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Zhang Y, Zhang P, Trivedi P, Riera N, Wang Y, et al. The structure and function of the global citrus rhizosphere microbiome. Nat Commun. 2018;9:4894. doi: 10.1038/s41467-018-07343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA amplicon sequences and metagenome-assembled genomes generated during the current study are available in the NCBI SRA repository, under the BioProject ID PRJNA902408. The full assemblies for each metagenome sample are publicly available at our in-house analysis platform, ggKbase (https://ggkbase.berkeley.edu).