Abstract
Background:
The use of ketamine, a controlled dissociative anesthetic, has become more widespread in recent years with recreational/nonmedical use increasing and ketamine becoming more widely available in clinics to treat depression.
Aims:
We examined recent trends in adverse effects related to ketamine use.
Methods:
US National Poison Control data were examined, focusing on ketamine exposures among those aged ⩾13 between 2019 and 2021 (n = 758). We examined quarterly trends in exposure and delineated correlates of patients experiencing a major adverse effect or death.
Results:
The number of reported exposures increased 81.1% from 2019 Quarter 1 through 2021 Quarter 4, from 37 to 67 (p = 0.018). The majority of patients were male (57.1%), and the plurality of cases involved intentional misuse or “abuse” (39.5%), followed by suspected suicide attempt (19.7%) and unintentional exposure (18.9%). A fifth (19.6%) experienced a major adverse effect or death. A third (33.4%) co-used other drugs; the drugs most commonly co-used were benzodiazepines (14.6%), alcohol (10.3%), and opioids (8.7%). Co-use of gamma-hydroxybutyrate (GHB; adjusted prevalence ratio (aPR) = 3.43, 95% confidence interval (CI): 1.57–7.46) and opioids (aPR = 2.44, 95% CI: 1.46–4.08) was associated with increased risk for a major adverse effect or death, as was injection-only administration (aPR = 2.68, 95% CI: 1.21–5.92).
Conclusions:
Although still rare, poisonings involving ketamine have increased in recent years. Polydrug use—particularly with opioids or GHB—appears to be a particular risk factor for more serious adverse effects. As prevalence of use increases, it is important to monitor adverse effects and co-occurring behaviors to inform timely prevention and harm reduction as needed.
Keywords: Ketamine, poisonings, polydrug use
Introduction
Ketamine, a dissociative drug with anesthetic, analgesic, and hallucinogenic properties, has an established history of both medical and nonmedical use. In addition to this NMDA receptor antagonist’s use as an anesthetic in human and veterinary medicine for half a century (Kohtala, 2021), ketamine has also been prevalent as a recreational drug in nightclub settings for decades (Halkitis et al., 2007; Palamar & Keyes, 2020). In recent years, ketamine use has become more widespread in the United States, which can be attributed, in part, to increasing availability of ketamine in both clinical and non-clinical settings and shifting prevalence of nonmedical use. Decades of research into ketamine’s rapid antidepressant properties led to the US Food and Drug Administration’s (US FDA, 2019) approval of the use of esketamine nasal spray for treatment-resistant depression in 2019, which has subsequently led to increased availability of ketamine in psychiatric treatment settings. Rates of ketamine seizures by US law enforcement also increased significantly from 2012 to 2019, suggesting increased availability outside clinical settings (Palamar et al., 2021). Along with indicators of availability, epidemiological research has shown that, while prevalence of non-medical use in the general US population has remained low, there was a quarterly increase in estimated past-year ketamine use from 2006 to 2019, which reached a peak of 0.9% in late 2019 (Palamar et al., 2021). Of note is the increasing prevalence of ketamine use among nightclub and dance festival attendees, which rose from 5.9% in 2016 to 15.3% in 2019 in a New York City sample (Palamar & Keyes, 2020).
While ketamine has a wide safety margin and is generally considered a less risky drug than various other types of drugs commonly used recreationally (Gable, 2004; Morgan et al., 2010; Nutt et al., 2010), both acute and chronic adverse effects associated with use have been described (Corkery et al., 2021; Fitzgerald et al., 2021), which appear to vary depending on dosage and frequency of use, co-use of ketamine with other substances, and interactions between the person using ketamine and the setting of use (Dillon et al., 2003). A recent systematic review found that most serious adverse effects, such as cognitive impairment, urinary cystitis, other urinary tract issues, and upper gastrointestinal problems (“K cramps”), were associated with chronic and “heavy” ketamine use, with several studies reporting a dose-effect relationship between duration of ketamine use and severity of adverse events (Van Amsterdam & Van Den Brink, 2022). Other studies have suggested that ketamine polysubstance use may be associated with adverse effects. For example, in a study of nightclub and festival attendees which found that nearly one-fifth (19.3%) of those who used ketamine in the past year experienced a harmful or very unpleasant effect, over half (56.3%) of those instances reportedly involved the use of other drugs—a quarter (25.0%) co-using ketamine with alcohol, 18.8% co-using with cocaine, and 18.8% co-using with ecstasy (Palamar et al., 2019). Impaired awareness or perception due to ketamine intoxication as well as the drug’s effects on coordination can also lead to a higher risk of acute physical harm and fatal accidents (Morgan et al., 2012).
Given the recent increases in both medical and nonmedical ketamine use and the potential for associated adverse effects, it is important for research to examine trends and co-occurring behaviors related to ketamine use and outcomes to inform prevention, intervention, and harm reduction efforts. Although ketamine-related deaths have been found to be relatively rare (Corkery et al., 2021; Darke et al., 2021; Dillon et al., 2003), the Centers for Disease Control and Prevention National Vital Statistics System (NVSS), which is the primary source of information on drug-related mortality in the United States, does not report on ketamine. One source of information which allows us to monitor trends in adverse effects related to ketamine use in the United States is the National Poison Control database. Unlike other national data sources such as NVSS, Poison Control data are uploaded in almost real time, including circumstances of exposure, and nonfatal overdose events, which may more effectively capture ketamine-related events. Previous studies have used US Poison Control data to examine national trends in ketamine poisonings (“exposures”) from 2000 to 2015 (Ni et al., 2018) and from 1991 to 2019 (Palamar et al., 2021); however, trends in exposures related to ketamine in more recent years following its FDA approval are unknown. In this analysis, we first examine trends in ketamine exposures (quarterly) from 2019 to 2021 and we then examine correlates of experiencing major (severe) adverse effects or death.
Methods
Procedure
Poison Control data were obtained through a collaboration between the National Institute on Drug Abuse National Drug Early Warning System (Cottler et al., 2020), and the Researched Abuse Diversion and Addiction-Related Surveillance (RADARS) System Poison Center Program. Participating Poison Control Centers (PCCs) provided cases involving pre-identified Micromedex codes to RADARS System staff who then reviewed the cases for accuracy by examining the case notes. PCCs provide treatment advice to the public and to healthcare staff treating individuals with suspected poisonings involving drugs, chemicals, and plants. Information about the patient and circumstances of the exposure are recorded by individual PCCs as per standards set by America’s Poison Center (APC) and stored in a database overseen by the National Poison Data System. Information is provided by the patient, healthcare provider, or other contact. RADARS System obtained data on ketamine poisonings reported between January 2019 and December 2021. Data were available from PCCs in all US states other than North Carolina (with coverage from 51 of the 55 US PCCs). The inclusion criteria of this analysis were that (1) cases reportedly involved a ketamine exposure (cases involving generic code 13800) and (2) cases were 13 years of age or older. As such, we identified 758 cases age ⩾13 (out of 799).
Variables
PCC staff collected data provided by the caller to the poison center on patient age and sex. With respect to characteristics of exposures, information was obtained regarding the reason or intention for exposure, whether other drugs were co-used, the route(s) of administration, and severity of the outcome. Reasons for use included intentional “abuse,” misuse, unintentional exposure (i.e., occupational, therapeutic error, unintentional unknown), suspected suicide (which may or may not have resulted in death), intentional use but unknown reason, adverse reaction, and other categories of exposure that were collapsed into other reason. “Abuse” is defined by APC as exposure resulting from intentional improper or incorrect use of a drug in which the patient was attempting to acquire a high, euphoric effect, or another psychotropic effect (Zosel et al., 2013). Misuse is defined as intentional improper or incorrect, or otherwise nonmedical use but for reasons other than acquiring a psychotropic effect. We combined “abuse” and misuse into a single category (Calcaterra et al., 2018). Information on reasons for use was collected by specialists in poison information (SPIs) from PCC contacts, who are instructed to determine whether exposures were due to intentional or unintentional actions based on coding guidelines provided by the APC. SPIs record the rationale for the selection of reason for use in cases notes.
Routes of administration included reported ingestion, injection, dermal administration, inhalation, and other method. Patients were able to report multiple routes. Based on past research (Palamar et al., 2016; Warrick et al., 2013), we recoded a variable indicating inhalation only, injection only, and ingestion only versus other routes or combinations of routes. Polydrug use was also queried, and we focused on co-use of alcohol, cannabis, cocaine, benzodiazepines, opioids (prescription opioids as well as heroin and fentanyl), gamma-hydroxybutyrate (GHB), methamphetamine, amphetamine, and other phenethylamines (e.g., ecstasy/ 3,4-methylenedioxymethamphetamine [MDMA], newer stimulant psychoactive drugs). Of note, benzodiazepines are drugs commonly used to treat anxiety, and methamphetamine, amphetamine, and other phenethylamines are stimulants. Drug use was based on self-report, although toxicology test results were considered when available.
Medical outcome was coded by PCC staff as none, mild, moderate, major, or death (Gummin et al., 2020). Exposure cases are followed by PCCs as appropriate to obtain the most precise medical outcome possible; while most cases are “closed” shortly after the initial contact, more medically complicated cases or cases involving death can remain open for months, in which data are continually collected (Gummin et al., 2020). Mild effects are defined as minimally bothersome effects, moderate effects are more pronounced or prolonged effects, and major effects are life threatening or permanently disabling effects. Deaths indicate that the patient was confirmed to have died in relation to use of the drug, which was either determined directly by PCC staff who were involved with case management or from death reports were obtained from a medical examiner or another source without the involvement of PCC staff. In cases which involved death reports obtained from another source, an APC faculty review team then judged whether the reported exposure was likely responsible or at least contributory to the death (Gummin et al., 2020).
Analyses
We first examined trends in the number of reported ketamine exposures by year/quarter using Joinpoint Regression version 4.8.0.1 (National Cancer Institute, 2020). Also known as piecewise, multi-phase, broken line, or segmented regression, Joinpoint fits weighted least-square regression models to counts on a log-transformed scale (Ingram et al., 2018; Kim et al., 2000). It also uses Monte Carlo permutation tests with a Bonferroni correction for multiple testing and further identifies models with the best fit set of joinpoints (we specified for a maximum of three). We specified Poisson models under the assumption of non-constant variance or heterogeneity over time. We then examined aggregate data to describe the prevalence of characteristics of exposures. This was done in a univariable manner, and then we examined bivariable and multivariable correlates of exposures resulting in a major effect or death. Chi-square and Fisher’s exact test were used to determine bivariable differences between each independent variable and whether the exposure resulted in a major effect or death or a less severe effect. Covariates were then fit into a multivariable generalized linear model using Poisson distribution and log link. This model allowed us to estimate adjusted prevalence ratios (aPRs) for each covariate. We imputed missing data for independent variables in the multivariable model. Multiple imputation was implemented using chained equations to handle missingness; predictors included variables in the case-complete model. In all, 10 datasets were imputed for the multivariable model and combined results (Rubin, 1987). All analyses other than trend analyses were conducted using Stata SE 17 (StataCorp, College Station, TX). This secondary analysis was exempt from review by New York University Langone Medical Center’s institutional review board.
Results
As shown in Figure 1, the number of reported ketamine exposures increased 81.1% from 2019 Quarter 1 through 2021 Quarter 4, from 37 to 67 (β = 0.05, standard error = 0.02, p = .018). This was an overall linear increase with no detected joinpoints. Table 1 presents the characteristics of the sample. The majority of patients with ketamine exposure were male (57.1%), and the plurality of cases involved intentional “abuse” or misuse (39.5%). This was followed by suspected suicide (19.7%), unintentional exposure (18.9%), adverse drug reaction (10.6%), unknown intentional exposure (4.5%), and 7.0% noted other reasons. A third (33.4%) reported co-use of other drugs. Among those reporting polydrug use, 60.5% reported use of one additional drug; 24.1%, 11.5%, 3.6%, and 0.4% reported the use of an additional two, three, four, and five drugs, respectively. The drugs most commonly co-used were benzodiazepines (14.6%), alcohol (10.3%), and opioids (8.7%). With respect to route of administration, 44.3% only ingested ketamine, 18.8% only injected, 17.6% only inhaled, and 19.3% used via another route or a combination of routes. The majority (85.3%) used via one route, and 14.7% used via multiple routes. Regarding medical outcome, of cases followed with a final determination of effect, 11.8% reported no effect, 25.8% reported a minor effect, 42.8% reported a moderate effect, 18.4% reported a major effect, and 1.2% had a reported death. As such, a fifth (19.6%) of cases followed experienced a major adverse effect or death.
Figure 1.
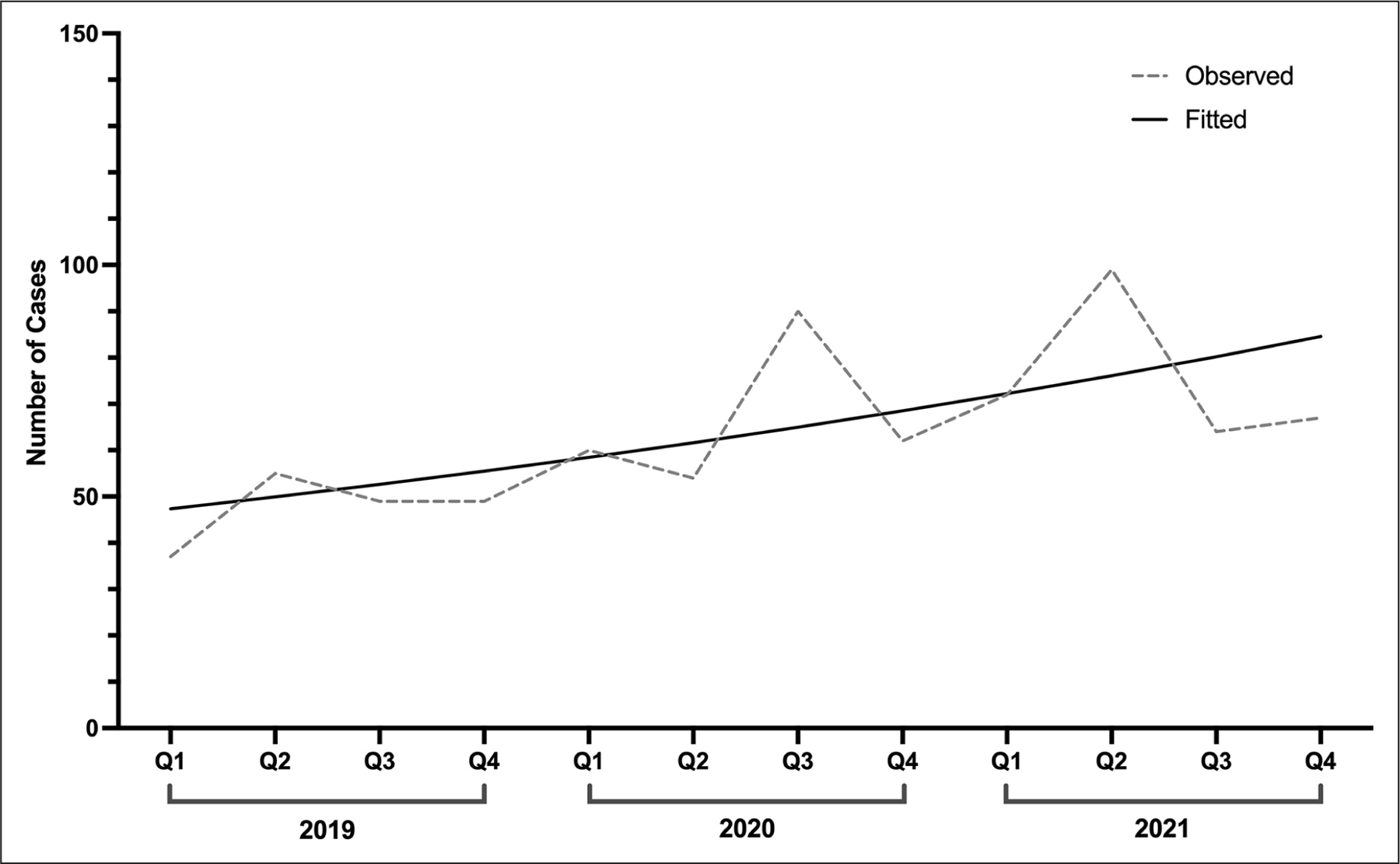
Quarterly trend in ketamine exposures in the United States, 2019–2021.
Table 1.
Characteristics of cases involving ketamine exposure (n = 758).
| n (%) | |
|---|---|
| Age | |
| 13–19 | 104 (14.8) |
| 20–29 | 247 (35.2) |
| 30–39 | 186 (26.5) |
| ⩾40 | 165 (23.5) |
| Sex | |
| Male | 420 (57.1) |
| Female | |
| Reason | |
| Intentional misuse or abuse | 299 (39.5) |
| Suspected suicide attempt | 149 (19.7) |
| Unintentional exposure | 143 (18.9) |
| Adverse reaction | 80 (10.6) |
| Intentional unknown | 34 (4.5) |
| Other reason | 53 (7.0) |
| Co-drug use | |
| Any polydrug use | 253 (33.4) |
| Benzodiazepines | 111 (14.6) |
| Alcohol | 78 (10.3) |
| Opioids | 66 (8.7) |
| Cocaine | 43 (5.7) |
| Cannabis | 37 (4.9) |
| Amphetamine | 18 (2.4) |
| Methamphetamine | 12 (1.6) |
| Other phenethylamines | 26 (3.4) |
| GHB | 12 (1.6) |
| Route of administration | |
| Ingestion only | 290 (44.3) |
| Injection only | 123 (18.8) |
| Inhalation only | 115 (17.6) |
| Other | 126 (19.3) |
| Medical outcome | |
| No effect | 69 (11.8) |
| Minor effect | 151 (25.8) |
| Moderate effect | 251 (42.8) |
| Major effect | 108 (18.4) |
| Death | 7 (1.2) |
Note. Percentages reflect case-complete data. Other route of administration consists of dermal or other route or routes in combination with inhalation, injection, or ingestion.
GHB: gamma-hydroxybutyrate.
Table 2 presents the correlates of patients experiencing a major adverse effect or death. Bivariable test results suggest that prevalence of experiencing a major adverse effect or death was higher among those reporting co-use of GHB (7.0% vs. 0.6%, p < 0.001) or opioids (20.0% vs. 7.6%, p < 0.001). There was also a detected significant difference regarding reason for use (p = 0.046) and route of administration (p = 0.002). In the multivariable model, co-use of GHB (aPR = 3.43, 95% confidence interval (CI: 1.57–7.46) and opioids (aPR = 2.44, 95% CI: 1.46–4.08) was associated with increased risk for experiencing a major adverse effect or death, as was injection-only route of administration (aPR = 2.68, 95% CI: 1.21–5.92).
Table 2.
Bivariable and multivariable correlates of major adverse effect or death.
| Less than major effect n (%) | Major effect or death n (%) | aPR (95% CI) | |
|---|---|---|---|
| Age | |||
| 13–19 | 71 (16.1) | 12 (10.4) | Ref |
| 20–29 | 149 (33.7) | 40 (34.8) | 1.30 (0.68–2.48) |
| 30–39 | 118 (26.7) | 29 (25.2) | 1.21 (0.62–2.38) |
| ⩾40 | 104 (23.5) | 34 (29.6) | 1.49 (0.75–2.99) |
| Sex | |||
| Male | 260 (57.5) | 77 (67.0) | Ref |
| Female | 192 (42.5) | 38 (33.0) | 0.72 (0.47–1.11) |
| Reason | |||
| Intentional misuse or abuse | 193 (41.0) | 50 (43.5)a | Ref |
| Suspected suicide attempt | 106 (22.5) | 30 (26.1) | 1.01 (0.63–1.63) |
| Unintentional exposure | 89 (18.9) | 11 (9.6) | 0.58 (0.29–1.16) |
| Adverse reaction | 40 (8.5) | 8 (7.0) | 0.67 (0.31–1.46) |
| Intentional unknown | 19 (4.0) | 11 (9.6) | 1.73 (0.92–3.25) |
| Other reason | 24 (5.1) | 5 (4.4) | 0.96 (0.38–2.39) |
| Co-drug use | |||
| Benzodiazepines | 81 (17.2) | 19 (16.5) | 0.70 (0.41–1.19) |
| Alcohol | 49 (10.4) | 16 (13.9) | 1.12 (0.62–2.03) |
| Opioids | 36 (7.6) | 23 (20.0)c | 2.44 (1.46–4.08)b |
| Cocaine | 24 (5.1) | 11 (9.6) | 1.43 (0.69–2.96) |
| Cannabis | 25 (5.3) | 7 (6.1) | 1.20 (0.53–2.69) |
| Amphetamine | 11 (2.3) | 4 (3.5) | 1.18 (0.43–3.20) |
| Methamphetamine | 9 (1.9) | 3 (2.6) | 1.88 (0.55–6.50) |
| Other phenethylamines | 18 (3.8) | 3 (2.6) | 0.59 (0.16–2.08) |
| GHB | 3 (0.6) | 8 (7.0)c | 3.43 (1.57–7.46)b |
| Route of administration | |||
| Other | 84 (20.2) | 12 (11.5)b | Ref |
| Ingestion only | 186 (44.7) | 59 (56.7) | 1.67 (0.83–3.35) |
| Injection only | 64 (15.4) | 24 (23.1) | 2.68 (1.21–5.92)a |
| Inhalation only | 82 (19.7) | 9 (8.7) | 0.87 (0.30–2.48) |
Note. Other route of administration consists of dermal or other route or routes in combination with inhalation, injection, or ingestion. Bivariable tests are based on case-complete data and the multivariable model is based on imputed data. The multivariable model controlled for year.
aPR: adjusted prevalence ratio; CI: confidence interval; GHB: gamma-hydroxybutyrate.
p < 0.05,
p < 0.01,
p < 0.001.
Discussion
In this analysis of fatal and nonfatal ketamine-related exposures reported to US PCCs, we found that, although ketamine exposures are still rare, there were significant increases in reported exposures from early 2019 through late 2021. Previous epidemiological research has estimated that rates of ketamine exposures in the United States increased in a cubic manner from 1991 through 2019, with an increase from 1991 through 2000, followed by a dip through 2008, and an increase through 2014, with use remaining stable through 2019 (Palamar et al., 2021). Our timelier quarterly analysis of trends from 2019 through 2021 indicates that exposures increased between early 2019 and late 2021, suggesting another uptick in ketamine use.
This study also delineated correlates of ketamine exposures involving major adverse effects or death. The co-use of ketamine with GHB or opioids was found to be a risk factor for more severe adverse outcomes. The use of ketamine with central nervous system depressants such as opioids increases the risk of complications like respiratory depression (Corkery et al., 2021; Wolff & Winstock, 2006), and opioids have been one of the most prevalently detected other drugs in ketamine-involved deaths (Corkery et al., 2021; Darke et al., 2021). GHB, like ketamine, is a popular club drug or party drug, and is also commonly used in nightclub settings (Halkitis et al., 2007; Palamar & Keyes, 2020). Importantly, both GHB and ketamine can be considered as central nervous system-sedating drugs, and inhibition of respiratory rates resulting from co-use could result in hippocampal hypoxia (Van Amsterdam et al., 2012). One study found that the risk of hospital treatment for GHB was almost three times higher when GHB was co-ingested with ketamine (Kim et al., 2007). Thus, though ketamine alone may be a less risky drug, these findings indicate that ketamine use becomes riskier when used concomitantly with other substances, specifically those which also increase the risk for respiratory depression.
Although co-use of other drugs with ketamine was not linked to increased risk of more severe effects in this study, combining other drugs with ketamine can still place individuals who use at risk. For example, a recent study found that a quarter (25.0%) of adverse effects experienced after using ketamine were tied to alcohol co-use (Palamar et al., 2019). Adverse effects were more common with alcohol co-use as compared to co-use of cocaine (18.8%) and ecstasy (18.8%). In nightclub scenes, amphetamines have been commonly combined with ketamine to balance out the effects of each drug (Degenhardt & Topp, 2003). While it is not fully known whether use of stimulants can ameliorate adverse effects from ketamine, we need to keep in mind that while drugs such as opioids, cocaine, amphetamines, and GHB have a higher risk for acute toxicity and addiction than ketamine (Gable, 1993; Morgan et al., 2010; Nutt et al., 2007), co-use with ketamine may have potential to increase such risk.
While prevalence of nasal and injection use was comparable in this sample, administration of ketamine through injection was associated with increased risk for experiencing a major adverse effect or death. Ketamine is typically obtained in a powder form and administered through snorting or inhalation when used nonmedically, while intravenous use has been found to be relatively rare (Morgan et al., 2012), even among persons who inject other drugs (Lankenau et al., 2010). Nonmedical use involving injection could thus be seen as an indicator of higher severity of use, and thus, this might have been why injection use was more prevalent in this sample of people reporting poisonings. Given that intravenous administration involves a more rapid duration and onset of ketamine (Corkery et al., 2021), and rapid administration can result in complications such as impairment of pharyngeal and laryngeal reflex, diaphragm rigidity, and/or transient respiratory depression (Darke et al., 2021), it is possible that injection may act as a risk factor primarily though the user’s lessened ability to titrate ketamine use. One study of ketamine-related deaths in Australia found that over 40% of decedents had a history of injection drug use, with over a third of deaths involving intravenous self-administration of ketamine (Darke et al., 2021).
While injection ketamine use was found to be a risk factor for more severe adverse effects, other routes of administration are not without risk. All routes can be safe given the correct dose and context of use (Kronenberg, 2002), but injection has the fastest drug effect with 100% bioavailability (Li & Vlisides, 2016), which can make use (e.g., recreational use) of large doses particularly dangerous. Intramuscular effects are slower (with 93% bioavailability), followed by intranasal (with 8–45% bioavailability) and oral effects (with 17–29% availability) (Li & Vlisides, 2016). Ketamine inhaled in powder form has a good safety profile in clinical settings (Matłoka et al., 2022), but given that most recreational use (e.g., in nightclubs) appears to be use in powder form, such nasal use can increase the risk of adverse effects given the contexts of use and higher likelihood of people using a less pure product (He et al., 2020; Palamar et al., 2019). The US FDA has also reported concerns about some nasal sprays (which can be obtained now in clinics), particularly take-home nasal sprays, as these appear to increase risk for misuse, “abuse,” and adverse effects (US FDA, 2022). Oral doses can be even safer than other products as effects are delayed, but when a full dose (e.g., pill) is administered, little can be done to prevent drug effect, as opposed to other routes in which doses can be more easily titrated (Andrade, 2019). Injection, though, appears to be the riskier mode of administration with regard to severity of adverse effects so prevention and harm reduction efforts should target this route of use in particular.
There are limitations to this study. Since calls to PCCs are based on a patient, medical professional, or other party calling to report an exposure or to ask for medical advice for treatment, these data are not generalizable to all ketamine poisonings. Likewise, these data are also not generalizable to ketamine use in the general population, as cases mostly involve adverse effects and reporting to PCCs is voluntary. However, these data can be useful in complementing other sources of national data on prevalence of ketamine use and ketamine-related mortality to monitor trends in adverse effects associated with use. Poison Control data are based on the caller or other contact’s reporting, which may or may not include the patient and could depend on second-hand information in some cases. In addition, toxicology testing was not always conducted to confirm ketamine exposures. Other studies, for example, have found that unintentional or underreported exposure to ketamine is common among nightclub attendees (Palamar et al., 2021).
In conclusion, this analysis of RADARS System Poison Center data suggests that, although still rare, poisonings involving ketamine have increased in recent years, with polysubstance use—particularly co-use with opioids or GHB—and injection acting as significant risk factors for more serious adverse effects. As prevalence of both medical and nonmedical use of ketamine increases in the United States, we believe these findings can be used to help monitor adverse effects associated with ketamine exposures and to inform more timely prevention and harm reduction efforts.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers U01DA051126, R01DA044207, and T32DA035167. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Palamar has consulted for Alkermes. The authors have no other potential conflicts to declare.
References
- Andrade C (2019) Oral ketamine for depression, 2: Practical considerations. J Clin Psychiatry 80: 19f12838. DOI: 10.4088/JCP.19f12838. [DOI] [PubMed] [Google Scholar]
- Calcaterra SL, Severtson SG, Bau GE, et al. (2018). Trends in intentional abuse or misuse of benzodiazepines and opioid analgesics and the associated mortality reported to poison centers across the United States from 2000 to 2014. Clin Toxicol 56: 1107–1114. DOI: 10.1080/15563650.2018.1457792. [DOI] [PubMed] [Google Scholar]
- Corkery JM, Hung W-C, Claridge H, et al. (2021) Recreational ketamine-related deaths notified to the national programme on substance abuse deaths, England, 1997–2019. J Psychopharmacol 35: 1324–1348. DOI: 10.1177/02698811211021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Goldberger BA, Nixon SJ, et al. (2020) Introducing NIDA’s new national drug early warning system. Drug Alcohol Depend 217: 108286. DOI: 10.1016/j.drugalcdep.2020.108286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Duflou J, Farrell M, et al. (2021) Characteristics and circumstances of death related to the self-administration of ketamine. Addiction 116: 339–345. DOI: 10.1111/add.15154. [DOI] [PubMed] [Google Scholar]
- Degenhardt L and Topp L (2003) ‘Crystal meth’ use among polydrug users in Sydney’s dance party subculture: Characteristics, use patterns and associated harms. Int J Drug Policy 14: 17–24. DOI: 10.1016/S0955-3959(02)00200-1. [DOI] [Google Scholar]
- Dillon P, Copeland J and Jansen K (2003) Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend 69: 23–28. DOI: 10.1016/S0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ND, Striley CW, Palamar JJ, et al. (2021) Test-retest reliability and cross-cultural applicability of DSM-5 adopted diagnostic criteria for ketamine use disorders. Drug Alcohol Depend 228: 109056. DOI: 10.1016/j.drugalcdep.2021.109056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable RS (1993) Toward a comparative overview of dependence potential and acute toxicity of psychoactive substances used nonmedically. Am J Drug Alcohol Abuse 19: 263–281. [DOI] [PubMed] [Google Scholar]
- Gable RS (2004) Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99: 686–696. DOI: 10.1111/j.1360-0443.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Gummin DD, Mowry JB, Beuhler MC, et al. (2020) 2019 Annual report of the American association of poison control centers’ national poison data system (NPDS): 37th annual report. Clin Toxicol 58: 1360–1541. DOI: 10.1080/15563650.2020.1834219. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Palamar JJ and Mukherjee PP (2007) Poly-club-drug use among gay and bisexual men: A longitudinal analysis. Drug Alcohol Depend 89: 153–160. DOI: 10.1016/j.drugalcdep.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Wang J, You X, et al. (2020). Classification of heroin, methamphetamine, ketamine and their additives by attenuated total reflection-Fourier transform infrared spectroscopy and chemometrics. Spectrochim Acta A Mol Biomol Spectrosc 241: 118665. DOI: 10.1016/j.saa.2020.118665. [DOI] [PubMed] [Google Scholar]
- Ingram DD, Malec DJ, Makuc DM, et al. (2018) National center for health statistics guidelines for analysis of trends. Vital Health Stat 2: 1–71. [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, et al. (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19: 335–351. DOI: . [DOI] [PubMed] [Google Scholar]
- Kim SY, Anderson IB, Dyer JE, et al. (2007). High-risk behaviors and hospitalizations among gamma hydroxybutyrate (GHB) users. Am J Drug Alcohol Abuse 33: 429–438. DOI: 10.1080/00952990701312316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S (2021) Ketamine—50 years in use: From anasthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol Rep 73: 323–345. DOI: 10.1007/s43440-021-00232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg RH (2002) Ketamine as an analgesic: parenteral, oral, rectal, subcutaneous, transdermal and intranasal administration. J Pain Palliat Care Pharmacother 16: 27–35. DOI: 10.1080/j354v16n03_03. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Bloom JJ and Shin C (2010) Longitudinal trajectories of ketamine use among young injection drug users. Intl J Drug Policy 21: 306–314. DOI: 10.1016/j.drugpo.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L and Vlisides PE (2016) Ketamine: 50 years of modulating the mind. Front Hum Neurosci 10: 612. DOI: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matłoka M, Janowska S, Gajos-Draus A, et al. (2022). Esketamine inhaled as dry powder: Pharmacokinetic, pharmacodynamic and safety assessment in a preclinical study. Pulm Pharmacol Ther 73–74: 102127. DOI: 10.1016/j.pupt.2022.102127. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV and Independent Scientific Committee on Drugs (2012) Ketamine use: A review. Addiction 107: 27–38. DOI: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Muetzelfeldt M, et al. (2010) Harms associated with psychoactive substances: Findings of the UK national drug survey. J Psychopharmacol 24: 147–153. DOI: 10.1177/0269881109106915. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2020) Joinpoint regression program (version 4.8.0.1).
- Ni A, Cantrell FL and Clark RF (2018) Ketamine exposure demographics and outcomes over 16 years as reported to US poison centers. Am J Emerg Med 36: 1459–1462. DOI: 10.1016/j.ajem.2018.04.066. [DOI] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, et al. (2007) Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369: 1047–1053. DOI: 10.1016/s0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA and Phillips LD (2010) Drug harms in the UK: A multicriteria decision analysis. Lancet 376: 1558–1565. DOI: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Acosta P, Le A, et al. (2019) Adverse drug-related effects among electronic dance music party attendees. Int J Drug Policy 73: 81–87. DOI: 10.1016/j.drugpo.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ and Keyes KM (2020) Trends in drug use among electronic dance music party attendees in New York City, 2016–2019. Drug Alcohol Depend 209: 107889. DOI: 10.1016/j.drugalcdep.2020.107889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Rutherford C and Keyes KM (2021) Trends in ketamine use, exposures, and seizures in the United States. Am J Public Health, e1–e4. DOI: 10.2105/AJPH.2021.306486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Rutherford C, et al. (2021). Extensive underreported exposure to ketamine among electronic dance music party attendees. J Gen Intern Med 36: 235–237. DOI: 10.1007/s11606-020-05672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Su MK and Hoffman RS (2016) Characteristics of Novel psychoactive substance exposures reported to New York City poison center, 2011–2014. Am J Drug Alcohol Abuse 42: 39–47. DOI: 10.3109/00952990.2015.1106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (1987) Multiple imputation for nonresponse in surveys. New York: Wiley & Sons. [Google Scholar]
- United States Food & Drug Administration (2019) FDA approves new nasal spray medication for treatment-resistant depression. Available only at a certified doctor’s office or clinic. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (accessed 5 March 2019).
- United States Food & Drug Administration (2022) FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray. Available at: https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-professionals-potential-risks-associated-compounded-ketamine-nasal-spray (accessed 16 February 2022).
- Van Amsterdam J, Brunt TM, McMaster MT, et al. (2012) Possible long-term effects of γ-hydroxybutyric acid (GHB) due to neurotoxicity and overdose. Neurosci Biobehav Rev 36: 1217–1227. DOI: 10.1016/j.neubiorev.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J and Van Den Brink W (2022) Harm related to recreational ketamine use and its relevance for the clinical use of ketamine: A systematic review and comparison study. Expert Opin Drug Saf 21: 83–94. DOI: 10.1080/14740338.2021.1949454. [DOI] [PubMed] [Google Scholar]
- Warrick BJ, Hill M, Hekman K, et al. (2013) A 9-state analysis of designer stimulant, “bath salt,” hospital visits reported to poison control centers. Ann Emerg Med 62: 244–251. DOI: 10.1016/j.annemergmed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Wolff K and Winstock AR (2006) Ketamine: From medicine to misuse. CNS Drugs 20: 199–218. DOI: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- Zosel A, Bartelson BB, Bailey E, et al. (2013) Characterization of adolescent prescription drug abuse and misuse using the researched abuse diversion and addiction-related surveillance (RADARS(®)) system. J Am Acad Child Adolesc Psychiatry 52: 196.e2–204.e2. DOI: 10.1016/j.jaac.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


