Abstract
The increasing incidence of methicillin-resistant S. aureus is a major public health concern. Recently, the performance of Citrus hystrix essential oil (CHEO) has been shown to contain broad-spectrum antibacterial activity. Therefore, this study aims to determine the antibacterial activity of CHEO alone and in combination with gentamicin against panels of clinical isolates of methicillin-susceptible S. aureus (MSSA, n = 45) and methicillin-resistant S. aureus (MRSA, n = 40). Antibiotic susceptibility testing revealed multidrug-resistant (MDR) patterns among 3 MSSA isolates and 39 MRSA isolates, indicating that the clinical MRSA isolates were associated with MDR (p < 0.05). For the drug resistant isolates, resistance was observed toward most antibiotics, except for chloramphenicol, trimethoprim-sulfamethoxazole, linezolid, and vancomycin. Antibacterial screening by disk diffusion demonstrated that CHEO alone had certain antibacterial activity toward all MSSA isolates (IZD: 16.0 ± 4.7 mm) and MRSA isolates (IZD: 16.5 ± 4.2 mm) (p > 0.05). The MIC values of CHEO are 18.3 ± 6.1 mg/mL in MSSA isolates and 17.9 ± 6.9 mg/mL in MRSA isolates (p > 0.05). The antibacterial activity of CHEO demonstrated the bactericidal effect with MIC index 1.0–1.4. Time-killing kinetics revealed that CHEO at 1 × MIC completely killed MSSA and MRSA within 12 h. Moreover, the checkerboard titration demonstrated the synergistic and additive interactions of CHEO with gentamicin with FIC index 0.012–0.625. CHEO against human epidermal keratinocyte; HaCaT cell line demonstrated the IC50 value at 2.15 mg/mL. The use of CHEO as an alternative antibacterial agent would reduce the emergence of resistant bacteria, especially MDR MRSA.
Keywords: Antibacterial activity, Cytotoxicity, Citrus hystrix, Multidrug-resistant, MRSA, S. aureus
1. Introduction
Staphylococcus aureus, a high priority resistant bacterium, is an organism of major public health concern worldwide. It can cause a wide variety of infections in the skin, respiratory tract, and surgical wounds, as well as life-threatening sepsis, particularly in immunocompromised hosts (Tong et al., 2015). Moreover, it is a major problem of both nosocomial and community-acquired infections (Li, 2018). Methicillin-resistant S. aureus (MRSA) is a resistant bacteria that has acquired resistance to β-lactam antibiotics associated with the resistance to most antibiotics. Methicillin resistance is caused by the acquisition of mecA gene carried by staphylococcal cassette chromosome mec (SCCmec), which produces penicillin-binding protein 2a (PBP2a) that contains a low β-lactam affinity (Lakhundi and Zhang, 2018). The emerging of this resistance is attributed to inappropriate use of β-lactam antibiotics. Therefore, several studies have attempted to seek out an alternative antibacterial agent against S. aureus, including herbal extracts.
Citrus plants belong to the family of Rutaceae containing around 1,300 species (Kamal et al., 2011). The citrus EOs are colorless to yellowish transparent liquids with a density of around 0.8 g/mL and a refractive index of approximately 1.46–1.47 (Yang et al., 2021). The major component is d-limonene accounting for 25–97% of the total component (Jing et al., 2014). Citrus hystrix DC. (or makrut lime) is a traditional plant which is commonly found in Thailand. Several biological properties of C. hystrix essential oil (CHEO) have been demonstrated, with antioxidant, anti-inflammatory, antibiofilm, and antimicrobial activities (Chanthaphon et al., 2008, Chao et al., 2008, Wongsariya et al., 2014, Md Othman et al., 2016, Kantawong et al., 2017, Aumeeruddy-Elalfi et al., 2018, Sreepian et al., 2019, Siti et al., 2022). For antibacterial activity, the efficacy of CHEO toward Gram-positive and Gram-negative bacteria have been reported. The greater activity of CHEO was observed against Gram-positive bacteria, especially S. aureus (Sreepian et al., 2019). The antibacterial activity of CHEO against the reference strain of MRSA has also been reported (Chao et al., 2008). However, there is still limited data on the antibacterial activity of CHEO towards clinical isolates of S. aureus, which contains a high variation of the antibiotic-resistant patterns, particularly in MDR S. aureus. This study hypothesized that CHEO could exhibit antibacterial activity toward clinical MDR MSSA and MRSA isolates. Therefore, the main objective of the present study is to evaluate the efficacy of CHEO as an alternative antibacterial agent for clinical S. aureus isolates including MRSA and MSSA, by disk diffusion and broth microdilution. The potential for synergistic interaction or additive effect of CHEO with gentamicin, a broad-spectrum antibiotic, was also investigated by checkerboard titration assay. The time-killing kinetic was also determined to quantify the growth of the bacteria in the presence of CHEO. Furthermore, cytotoxicity was evaluated on a human epidermal keratinocyte cell line by MTT assay.
2. Materials and methods
2.1. Citrus hystrix essential oil
The fresh fruit of C. hystrix DC. were collected from the northern part of Thailand, Chiang Rai Province. The plant sample was identified by the Department of Botany, Faculty of Science, Chulalongkorn University, Thailand (BCU No. 015826). The fruit peels were harvested, then processed through hydrodistillation. The percentage yield of C. hystrix essential oil (CHEO) was 1.35% (w/w) with a density of 0.9 g/mL. The chemical compositions were analyzed by Gas Chromatography-Mass Spectrometry (GC–MS) in a previous study; 3 major components composed of d-limonene (25.28%), β-pinene (21.10%), and sabinene (14.99%) (Sreepian et al., 2019). The CHEO was stored in a brown bottle at 4 °C. A working solution of CHEO was prepared at the concentration of 400 mg/mL in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) prior to MIC determination.
2.2. Bacterial strains and culture conditions
Two reference strains used in the present study composed of S. aureus ATCC 25923 (methicillin-susceptible S. aureus; MSSA) and S. aureus ATCC 43300 (methicillin-resistant S. aureus; MRSA) were received from the Faculty of Medical Technology, Rangsit University, Thailand. The 85 clinical S. aureus isolates were collected from the Division of Microbiology, Department of Central Laboratory and Blood bank, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Thailand. All bacteria were stored at −70 °C and sub-cultivated on blood agar at 37 °C overnight prior to the assay.
2.3. Antibiotic susceptibility test
Antibiotic susceptibility patterns of reference strains and clinical isolates were investigated by using Kirby-Bauer disk diffusion according to Clinical and Laboratory Standards Institute (CLSI) 2020. Totally, 12 different standard antibiotic disks (Oxoid, England) containing ampicillin (AMP, 10 µg), amoxicillin/clavulanic acid (AMC, 30 µg), penicillin (P, 10 units), chloramphenicol (C, 30 µg), cefoxitin (FOX, 30 µg), gentamicin (CN, 10 µg), erythromycin (E, 5 µg), clindamycin (DA, 2 µg), ciprofloxacin (CIP, 5 µg), levofloxacin (LEV, 5 µg), trimethoprim-sulfamethoxazole (SXT, 1.25/23.75 µg), and linezolid (LZD, 30 µg) were selected to investigate the susceptibility profile. The MIC of vancomycin (VA) was also analyzed by E-test strip (Oxoid, England). The inhibition zone diameter (IZD) and MIC value of these standard antibiotics were interpreted according to CLSI (2020). D-test was also investigated for detecting erythromycin inducible clindamycin resistance. In brief, a bacterial suspension (McFarland no. 0.5) was inoculated on Mueller Hinton agar (MHA) plate (Oxoid, England). E and DA disks were placed nearby around 20 mm and incubated at 37 °C for 16–18 h. The D-shaped zone surrounding the DA disk was defined as erythromycin inducible clindamycin resistance S. aureus.
2.4. Antibacterial activity screening for CHEO
To screen whether CHEO has in vitro antibacterial activity against clinical isolates of S. aureus, disk diffusion was performed according to Sreepian et al. (2019). In brief, a bacterial suspension (1.5 × 108 CFU/mL) was inoculated on MHA. After that, a sterile disk (6 mm in diameter) impregnated with 10 µL of undiluted CHEO was placed on MHA plate which contained inoculum bacteria. The disk impregnated with 10 µL of 4% DMSO and gentamicin disk (10 µg) were included as negative and positive controls. After incubation at 37 °C for 18–24 h, the IZDs were measured in diameter (mm) and interpreted by the following criteria described by Lv et al. (2011); no activity (IZD = 6 mm), weak activity (6 mm < IZD ≤ 12 mm), moderate activity (12 mm < IZD < 20 mm), and strong activity (IZD ≥ 20 mm).
2.5. Broth microdilution
The minimum inhibitory concentration (MIC) of CHEO and gentamicin were evaluated by resazurin-based microdilution (Elshikh et al., 2016). Briefly, 50 µL of various concentrations of CHEO were prepared by serial 2-fold dilution with cation-adjusted Mueller Hinton broth (CAMHB) to obtain the final concentrations of 0.1–32.0 mg/mL in a sterile 96-well microplate. Gentamicin was prepared with the final concentrations of 0.1–256.0 µg/mL. Afterward, 50 µL of bacterial suspension (5 × 105 CFU/mL) was added into each well. The oil control (CHEO in CAMHB), bacterial control (bacterial suspension in CAMHB) and diluent control (4% DMSO with bacterial suspension) were also included in the experiment. After incubation at 37 °C for 24 h, 5 µL of 0.015% resazurin (Sigma-Aldrich, St. Louis, MO, USA) was added into each well. The bacterial growth was measured after incubation for 2 h in the dark by the change of blue-purple to red-pink color. The MIC was defined as the lowest concentration that had no color change corresponding to no bacterial cell growth. Consequently, one loop of the MIC suspension that showed no color change was sub-cultivated on MHA and further incubated at 37 °C for 18–24 h. Then, the minimum bactericidal concentration (MBC) was defined as the lowest concentration that no bacterial growth on the agar plate. The MIC index was calculated by MBC/MIC ratio to classify the effect of antimicrobial activity. It is considered a bactericidal effect if the MIC index ≤ 4, bacteriostatic effect if MIC index > 4, and resistant effect if MIC index ≥ 32 (Gatsing et al., 2009).
2.6. Checkerboard titration assay
The checkerboard titration assay was performed to evaluate the synergistic interaction between CHEO and gentamicin against the clinical isolates of S. aureus. The method is based on the resazurin-based microdilution method with a final volume of 100 µL (Sreepian et al., 2022). Briefly, 25 µL of various concentrations of CHEO (0.3–32.0 mg/mL) were prepared by serial 2-fold dilution in 96-well microplate. Meanwhile, various concentrations of gentamicin were prepared at 0.001–128.0 µg/mL. Then, 25 µL of each concentration of gentamicin was added into each concentration of CHEO to perform checkerboard testing. Fifty microliters of bacterial suspension (5 × 105 CFU/mL) were added to each well. After incubation at 37 °C for 18–24 h, 5 µL of 0.015% resazurin was added to each well and further incubated for 2 h in the dark. The bacterial growth was measured by the color change of resazurin. The combination effect of CHEO with gentamicin was determined by calculating fractional inhibitory concentration (FIC) and fractional inhibitory concentration index (FICI) by the following equations below:
The interaction of CHEO and gentamicin was interpreted by using the following criteria: synergistic effect (FICI ≤ 0.5), additive effect (0.5 < FICI ≤ 1), indifferent effect (1 < FICI ≤ 4), and antagonistic effect (FICI > 4) (Mulyaningsih et al., 2010).
2.7. Time-killing kinetics assay
The 24-h time-killing kinetics of CHEO against S. aureus ATCC 25923 and ATCC 43300 were performed using a previously described procedure with some modifications (Xu et al., 2016). One milliliter of bacterial cell suspension (5 × 105 CFU/mL) was exposed to 1 mL of CHEO at final concentrations of 1 × MIC, 2 × MIC, and 4 × MIC, and then incubated at 37 °C. The suspension was sampling at different time points; 0–24 h. The bacterial suspensions in either CAMHB or 4% DMSO were included as bacterial and diluent controls. At a particular timepoint, the absorbance of bacterial turbidity at a wavelength of 600 nm was measured. The bacterial kinetic growth curves were constructed by the exposure time as the x-axis and the optical density as the y-axis. Consequently, the timepoint for bactericidal effect was determined by sub-cultivated with one loop of a bacterial suspension at each timepoint on MHA plate and further incubated at 37 °C for 18–24 h. After that, the bactericidal timepoint was classified as a particular time with no bacterial colony observed on MHA plate.
2.8. Cell culture and conditions
Human epidermal keratinocyte: HaCaT cell line, was maintained in Dulbecco’s Modified Eagle Medium (DMEM) with high glucose (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and penicillin (100 IU/mL)/streptomycin (100 µg/mL) (Gibco, USA). The supplemented cells were incubated at 37 °C with 5% CO2 for 24 h prior to the MTT assay. The healthy HaCaT cell line is shown in Fig. 1.
Fig. 1.
The supplemented HaCaT cell line without treatments of CHEO and d-limonene.
2.9. Cell cytotoxicity by MTT assay
The cell cytotoxicity of CHEO against human keratinocyte was evaluated by MTT assay following the previous study with some modification (López-García et al., 2014). HaCaT cells were seeded in a sterile 96-well microplate at a density of 1.8 × 104 cells/well. The cell line was incubated at 37 °C in a humidified atmosphere with 5% CO2 for 24 h prior to be treated with CHEO or limonene at 0.5–32 mg/mL. The mixture of ethyl acetate (EtOAc) and ethanol (EtOH) which is a solvent for dissolving CHEO and d-limonene, at the concentrations of 0.01–1.0% EtOAc and 0.04–5.4% EtOH, were used as a comparator. HaCaT cells in a mixture of EtOAc and EtOH was included as diluent control. After treatment for 24 h, 10 µL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL) was added into each well and further incubated at 37 °C for 4 h. After that, 200 µL of DMSO was added to each well to solubilize the water-insoluble purple formazan crystals in a living cell. The absorbance was measured at a wavelength of 540 nm by a microplate reader (Multiskan; Thermo Fisher Scientific, USA). The percentage of cell inhibition was calculated based on the untreated cell using the following equation.
Where AbsX and Abs (control) are the absorbances at a wavelength of 540 nm of HaCaT cells treated with and without either CHEO or d-limonene, respectively.
The IC50 values were analyzed using the non-linear sigmoidal curve equation in KaleidaGraph (Synergy Software, Reading, PA, USA) according to the following equation.
Where m1 is the maximum value on the y-axis, m2 is the minimum value on the y-axis, m3 is the X value at mid-point of the Y range, and m4 is the slope of the curve at the midpoint (Sopitthummakhun et al., 2021).
2.10. Statistical analysis
All experiments were performed in triplicate. Analysis of data was carried out by the descriptive statistic, Mann-Whitney U test and Chi-square test using the IBM Statistical Package for Social Services (SPSS) version 21.0 (IBM, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Antibacterial susceptibility of clinical S. Aureus
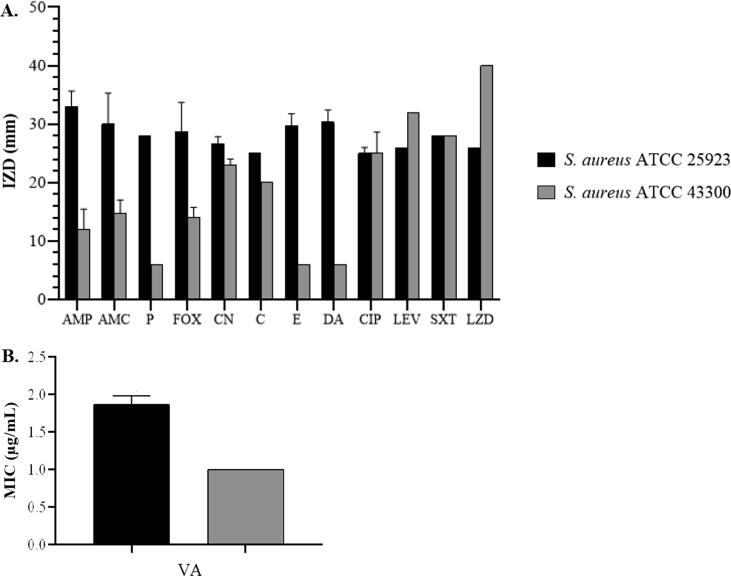
In the present study, the reference strains of S. aureus; ATCC 25923 and ATCC 43300 were included as FOX-susceptible and FOX-resistant control strains according to CLSI 2020. In Fig. 2, the results indicated that they were methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) with IZDs at 28.7 ± 5.0 mm and 14.0 ± 1.7 mm, respectively. In addition, MSSA ATCC 25923 was susceptible to all tested antibiotics including P (IZD 29.0 ± 0.0 mm), CN (IZD 25.3 ± 1.2 mm), C (IZD 25.0 ± 0.0 mm), E (IZD 29.7 ± 2.1 mm), DA (IZD 30.3 ± 2.1 mm), CIP (IZD 25.0 ± 1.0 mm), LEV (IZD 26.0 ± 0.0 mm), SXT (IZD 28.0 ± 0.0 mm), LZD (IZD 26.0 ± 0.0 mm), and VA (MIC 1.9 ± 0.1 µg/mL). Meanwhile, MRSA ATCC 43300 was susceptible to CN (IZD 22.3 ± 0.6 mm), C (IZD 20.0 ± 0.2 mm), CIP (IZD 25.0 ± 3.6 mm), LEV (IZD 32.0 ± 0.0 mm), SXT (IZD 28.0 ± 0.0 mm), LZD (IZD 40.0 ± 0.0 mm), and VA (MIC 1.0 ± 0.0 µg/mL), but no of inhibition zone was observed in P, E, and DA (IZD = 6 mm). The IZDs of AMC and AMP were also reported but there were no interpreting criteria for their susceptibility patterns. However, the IZDs for S. aureus ATCC 25923 (IZDs for AMC: 30.0 ± 5.3 mm; AMP: 33.0 ± 2.6 mm) were greater than those of S. aureus ATCC 43300 (IZDs for AMC: 14.7 ± 2.3 mm; AMP: 12.0 ± 3.5 mm). The antibiotic susceptibility pattern of S. aureus clinical isolates is displayed in Fig. 3. All isolates were further screened for methicillin resistance by FOX disk and then interpreted according to CLSI 2020. It indicated that 45 isolates were MSSA (IZD 30.0 ± 2.3 mm), and the other 40 isolates were MRSA (IZD 7.2 ± 2.4 mm) (Fig. 2A). The IZDs of AMC, AMP, and P against MSSA isolates were 25.6 ± 6.9 mm, 21.9 ± 8.6 mm, and 22.0 ± 10.4 mm, while those of MRSA isolates were 8.9 ± 3.9 mm, 8.3 ± 2.4 mm, and 8.5 ± 5.0 mm (p-value < 0.05) (Fig. 3B − 3D). The resistance rates of penicillin were 68.9% in MSSA isolates and 97.5% in MRSA isolates (Fig. 4).
Fig. 2.
Antibiotic susceptibility patterns of S. aureus ATCC 25923 and S. aureus ATCC 43300 by disk diffusion (A) and E test (B). Values are presented as mean ± SD of triplicate experiments. AMP, ampicillin; AMC, amoxicillin/clavulanic acid; P, penicillin; FOX, cefoxitin; CN, gentamicin; C, chloramphenicol; E, erythromycin; DA, clindamycin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim-sulfamethoxazole; LZD, linezolid; VA, vancomycin.
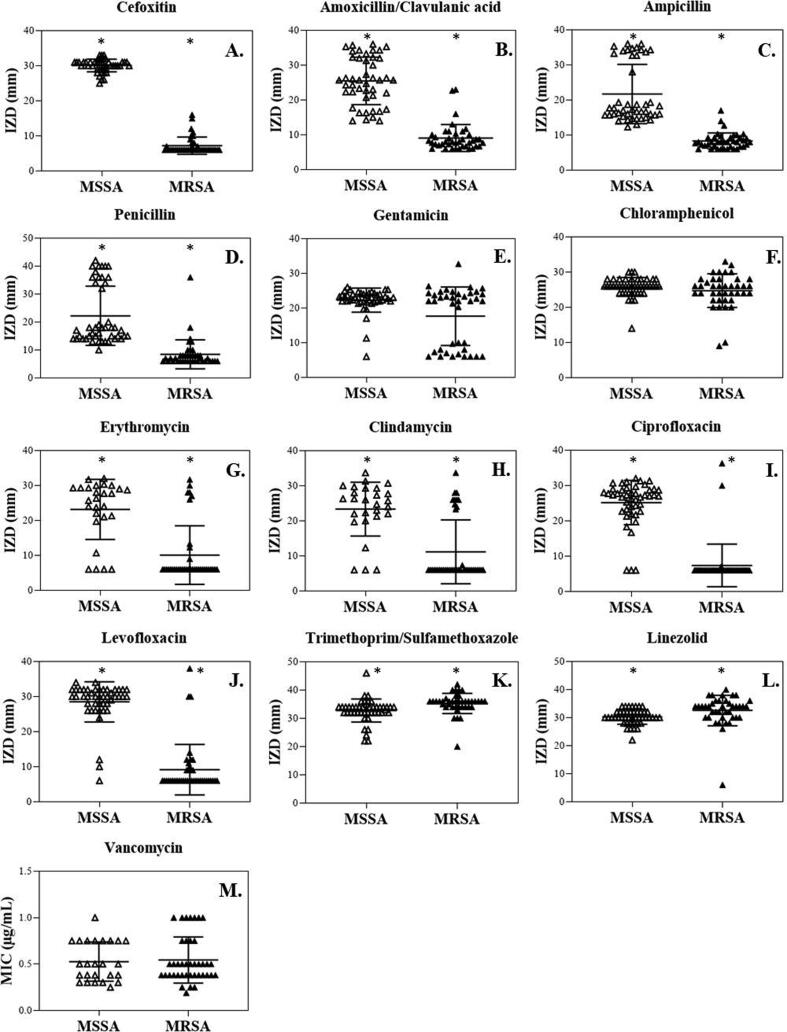
Fig. 3.
Antibiotic susceptibility patterns of 13 antibiotics against MSSA and MRSA isolates. * Significant difference in the IZDs between MSSA and MRSA isolates (p-value < 0.05). The means are displayed by the middle horizontal line, and the SD values are displayed by the lower and the upper lines.
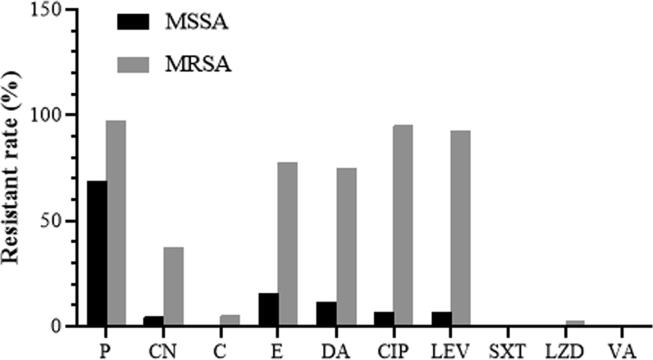
Fig. 4.
Resistant rates of MSSA and MRSA clinical isolates toward 10 standard antibiotics. P, penicillin; CN, gentamicin; C, chloramphenicol; E, erythromycin; DA, clindamycin; CIP, ciprofloxacin; LEV, levofloxacin; SXT, trimethoprim-sulfamethoxazole; LZD, linezolid; VA, vancomycin.
For antibiotics that inhibit protein synthesis, the IZDs of CN, C, E, and DA for MSSA isolates were 22.3 ± 3.5 mm, 25.8 ± 2.7 mm, 23.8 ± 8.4 mm, and 24.0 ± 7.5 mm, while those of MRSA isolates were 17.7 ± 8.5 mm, 24.9 ± 4.8 mm, 9.7 ± 7.9 mm, and 9.7 ± 8.1 mm, respectively. The results indicated that the IZDs of these standard antibiotics against MSSA were significantly higher than those of MRSA (p-value < 0.05), except for CN and C (p-value > 0.05) (Fig. 3E−H). According to CLSI 2020, among MRSA isolates, the resistance rates of CN, C, E, and DA were 37.5%, 5.0%, 77.5%, and 75.0%. Only one MRSA isolate was D-test positive indicating a D-phenotype. Among MSSA isolates, the resistance rates of CN, E, and DA were 4.4%, 15.5%, and 11.1%, but the resistance was not observed against C (Fig. 4). These results indicated a relatively low resistance rate of C among both MRSA and MSSA isolates in the present study.
For the fluoroquinolone antibiotics, the IZDs of CIP and LEV for MSSA isolates were 25.2 ± 6.3 mm and 28.6 ± 5.6 mm, while those of MRSA isolates were 7.4 ± 6.0 mm and 9.7 ± 8.2 mm, respectively (p-value < 0.05) (Fig. 3I and J). In MSSA isolates, the resistance rates of CIP and LEV were equal to 6.7%. However, the higher rates of resistance against CIP and LEV were observed in MRSA at 95.0% and 92.5%, respectively (Fig. 4).
The IZDs of SXT (an inhibitor of tetrahydrofolic acid synthesis) and LZD (an oxazolidinone antibiotic) against MSSA isolates were 32.8 ± 4.0 mm and 30.3 ± 2.6 mm, and those of MRSA isolates were 35.4 ± 3.6 mm and 32.8 ± 5.4 mm, respectively (p-value < 0.05) (Fig. 3K and L). The MICs of VA (a glycopeptide class-antibiotic) against MSSA and MRSA isolates were equal to 0.5 ± 0.2 µg/mL (p-value > 0.05) (Fig. 3M). A relatively low resistance rate against LZD (2.5%) was observed in MRSA isolates, with no resistance to SXT and VA (Fig. 4).
Overall, the IZDs of all tested antibiotics (AMC, AMP, P, E, DA, CIP, LEV, SXT, and LZD) against the MRSA isolates were significantly different from the MSSA isolates (p-value < 0.05), except for CN and C, (p-value > 0.05). In addition, the MICs of VA were not significantly different between MSSA and MRSA isolates (p-value > 0.05). These results demonstrated that the MSSA and MRSA isolates were highly susceptible to C, SXT, LZD, and VA. However, the MRSA isolates were less susceptible to several classes of antibiotics with different mechanisms of action compared to the MSSA isolates.
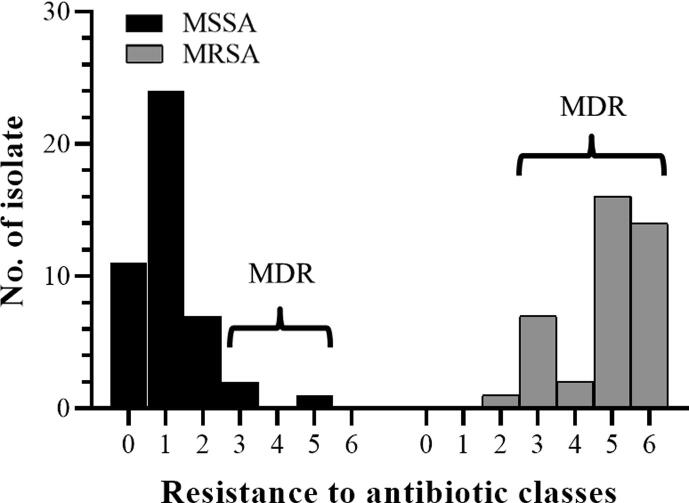
The multidrug-resistant (MDR) profile of the S. aureus isolates is shown in Fig. 5. MDR is designated as bacteria with no susceptibility to at least 1 in 3 or more antibiotic classes. It showed that 3/45 (8.9%) isolates of MSSA and 39/40 (97.5%) isolates of MRSA were MDR MSSA and MDR MRSA, respectively. This result indicated that clinical MRSA associated with MDR resisted to several classes of antibiotics (p-value < 0.05). The highest resistance rate of MDR was revealed in P (97.7%), followed by FOX (93.0%), and CIP (90.7%).
Fig. 5.
Multidrug-resistant profile of the clinical isolates; MSSA (n = 45) and MRSA (n = 40). MDR is defined as non-susceptibility to at least one agent in three or more antibiotic classes. Overall, 3 isolates of MSSA and 39 isolates of MRSA were classified as MDR.
3.2. Antibacterial activity of CHEO
The results of the antibacterial activity of CHEO against 2 reference strains and 85 clinical isolates of S. aureus are shown in Table 1. The negative control (4% DMSO) demonstrated no inhibitory effect (IZD = 6 mm), whereas the positive control (CN disk) indicated an inhibitory effect toward all tested bacteria. The results showed that CHEO exhibited moderate activity against both reference strains of S. aureus ATCC 25923 and ATCC 43300 (IZD 16.3 ± 4.0 mm and 12.3 ± 1.5 mm, respectively). Among the clinical isolates, the IZDs of CHEO were 16.0 ± 4.7 mm for MSSA isolates and 16.5 ± 4.2 mm for MRSA isolates (p-value > 0.05). It indicated that CHEO had an inhibitory effect against both MSSA and MRSA isolates, which is in accordance with a previous study (Lv et al., 2011). The inhibitory effect of CHEO on the growth of MSSA and MRSA isolates was observed in different extents; strong, moderate, and weak effectiveness. The trend of activity was most likely to be moderately effective (77.8% and 60.0%), followed by strongly effective (8.9% and 25.0%), and weakly effective (13.3% and 15.0%) against MSSA and MRSA isolates, respectively.
Table 1.
Antibacterial activity of CHEO against the reference strains and the clinical isolates of MSSA and MRSA by disk diffusion and broth microdilution.
| Bacterial strains | CHEO |
Gentamicin |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) |
MIC (mg/mL) |
MBC (mg/mL) |
MIC index | IZD (mm) |
MIC (µg/mL) |
MBC (µg/mL) |
MIC index | ||
| S. aureus ATCC 25923 (MSSA) | 16.3 ± 4.0 (M) | 6.0 ± 2.8 | 6.0 ± 2.8 | 1.0 ± 0.0 | 25.3 ± 1.2 (S) | 3.0 ± 1.4 (S) | 3.0 ± 1.4 | 1.0 ± 0.0 | |
| S. aureus ATCC 43300 (MRSA) | 12.3 ± 1.5 (M) | 8.0 ± 0.0 | 8.0 ± 0.0 | 1.0 ± 0.0 | 22.3 ± 0.6 (S) | 3.0 ± 1.4 (S) | 8.0 ± 0.0 | 3.0 ± 1.4 | |
| MSSA isolates (n = 45) | 16.0 ± 4.7 | 18.3 ± 6.1 | 19.0 ± 6.5 | 1.2 ± 0.5 | 22.3 ± 3.5 | 6.3 ± 37.9 | 15.4 ± 77.6 | 9.4 ± 23.0 | |
| MRSA isolates (n = 40) | 16.5 ± 4.2 | 17.9 ± 6.9 | 21.9 ± 6.6 | 1.4 ± 0.6 | 17.7 ± 8.5 | 46.3 ± 77.4 | 61.2 ± 101.9 | 9.4 ± 21.6 | |
| MSSA isolates | |||||||||
| Weak, n (%) | 6/45 (13.3) | ||||||||
| Moderate, n (%) | 35/45 (77.8) | ||||||||
| Strong, n (%) | 4/45 (8.9) | ||||||||
| MRSA isolates | |||||||||
| Weak. n (%) | 6/40 (15.0) | ||||||||
| Moderate, n (%) | 24/40 (60.0) | ||||||||
| Strong, n (%) | 10/40 (25.0) | ||||||||
IZD and MIC values are expressed by mean ± SD of triplicate experiments. The IZD of the CHEO was measured and interpreted following the criteria: no activity, IZD = 6 mm; weak activity, 6 mm < IZD ≤ 12 mm; moderate activity, 12 mm < IZD < 20 mm; and strong activity, IZD > 20 mm (Lv et al., 2011).
According to the resazurin-based microdilution assay, all negative controls including oil control, bacterial control, and diluent control indicated no contamination in the tested CHEO, growth ability of the tested bacteria, and no inhibitory effect according to 4% DMSO diluent, respectively. As expected, CHEO had antibacterial activity toward all tested bacteria. The MIC values of CHEO against the reference strains; MSSA ATCC 25923 and MRSA ATCC 43300 were 6.0 ± 2.8 mg/mL and 8.0 ± 0.0 mg/mL, while those of the MSSA and MRSA isolates were 18.3 ± 6.1 mg/mL and 17.9 ± 6.9 mg/mL, respectively (p-value > 0.05). The MIC indexes of CHEO for the reference strains and clinical isolates of MSSA and MRSA were 1.0–1.4, indicating a bactericidal effect in manner.
3.3. Determination of time-killing kinetics
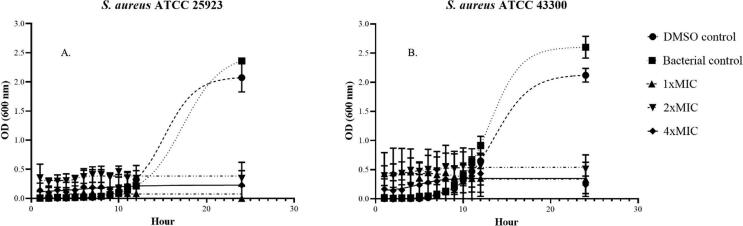
Time-killing kinetic curves were performed to evaluate the duration of CHEO to inhibit the growth of MSSA and MRSA. The growth curves of S. aureus ATCC 25923 (MSSA) and ATCC 43300 (MRSA) after exposure to CHEO are shown in Fig. 6. In the present study, the bacterial and diluent controls of both MSSA ATCC 25923 and MRSA ATCC 43300 demonstrated growth curves as S trended lines indicating normal growth of bacteria, while those bacterial suspension exposures to different MIC of CHEO tend to be a horizontal straight trended line. The bacterial growth curves in the controls and CHEO treatments had no obvious change within 5 h. However, after 6 h, increasing turbidity was observed in both bacterial and diluent controls indicating the initiation of the exponential phase of bacterial growth. Afterward, the maximum turbidity was observed after 20 h indicating the stationary phase. On the contrary, the bacterial suspension exposure to various MIC of CHEO led to no increase in bacterial turbidity within 24 h, indicating the growth of bacteria was inhibited by CHEO. The bactericidal effect of CHEO was also observed after subculture on MHA plates. MSSA ATCC 25923 was completely killed after exposure to CHEO at 1 × MIC, 2 × MIC, and 4 × MIC within 12, 5, and 1 h, while MRSA ATCC 43300 was completely killed in 12, 6, and 1 h, respectively. For bacterial and diluent controls, the bacterial growths on the MHA plate were observed in all experimental timepoints.
Fig. 6.
The time-killing kinetics of S. aureus ATCC 25923 (MSSA) (A) and S. aureus ATCC 43300 (MRSA) (B) after exposure to CHEO at 1 × MIC, 2 × MIC, and 4 × MIC from 0 to 24 h.
3.4. Synergistic interaction of CHEO in combination with gentamicin
The interaction of CHEO in combination with gentamicin against the clinical isolates of MDR MSSA and MDR MRSA was evaluated by checkerboard titration assay. As shown in Table 2, the indifferent interaction in the CHEO-gentamicin combination was found in all MDR MSSA isolates (FIC index: 1.001–1.335), and only in one isolate of MDR MRSA (FIC index: 1.031). However, no antagonistic effect was observed in both MDR MRSA and MDR MSSA isolates. Interestingly, the CHEO-gentamicin combination produced predominantly synergistic and additive interactions (FIC indexes: 0.012–0.016 and 0.531–0.625) against MDR MRSA isolates, with a substantial reduction in the MIC values of gentamicin. These findings demonstrated the synergistic and additive interactions of CHEO with gentamicin, affecting the growth inhibition of clinical MDR MRSA isolates.
Table 2.
The interaction of CHEO with gentamicin on MDR MSSA and MDR MRSA isolates by checkerboard titration method.
| Isolate No. | Compounds | MIC |
FIC | FIC index | Interaction | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| MSSA01 | CHEO | 12.0 ± 5.7 | 16.0 | 1.333 | 1.335 | Indifference |
| Gentamicin | 256.0 ± 0.0 | 0.5 | 0.002 | |||
| MSSA02 | CHEO | 16.0 ± 0.0 | 16.0 | 1.000 | 1.001 | Indifference |
| Gentamicin | 48.0 ± 22.6 | 0.063 | 0.001 | |||
| MRSA01 | CHEO | 32.0 ± 0.0 | 0.25 | 0.008 | 0.012 | Synergy |
| Gentamicin | 0.5 ± 0.0 | 0.002 | 0.004 | |||
| MRSA02 | CHEO | 32.0 ± 0.0 | 0.25 | 0.008 | 0.016 | Synergy |
| Gentamicin | 0.3 ± 0.0 | 0.002 | 0.008 | |||
| MRSA03 | CHEO | 16.0 ± 0.0 | 8.0 | 0.500 | 0.531 | Additive |
| Gentamicin | 64.0 ± 0.0 | 2.0 | 0.031 | |||
| MRSA04 | CHEO | 16.0 ± 0.0 | 8.0 | 0.500 | 0.625 | Additive |
| Gentamicin | 128.0 ± 0.0 | 16.0 | 0.125 | |||
| MRSA05 | CHEO | 8.0 ± 0.0 | 8.0 | 1.000 | 1.031 | Indifference |
| Gentamicin | 256.0 ± 0.0 | 8.0 | 0.031 | |||
Values are expressed by mean ± SD of triplicate experiments. MIC and FIC of CHEO are expressed in mg/mL whereas that of gentamicin is expressed in µg/mL.
3.5. In vitro cell cytotoxicity of CHEO and d-limonene toward human keratinocytes
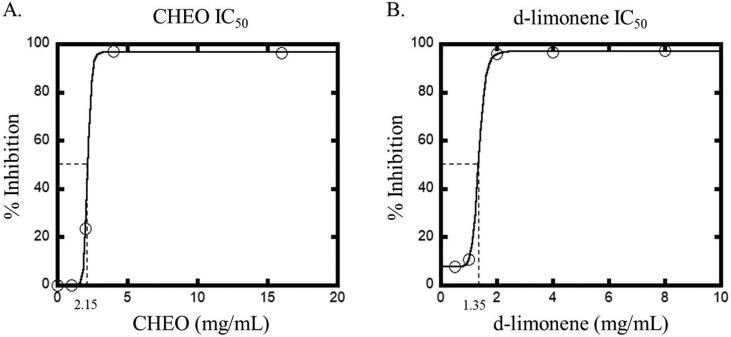
The cytotoxicity of CHEO and d-limonene, a major component in CHEO, were evaluated against HaCaT cell line by using the MTT assay. The concentrations of CHEO and d-limonene ranged from 0.5−32.0 mg/mL. As shown in Fig. 7A, the results revealed that CHEO exhibited cytotoxicity against HaCaT cells with an IC50 value of 2.15 mg/mL. However, d-limonene exhibited higher cytotoxicity with an IC50 value of 1.35 mg/mL (Fig. 7B), indicating that the cytotoxicity of CHEO might be influenced by the d-limonene compound.
Fig. 7.
In vitro cytotoxic effect on HaCaT cell line after 24 h treatment with CHEO (A) and d-limonene (B).
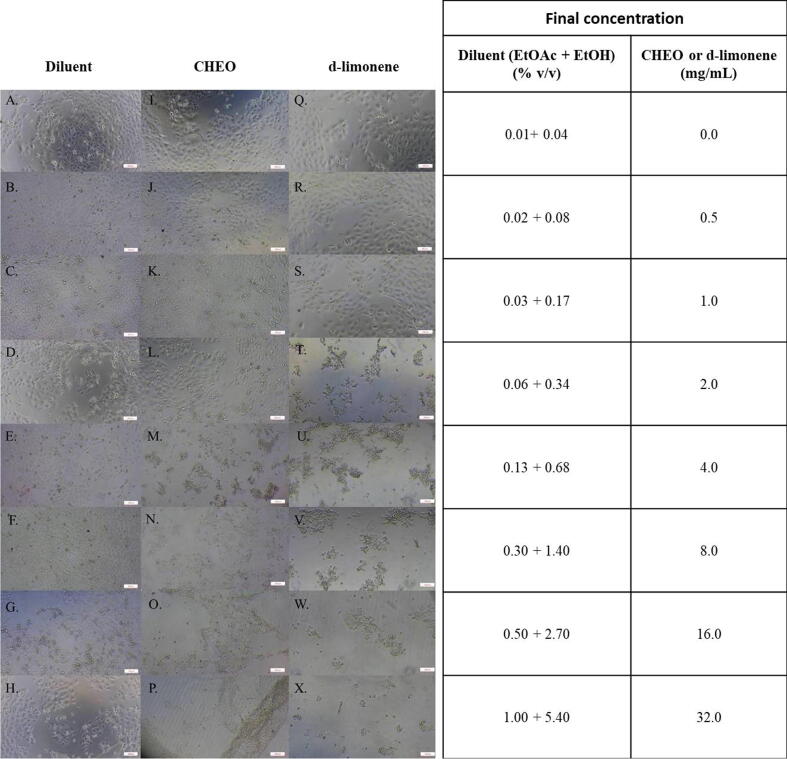
The morphology of HaCaT cells after treatment with CHEO and d-limonene was shown in Fig. 8. After 24 h treatment with CHEO and d-limonene at concentrations greater than 2 mg/mL and 1 mg/mL, respectively, the alterations in cell morphology were observed including cell debris, round cells, and floating cells in the medium (Fig. 8M−P, T−X). The HaCaT cells untreated with CHEO or d-limonene demonstrated normal fibroblastic-like shape with high confluence and adherence to a 96-well microplate (Fig. 8I and 8Q). In addition, the HaCaT cells treated with a mixture of EtOAc and EtOH, which is a diluent of CHEO and d-limonene in the experiment, also exhibited a fibroblastic-like shape (Fig. 8A−H). This result indicated that the diluent at various concentrations did not affect the morphology of the HaCaT cells.
Fig. 8.
The morphology of HaCaT cell line at 24 h post-exposure by MTT assay. A − H, HaCaT cells in the diluent. I − P, HaCaT cells exposed to CHEO at 0–32 mg/mL. Q − X, HaCaT cells exposed to d-limonene at 0–32 mg/mL.
4. Discussion
Methicillin-resistant S. aureus established resistance to several classes of antibiotics including the β-lactams and are considered to be multidrug resistant. Therefore, it is necessary to develop alternative antibacterial agents to combat these currently circulating resistant bacterial strains. C. hystrix or makrut lime, is a medicinal plant prevalent in several regions worldwide including Thailand. It is used as an ingredient in food and hair products. In addition, it also contains several biological properties, including antibacterial activity, with fewer side effects than standard antibiotics. Therefore, the present study was carried out to evaluate the antibacterial activity and the possible synergistic interaction with antibiotic drugs with CHEO extracted from its peel against S. aureus isolates from various sources of the clinical specimens using disk diffusion, microdilution, checkerboard titration, and time-killing assays. Our preliminary study of the antibacterial activity on a broad range of pathogenic bacteria demonstrated greater activity to Gram-positive bacteria, particularly S. aureus (Sreepian et al., 2019). In this study, clinical MRSA isolates of which almost all of them were MDR (97.5%) were investigated with CHEO. MRSA is a serious threat to public health due to its extensive antibiotic resistance. In addition, it is associated with an increasing resistance rate to other antibiotics such as ciprofloxacin (fluoroquinolones; DNA synthesis inhibitor). In the present study, we estimated the prevalence of S. aureus resistance to 13 different antibiotics and showed a high degree of resistance to most antibiotics. This finding is consistent with previous studies that demonstrated clinical MRSA isolates were MDR against various kinds of antibiotics (Gurung et al., 2020, Mekuriya et al., 2022). To a lower extent resistance was displayed against chloramphenicol, trimethoprim/sulfamethoxazole, and vancomycin. It has been acknowledged that vancomycin is the last resort for Gram-positive bacteria (Cheng et al., 2015). One possible mechanism of MDR MRSA might be the production of biofilm (McCarthy et al., 2015). In addition, the indiscriminate use of these antibiotics might lead to the emergence of resistant bacteria such as vancomycin-resistant S. aureus (VRSA). Under these circumstances, the utility of alternative medicinal plants for the treatment of antibiotic-resistant bacteria is being explored. Although this study found that the clinical MRSA isolates are resistant to several antibiotics, the essential oil extracted from the peel of C. hystrix has antibacterial activity against them, including MSSA isolates, in a bactericidal manner. On the basis of the results obtained from the present study, CHEO can be used as a medicinal product to fight MRSA. CHEO contains various volatile components which can be divided into 3 major groups: monoterpenes, sesquiterpenes, and their oxygenated derivatives (Nannapaneni et al., 2009). Our previous study showed that CHEO was rich in monoterpenes with the major component being d-limonene, which is in accordance with the previous study (Lin et al., 2021). The monoterpenes can be found in various citrus plants and contain anti-staphylococcal properties (Badawy et al., 2019). In addition, d-limonene has been reported to have antibacterial activity against MRSA (Sreepian et al., 2022). However, the antibacterial activity of CHEO might result from the interaction between its various components which produces a synergistic effect (Bassolé and Juliani, 2012, Cox et al., 2000).
Previously, various EOs and their components possessing various antibacterial activities showed synergistic or additive effects with antibiotics. For example, Uzair et al. (2017) exhibited the synergism of EO with some β-lactam against S. aureus. However, no studies have been reported on the synergistic effect of EO with gentamicin. In the present study, synergistic interaction between CHEO and gentamicin was observed. This indicated that CHEO could be used alone or in combination with an antibiotic against MRSA. The synergistic interaction between essential oil and standard antibiotics might be attributed to the membrane permeability facilitating the penetration of standard antibiotics and consequently their action inside the bacterial cells in a specific target such as gentamicin (Kohanski et al., 2010). Gentamicin is a bactericidal antibiotic of aminoglycoside which inhibits bacterial protein synthesis by binding to 30S ribosomal subunit while CHEO causing the loss of cell membrane permeability and eventually cell death (Filoche et al., 2005). The strategy of combining CHEO and antibiotics is a creative strategy to enhance antibacterial activity. It could be applied in various forms of products such as a topical application or oral spray to reduce the usage of antibiotics (Srifuengfung et al., 2020).
To assess biological safety, the cytotoxicity of CHEO was evaluated against human keratinocytes. HaCaT cell line is considered to be a model to assess the safety of CHEO regarding the topical application. HaCaT cells were treated with different concentrations of CHEO for 24 h and evaluated in terms of IC50 and cell morphological change. In addition, to better understand the complexity in the action of CHEO, the present study also included an evaluation of the cytotoxicity of d-limonene. Unfortunately, the results revealed that both CHEO and d-limonene had cytotoxicity on HaCaT cell line at 24 h post-exposure. This observation indicated that the cytotoxicity of CHEO might be attributed to a high content of d-limonene. In addition, it revealed that CHEO and d-limonene had cytotoxicity on HaCaT cells at the active concentration on MRSA. The previous study also reported that although d-limonene had no acute toxicity, nephrotoxic, and carcinogenic effects, it can be an irritant at concentrations of 50% or above. The acute dermal LD50 of d-limonene was greater than 5 g/kg in rabbits, while the acute oral LD50 was greater than 5 g/kg in rats (Dosoky and Setzer, 2018). Another previous study carried out an experiment in human fibroblasts, to assess the safety of CHEO for topical application on the skin (Kulig et al., 2022). It revealed that CHEO and its major component were not cytotoxic to the normal cells after prolonged exposure to 72 h. Although CHEO has a perspective as an antibacterial agent, pure CHEO might be unsafe when applied via a topical route. Therefore, further in vitro studies on the cytotoxic effect of CHEO alone and in combination with either antibiotics or bioactive compounds on various types of human cells, as well as in-depth in vivo efficacy and safety should be investigated.
5. Conclusions
This study demonstrated that CHEO exhibited a high efficacy of bactericidal activities against clinical isolates of S. aureus, particularly MDR MRSA. The synergistic and additive effects of CHEO with gentamicin, an aminoglycoside, toward clinical isolates of MDR MRSA were also revealed. These findings indicated that CHEO might be a candidate for use as an antibacterial agent against MDR MRSA. This alternative medicinal plant might reduce the indiscriminate use of antibiotics and indirectly delay the spread of multidrug resistance. Further in vitro studies on cytotoxic effect of CHEO alone and in combination with either antibiotics or bioactive compounds on various types of human cells should be investigated.
Ethical approval
This study, including the collection of clinical bacterial isolates, was approved by the Research Ethics Committee of the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Thailand (No. COE011/2019) and the Research Ethics Committee of Rangsit University, Thailand (No. RSUERB2019-027).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Faculty of Medical Technology, Rangsit University, Thailand for providing research facilities and the Division of Microbiology, Department of Central Laboratory and Blood bank, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Thailand for their facilitating in collection of the bacterial isolates in this study. Also grateful thank to Prof. Praneet Opanasopit, Silpakorn University, Thailand for providing HaCaT cell line and Dr. Brian Andrew Vesely for proofreading this manuscript. This study was funded by the Research Institute of Rangsit University, Thailand [grant number 1-2019].
References
- Aumeeruddy-Elalfi Z., Ismaël I.S., Hosenally M., Zengin G., Mahomoodally M.F. Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech. 2018;8(9):395. doi: 10.1007/s13205-018-1413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy M.E.I., Marei G.I.K., Rabea E.I., Taktak N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019;158:185–200. doi: 10.1016/j.pestbp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17(4):3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanthaphon S., Chanthachum S., Hongpattarakere T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin. J. Sci. Technol. 2008;30(Suppl. 1):125–131. [Google Scholar]
- Chao S., Young G., Oberg C., Nakaoka K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008;23:444–449. [Google Scholar]
- Cheng J., Chin W., Dong H., Xu L., Zhong G., Huang Y., Li L., Xu K., Wu M., Hedrick J.L., Yang Y.Y., Fan W. Biodegradable antimicrobial polycarbonates with in vivo efficacy against multidrug-resistant MRSA systemic infection. Adv. Healthc. Mater. 2015;4(14):2128–2136. doi: 10.1002/adhm.201500471. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), 2020. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100, 30th edition. Wayne, PA, USA.
- Cox S.D., Mann C.M., Markham J.L., Bell H.C., Gustafson J.E., Warmington J.R., Wyllie S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J. Appl. Microbiol. 2000;88(1):170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Dosoky N.S., Setzer W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018;19(7):1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., Banat I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38(6):1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoche S.K., Soma K., Sissons C.H. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral microbiol. immunol. 2005;20(4):221–225. doi: 10.1111/j.1399-302X.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- Gatsing D., Tchakoute V., Ngamga D., Kuiate J.R., Tamokou J.D.D., Nji-Nkah B.F., Tchouanguep F.M., Fodouop S.P.C. In vitro antibacterial activity of Crinum purpurascens herb leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009;34(2):126–136. [Google Scholar]
- Gurung R.R., Maharjan P., Chhetri G.G. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Future Sci. OA. 2020;6(4):FSO464. doi: 10.2144/fsoa-2019-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L., Lei Z., Li L., Xie R., Xi W., Guan Y., Sumner L.W., Zhou Z. Antifungal activity of citrus essential oils. Agric. Food Chem. 2014;62(14):3011–3033. doi: 10.1021/jf5006148. [DOI] [PubMed] [Google Scholar]
- Kamal G.M., Anwar F., Hussain A.I., Sarri N., Ashraf M.Y. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Inter. Food Res. J. 2011;18:1275–1282. [Google Scholar]
- Kantawong F., Singhatong S., Srilamay A., Boonyuen K., Mooti N., Wanachantararak P., Kuboki T. Properties of macerated herbal oil. Bioimpacts. 2017;7(1):13–23. doi: 10.15171/bi.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulig M., Galanty A., Grabowska K., Podolak I. Assessment of safety and health-benefits of Citrus hystrix DC. peel essential oil, with regard to its bioactive constituents in an in vitro model of physiological and pathological skin conditions. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113151. [DOI] [PubMed] [Google Scholar]
- Lakhundi S., Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018;31(4):e00020–e28. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. A review of Staphylococcus aureus and the emergence of drug-resistant problem. Adv Microbiol. 2018;8(1):65–76. [Google Scholar]
- Lin X., Cao S., Sun J., Lu D., Zhong B., Chun J. The chemical compositions, and antibacterial and antioxidant activities of four types of citrus essential oils. Molecules. 2021;26(11):3412. doi: 10.3390/molecules26113412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-García J., Lehocký M., Humpolíček P., Sáha P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: extent of cytotoxicity, cell viability and proliferation. J Funct Biomater. 2014;5(2):43–57. doi: 10.3390/jfb5020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv F., Liang H., Yuan Q., Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011;44(9):3057–3064. [Google Scholar]
- McCarthy H., Rudkin J.K., Black N.S., Gallagher L., O'Neill E., O'Gara J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015;5:1. doi: 10.3389/fcimb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Othman S.N.A., Hassan M.A., Nahar L., Basar N., Jamil S., Sarker S.D. Essential oils from the Malaysian citrus (Rutaceae) medicinal plants. Medicines (Basel). 2016;3(2):13. doi: 10.3390/medicines3020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekuriya E., Manilal A., Aklilu A., Woldemariam M., Hailu T., Wasihun B. Methicillin-resistant Staphylococcus aureus colonization among medicine and health science students, Arba Minch University. Ethiopia. Sci. Rep. 2022;12:10161. doi: 10.1038/s41598-022-14212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17(13):1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Nannapaneni R., Chalova V.I., Crandall P.G., Ricke S.C., Johnson M.G., O’Bryan C.A. Campylobacter and Arcobacter species sensitivity to commercial orange oil fractions. Int. J. Food Microbiol. 2009;129(1):43–49. doi: 10.1016/j.ijfoodmicro.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Siti H.N., Mohamed S., Kamisah Y. Potential therapeutic effects of Citrus hystrix DC and its bioactive compounds on metabolic disorders. Pharmaceuticals. 2022;15(2):167. doi: 10.3390/ph15020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopitthummakhun K., Rattanasinganchan P., Nimmanee P., Paungmoung P., Moonthiya P., Thitapakorn V. Antioxidant capacity, antibacterial activity, and cell cytotoxicity in cholangiocarcinoma (CCA) from Boesenbergia rotunda (L.) Mansf. Asia Pac. J. Sci. Technol. 2021;26(02):6. doi: 10.14456/apst.2021.16. Article ID: APST-26-02-06. [DOI] [Google Scholar]
- Sreepian A., Sreepian P.M., Chanthong C., Mingkhwancheep T., Prathit P. Antibacterial activity of essential oil extracted from Citrus hystrix (kaffir lime) peels: An in vitro study. Trop Biomed. 2019;36(2):531–541. [PubMed] [Google Scholar]
- Sreepian A., Popruk S., Nutalai D., Phutthanu C., Sreepian P.M. Antibacterial activities and synergistic interaction of citrus essential oils and limonene with gentamicin against clinically isolated methicillin-resistant Staphylococcus aureus. Sci. World J. 2022;2022:8418287. doi: 10.1155/2022/8418287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srifuengfung S., Bunyapraphatsara N., Satitpatipan V., Tribuddharat C., Junyaprasert V.B., Tungrugsasut W., Srisukh V. Antibacterial oral sprays from kaffir lime (Citrus hystrix DC.) fruit peel oil and leaf oil and their activities against respiratory tract pathogens. J. Tradit. Complement. Med. 2020;10(6):594–598. doi: 10.1016/j.jtcme.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G., Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzair B., Niaz N., Bano A., Khan B.A., Zafar N., Iqbal M., Tahira R., Fasim F. Essential oils showing in vitro anti MRSA and synergistic activity with penicillin group of antibiotics. Pak. J. Pharm. Sci. 2017;30(5(Supplementary)):1997–2002. [PubMed] [Google Scholar]
- Wongsariya K., Phanthong P., Bunyapraphatsara N., Srisukh V., Chomnawang M.T. Synergistic interaction and mode of action of Citrus hystrix essential oil against bacteria causing periodontal diseases. Pharm. Biol. 2014;52(3):273–280. doi: 10.3109/13880209.2013.833948. [DOI] [PubMed] [Google Scholar]
- Xu J.G., Liu T., Hu Q.P., Cao X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules. 2016;21(9):1194. doi: 10.3390/molecules21091194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Zhang H., Tian G., Ren W., Li J., Xiao H., Zheng J. Effects of molecular distillation on the chemical components, cleaning, and antibacterial abilities of four different citrus oils. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.731724. [DOI] [PMC free article] [PubMed] [Google Scholar]