Abstract
INTRODUCTION:
Vitamin D purportedly protects against cognitive decline and dementia based on observational data using circulating 25-hydroxyvitamin D3 (25(OH)D3). Little is known about vitamin D in the human brain and the association with dementia or neuropathology.
METHODS:
Decedents of the Rush Memory and Aging Project (n=290) had vitamin D concentrations measured in four brain regions. Associations with cognitive and neuropathological outcomes were estimated using linear and logistic regression.
RESULTS:
The main form of vitamin D in all brain regions was 25-hydroxyvitamin D3 (25(OH)D3). Higher brain 25(OH)D3 concentrations were associated with a 25% to 33% lower odds of dementia or mild cognitive impairment at the last visit before death (all p≤0.031). However, brain 25(OH)D3 concentrations were not associated with any post-mortem neuropathology outcome studied.
DISCUSSION:
Higher brain 25(OH)D3 concentrations were associated with better cognitive function prior to death. Additional research is needed to clarify the specific mechanisms underlying this potential protective relationship.
Keywords: nutrition, vitamin D, aging, cognitive decline, dementia, neuropathology, Alzheimer’s disease
INTRODUCTION:
By 2050, the global dementia prevalence is projected to exceed 150 million 1, representing a six-fold increase from 2019. Hence, there is an urgent need for preventive strategies to reduce the burden of AD and dementia as the population ages. Evidence is accumulating that nutritional strategies play a key role in delaying or preventing the onset of cognitive decline and dementia, either through directly affecting neuropathology or by fostering resilience to pathology 2. One nutritional factor that has received considerable attention is vitamin D, an essential fat-soluble vitamin and pro-hormone acquired through the diet and sun exposure.
The 1α-hydroxylase enzyme (CYP27B1) is required to convert 25-hydroxyvitamin D (25(OH)D), the main circulating form of vitamin D, to the biologically active 1,25-dihydroxyvitamin D (1,25(OH)2D), the form that binds to the nuclear vitamin D receptor (VDR) to exert its biological function 3,4. The resultant vitamin D signaling in the brain is purportedly involved in neurodegeneration 5,6. Several 7–11, but not all 12,13, epidemiological studies have associated low vitamin D intake or circulating 25(OH)D3 levels with cognitive decline and dementia. Whether low vitamin D represents an independent risk factor for cognitive decline and dementia is controversial 14. Several randomized controlled trials have tested the effect of vitamin D supplementation on cognitive performance 15–19, some with reported null findings 15–17. However, the limitations of these trials include not being designed to study cognitive decline as a primary outcome 16,17 and/or studying participants not necessarily at risk for cognitive decline or vitamin D insufficiency 15–17. The observation of change in cognitive performance has often been limited to a period of less than 3 years. Important outstanding questions remain including: (1) are brain levels of vitamin D metabolites associated with cognitive decline or underlying neuropathologies; and (2) do circulating 25(OH)D levels reflect the vitamin D in the human brain? The purpose of this study was to analyze human brain concentrations of vitamin D and related metabolites and determine the associations with antemortem measures of cognitive function and postmortem neuropathologic outcomes in participants of the well-characterized Rush Memory and Aging Project.
In circulation nearly 99% of 25(OH)D is bound, mostly to vitamin D binding protein (DBP) or, to a lesser extent, albumin. A small amount (~1%) is unbound (free). Because free 25(OH)D is more readily taken up by some tissues, it has been suggested free 25(OH)D levels may be more relevant to some health outcomes 20, including AD and dementia 21. Therefore, to identify optimal circulating biomarkers of vitamin D status in the brain, the associations of antemortem circulating total 25(OH)D, free 25(OH)D, and DBP concentrations with antemortem cognitive status and with postmortem neuropathology outcomes were also evaluated.
METHODS
Participants:
Ante- and postmortem measures were conducted in Rush Memory and Aging Project (MAP) participants. MAP is an ongoing community-based longitudinal study designed to identify risk factors for Alzheimer’s Disease (AD) and Related Disorders and cognitive decline 22. At enrollment, MAP participants are free of known dementia and agree to participate in detailed clinical evaluations annually and organ donation upon death. All participants signed an informed consent and Anatomic Gift Act. The Institutional Review Boards of Rush University Medical Center and Tufts University approved this study. Vitamin D concentrations were measured in brain tissue samples obtained from 499 MAP decedents who died between 2005 and 2019. We previously reported that prolonged freezer storage time reduced brain vitamin D concentrations 23 so we excluded decedents whose brains were stored more than 6 years (n=207) or missing data on APOE genotype (n=2), leaving 290 decedents available for statistical analysis (Supplemental Figure 1). Plasma total 25(OH)D3, free 25(OH)D3, and DBP were measured in 270 of these participants.
Vitamin D Measurements:
Brain:
Vitamin D3, 25(OH)D3 and 1,25(OH)D3 were measured in 4 brain regions [midtemporal cortex (MT), midfrontal cortex (MF), cerebellum (CR), and anterior watershed white matter (AWS)] as previously described 24. The lower limits of detection (LOD) for this assay are as follows: vitamin D3 0.06 pmol/g; 25(OH)D3 0.1 pmol/g, and 1,25(OH)2D3 0.06 pmol/g.
Blood:
Total plasma 25(OH)D3 was measured using LC-MS/MS (Waters Acquity UPLC with TQD triple quadrupole mass spectrometer; CV: 6%) and NIST-traceable standards for assay calibration at Tufts Medical Center. Free 25(OH)D3 was measured by Heartland Assays, LLC directly by immunoassay (Future Diagnostics, Wijchen, Netherlands) with an inter-assay CV of 5.6-6.9%. DBP was measured by a polyclonal ELISA (GenWay Biotech) at Heartland assays. Circulating measures of vitamin D were sampled an average of 2.1 (SD=1.6) and 3.4 (SD=1.9) years before the last assessment of global cognitive function and death, respectively.
Outcome Measurements:
Cognitive Function:
As described elsewhere, MAP participants are enrolled without known dementia and followed annually 25,26. At each visit, global cognitive function was determined using scores from a battery of 19 cognitive tests that evaluated the following cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and perceptual orientation 27. The person-specific rate of change in the global cognition variable over time was previously determined to estimate individual trajectories of cognitive decline, as described 28. Annual diagnosis was assessed using computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and ultimately by diagnostic classification by a clinician and classified as dementia, mild cognitive impairment (MCI) or no cognitive impairment (NCI) 29. At the time of death and blinded to the results of autopsy, a final cognitive diagnosis was made based on all available clinical data reviewed by a neurologist with expertise in dementia, and classified as dementia, MCI, or NCI 29,30.
Neuropathologic Evaluation:
After death, brains were removed and dissected during rapid autopsy using following established protocols as described 31 and evaluated histologically by examiners blinded to clinical information. The median (interquartile range) post-mortem interval was 7.4 (2.8) hours. A quantitative summary of global AD pathology was derived from counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles in the Bielschowski stained sections of the midfrontal cortex, midtemporal cortex, inferior parietal cortex, entorhinal cortex, and hippocampus 26. Braak stages were based upon the distribution and severity of neurofibrillary tangle pathology 31. CERAD scores were based on neuritic plaques 31. The Braak stages for neurofibrillary pathology and the CERAD estimate of neuritic plaques was used to derive the NIA–Reagan diagnosis of AD 31. The percent area occupied by amyloid β protein in eight cortical regions was identified by molecular-specific immunohistochemistry and calculated as described 26. Neuronal PHF-tau tangle density and burden were identified by immunohistochemistry in eight regions and quantified as described 26. The age, volume (in mm3), side, and location of macroscopic and microscopic cerebral infarctions were identified as described 32,33. Lewy bodies were identified using immunohistochemistry 34.
Covariates:
At the baseline evaluation, date of birth, sex, and years of education were assessed by self-report. APOE genotype was evaluated as described 35, given the association between APOE genotype and dementia risk.
Statistical Analyses:
Linear and logistic regressions were used to estimate associations of brain 25(OH)D3 and plasma total 25(OH)D3, free 25(OH)D3 and DBP concentrations with continuous and categorical cognitive and neuropathological outcomes. Statistical analyses of the brain regions focused on 25(OH)D3 because 25(OH)D3 was main form of vitamin D in all human brain regions evaluated and was detected in all participants’ brains. Vitamin D3 and 1,25(OH)2D3 were also detected but were below the assay LOD in 22% and 58% of participants respectively 23. Clinical cognitive diagnosis and final cognitive diagnosis were analyzed with ordinal logistic regression using dementia, MCI, and NCI categories. Participants who had MCI or AD diagnosis with another condition contributing to cognitive impairment were included in the MCI and AD groups, respectively; participants with other primary cause of dementia were excluded (9 in clinical cognitive diagnosis and 3 in final cognitive diagnosis). Ordinal logistic regression with proportional odds was used for ordered categories, as we saw no evidence for non-proportional odds. Global cognitive function and person-specific rate of change in global cognitive function (slope of global cognition) were analyzed as continuous outcomes. Domain-specific cognitive function and rate of change in domain-specific cognitive function were analyzed as exploratory outcomes using a parallel approach. AD neuropathology was considered as present or absent based on NIA-Reagan criteria and CERAD scores 31,36. Braak stage was categorized as ≤ III or ≥ IV. Lewy Body disease and infarcts were considered present or absent 32. Global pathology, amyloid burden, diffuse and neuritic plaques, neurofibrillary tangle density and burden were analyzed as continuous outcomes. Appropriate variable transformations were applied to continuous neuropathology outcomes as indicated by Box-Cox transformations and visual inspection of residuals. The 25(OH)D3 concentrations in the midtemporal and midfrontal cortexes were averaged (since the 25(OH)D3 concentrations in these two cortical regions was similar 23) and the anterior watershed and cerebellum were analyzed as separate regions. Covariates included age at death, sex, education (3 levels; ≤12 years, 12–16 years, >16 years), presence of APOE4 allele (2 levels; E4 present or E4 not present), and season of death (for brain analyses) or season of blood draw (for blood measures analyses). When applicable, models were adjusted for post-mortem interval and/or time between last clinic visit and death. A log2 transformation was used for brain 25(OH)D3 to satisfy linearity assumptions. Estimated associations are reported as beta coefficients or odds ratios, OR=exp(β). The 25(OH)D3 concentrations between brain regions were compared using repeated measures ANOVA to model correlation on within-subject measures. Spearman rank coefficients were reported for pairwise correlations among brain regions and between brain and circulating vitamin D levels. Analyses were performed in R v 4.0 (R Core Team, 2020) and the VGAM package 37. An alpha level of 0.05 was used to determine statistical significance.
RESULTS
Participants were, on average, 92±6 years old at the time of death. Seventy-seven percent (77%) were female and 72% had at least 12 years of education Overall, the mean rate of decline in global cognitive scores before death was −0.007 standard units per year and 40% of participants had diagnosed dementia at their last clinic visit (Table 1).
Table 1:
Participant characteristics (n=290) *
| Age at death, mean (SD) years | 92 (6) |
|
| |
| Female, n (%) | 223 (77%) |
|
| |
| Education, n (%) ≤12 years | 81 (28%) |
| 12–16 years | 141 (49%) |
| >16 years | 68 (23%) |
|
| |
| APOE4 allele, n (%) 1 or more | 66 (23%) |
| no alleles | 224 (77%) |
|
| |
| Season at death Spring | 73 (25%) |
| Summer | 66 (23%) |
| Fall | 77 (27%) |
| Winter | 74 (26%) |
|
| |
| Season of blood draw Spring | 57 (21%) |
| Summer | 78 (29%) |
| Fall | 88 (33%) |
| Winter | 47 (17%) |
|
| |
| Post-mortem interval, hours (median (IQR)) | 7.4 (2.8) |
|
| |
| Brain 25(OH)D3 mean (SD) pmol/g † | |
| Midfrontal and midtemporal cortex ‡ | 1.2 (0.8) |
| Anterior watershed | 1.0 (0.8) |
| Cerebellum | 1.2 (0.9) |
|
| |
| Plasma total 25(OH)D3, mean (SD) ng/ml † | 35.2 (16.4) |
|
| |
| Plasma free 25(OH)D3, mean (SD) pg/ml † | 8.6 (4.4) |
|
| |
| Plasma Vitamin D binding protein, mean (SD) ug/mL † | 283.6 (73.0) |
|
| |
| Global cognitive function score (last visit), mean (SD) | −0.96 (1.13) |
|
| |
| Slope of global cognition, mean (SD) | −0.007 (0.090) |
|
| |
| Clinical diagnosis at last clinic visit, n (%) Dementia | 113 (40%) |
| MCI | 68 (24%) |
| NCI | 100 (36%) |
|
| |
| Final cognitive diagnosis, n (%) Dementia | 119 (41%) |
| MCI | 68 (24%) |
| NCI | 100 (35%) |
|
| |
| Global AD pathology, mean (SD) | 0.79 (0.61) |
|
| |
| Braak stage, n (%) IV-VI | 195 (67%) |
| 0-III | 95 (33%) |
|
| |
| CERAD neuritic plaque score, n(%) moderate-frequent | 212 (73%) |
| none or sparse | 78 (27%) |
|
| |
| NIA-Reagan diagnosis, n(%) AD | 202 (70%) |
| No AD | 88 (30%) |
|
| |
| Amyloid-beta, mean (SD) % area | 4.27 (3.71) |
|
| |
| Gross chronic cerebral infarcts, n(%) 1 or more | 113 (39%) |
| none | 177 (61%) |
|
| |
| Chronic microinfarcts, n(%) 1 or more | 110 (38%) |
| none | 180 (62%) |
|
| |
| Lewy body disease, n(%) Present | 76 (27%) |
| Absent | 208 (73%) |
|
| |
| Diffuse plaques, mean (SD) | 0.74 (0.70) |
|
| |
| Neuritic plaques, mean (SD) | 0.93 (0.83) |
|
| |
| Neurofibrillary tangle burden, mean (SD) | 0.70 (0.77) |
|
| |
| PHF-tau tangle density, mean (SD) count per mm2 | 8.95 (9.40) |
Season of blood draw, plasma total 25(OH)D3, free 25(OH)D3, vitamin D binding protein n=270; global cognitive function n=289; slope of global cognition n=286; clinical diagnosis at last clinic visit n=281; final cognitive diagnosis n=287; Lewy body disease n=284.
Geometric mean reported
Geometric mean of the mean across the midfrontal and midtemporal cortical regions
While variable within regions, brain 25(OH)D3 concentrations were correlated across the four regions (intra-class correlation coefficient =0.87) (Figure 1). Plasma total 25(OH)D3 and free 25(OH)D3 were correlated with the brain 25(OH)D3 in the 4 regions measured (r= 0.32–0.39, p≤0.0001). Plasma total 25(OH)D3 and free 25(OH)D3 were correlated with one another (r=0.73, p≤0.0001), but were not correlated with DBP concentrations (r=0.08–0.10, p≥0.09).
Figure 1:
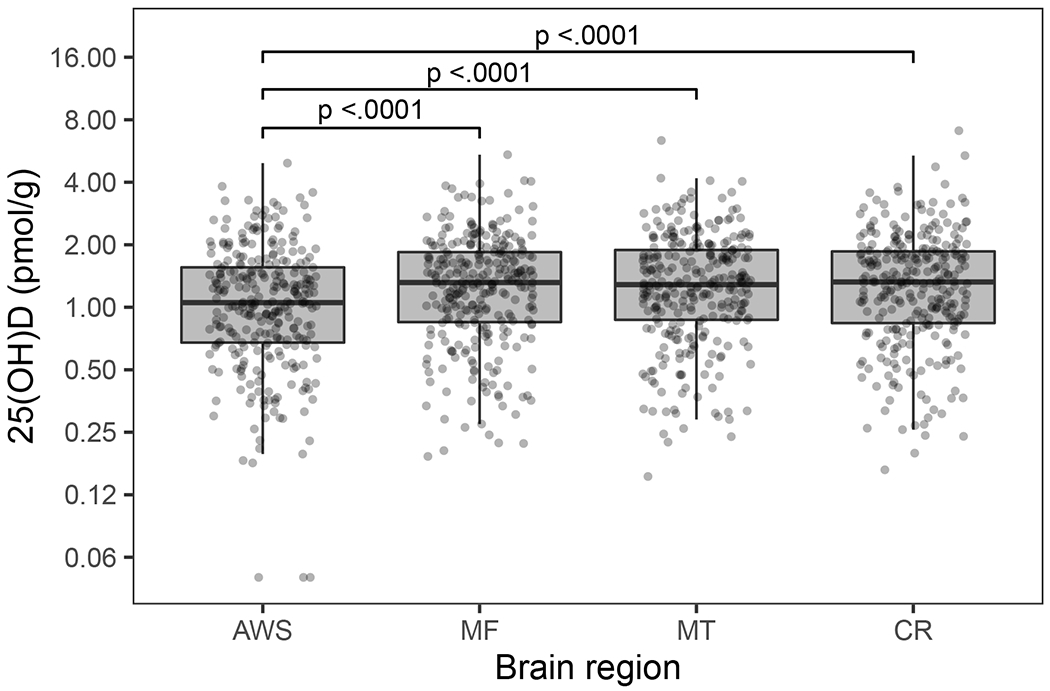
Boxplots of 25(OH)D3 concentrations in 4 human brain regions (AWS anterior watershed, MF midfrontal cortex, MT midtemporal cortex, CR cerebellum) (n=290). Boxplot indicates the median (middle line of the box), first quartile (lower boundary of the box), and third quartile (upper boundary of the box) of 25(OH)D3 concentrations in each brain region. Brackets indicate post-hoc pairwise comparison tests with Tukey adjustment that have significant p values.
The odds of having dementia or MCI at the last cognitive assessment before death were 25% to 33% lower per doubling of 25(OH)D3 in the four brain regions measured (Table 2) (ORs 0.669 to 0.754 all p≤0.031.) These odds were generally consistent with the odds for dementia or MCI at the final cognitive diagnosis. Higher brain 25(OH)D3 concentrations in all regions were also associated with better ante mortem global cognitive function scores (all p≤0.025), and in the AWS, was also associated with a slower rate of cognitive decline (p=0.044) (Table 2). Higher concentrations of 25(OH)D3 in all regions measured were associated with better semantic and working memory and higher 25(OH)D3 concentrations in the AWS were additionally associated with better episodic memory and perceptual speed (Supplemental Table 1). Brain 25(OH)D3 concentrations were not associated with any neuropathology outcome evaluated (Table 3).
Table 2:
Associations of brain 25(OH)D3 concentrations with cognitive function
| MT & MF cortex | AWS | CR | ||||
|---|---|---|---|---|---|---|
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Cognitive diagnosis at last clinic visit before death (n=281) | −0.288 (0.133) | 0.031 | −0.402 (0.124) | 0.001 | −0.282 (0.127) | 0.027 |
|
| ||||||
| Final cognitive diagnosis (n=287) | −0.234 (0.133) | 0.080 | −0.354 (0.124) | 0.004 | −0.236 (0.127) | 0.064 |
|
| ||||||
| Global cognitive function (n=289) | 0.165 (0.073) | 0.025 | 0.198 (0.065) | 0.002 | 0.159 (0.070) | 0.025 |
|
| ||||||
| Slope of cognitive function (n=286) | 0.008 (0.006) | 0.176 | 0.010 (0.005) | 0.044 | 0.009 (0.006) | 0.124 |
Regression coefficients are reported from ordinal logistic regression for cognitive diagnosis outcomes (categorized as AD, MCI, or NCI) and multiple linear regression (for global cognitive function and slope of cognitive function). Brain 25(OH)D3 are expressed as log2. Models are adjusted for age at death, sex, education, presence of APOE4 allele, and season of death. Time between last clinic visit and death is additionally adjusted in model of global cognitive function and cognitive diagnosis at last clinic visit before death. Regression coefficients from ordinal logistic regression indicate cumulative log odds of AD. Boldface indicates p<0.05.
Abbreviations: MT midtemporal cortex, MF midfrontal cortex, AWS anterior watershed, CR cerebellum,
Table 3:
Associations of brain 25(OH)D3 concentrations with brain pathology (n=290)
| MT & MF cortex | AWS | CR | ||||
|---|---|---|---|---|---|---|
| β (SE) | p-value | β (SE) | p-value | β (SE) | p-value | |
| Dichotomous outcomes: | ||||||
|
| ||||||
| Braak ≥ IV | −0.178 (0.155) | 0.25 | −0.149 (0.142) | 0.29 | −0.184 (0.150) | 0.22 |
|
| ||||||
| CERAD neuritic plaque score | −0.115 (0.162) | 0.48 | −0.118 (0.148) | 0.42 | −0.168 (0.158) | 0.29 |
|
| ||||||
| AD based on NIA Reagan | −0.150 (0.160) | 0.35 | −0.153 (0.147) | 0.30 | −0.189 (0.155) | 0.22 |
|
| ||||||
| Presence of gross chronic infarcts | −0128 (0.141) | 0.37 | −0.233 (0.125) | 0.06 | −0.188 (0.136) | 0.17 |
|
| ||||||
| Presence of chronic microinfarcts | −0.099 (0.142) | 0.48 | −0.146 (0.126) | 0.25 | −0.117 (0.137) | 0.39 |
|
| ||||||
| Presence of Lewy body disease * | −0.146 (0.158) | 0.36 | −0.108 (0.141) | 0.44 | −0.162 (0.151) | 0.28 |
|
| ||||||
| Continuous outcomes: | ||||||
|
| ||||||
| Global AD pathology | −0.030 (0.024) | 0.21 | −0.018 (0.021) | 0.41 | −0.037 (0.023) | 0.12 |
|
| ||||||
| Amyloid-beta | −0.075 (0.068) | 0.27 | −0.050 (0.060) | 0.40 | −0.085 (0.065) | 0.19 |
|
| ||||||
| Diffuse plaques | −0.029 (0.031) | 0.35 | −0.005 (0.027) | 0.86 | −0.036 (0.029) | 0.21 |
|
| ||||||
| Neuritic plaques | −0.053 (0.033) | 0.11 | −0.034 (0.029) | 0.25 | −0.057 (0.032) | 0.07 |
|
| ||||||
| PHF-tau Tangle density | −0.034 (0.029) | 0.25 | −0.033 (0.026) | 0.21 | −0.045 (0.028) | 0.11 |
|
| ||||||
| Neurofibrillary tangle burden | −0.020 (0.016) | 0.24 | −0.019 (0.014) | 0.18 | −0.024 (0.016) | 0.13 |
Regression coefficients are reported from multiple logistic regression (for dichotomous outcomes) and multiple linear regression (for continuous outcomes). Continuous outcomes are square root transformed, except for tangle density and neurofibrillary tangle burden which are quartic root transformed. Brain 25(OH)D3 are expressed as log2. Models are adjusted for age at death, sex, education, presence of APOE4 allele, season of death, and post-mortem interval.
Abbreviations: MT midtemporal cortex, MF midfrontal cortex, AWS anterior watershed, CR cerebellum, AD Alzheimer’s dementia
n=284 for analysis of Lewy Body due to missing values
Plasma 25(OH)D3, free 25(OH)D3 and DBP concentrations were not significantly associated with global cognitive function or global cognitive decline (all p>0.06) (Supplemental Table 2). Higher DBP concentrations were associated with less decline in semantic memory and perceptual orientation (Supplemental Table 2). Otherwise there were no significant associations between any plasma measure and domain-specific cognitive function. Among the post-mortem neuropathologically-defined outcomes, higher plasma total 25(OH)D3 was associated with fewer chronic microinfarcts (β=0.025 (0.009), p=0.004). Otherwise, the associations of plasma total and free 25(OH)D3 and DBP concentrations with the neuropathologically-defined outcomes evaluated were not statistically significant (all p≥0.12).
DISCUSSION
In this study of older community-dwelling adults, higher brain 25(OH)D3 concentrations were associated with better global cognitive function prior to death but were not associated with any post-mortem neuropathology outcome studied. The 25(OH)D3 was the predominate form of vitamin D in human brain 23. To the best of our knowledge, this is the first study to quantify vitamin D in human brain tissue and evaluate the associations with cognitive and neuropathological outcomes. That brain 25(OH)D3 was associated with global cognitive status but not with any neuropathology may indicate the 25(OH)D3 in the brain is relevant to neuropathologies not studied here. For example, circulating vitamin D has been associated with white matter hyperintensities 38 and regional brain volumes 39, both of which have been linked to cognitive impairment 40,41. Alternatively, it is plausible that brain 25(OH)D3 concentrations may be an indicator of cognitive resilience, such that individuals with higher levels may display fewer signs of cognitive impairment despite a high neuropathological burden 42. It is also possible 25(OH)D3 may be involved in cognition through pathologies not studied here. Results of our analyses suggest brain 25(OH)D3 concentrations may be more relevant to semantic and working memory. However, given the exploratory nature of these results, it is premature to infer any domain-specific role of 25(OH)D3 in cognitive health.
In this study, plasma total 25(OH)D3 concentrations were moderately correlated with the brain 25(OH)D3 concentrations, but not with cognitive status or cognitive decline. A recent meta-analysis reported that only vitamin D insufficiency (defined as circulating 25(OH)D3 10–19 ng/ml) and deficiency (defined as circulating 25(OH)D <10 ng/ml) were associated with a significantly higher risk for AD and dementia 10. The mean±SD plasma 25(OH)D3 in our sample was 35.2±16.4 ng/ml, which is >10ng/ml above the threshold considered sufficient by the Institute of Medicine (20 ng/ml), and only 12% of our participants had plasma 25(OH)D below this threshold 43, which may have blunted our ability to detect associations. A similar criticism has been made of randomized clinical trials in which the study participants were vitamin D sufficient at the onset of the trial, hence vitamin D supplementation would have little benefit on outcomes, such as cognitive performance 16. Alternatively, circulating 25(OH)D3 may not be a robust biomarker of vitamin D status in the brain, which presents a conundrum that could challenge the utility of basing vitamin D recommendations for cognitive outcomes on circulating 25(OH)D. Of note, we did detect an inverse association between plasma total 25(OH)D3 and chronic microinfarcts. The association with microinfarcts is not strong enough to be maintained if corrections for multiple testing were employed, but we consider this observation to be hypothesis-generating since we did not observe the same associations with brain 25(OH)D3 concentrations.
The majority of circulating 25(OH)D3 is bound to DBP for transport to target tissues. In vivo and in vitro experiments have implicated DBP in reducing amyloid-β aggregation and neuronal cell death 44. In a case-control study of adults ≥60 year old, circulating DBP concentrations were reported to be higher in those with AD than in cognitively normal controls 21. In an analysis of ~2000 older adults (mean age 73 yrs; 920 with AD, 277 with MCI, and 819 controls), higher circulating DBP concentrations were associated with poorer cognitive performance 45. In contrast, plasma DBP concentrations were not significantly associated with global cognitive function or any neuropathology outcome in our study. Given the overall paucity of studies in this area, the involvement of DBP in AD, dementia and cognitive function remains to be determined.
A novel finding in our study was that brain 25(OH)D3 concentrations were also moderately correlated with plasma free 25(OH)D3. However, free 25(OH)D3, while thought to be more readily available to some tissues, including brain 46, was not significantly associated with cognitive function or with any neuropathology outcome evaluated. The results of our study, in which plasma free 25(OH)D3 was measured directly, are in discordance with the only known study to link free circulating 25(OH)D to cognitive status 21. The latter study used the less precise approach of calculating free 25(OH)D3 47 to compare concentrations between adults ≥60 years old diagnosed with AD and those with normal cognitive function 21. It is now known that megalin, which is integral for cellular uptake of bound vitamin D, is expressed in neuronal tissues and has been implicated in neurodegenerative processes 48,49, so the importance of free 25(OH)D3 specifically to brain health remains to be clarified.
This study’s findings should be interpreted in context of its strengths and limitations. The unique application of antemortem biomarker and cognition measures combined with postmortem brain and neuropathologically-defined measures that were obtained from a well-characterized community-based cohort is a notable strength. We had previously conducted a sensitivity analysis that revealed freezer storage time >6 years lowered 25(OH)D3 concentrations across all four brain regions measured 23, so we selected decedents whose brains were stored ≤6 years to enhance confidence that the measures reflected the 25(OH)D3 concentrations at time of death. However, this reduced our statistical power, which may account for detecting some associations that approached, but did not reach, statistical significance. The 1,25(OH)2D3 was below the assay LLD in over half of the brains analyzed, which limited our ability to evaluate the associations of the biologically active form of vitamin D with cognitive or neuropathological outcomes. Most decedents in this study were white, non-Hispanic, so generalizability to other race-ethnic groups is uncertain. Given the observational design, causation cannot be proven. There is the potential for residual confounding, as well as for reverse causation, although time ordering of ante-mortem exposures mitigates this limitation for some of our analyses.
In conclusion, the results of this study suggest vitamin D in the brain may be involved in cognitive decline. However, given the study’s results and acknowledged limitations, steps for future research include: (1) Developing and testing a priori hypotheses about potential domain-specific roles of brain 25(OH)D3 in cognitive health in cohorts with robust measures of performance across multiple cognitive domains; (2) Leveraging technology to develop assays with better sensitivity that more precisely quantify 1,25(OH)2D3 in the brain to advance our knowledge of how the active form of vitamin D is involved in human neuropathology; (3) Applying the design and methodologies of the present study to race-ethnically diverse cohorts, such as the Minority Aging Research Study 50, since vitamin D status is known to differ by race and ethnicity 51 and lower vitamin D intake has been associated with a slower rate of cognitive decline in older black adults 52; and (4) Expanding the analyses of brain structure outcomes, for example using ex-vivo neuroimaging to determine if vitamin D is involved in changes in brain tissue integrity not studied here and/or whether it is an indicator of cognitive resilience.
Supplementary Material
Systematic Review:
Observational studies report higher circulating 25-hydroxyvitamin D (25(OH)D) concentrations were associated with better cognitive function in older adults. Little is known about vitamin D in the human brain. The goal of this study was to analyze postmortem human brain concentrations of vitamin D and related metabolites and determine their association with cognitive function. We also evaluated the association of brain vitamin D concentrations with dementia-related neuropathologies.
Interpretation:
In this study of Rush Memory and Aging Project participants, higher post-mortem brain concentrations of the vitamin D metabolite, 25(OH)D, were associated with better cognitive function prior to death. However, brain 25(OH)D concentrations were not significantly associated with any dementia-related neuropathology outcome studied.
Future Directions:
Additional research is needed to clarify the mechanisms by which 25(OH)D has a cognitive protective effect.
Author Disclosures:
MKS, KB, BDH, XF, SLB are supported by NIH grants (to Tufts University) and a USDA Cooperative Agreement (with Tufts University). SEL is supported by NIH grants (to Rush University) and serves on data safety monitoring and advisory boards for NIH funded studies. BDJ is supported by NIH grants (to Rush University), serves on a data safety monitoring board for Eisai, is involved in the Alzheimer’s Association Facts & Figures annual report, and stock/stock options in Vivid Genomics. TMH is a member of the U.S. Against Alzheimer’s non-profit risk reduction working group. PA reports support from the Michael J Fox Foundation and the NIA (both to Rush University), the Alzheimer’s Association (to Rush University and self), honoraria from Wake Forest Baptist Medical Center and the Swedish Medical Center Seattle, and is Communications Chair, Nutrition Metabolism Dementia PIA, ISTRAART Alzheimer’s Association; JAS is supported by NIH grants (to Rush University), receives support from Apellis, Alnylam, Eli Lily, for expert legal testimony (for the NHL), serves on Observational Monitoring Boards for the Framingham Heart study and NINDS Discovery at Boston University, External Advisory Board for Alzheimer’s Centers, and involvement in the Alzheimer’s Foundation France. All other co-authors report no disclosures.
Study supported by National Institute on Aging R01AG051641 and R01AG17917 and USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
The authors would like to acknowledge the contribution of the late Dr. Martha Clare Morris in the development of this project.
Reference List
- 1.Global Burden of Disease Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. [DOI] [PubMed] [Google Scholar]
- 3.Landel V, Stephan D, Cui X, Eyles D, Feron F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J Steroid Biochem Mol Biol. 2018;177:129–134. [DOI] [PubMed] [Google Scholar]
- 4.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. [DOI] [PubMed] [Google Scholar]
- 5.Keeney JT, Butterfield DA. Vitamin D deficiency and Alzheimer disease: Common links. Neurobiol Dis. 2015;84:84–98. [DOI] [PubMed] [Google Scholar]
- 6.Gezen-Ak D, Dursun E. Molecular basis of vitamin D action in neurodegeneration: the story of a team perspective. Hormones (Athens). 2019;18(1):17–21. [DOI] [PubMed] [Google Scholar]
- 7.Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beydoun MA, Hossain S, Fanelli-Kuczmarski MT, et al. Vitamin D Status and Intakes and Their Association with Cognitive Trajectory in A Longitudinal Study of Urban Adults. J Clin Endocrinol Metab. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JW, Harvey DJ, Beckett LA, et al. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015;72(11):1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra A, Teixeira AL, Diniz BS. Association of Vitamin D Levels with Incident All-Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-analysis. J Prev Alzheimers Dis. 2020;7(1):14–20. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Tsapanou A, Manly J, Schupf N, Brickman AM, Gu Y. Vitamin D intake is associated with dementia risk in the Washington Heights-Inwood Columbia Aging Project (WHICAP). Alzheimers Dement. 2020;16(10):1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchaine CS, Talbot D, Nafti M, et al. Vitamin D status, cognitive decline and incident dementia: the Canadian Study of Health and Aging. Can J Public Health. 2020;111(3):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson E, Byberg L, Karlstrom B, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr. 2017;105(4):936–943. [DOI] [PubMed] [Google Scholar]
- 14.Bivona G, Lo Sasso B, Gambino CM, et al. The Role of Vitamin D as a Biomarker in Alzheimer’s Disease. Brain Sci. 2021;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owusu JE, Islam S, Katumuluwa SS, et al. Cognition and Vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc. 2019;67(1):81–86. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Vyas CM, Okereke OI, et al. Effect of vitamin D on cognitive decline: results from two ancillary studies of the VITAL randomized trial. Sci Rep. 2021;11(1):23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J Am Geriatr Soc. 2012;60(12):2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T, Wang H, Xiong Y, et al. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J Alzheimers Dis. 2020;78(4):1509–1518. [DOI] [PubMed] [Google Scholar]
- 19.Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Abeta-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(12):1347–1352. [DOI] [PubMed] [Google Scholar]
- 20.Premer C, Schulman IH. Have We Been Measuring the Wrong Form of Vitamin D? Circ Res. 2018;123(8):934–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertilav E, Barcin NE, Ozdem S. Comparison of Serum Free and Bioavailable 25-Hydroxyvitamin D Levels in Alzheimer’s Disease and Healthy Control Patients. Lab Med. 2021;52(3):219–225. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X, Shea MK, Dolnikowski GG, et al. Vitamin D and Vitamin K Concentrations in Human Brain Tissue Are Influenced by Freezer Storage Time: The Memory and Aging Project. J Nutr. 2021;151(1):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Dolnikowski GG, Patterson WB, et al. Determination of Vitamin D and Its Metabolites in Human Brain Using an Ultra-Pressure LC-Tandem Mass Spectra Method. Curr Dev Nutr. 2019;3(7):nzz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett DA, Schneider JA, Buchman AS, Mendes de LC, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. [DOI] [PubMed] [Google Scholar]
- 26.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85(11):984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Jager PL, Shulman JM, Chibnik LB, et al. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging. 2012;33(5):1017 e1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. [DOI] [PubMed] [Google Scholar]
- 31.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. [DOI] [PubMed] [Google Scholar]
- 32.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke. 2005;36(5):954–959. [DOI] [PubMed] [Google Scholar]
- 34.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135(Pt 10):3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oveisgharan S, Buchman AS, Yu L, et al. APOE epsilon2epsilon4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90(24):e2127–e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. [DOI] [PubMed] [Google Scholar]
- 37.Yee TW. The VGAM Package for Categorical Data Analysis. The Journal of Statistical Software. 2010;32(10):1–34. [Google Scholar]
- 38.Schramm S, Schliephake L, Himpfen H, et al. Vitamin D and white matter hyperintensities: results of the population-based Heinz Nixdorf Recall Study and 1000BRAINS. Eur J Neurol. 2021;28(6):1849–1858. [DOI] [PubMed] [Google Scholar]
- 39.Soares JZ, Pettersen R, Benth JS, et al. Vitamin D Levels, APOE Allele, and MRI Volumetry Assessed by NeuroQuant in Norwegian Adults with Cognitive Symptoms. J Alzheimers Dis. 2021;79(1):311–321. [DOI] [PubMed] [Google Scholar]
- 40.Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct. 2016;221(4):2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyle PA, Yu L, Fleischman DA, et al. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3(10):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James BD, Bennett DA. Causes and Patterns of Dementia: An Update in the Era of Redefining Alzheimer’s Disease. Annu Rev Public Health. 2019;40:65–84. [DOI] [PubMed] [Google Scholar]
- 43.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: 2010 2010. [Google Scholar]
- 44.Moon M, Song H, Hong HJ, et al. Vitamin D-binding protein interacts with Abeta and suppresses Abeta-mediated pathology. Cell Death Differ. 2013;20(4):630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishnoi RJ, Palmer RF, Royall DR. Vitamin D binding protein as a serum biomarker of Alzheimer’s disease. J Alzheimers Dis. 2015;43(1):37–45. [DOI] [PubMed] [Google Scholar]
- 46.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144 Pt A:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 48.Alvira-Botero X, Perez-Gonzalez R, Spuch C, et al. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol Cell Neurosci. 2010;45(3):306–315. [DOI] [PubMed] [Google Scholar]
- 49.Dietrich M, Antequera D, Pascual C, Castro N, Bolos M, Carro E. Alzheimer’s disease-like impaired cognition in endothelial-specific megalin-null mice. J Alzheimers Dis. 2014;39(4):711–717. [DOI] [PubMed] [Google Scholar]
- 50.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhana K, Barnes LL, Agarwal P, et al. Vitamin D intake and cognitive decline in Blacks and Whites: The role of diet and supplements. Alzheimers Dement. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


