Abstract
Rutin (RUT) is a phytochemical flavonoid with numerous therapeutic potentials including antihypertension, cardioprotective, neuroprotective, and anti-cancer activities. Its clinical use is inhibited due to its poor aqueous solubility and permeability over oral administration. The present study aimed to overcome these problems through micellization and entrapment of RUT in solid dispersion (SD) using Poloxamer (POL) 407 and 188 as surfactant-based matrices. The RUT/SD formulations were prepared in serial drug loading concentrations in weight percentage to the total solid. The physical properties of the formed RUT/SD solids were characterized by several methods including polarizing microscopy, differential thermal analysis (DTA), X-ray diffractometry (XRD), scanning electron microscopy (SEM) and dissolution study. The dissolution test was performed using a paddle dissolution apparatus and samples were analyzed using UV spectrophotometry. Polarized microscope confirmed that the optical behaviors of the RUT/SD implied a formation of miscible RUT with POL matrices. The morphology of RUT/SDs varied from porous matrices with craters to smoother surfaces as a function of RUT concentrations. XRD and DTA data exhibited that RUT existed as partially amorphous. These data indicated that the higher concentration of RUT in the RUT/SD formulations, the higher amorphous proportion of the RUT in the solid state. Henceforth, this led to an increase in the percentage of dissolved RUT from the developed RUT/SD formulations at 94 to 100% compared to pure RUT at only < 35% within an hour. The present study disclosed the successful improvements in the physical characteristics of the RUT/SD formulations and their potencies for the future development for oral formulation.
Keywords: Rutin, Lyophilization, Surfactant, Solid dispersion, Poloxamer
1. Introduction
RUT, chemically named as 3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside is one of flavonoids largely found in medicinal plants such as apple and tea. RUT is a family member of flavonoids, natural polyphenolic compound that possesses a number of pharmacological activities including antihypertension, cardioprotective, neuroprotective and anticancer activities (Oyagbemi et al., 2020, Wang et al., 2018, Budzynska et al., 2019, Imani et al., 2021). The challenges in bringing RUT into clinical use have been addressed to its poor water solubility (<2 μg/ml) that limits its oral bioavailability (Ravi et al., 2018, Liu et al., 2020). The biopharmaceutical classification system (BCS) has classified RUT as a Class II drug, characterized by poor solubility in aqueous environment, yet good in permeability through cellular membrane. Furthermore, dissolution related problems have been addressed to drugs in this class which cause a hindered absorption in the gastrointestinal tract. These conditions may close the drugs potency to the production of dosage form in the pharmaceutical industry. Thus, improving the solubility and dissolution especially for oral administration is one of particular challenges (Saha and Mishra, 2020, Popović et al., 2022). Various approaches have been castoff to improve the aqueous solubility of such drugs. Among those approaches such as cocrystallization (Chadha et al. 2015), cyclodextrins complexations (Pham et al. 2020), and in particular solid dispersions that have brought a more promising results for the ease on scaling up challenges for mass production. Solid dispersion technique is simple and cost-effective that made it the most favorable method (Bhujbal et al. 2021). The active drug is simply dispersed in inert and hydrophilic matrices and form an amorphous solid state. The matrices must be easily dissolved in water and hydrate the active drugs. Preparation of hydrophobic drugs in the solid dispersions using hydrophilic matrices such as HPMC, PEG, PVP has been reported previously (Yu et al., 2020, Reginald-Opara et al., 2015, LaFountaine et al., 2016).
In line with this, entrapment in surfactant-based solid dispersion matrices such as POL 407 and 188 to improve the solubility and bioavailability is one of particular interests (Jin et al., 2021, Pardhi and Jain, 2021). POL is an excellent polymer that fits such properties. In solid dispersions, the amorphous drug exhibits better solubility and dissolution compared to a crystalline state (Kanaujia et al. 2015). Solid dispersion is not only converting drug crystal into amorphous form, but also reducing the particle size of the drugs to a molecular level (Sareen, Mathew, and Joseph 2012). POL has been used to produce homogenous, stable, amorphous drug and reduce the particle size, thus making this polymer a promising matrix for solid dispersion formulations (Medarević et al., 2016, Vasconcelos et al., 2021). Beside the good performance of POL as a surfactant-based matrix for enhancing the dissolution of drugs, it could improve the wettability of the solid as well as solubilizing agent. POL reduces the surface tension that is useful in improving the dissolution rate (Percival et al. 2018). In this case, POL can form micelle structure that entrapped the hydrophobic compounds, owing to its good solubilizing properties during contact with aqueous environment (Bodratti and Alexandridis 2018). Moreover, the RUT molecular structure exhibits large number of hydroxyl groups that may form hydrogen bonds with POL 407 and 188, thus increase the water solubility (Giuliano et al., 2018, Zhang et al., 2021). In the presented study, POL 407 and 188 were selected as surfactant-based matrices, as micelle-forming agents, as well as the amphiphilic matrices for the developed solid dispersion system to resolve the solubility problem of RUT. Physicochemical characterizations are key aspects as scientific evidences for further development. The study can be a steppingstone to cope with rapid formulation development. Hence, in this study, RUT solid dispersions obtained from freeze-drying of ethanolic solutions with different ratios of drug loading to a fixed proportion of combination matrices of POL 407 and 188 were investigated.
2. Materials and methods
2.1. Materials
RUT, POL 407 and 188 were purchased from Sigma-Aldrich (Singapore). Ethanol was obtained from Merck (Germany). All other solvents and chemicals were of analytical grade.
2.2. Methods
2.2.1. Preparation of RUT/SD by freeze-drying
POL 407 and 188 were made at a fixed weight ratio of 1:1. Next, an accurate amount of RUT was weighed to make gradient concentrations of RUT in the mixtures at 20%, 30% and 40%w/w. The aforementioned ratios of RUT and POLs were dissolved in a specific amount of 96% ethanol since RUT is soluble in ethanol. The ethanolic solutions were divided into vials and freeze-dried using Büchi Labortechnik AG (Flawil, Switzerland). The freezing temperature was conducted in −80°C for 12 h and continued by drying at −20°C for 12 h. The freeze-dried cakes were stored in closed vials at 4°C until further analysis.
2.2.2. Preparation of physical mixtures (PM)
The physical mixtures of RUT and POLs were prepared at the same ratios as those used in the RUT/SD formula. The mixtures were simply mixed using mortar and pestle.
2.2.3. Scanning electron microscope (SEM)
The crude RUT, POL and the freeze-dried samples were examined by SEM (Philips X series, Netherlands) to reveal their morphology. A sample size of 25 – 50 mg were used for the analysis. The morphology was captured at magnifications of 250x and 2500x at an acceleration voltage of 20 kV. Previously, all samples were coated with a thin gold–palladium layer by sputter coater (SC 7620, England).
2.2.4. Polarization microscopy
The effect of POL 407 and 188 as matrix on RUT crystals was evaluated via polarized light microscope (Novel), images captured by Optilab viewer version 2.2. All samples of raw materials and RUT/SD formulations in amount of 2–5 mg of each sample was placed on a microscopy slide with a cover glass and mounted under the polarized light microscope. Subsequently, the visual images of the crystals were captured at magnification of 40x. In all cases, several visual images were obtained for each sample.
2.2.5. Differential thermal analysis (DTA)
Thermal analysis was performed using a DTA of Mettler Toledo (Switzerland). Samples of RUT/SD and the physical mixtures at 3–5 mg was placed in a lid-sealed aluminum crucible pan. The aluminum pans were crimped prior to placement inside the DTA sample holder. They were scanned at a rate of 10 °C/min from 30 to 250°C. Thermodynamic events including melting points were determined using the Mettler software (Switzerland).
2.2.6. Powder X-ray diffraction (PXRD)
The XRD patterns of all raw materials, RUT/SD formulations prepared by freeze-drying, and the PM samples were recorded using X-ray diffractometer (XRD) Philips X'Pert PRO (Panalytical, The Netherlands). The measurement setting was using a 30 mA current and a voltage of 40 kV. The scanned range of 5–50° (2θ) was employed for each sample.
2.2.7. Dissolution studies
To investigate the dissolution profiles of the prepared RUT/SD formulations, PBS buffer at pH of 6.8 was used as a dissolution media in the USP Apparatus 2 of dissolution test (paddle type). The selected pH of 6.8 was widely accepted for a discriminative in vitro release of a dissolution study although the use of pH 2 was also possible and advantageous for future perspective. However, as the drug may undergo ionization at lower pH, then a medium with pH of 6.8 was selected in the study. The dissolution apparatus vessel was filled with 900 mL of PBS (pH = 6,8), the temperature was kept at 37°C and the paddles rotation was set at 100 rpm to maintain the sink condition. Samples were accurately weighed equivalent to 50 mg of RUT powder and added to the dissolution medium. Samples were withdrawn at a volume of 5 mL from the vessels and immediately replaced with fresh dissolution medium at equal volume. Withdrawn samples were filtered through 0.22-μm nylon filter (Whatman, Germany) prior to analysis. The concentration of RUT was determined using spectrophotometer (Shimadzu, Japan) at 359 nm where POL 407 and 188 showed no interference at this wavelength. The amount of dissolved RUT was analyzed at each sampling interval (5, 10, 20, 30, 40, 50 and 60 min).
3. Results and discussions
3.1. Optical characteristics
To obtain information about the crystallinity of the freeze-dried RUT as compared to its raw materials, a polarized microscope was employed. The microscopy observation can investigate the changes in the crystal’s morphology and the optical characteristics of the mixture, permitting for the assessment whether the presence of polymeric matrices affect the nature of the RUT crystals. The practicality of such technique has been demonstrated in several investigations of various drugs and their mixtures with many hydrophilic matrices (Aho et al., 2017, Herrera et al., 2016). The microscopic images of all samples were provided in Fig. 1.
Fig. 1.
Polarizing microscope images of: A) RUT, B) POL 188, C) POL 407, D) RUT/SD20, E) RUT/SD30, F) RUT/SD40. Abbreviations: RUT = Rutin; POL 188 = Poloxamer 188; POL 407 = Poloxamer 407; RUT/SD 20, RUT/SD 30, RUT/SD 40 = formulations containing RUT of 20%, 30% and 40%, respectively.
The miscibility of RUT with POL 407 and 188 is important during the preparation of the RUT/SD as it is strongly related to the physical stability of the formulations (Marsac et al., 2009, Liu, 2006). Phase separation that leads to higher tendency of recrystallization of the active pharmaceutical ingredients (APIs) may be resulted from immiscible mixtures (Pajula et al., 2014, Luebbert and Sadowski, 2017). To predict the miscibility of the mixture, polarized microscopy is considered as the simplest to implement (Kapourani et al. 2021). With this technique, miscibility of the components in the formulation can be easily detected where miscible compounds will form a uniform optical pattern. Fig. 1 showed the obtained polarized images of all samples. In all the developed RUT/SD formulations, RUT crystals were observed as fully miscible with the POL 407 and 188, indicating that RUT is homogenously dispersed in the matrices. De-mixing zones were not observed among them, a strong suggestion of good miscibility among all RUT/SD components.
3.2. Morphology
The influence of the freeze-drying process on the morphology of the RUT/SD formulations was examined using SEM. SEM provides useful information about morphology of solid dispersions, shape and size of particle and while it can specifically portray the topography of sample surfaces without see-through the internal structure of samples. In this study, it is expected that the RUT was in amorphous state, miscible and well dispersed into the POL 407 and 188 matrices. SEM images of raw components and RUT/SD formulations are shown in Fig. 2; Fig. 2A, B and C depict the micrograph of raw materials whilst Fig. 2D to 2F depict the SEM micrographs of solid dispersions using POL 407 and 188, containing RUT of 20%, 30% and 40%, respectively.
Fig. 2.
SEM images of: A) Rutin, B) Poloxamer 188, C) Poloxamer 407, D) RUT/SD20, E) RUT/SD30, F) RUT/SD40, G) Physical mixture of all materials. RUT/SD20, RUT/SD30, RUT/SD40 were formulations containing RUT of 20%, 30% and 40%, respectively. The images of raw materials were captured at magnification of 250X, while the images of the developed formulations and their physical mixture were captured at magnification of 2500X.
Pure RUT crystals exhibited morphology of irregular form and fragmentary-shape (Fig. 2A) while the POL 407 and 188 particles have a spherical and round-shape (Fig. 2B and 2C). In the RUT/SD formulations (Fig. 2D, E, and F), small portion of the RUT particles observed as irregular-shaped crystals on the surface of the RUT/SD matrices, whilst the solid matrices were porous with nearly smooth surfaces (Fig. 2D, E and F). On increasing the RUT concentration in the formulations from 20%, 30% and 40%, this property becomes more pronounced. However, the physical mixture showed most scattering of the RUT crystals, indicating that all RUT still existed as crystalline material (Fig. 2G).
The desirable properties of POL 407 and 188 for the freeze-drying process have been demonstrated previously in numerous studies (Jin et al., 2021, Pardhi and Jain, 2021). As shown in Fig. 2D, E and F, RUT existed as irregular-like solid present on the surfaces of the POL matrices. This might be resulted by the prompt evaporation of solvent during freeze drying. In this case, a fast concentrated RUT was occupying the surface of the matrices and after rapid evaporation of the solvent, some portions of RUT molecules remained on the surface of the POL matrices. After a while, RUT molecules might be recrystallized and exhibited a fragmentary-like crystal form.
3.3. Crystallinity
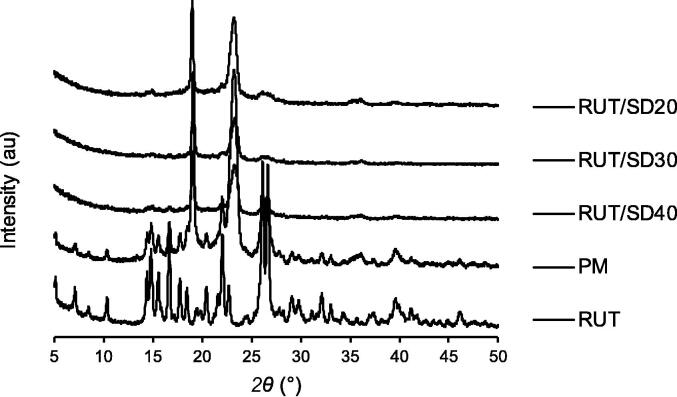
Fig. 3 displays the XRD patterns for all raw materials. Pure RUT exhibited sharp characteristic peaks at several angles, which designate the crystalline nature of RUT (Remanan and Zhu, 2021, Savic et al., 2016). POL 407 and 188 exhibit a few of sharp peaks due to its crystal-like nature, where both POLs showed identical distinct peaks related to its semi-crystalline structure (Swain and Subudhi, 2019, Gumaste et al., 2016).
Fig. 3.
XRD diffractograms of single materials: rutin (RUT), poloxamer 188 (POL 188) and poloxamer 407 (POL 407).
The physical mixture sample of RUT-POL 1:1 exhibited sharp peaks referring to RUT and POL that were still visible, yet slightly decreased in the intensity (Fig. 4). However, in the samples prepared by freeze drying, the RUT/SD formulations showed that the intensity of these peaks has substantially decreased compared to the respective PM sample. All peaks identical to RUT were no longer present. Only few peaks relating to POL were still existed and the results were identical regardless the concentration of RUT in the formulations. Almost all the RUT characteristic peaks were diminished, indicating that the RUT/SD formulations exists in the amorphous state. The amorphous nature could be attributed to the amorphous nature of POL 407 and 188 themselves.
Fig. 4.
XRD diffractograms of the developed RUT/SD formulations and their physical mixture. RUT/SD20, RUT/SD30, RUT/SD40 were formulations containing RUT of 20%, 30% and 40%, respectively; PM = physical mixture of all materials; RUT = raw rutin.
The two POLs (due to their hydrophilic characteristics) increased the solubility and dissolution of drugs in the solid dispersion formulations. As POL 407 and 188 are hydrophilic polymers, they surrounded the surface of hydrophobic RUT, causing the surface of hydrophobic RUT more hydrophilic. Therefore, it may enhance the wettability of the RUT and improve the dissolution (Yusuf et al. 2019). Moreover, the RUT/SD formulations were present in the amorphous state that improve the dissolution compared to corresponding pure drugs or the physical mixtures. Moreover, RUT is a polyhydroxy compound that may form hydrogen bonding with the matrices, thus increase the drugs solubility (Shi et al. 2021).
3.4. Thermal properties
The DTA thermograms of pure RUT, POL 188 and POL 407 were presented in Fig. 5. Pure RUT exhibited a sharp endothermic peak at around 189°C, which was ascribed to its melting point and is in accordance with the similar published data (Ahmad et al. 2016). This data indicated that RUT is crystalline material. As shown in Fig. 5, both POL 407 and 188 displayed an endothermic peak at 69°C and an exothermic peak at 167°C, indicating that the material was recrystallized upon heating.
Fig. 5.
DTA Thermograms of single materials: Rutin (RUT), poloxamer 188 (POL 188), poloxamer 407 (POL 407).
The obtained RUT/SD formulations still showed endothermic peaks at around 60°C and around 195°C referring to POL and RUT respectively (Fig. 6). All developed RUT/SD formulations still have endothermic peaks that attributed to the POL. The endothermic peaks attributed to RUT were sharper in RUT/SD20 that containing RUT of 20% and RUT/SD30 with 30%. However, such peak was not obviously present in the RUT/SD40 that contain 40% of RUT. The two peaks referring to POL and RUT observed in the RUT/SD formulations indicated that the RUT were not homogenously dispersed in the matrices, leaving the matrices partially phase separated from the mixture. Increasing the drug concentration in these formulations resulted in an enhancement of the RUT fusion for the second peak as shown by a reduction in the intensity of the corresponding peak. In the RUT/SD40, the intensity of the second peak decreased significantly compared to that for the RUT/SD20 and RUT/SD30 where the peaks were more pronounced. The DTA data indicated that all RUT/SD formulations were partially amorphous as the RUT was no longer pronounced in the mixtures. The resulted thermogram for PM sample indicated three notable peaks referring to the individual components, though the height of the peaks were significantly reduced. As the sample heated during analysis, all materials were melted and mixed altogether resulted a pattern that looked like a solid dispersion sample. Henceforth, applying PM sample for thermal analysis sometimes intriguing. As such, this was generally accepted result as the impact of heat during the analysis.
Fig. 6.
DTA thermograms of the developed RUT/SD formulations and the physical mixture. RUT/SD20, RUT/SD30, RUT/SD40 were formulations containing RUT of 20%, 30% and 40%, respectively; PM = physical mixture of all materials; RUT = raw rutin.
Considering that RUT was dissolved in ethanol and subjected to a freeze-drying process, then POL generated matrices with RUT molecularly dispersed in their structures. The XRD results have clearly shown the amorphous state of RUT in these matrices. However, the DTA results were not fully supported the conclusion. As the RUT concentration decreases, the second peak at around 190°C attributed to the melting point of RUT becomes more pronounced. These results indicated that the presence of RUT in the matrices was partially crystalline. It is common that drugs can be transformed into partially amorphous state following lyophilization results (Jin et al., 2021, Singh et al., 2017).
3.5. Dissolution study
The dissolution apparatus type 2 with phosphate buffer as dissolution media were used to investigate the dissolution profiles of the RUT formulations. As shown in Fig. 7, it is obvious that the presence of POL 407 and 188 in the RUT/SD formulations improved the dissolution of RUT. Moreover, it was interesting that the samples containing higher RUT show higher percentage of the dissolved drug. The RUT/SD40 exhibited the highest percentage of dissolved RUT followed by RUT/SD30 and RUT/SD20, respectively. Nevertheless, all the developed RUT/SD formulations showed clear increases in the percentage of RUT dissolved at 94 to 100% compared to the raw RUT at only < 35%. The partially amorphous drug in these developed formulations tends to recrystallize, which might be accelerated by the presence of humidity during storage (Luebbert and Sadowski, 2017, Prudic et al., 2015). Such problem has been the major challenges for an amorphous drug in solid dispersion formulations.
Fig. 7.
Dissolution profiles of RUT and the developed RUT/SD formulations. RUT/SD20, RUT/SD30, RUT/SD40 were formulations containing RUT of 20%, 30% and 40%, respectively.
As shown in Fig. 7, the lower percentage of dissolved raw RUT (crystalline form) was noteworthy. This could be attributed to nature poor solubility of RUT in aqueous solution. The freeze-dried RUT/SD exhibited a higher percentage of dissolved RUT. Fully or partially amorphous RUT could contribute in intermolecular hydrogen bonding with the hydrophilic POLs and forms a homogeneous solid dispersion mixture. This may accelerate the hydration of RUT molecules, thus, enhanced the dissolution of RUT (Yusuf et al. 2022). Moreover, the percentage of dissolved RUT was also increased as the RUT concentration increases in the POL matrices. The dissolution enhancement for RUT/SD40 containing 40% RUT was obvious, in which after 5 min more than 60% of the RUT was dissolved, compared to < 10% of pure RUT at the same time point. The raw RUT was only dissolved < 35% after 60 min (Fig. 7). In contrast, it is obvious that the RUT dissolution has been enhanced in almost all of the developed RUT/SD formulations.
4. Conclusion
The presence of POL 407 and 188 in RUT solid dispersion produced by freeze drying technique was able to change the physicochemical characteristics of RUT. The RUT/SD formulations showed a porous structure and smooth surface (SEM data). The mixtures indicated metastable properties where the dry product was partially amorphous (XRD and DTA data). The RUT/SD formulations were clearly increased the percentage of dissolved RUT as revealed by dissolution results. The present study disclosed the improved physical characteristics and potency of RUT/SD formulations for future development of RUT oral formulation.
CRediT authorship contribution statement
Helmy Yusuf: Conceptualization, Methodology, Visualization, Writing – review & editing. Orchidea Meidy Nurintan Savitri: Data curation, Writing – original draft. Riesta Primaharinastiti: Validation. M Agus Syamsur Rijal: Data curation, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Directorate General of Higher Education of the Republic of Indonesia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Ashafaq M., Samim M., Iqbal Z., Ahmad F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Aho J., Van Renterghem J., Arnfast L., De Beer T., Rantanen J. The flow properties and presence of crystals in drug-polymer mixtures: rheological investigation combined with light microscopy. Int. J. Pharm. 2017;528:383–394. doi: 10.1016/j.ijpharm.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Bhujbal S.V., Mitra B., Jain U., Gong Y., Agrawal A., Karki S., Taylor L.S., Kumar S., Zhou Q.T. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm. Sin. B. 2021;11:2505–2536. doi: 10.1016/j.apsb.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodratti A.M., Alexandridis P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018;9:11. doi: 10.3390/jfb9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynska B., Faggio C., Kruk-Slomka M., Samec D., Nabavi S.F., Sureda A., Devi K.P., Nabavi S.M. Rutin as neuroprotective agent: from bench to bedside. Curr. Med. Chem. 2019;26:5152–5164. doi: 10.2174/0929867324666171003114154. [DOI] [PubMed] [Google Scholar]
- Chadha R., Bhalla Y., Vashisht M.K., Chadha K. Cocrystallization in nutraceuticals. Recryst. Mater. Process. 2015;35–50 doi: 10.5772/59365. [DOI] [Google Scholar]
- Giuliano E., Paolino D., Fresta M., Cosco D. Mucosal applications of poloxamer 407-based hydrogels: an overview. Pharmaceutics. 2018;10:159. doi: 10.3390/pharmaceutics10030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaste S.G., Gupta S.S., Serajuddin A. Investigation of polymer-surfactant and polymer-drug-surfactant miscibility for solid dispersion. AAPS J. 2016;18:1131–1143. doi: 10.1208/s12248-016-9939-5. [DOI] [PubMed] [Google Scholar]
- Herrera N., Salaberria A.M., Mathew A.P., Oksman K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: effects on mechanical, thermal and optical properties. Compos. Part A Appl. Sci. Manuf. 2016;1(83):89–97. doi: 10.1016/j.compositesa.2015.05.024. [DOI] [Google Scholar]
- Imani A., Maleki N., Bohlouli S., Kouhsoltani M., Sharifi S., Dizaj S.M. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021;35:2500–2513. doi: 10.1002/ptr.6977. [DOI] [PubMed] [Google Scholar]
- Jin G., Ngo H.V., Cui J.-H., Wang J., Park C., Lee B.-J. Role of surfactant micellization for enhanced dissolution of poorly water-soluble cilostazol using poloxamer 407-based solid dispersion via the anti-solvent method. Pharmaceutics. 2021;13:662. doi: 10.3390/pharmaceutics13050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaujia P., Poovizhi P., Ng W.K., Tan R.B.H. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015;285:2–15. doi: 10.1016/j.powtec.2015.05.012. [DOI] [Google Scholar]
- Kapourani A., Tzakri T., Valkanioti V., Kontogiannopoulos K.N., Barmpalexis P. Drug crystal growth in ternary amorphous solid dispersions: effect of surfactants and polymeric matrix-carriers. Int. J. Pharm. X. 2021;3 doi: 10.1016/j.ijpx.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountaine J.S., Prasad L.K., Brough C., Miller D.A., McGinity J.W., Williams R.O. Thermal processing of PVP-and HPMC-based amorphous solid dispersions. AAPS PharmSciTech. 2016;17:120–132. doi: 10.1208/s12249-015-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Physical characterization of pharmaceutical formulations in frozen and freeze-dried solid states: techniques and applications in freeze-drying development. Pharm. Dev. Technol. 2006;11:3–28. doi: 10.1080/10837450500463729. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhao X., Zhang Q., Wang L., Li Y., Li Y. Characterization and evaluation of the solubility and oral bioavailability of Rutin-Ethanolate solvate. AAPS PharmSciTech. 2020;21:1–12. doi: 10.1208/s12249-020-01779-w. [DOI] [PubMed] [Google Scholar]
- Luebbert C., Sadowski G. Moisture-induced phase separation and recrystallization in amorphous solid dispersions. Int. J. Pharm. 2017;532:635–646. doi: 10.1016/j.ijpharm.2017.08.121. [DOI] [PubMed] [Google Scholar]
- Marsac P.J., Li T., Taylor L.S. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm. Res. 2009;26:139–151. doi: 10.1007/s11095-008-9721-1. [DOI] [PubMed] [Google Scholar]
- Medarević D.P., Kachrimanis K., Mitrić M., Djuriš J., Djurić Z., Ibrić S. Dissolution rate enhancement and physicochemical characterization of carbamazepine-poloxamer solid dispersions. Pharm. Dev. Technol. 2016;21:268–276. doi: 10.3109/10837450.2014.996899. [DOI] [PubMed] [Google Scholar]
- Oyagbemi A.A., Bolaji-Alabi F.B., Ajibade T.O., Adejumobi O.A., Ajani O.S., Jarikre T.A., Omobowale T.O., Ola-Davies O.E., Soetan K.O., Aro A.O. Novel antihypertensive action of rutin is mediated via inhibition of angiotensin converting enzyme/mineralocorticoid receptor/angiotensin 2 type 1 receptor (ATR1) signaling pathways in uninephrectomized hypertensive rats. J. Food Biochem. 2020;44:e13534. doi: 10.1111/jfbc.13534. [DOI] [PubMed] [Google Scholar]
- Pajula K., Wittoek L., Lehto V.-P., Ketolainen J., Korhonen O. Phase separation in coamorphous systems: in silico prediction and the experimental challenge of detection. Mol. Pharm. 2014;11:2271–3229. doi: 10.1021/mp400712m. [DOI] [PubMed] [Google Scholar]
- Pardhi V.P., Jain K. Impact of binary/ternary solid dispersion utilizing poloxamer 188 and TPGS to improve pharmaceutical attributes of bedaquiline fumarate. J. Drug Deliv. Sci. Technol. 2021;62 doi: 10.1016/j.jddst.2021.102349. [DOI] [Google Scholar]
- Percival S.L., Chen R., Mayer D., Salisbury A.-M. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int. Wound J. 2018;15:749–755. doi: 10.1111/iwj.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, Thi Lan, TR Usacheva, IA Kuz'mina, Thi Ngoan Nguyen, Hoang Thai, MA Volkova, Hai Khoa Le, Tuan Dung Nguyen, and VA Volynkin. 2020. Effect of cyclodextrin types and reagents solvation on the stability of complexes between B-cyclodextrins and rutin in water-ethanol solvents. J. Mol. Liq. 318: 114308. 10.1016/j.molliq.2020.114308
- Popović B.M., Uka D., Alioui O., Pavlović R.Ž., Benguerba Y. Experimental and COSMO-RS theoretical exploration of rutin formulations in natural deep eutectic solvents: solubility, stability, antioxidant activity, and bioaccessibility. J. Mol. Liq. 2022;359 doi: 10.1016/j.molliq.2022.119266. [DOI] [Google Scholar]
- Prudic A., Ji Y., Luebbert C., Sadowski G. Influence of humidity on the phase behavior of API/polymer formulations. Eur. J. Pharm. Biopharm. 2015;94:352–362. doi: 10.1016/j.ejpb.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Ravi G.S., Narayana Charyulu R., Dubey A., Prabhu P., Hebbar S., Mathias A.C. Nano-lipid complex of rutin: development, characterisation and in vivo investigation of hepatoprotective, antioxidant activity and bioavailability study in rats. AAPS Pharmscitech. 2018;19:3631–3649. doi: 10.1208/s12249-018-1195-9. [DOI] [PubMed] [Google Scholar]
- Reginald-Opara J.N., Attama A., Ofokansi K., Umeyor C., Kenechukwu F. Molecular interaction between glimepiride and Soluplus®-PEG 4000 hybrid based solid dispersions: characterisation and anti-diabetic studies. Int. J. Pharm. 2015;496:741–750. doi: 10.1016/j.ijpharm.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Remanan M.K., Zhu F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021;353 doi: 10.1016/j.foodchem.2020.128534. [DOI] [PubMed] [Google Scholar]
- Saha S., Mishra A. A facile preparation of rutin nanoparticles and its effects on controlled growth and morphology of calcium oxalate crystals. J. Cryst. Growth. 2020;540 doi: 10.1016/j.jcrysgro.2020.125635. [DOI] [Google Scholar]
- Sareen, Swati, George Mathew, and Lincy Joseph. 2012. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2: 12. 10.4103%2F2230-973X.96921. [DOI] [PMC free article] [PubMed]
- Savic I.M., Savic-Gajic I.M., Nikolic V.D., Nikolic L.B., Radovanovic B.C., Milenkovic-Andjelkovic A. Enhencemnet of solubility and photostability of rutin by complexation with β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016;86:33–43. doi: 10.1007/s10847-016-0638-8. [DOI] [Google Scholar]
- Shi X., Fan B., Zhou X., Chen Q., Shen S., Xing X., Deng Y.u. Preparation and characterization of Ibrutinib amorphous solid dispersions: a discussion of interaction force. J. Pharm. Innov. 2021;1–10 doi: 10.1007/s12247-021-09585-y. [DOI] [Google Scholar]
- Singh G., Sharma S., Gupta G.D. Extensive diminution of particle size and amorphization of a crystalline drug attained by eminent technology of solid dispersion: a comparative study. AAPS PharmSciTech. 2017;18:1770–1784. doi: 10.1208/s12249-016-0647-3. [DOI] [PubMed] [Google Scholar]
- Swain R.P., Subudhi B.B. Effect of semicrystalline copolymers in solid dispersions of pioglitazone hydrochloride: in vitro-in vivo correlation. Drug Dev. Ind. Pharm. 2019;45:775–786. doi: 10.1080/03639045.2019.1572183. [DOI] [PubMed] [Google Scholar]
- Vasconcelos T., Prezotti F., Araújo F., Lopes C., Loureiro A., Marques S., Sarmento B. Third-generation solid dispersion combining Soluplus and poloxamer 407 enhances the oral bioavailability of resveratrol. Int. J. Pharm. 2021;595 doi: 10.1016/j.ijpharm.2021.120245. [DOI] [PubMed] [Google Scholar]
- Wang Y.-D., Zhang Y., Sun B.o., Leng X.-W., Li Y.-J., Ren L.-Q. Cardioprotective effects of rutin in rats exposed to pirarubicin toxicity. J. Asian Nat. Prod. Res. 2018;20:361–373. doi: 10.1080/10286020.2017.1394292. [DOI] [PubMed] [Google Scholar]
- Yu J.Y., Kim J.A., Joung H.J., Ko J.A., Park H.J. Preparation and characterization of curcumin solid dispersion using HPMC. J. Food Sci. 2020;85:3866–3873. doi: 10.1111/1750-3841.15489. [DOI] [PubMed] [Google Scholar]
- Yusuf H., Nugraheni R.W., Setyawan D. Effect of cellulose derivative matrix and oligosaccharide on the solid state and physical characteristics of dimethyldioctadecylammoniumliposomes for vaccine. Res. Pharm. Sci. 2019;14(1):1. doi: 10.4103/1735-5362.251847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf H., Novitasari E.K., Purnami N.LW., Mahbub A.W., Sari R., Setyawan D. Formulation design and cell cytotoxicity of curcumin-loaded liposomal solid gels for anti-Hepatitis C virus. Adv. Pharmacol. Pharm. Sci. 2022;2022 doi: 10.1155/2022/3336837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Chen S., Dou H., Liu Q., Shu G., Lin J., Zhang W., Peng G., Zhong Z., Hualin F.u. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111352. [DOI] [PubMed] [Google Scholar]